Abstract
The visual system relies on both the integration and interocular inhibitory processes to achieve single vision from different images in the two eyes. It is generally assumed that the integration process first searches for matching local features between the two eyes. If the matching fails, an interocular inhibitory process is triggered to suppress the image representation of one eye, leading to visual perception that is essentially contributed by the other eye. Here, using a stimulus comprising of binocularly corresponding features (vertical gratings) but incompatible surface border information, we found evidence to the contrary. In one half-image, a circular patch of vertical grating was phase-shifted relative to the surrounding vertical grating to create a circular, monocular boundary contour (MBC), while the other half-image had a similar vertical grating. The two half-images had a binocular disparity at the circular grating patch area, leading to the percept of a disc in depth. Concurrent with the stereo percept, threshold for detecting a Gabor probe on the half-image without the MBC was higher than that on the corresponding area with the grating disc, indicating binocular suppression. These findings reveal that when we perceive depth, which requires the integration process to obtain binocular disparity from the two eyes, one eye's image could simultaneously be suppressed from visual awareness by the interocular inhibitory process. Our study also presents a provocative example of where the brain selectively binds some, but not all, features of the images from the two eyes for visual perception.
Keywords: awareness, binocular fusion, boundary contours, contrast threshold, stereopsis
The lateral separation between our eyes causes 3-D scenes to be seen from slightly different vantage points of view, providing the basis for binocular depth perception (stereopsis). The visual system ensures that the disparate images from the two eyes are experienced as a single, coherent percept predominantly through: (1) the integration process, which analyzes signals from the two eyes to create binocular representations of the images and extracts the images' binocular disparity for depth perception, and (2) the interocular inhibitory process, which suppresses all but one binocular image representation to provide a coherent percept. Much of our knowledge of the interocular inhibitory process comes from studies of binocular rivalry (BR).
The typical BR stimuli have two half-images of the same overall shape but whose simple features differ in orientation (Fig. 1A), motion direction, color, etc. This local difference in simple features prevents the fusion/stereopsis process from integrating the two half-images. If the features of the two half-images are made more similar, the BR between the two half-images is reduced and/or is replaced by the fusion phenomenon (1). Meanwhile, Hochberg found that adding contours (grid lines) to one half-image of a correlated stereogram eliminates the depth percept (2). This suggests the added contours suppress the information of the other half-image. Observations like these lead to the notion that the visual system triggers the interocular inhibitory process to suppress one of the two half-images when the integration process fails to make a match (3, 4). We coin this the fusion-preceding-rivalry hypothesis. Opposing the fusion-preceding-rivalry hypothesis is what we coin the co-existence hypothesis, which claims that the integration and the interocular inhibitory processes operate independently (5–10). Support for the co-existence hypothesis includes the observations that one can perceive binocular depth even as BR is experienced. For instance, using bandpass filtered random-dot stereograms with masking noise of other spatial frequency bands, Julesz and Miller found observers simultaneously experienced stereopsis and BR (11). Others used half-images with dissimilar features (e.g., different colors, opposite contrast, or orthogonal grid lines) to induce BR while having correlated contours with binocular disparity to induce stereopsis (6, 7, 12, 13). Although these studies provide support for the co-existence hypothesis several arguments against them could be made. For example, the depth and BR percepts are probably carried by different spatial frequency channels (11), or separately via the chromatic vs. achromatic channels (7), and these percepts may not occur at the same spatial location (11, 13). Thus, the fusion-preceding-rivalry hypothesis could still prevail. According to this view, that stereopsis and BR occur simultaneously at different spatial locations, or different channels, simply reflects the spatial and modular independence of visual processing.
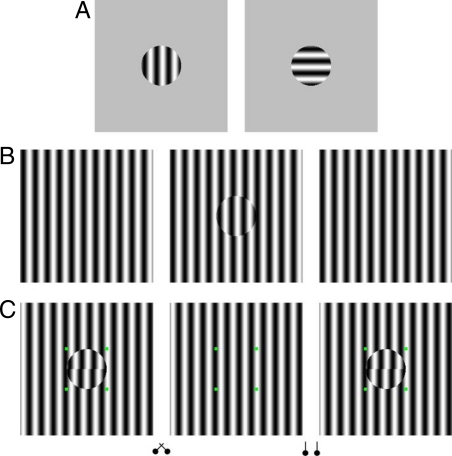
Fig. 1.
(A) Typical BR stimulus. (B) MBC phase-shift stereo/rivalry stimulus. The half-image with the disc grating is phase-shifted 45° relative to the surrounding background grating. With crossed fusion of the left and middle half-images, one perceives the grating within the disc as behind the surrounding grating. (C) The stimulus used in Experiment 1, where observers judged the relative depth between the upper and lower half-discs. With crossed fusion of the left and middle half-images, the grating within the disc is seen behind the surrounding grating (far condition) while the lower half-disc is seen in front of the upper half-disc. And by crossed fusion of the right and middle half-images, the entire disc grating is seen in front of the surrounding grating, with the upper half-disc being seen in front of the lower half-disc.
Our study approaches the issue behind these two hypotheses, namely, how the visual system integrates information from the two eyes to support stereopsis while suppressing incompatible images, from a different perspective. We created a stereo/rivalry stimulus with one half-image having a homogeneous vertical grating (right or left, Fig. 1B) and the other half-image having the same vertical grating but with an additional disc in the center (middle, Fig. 1B). The central disc is defined by a monocular boundary contour (MBC) created by phase shifting (45°) a circular area of the vertical grating. This circular disc area, relative to the homogeneous grating half-image also creates binocular disparity. Thus, with free fusion of the two half-images in Fig. 1B, one perceives a stable depth separation between the central grating disc and the surrounding grating (disc is seen behind with crossed fusion of the left and middle half-images), indicating the working of the integration process. Our first experiment quantified this observation using a simultaneous depth discrimination design. We further inserted a phase-shift between the gratings in the upper and lower half-disc areas and measured observers' ability to discriminate the relative depth of the two half-discs in two conditions (Fig. 1C). In a back depth condition (Fig. 1C, crossed fusers should fuse the left and middle half-images) the grating of the disc is seen behind the surrounding homogeneous grating, while in a front depth condition it is seen in front of the surrounding grating. Notice also (with crossed fusion), that in the back depth condition, the lower half-disc is seen in front of the upper half-disc. In the front depth condition, the upper half-disc is seen in front of the lower half-disc.
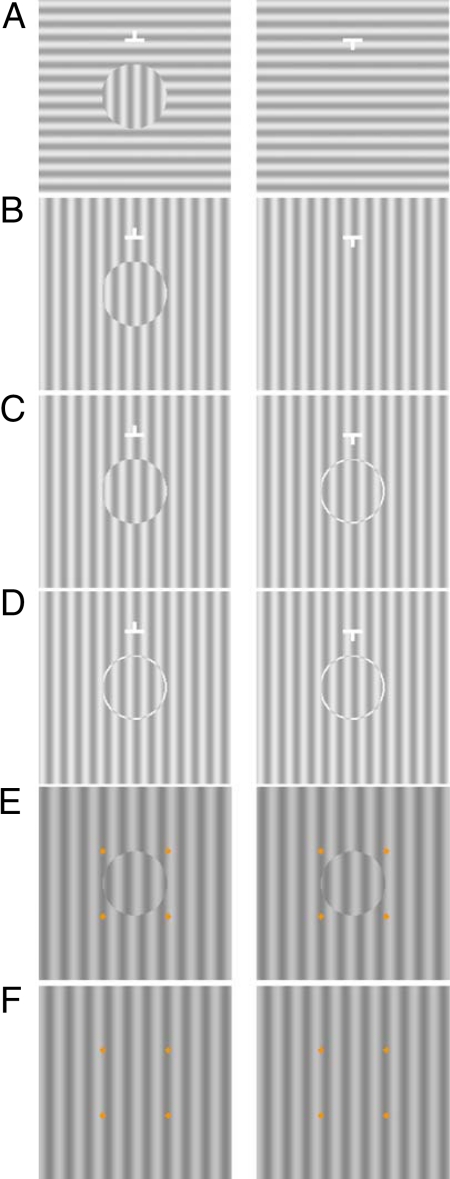
Accompanying the depth percept with the MBC phase-shift stimuli (Fig. 1 B and C) is the observation that the perceived MBC disc is stable, suggesting that the corresponding homogeneous grating is suppressed. This observation agrees with our previous findings showing that the MBC strongly affects BR (14–16). For example, Fig. 2A (MBC from orientation-difference) shows an MBC disc in the left half-image created by an orientation difference between the central and surrounding areas. With free-fusion of the half-images, one perceives a stable vertical disc in front (17). This is unlike the frequent alternating percepts experienced with the typical BR stimulus in Fig. 1A even though the central corresponding areas in both stimuli have orthogonal gratings. Thus, the MBC orientation-difference stimulus suggests the strong dominance of the MBC disc is mainly attributed to the MBC. To reveal the suppression of the homogeneous grating half-image by the MBC, our second experiment measured the contrast increment threshold of a Gabor probe on the MBC phase-shift stimulus (Fig. 2B). The Gabor probe was either added to the center of the left half-image with the MBC disc, or that of the right half-image with the homogeneous grating. We found a higher threshold on the homogeneous grating. To further reveal that it is the MBC that triggers the interocular inhibition, we tested two additional conditions where both half-images have boundary contours. These are the ring/disc condition where we added a ring onto the homogeneous grating half-image (right half-image in Fig. 2C) to correspond to the MBC in the left half-image, and the ring/ring condition where the MBC phase-shift disc was replaced by the same ring as that in the other half-image (Fig. 2D). We found that with such binocular boundary contour (BBC) stimuli, thresholds on the two half-images are similar, that is, binocular suppression is absent without the MBC.
Fig. 2.
(A) MBC stimulus created by orientation difference between the grating disc and surrounding grating. The stimuli used in Experiment 2: (B) MBC phase-shift, (C) ring/disc, (D) ring/ring, (E) binocular disc, and (F) binocular background.
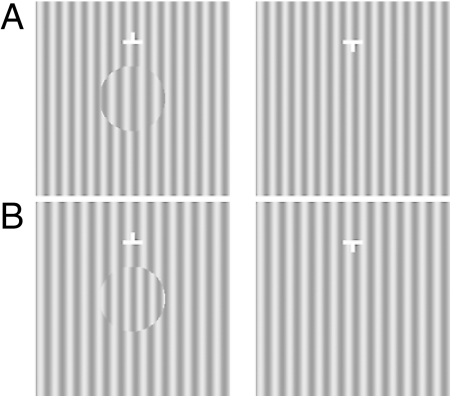
To generalize the notion that MBC alone can induce interocular inhibition, our third experiment measured thresholds on a variant of the above stimulus (Fig. 3 A and B). Here, the MBC and relative binocular disparities are created by a difference in spatial frequency, which render the grating disc to be perceived as tilted in depth (18). We obtained similar threshold results as with the MBC phase-shift stimulus.
Fig. 3.
MBC spatial-frequency-difference stimuli used in Experiment 3. The spatial frequency difference between the disc grating and the corresponding grating in the other half-image [3.5 cpd vs. 3 cpd in (A) and 3 cpd vs. 3.5 cpd in (B)] creates a gradient binocular disparity. With crossed fusion, the MBC disc in stimulus (A) is seen as rotated around the vertical axis with the right side in back. The MBC disc in stimulus (B) is seen as rotated around the vertical axis with the left side in back.
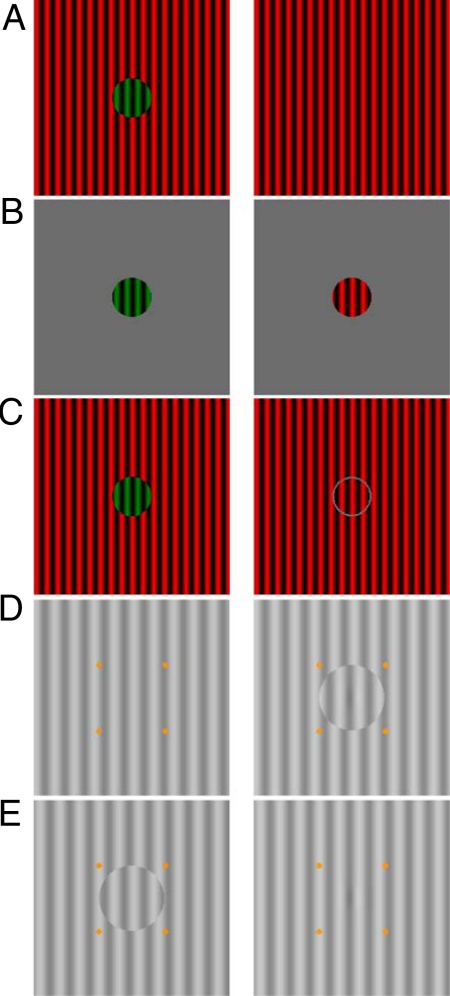
To further demonstrate the co-existence of integration and binocular suppression, our fourth experiment had observers tracked their percepts of a colored MBC phase-shift stimulus (Fig. 4A). With color labeling, we can readily observe the alternating dominance and suppression percepts of the two half-images. From the foregoing, we can predict that the MBC disc remains dominant most of the time while the corresponding homogeneous grating is suppressed. One can confirm this by free fusing Fig. 4A and observing that a stable green disc is seen most of the time. Also, one can verify that the strong green dominance is not due to a stronger perceptual salience of the green color, because a robust BR alternation between the red and green half-images occurs with the typical rivalry (red/green discs) stimulus in Fig. 4B. Similarly, when we tested observers with a BBC (disc/ring) stimulus (Fig. 4C), we found the frequency of BR alternation is almost equal. Finally, to reinforce our finding, we conducted a control experiment where observers tracked a monocular Gabor probe on grayscale MBC phase-shift stimuli (Fig. 4 D and E). We found higher predominance for seeing the Gabor probe on the half-image with the MBC.
Fig. 4.
Stimuli used in Experiment 4. (A) MBC phase-shift stimulus with a green MBC disc. (B) Typical BR stimulus with red/green discs. (C) Similar to (A) except a gray ring is added to the half-image with the homogeneous grating. Control Experiment 2: The MBC phase-shift stimuli in (D, dominance condition) and (E, suppression condition) each has a Gabor probe in the center.
Results
1. Experiment 1: Relative Depth Perception.
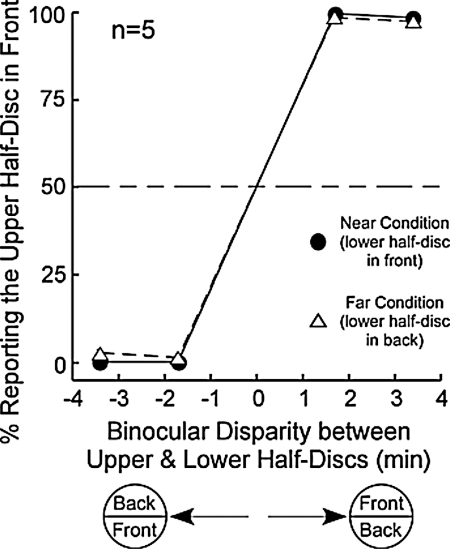
Fig. 5 depicts the average responses in perceiving the relative depth between the upper and lower half-discs in the near (circles) and far conditions (triangles) in Fig. 1C. Clearly, observers correctly perceived the depth of the upper half-disc when it is either nearer (>50%) or farther (<50%) than the lower half-disc. This indicates they ably integrated the MBC phase-shift stimulus for binocular depth perception.
Fig. 5.
Results of Experiment 1.
2. Experiment 2: Contrast Increment Threshold (Phase-Shift) and Control Experiment 1.
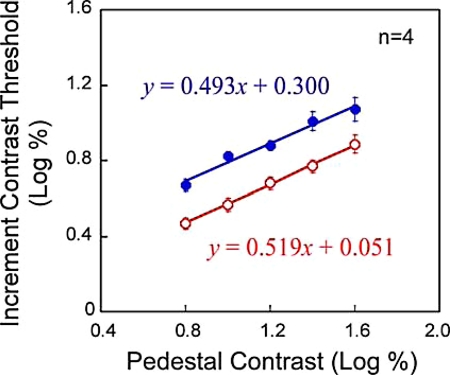
We measured contrast increment thresholds of monocular Gabor probes that were presented on either half-image (that acted as the pedestal for the probe) of the MBC phase-shift stimulus (Fig. 2B). We found contrast thresholds on the homogeneous grating half-image (filled circles, Fig. 6) are significantly higher than those on the MBC disc (open circles) [F(1,3) = 61.7, P < 0.005; two-way ANOVA with repeated measures for the average data]. This confirms that MBC alone, in the absence of locally conflicting simple features, can initiate the interocular inhibitory process to suppress the half-image with the homogeneous grating.
Fig. 6.
Results of Experiment 2.
Our data also reveal that thresholds on both half-images increase with the contrast of the pedestal grating in a similar linear pattern [F(4,12) = 70.1, P < 0.001; F(4,12) = 1.40, P > 0.25]. This suggests that binocular suppression does not affect the contrast gain control mechanism, just like in other types of BR stimuli (19).
The data for the ring/disc condition show that the thresholds on the phase-shifted disc (0.857 ± 0.059 log unit) and on the gray-ring disc (0.865 ± 0.051 log unit) are not significantly different [t(9) = 0.100; P = 0.922, ANOVA contrast analysis]. These thresholds are quite close to the thresholds in the ring/ring condition, which average 0.835 ± 0.065 log unit [t(9) = 0.367; P = 0.722, ANOVA contrast analysis]. Thus both conditions reveal that when the MBC in each half-image corresponds as a pair of BBC interocular inhibition no longer exerts its influence.
Arguably, our findings in Fig. 6 need not necessarily indicate interocular suppression, but rather that probe detection threshold on a figure (MBC disc grating) is lower than that on a large grating background. Presumably, the MBC disc configuration itself could cause less spatial uncertainty and/or attract stronger focal attention, leading to a lower threshold. To explore this alternative argument, we compared probe detection thresholds of the MBC phase-shift condition with two new conditions. The binocular disc condition (Fig. 2E) had the MBC disc half-image stimulating both eyes during threshold measurement, and the binocular background condition (Fig. 2F) had the homogeneous background half-image stimulating both eyes. We found the average threshold in the binocular disc condition (0.98 ± 0.02 log%) to be slightly higher than that in the binocular background condition (0.91 ± 0.02 log%) [F(1,3) = 37.376, P = 0.009]. Clearly, this finding rejects the alternative argument. Moreover, in the MBC phase-shift condition (measured in the same test block), we found the average threshold on the MBC disc (0.89 ± 0.02) lower than that on the homogeneous grating (1.08 ± 0.05 log%) [F(1,3) = 12.076, P = 0.040].
3. Experiment 3: Contrast Increment Threshold (Spatial-Frequency-Difference).
We measured contrast increment thresholds on each half-image of the MBC spatial-frequency-difference stimuli in Fig. 3. We found the average threshold on the 3 cpd homogeneous grating (1.016 ± 0.099 log unit) higher than that on the 3.5 cpd disc (0.821 ± 0.072l) [Fig. 3A; t(3) = 3.704, P = 0.034]. Similar results were found for the stimulus in Fig. 3B (3.5 cpd homogeneous grating: 1.013 ± 0.084 log unit; 3 cpd disc: 0.792 ± 0.069) [t(3) = 3.638, P = 0.036]. The higher threshold on the homogeneous grating half-image in both stimuli indicates binocular suppression, even as stereopsis is experienced, further supporting the co-existence hypothesis.
4. Experiment 4: Perceptual Tracking and Control Experiment 2.
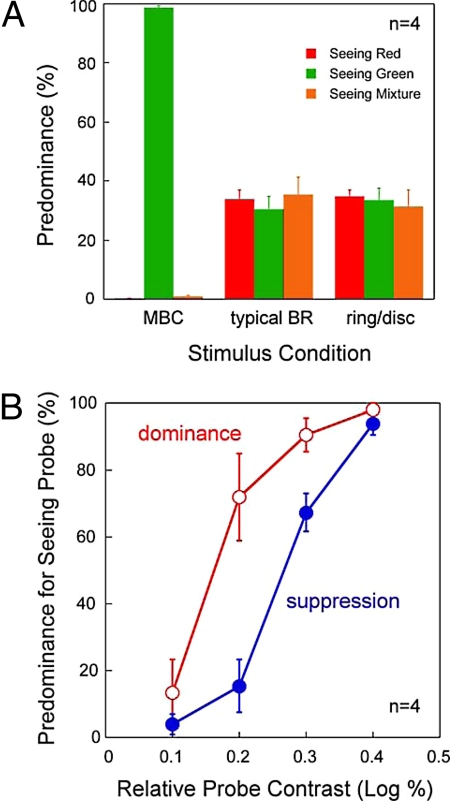
To reveal the binocular suppression induced by the MBC alone is sufficiently strong to prevent the homogeneous grating from perception, observers indicated their instantaneous percepts of the stimuli in Fig. 4 A–C. For the colored-MBC phase-shift stimulus (Fig. 4A), the predominance (Fig. 7A) for seeing the MBC disc (green) is much higher than for seeing the homogeneous grating [F(1.012, 4.407) = 10011.296, P < 0.001, ANOVA with repeated measures, Greenhouse-Geisser correction]. This indicates MBC induced binocular suppression causes the homogeneous grating to be unperceived most of the time. In contrast, for the red/green disc (Fig. 4B) and the disc/ring (Fig. 4C) stimuli, the predominance for seeing red, green or mixed colored disc is quite similar [red/green disc stimulus: F(2,8) = 0.192, P = 0.829; disc/ring stimulus, F(2,8) = 0.11, P = 0.897, ANOVA with repeated measures].
Fig. 7.
Results of Experiment 4. (A) The average predominance for the stimuli in Fig. 4 A–C. (B) The average predominance as a function of relative probe contrast for seeing the monocular Gabor probe for the stimuli in Fig. 4 D and E. Due to individual differences, we aligned all four observers' data at the probe contrast with the maximum predominance difference between the two stimulus conditions (this occurred at 1.2 log% for two observers, and 1.1 log% and 1.0 log%, respectively, for the remaining two observers).
Instead of color labeling the MBC phase-shift stimulus to measure binocular suppression, our control experiment tagged a Gabor probe onto either half-image of the grayscale stimulus (Fig. 4 D and E). Observers tracked the probe's visibility (seen or unseen), which reflects the dominance or suppression state of the half-images. Fig. 7B plots the average predominance for seeing the probe on the MBC disc (open circles) and on the homogeneous grating (filled circles) as a function of relative probe contrast. As expected, for an extremely low contrast probe that is barely above detection threshold, or an extremely high contrast probe that is sufficiently strong to resist suppression by the MBC, the predominance for seeing the probe is similar on both half-images. But for intermediate probe contrast levels, the predominance is significantly higher on the MBC disc than on the homogeneous grating, indicating binocular suppression of the former on the latter half-image. This is confirmed by a statistical analysis of the average data [main effect of stimulus condition: F(1,3) = 35.103, P = 0.010; main effect of contrast: F(3,9) = 95.187, P < 0.001; interaction: F(3,9) = 6.770; P = 0.011, two-way ANOVA with repeated measures], further confirming the existence of interocular inhibition with the MBC phase-shift stimulus. Finally, we found the binocular suppression does not affect the stability of the perceived depth of the MBC disc, as the observers continuously experienced the depth percept throughout the 30-s stimulus interval.
Discussion
Using the MBC phase-shift stimulus, we showed both stereopsis and binocular suppression are experienced simultaneously at the same location. This suggests even as the integration process extracts binocular depth from both eyes, the interocular inhibitory process suppresses the homogeneous grating half-image leading to the selection of the MBC grating disc for perception. In general, our findings agree with the notion that the integration process and the interocular inhibitory process operate independently (co-existence hypothesis) (5–10). However, we recognize that our observation does not necessarily exclude the fusion-preceding-rivalry hypothesis (1–4, 20). This is because the observed binocular integration and binocular suppression are intimately related, respectively, to the surface feature (grating) and boundary contour (MBC) information that are processed by different neural mechanisms. Specifically, with the MBC phase-shift stimulus, the interocular inhibitory process is activated when the visual system cannot find a matching boundary contour in the homogeneous grating half-image, i.e., agreeing with the fusion-preceding-rivalry hypothesis. In this regard, the two hypotheses are reconcilable when we consider that BR is processed by a distributed intercortical network (21–23).
Equally significant, our study reveals the important role of the boundary contour. In earlier studies (14, 15) we showed using an MBC stimulus with conflicting local features (orthogonal gratings in Fig. 2A), the critical contribution of the boundary contour to BR. Here, with the MBC phase-shift stimulus, we found that the MBC can induce binocular suppression even when there are no conflicting local features (same vertical grating in Fig. 2B). This indicates that the MBC alone can trigger the interocular inhibitory process.
Research shows that the boundary contour representation provides the basis for representing surfaces and objects, and that boundary contours are extracted in the early visual cortices (e.g., 24–31). Importantly, single unit recording in monkeys have revealed a significant proportion of V2 cortical neurons with selectivity for the side of the contour, that is, border ownership (BO) (31, 32). Border ownership (BO) signals are critical for segregating surfaces in depth, as front (figure) or back (ground) (28, 29, 31, 33–35). We believe the crucial role of the BO neurons is reflected in our study. It is possible that the MBC stimulus (e.g., Fig. 2 A and B) triggers activities in the BO selective neurons, which in turn initiates the boundary contour based surface representation process to construct the MBC defined texture surface (disc). Meanwhile, the BO selective neurons also trigger the interocular inhibitory network to suppress the homogeneous grating half-image at the corresponding retinal area. This ensures the homogeneous grating image does not interfere with the surface representation of the MBC disc.
Given the modular organization of the early visual cortices (36, 37), our findings also suggest that the perceived 3-D MBC disc is obtained from the binding of different modular inputs. The first is the monocular texture information within the MBC disc. The second is the quantitative binocular depth derived from the binocular disparity process (38). This explanation, if correct, advances our understanding of feature-binding in vision (39). Interestingly, for binocular surface perception, the visual system can bind selective features from the two retinal images based on projection geometry constraints. This reinforces the notion that the visual system can solve the feature-binding problem by relying on perceptual rules that are derived from its past experiences of interacting with the ecological environment (40–42).
Methods
Observers.
One author participated in all four main experiments, and another in Control Experiment 2. Fifteen naïve observers with informed consent were recruited. Four participated in Experiment 1, three in Experiments 2 and 3, four in Control Experiment 1, and the remaining four in Experiment 4. Three of the four naive observers in Control Experiment 1 participated in Control Experiment 2. All observers had normal or corrected-to-normal visual acuity and a stereoscopic resolution of 40-s arc or better.
Stimuli.
MATLAB and Psychophysics Toolbox (43, 44) on a Macintosh were used to present stimuli on a CRT monitor (1280 × 1024, 100 Hz). Observers viewed the stimuli (75 cm) through a mirror haploscopic system attached to a chin-and-headrest.
Experiment 1: Stimuli and Procedures.
A 0.45° × 0.45° white nonius fixation target (76 cd/m2) on a gray background (40 cd/m2) preceded the 5° × 5° MBC half-images with grayscale vertical sinusoidal grating (2.2 cpd, 40 cd/m2; 90% Michelson contrast) (Fig. 1C). The half-image presented to the test eye had a 1.5° circular MBC disc, which was divided into two halves. The upper half-disc grating was phase-shifted horizontally relative to the surrounding grating by 54°, 72°, 108°, or 126°, while the lower half-disc grating was shifted by 90°. Depending on the eye viewing the MBC disc and the direction of the shift (left/right), the MBC disc grating had either positive or negative horizontal disparities relative to the background grating (−3.4, −1.7, 1.7, and 3.4 min of arc). Four binocular green dots (≈0.1° × 0.1°) in the vicinity of the MBC disc aided eye alignment. A 500-ms black and white square-wave checkerboard mask (5° × 5°; 2.2 cpd; 40 cd/m2; 90% contrast) terminated a trial. Each test condition was run over 40 blocks of trials in four sessions [5 contrast levels × 2 eyes × 2 probe types (dominance vs. suppression) × 2 repeats].
Task.
The observer judged whether the upper or lower half-disc was perceived as nearer.
Experiment 2: Stimuli and Procedures.
The MBC phase-shift stimulus (4.5° × 4.5°, Fig. 2B) had 3 cpd vertical sinusoidal gratings (72.4 cd/m2), with the central 1.5° disc region of one half-image being phase-shifted by 180°. The contrast of the gratings was set at one of five levels (0.8, 1.0, 1.2, 1.4, and 1.6 log%). A white nonius fixation target (0.4° × 0.4°) was located 0.91° above the disc. A Gabor probe (Gaussian kernel FWHM = 0.75°, 160 ms) was presented either on the half-image with the MBC disc (dominant condition) or the half-image with the homogeneous grating (suppression condition). The trial ended with a 500 ms, 95% contrast, 3 cpd black and white checkerboard mask. For the ring/disc condition (Fig. 2C), a 1.5° counterphase annulus (width = 0.046°) was added to the half-image with the homogeneous grating (72.4 cd/m2, 1.4 log% contrast). For the ring/ring condition (Fig. 2D), the half-image with the MBC disc was replaced by the half-image with the annulus and homogeneous grating.
Task.
Monocular contrast increment thresholds were obtained using a 2AFC-staircase method. The Gabor probe was either presented 1 or 2 s after the onset of the MBC stimulus. Four blocks of trials over four sessions were tested for each stimulus.
Control Experiment 1.
The MBC phase-shift stimulus (2.2 cpd, 60 cd/m2, 1.5 log% contrast, 90° phase-shift) was centrally fixated. Four surrounding orange dots (0.13°) served as fusion lock. Monocular increment thresholds on the MBC phase-shift stimulus were compared with those on the binocular disc and binocular background conditions (Fig. 2 E and F). The Gabor probe (Gaussian kernel FWHM = 0.25° × 0.43°, 250 ms) was either presented to a location corresponding to the upper half or lower half of the MBC disc. Four blocks of thresholds were measured in each condition using a 2AFC-QUEST design.
Experiment 3.
All aspects of the experiment were the same to those of Experiment 2 (72.4 cd/m2, 1.4 log% contrast) except for the MBC stimulus design (Fig. 3). The MBC disc was generated by a circular area of grating with a different spatial frequency from that of the surrounding grating (3 cpd vs. 3.5 cpd and vice versa).
Experiments 4: Stimuli and Procedures.
The MBC phase-shift stimulus (5° × 5°, Fig. 4A) comprised of 4 cpd red/black vertical sinusoidal grating (12.6 cd/m2, 79.7% contrast, CIE: 0.564, 0.348) with a central 1° circular region of green/black grating (phase-shift = 180°, 15.3 cd/m2, 83.4% contrast, CIE: 0.302, 0.553) in one half-image. For the disc/ring stimulus (Fig. 4C), a 1° gray annulus (width = 0.05°, 29.7 cd/m2) was added to the half-image with the homogeneous red/black grating. For the red/green disc stimulus (typical BR, Fig. 4B), 1° red/black and green/black vertical grating discs with 180° phase difference were presented against a gray background (29.7 cd/m2). All three stimuli were randomly interspersed within a block of 18 trials (2 test eyes × 3 stimulus types × 3 repeats). Each observer was tested over four blocks. A 0.5° × 0.5° white nonius fixation target (76 cd/m2) was presented between each trial and removed 250 ms before the stimulus display of the upcoming trial, whose duration was 30 s. To eliminate the afterimages at the end of the 30-s trial, a 0.25-Hz anti-phase, 4 cpd black and white checkerboard mask (luminance = 52.3 cd/m2, contrast = 95.1%) was presented for 8 s, and followed by a 2-s blank screen.
Task.
The observer reported his/her instantaneous percepts (whole disc, no disc, mixture) by continuously depressing one of three keys on the keyboard.
Control Experiment 2.
The MBC phase-shift stimulus was the same as that in Control Experiment 1 (60 cd/m2, 1.5log% contrast, 4.5° × 4.5°). A Gabor probe (Gaussian kernel FWHM = 0.4°) with variable contrast (0.1 log% interval) was presented either to the center of the MBC disc (Fig. 4D, dominance condition) or its corresponding area on the homogeneous grating (Fig. 4E, suppression condition). Observers reported either seeing or not seeing the Gabor probe throughout the 30-s stimulus duration by pressing either the left or right arrow key. At least six Gabor probe contrast levels, with four repeat trials per probe contrast, were tested on each observer.
Statistical Analysis.
ANOVA was performed on the data in Experiments 2–4 and the control experiments.
Acknowledgments.
We thank the two reviewers for their helpful comments. This study was supported by National Institutes of Health Grant EY015804 (to T.L.O. and Z.J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Blake R, Boothroyd K. The precedence of binocular fusion over binocular rivalry. Percept Psychophys. 1985;37:114–124. doi: 10.3758/bf03202845. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg J. Depth perception loss with local monocular suppression: A problem in the explanation of stereopsis. Science. 1964;145:1334–1336. doi: 10.1126/science.145.3638.1334. [DOI] [PubMed] [Google Scholar]
- 3.Blake R, O'Shea RP. “Abnormal fusion” of stereopsis and binocular rivalry. Psychol Rev. 1988;95:151–154. doi: 10.1037/0033-295x.95.1.151. [DOI] [PubMed] [Google Scholar]
- 4.O'Shea RP. Chronometric analysis supports fusion rather than suppression theory of binocular vision. Vision Res. 1987;27:781–791. doi: 10.1016/0042-6989(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 5.Blake R, Westendorf DH, Overton R. What is suppressed during binocular rivalry? Perception. 1980;9:223–231. doi: 10.1068/p090223. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman L. Sight and Mind. New York, New York: Oxford Univ Press; 1974. [Google Scholar]
- 7.Treisman A. Binocular rivalry and stereoscopic depth perception. Q J Exp Psychol. 1962;14:23–37. [Google Scholar]
- 8.Wolfe JM. Influence of spatial frequency, luminance, and duration on binocular rivalry and abnormal fusion of briefly presented dichoptic stimuli. Perception. 1983;12:447–456. doi: 10.1068/p120447. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe JM. Stereopsis and binocular rivalry. Psychol Rev. 1986;93:269–282. [PubMed] [Google Scholar]
- 10.Wolfe JM. Parallel ideas about stereopsis and binocular rivalry: A reply to Blake and O'Shea. Psychol Rev. 1988;95:155–158. [Google Scholar]
- 11.Julesz B, Miller JE. Independent spatial-frequency-tuned channels in binocular fusion and rivalry. Perception. 1975;4:125–143. [Google Scholar]
- 12.Kaufman L. Suppression and fusion in viewing complex stereograms. Am J Psychol. 1964;77:193–205. [PubMed] [Google Scholar]
- 13.Ogle KN, Wakefield JM. Stereoscopic depth and binocular rivalry. Vision Res. 1967;7:89–98. doi: 10.1016/0042-6989(67)90029-6. [DOI] [PubMed] [Google Scholar]
- 14.Ooi TL, He ZJ. Surface representation and attention modulation mechanisms in binocular rivalry. In: Alais D, Blake R, editors. Binocular Rivalry. Cambridge, MA: MIT Press; 2005. pp. 117–135. [Google Scholar]
- 15.Ooi TL, He ZJ. Binocular rivalry and surface-boundary processing. Perception. 2006;35:581–603. doi: 10.1068/p5489. [DOI] [PubMed] [Google Scholar]
- 16.van Bogaert EA, Ooi TL, He ZJ. The monocular-boundary-contour mechanism in binocular surface representation and suppression. Perception. 2008;37:1197–1215. doi: 10.1068/p5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisby JP, Mayhew JE. The relationship between apparent depth and disparity in rivalrous-texture stereograms. Perception. 1978;7:661–678. doi: 10.1068/p070661. [DOI] [PubMed] [Google Scholar]
- 18.Blakemore C. A new kind of stereoscopic vision. Vision Res. 1970;10:1181–1199. doi: 10.1016/0042-6989(70)90036-2. [DOI] [PubMed] [Google Scholar]
- 19.Ooi TL, He ZJ, Su Y. Binocular rivalry is affected by surface boundary contours. J Vis. 2005;5:5a. [Google Scholar]
- 20.Blake R, Camisa J. Is binocular vision always monocular? Science. 1978;200:1497–1499. doi: 10.1126/science.663633. [DOI] [PubMed] [Google Scholar]
- 21.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 22.Ooi TL, He ZJ. Binocular rivalry and visual awareness: The role of attention. Perception. 1999;28:551–574. doi: 10.1068/p2923. [DOI] [PubMed] [Google Scholar]
- 23.Ooi TL, He ZJ. A distributed intercortical processing of binocular rivalry: Psychophysical evidence. Perception. 2003;32:155–166. doi: 10.1068/p3467. [DOI] [PubMed] [Google Scholar]
- 24.Bakin JS, Nakayama K, Gilbert CD. Visual responses in monkey areas V1 and V2 to three-dimensional surface configurations. J Neurosci. 2000;20:8188–8198. doi: 10.1523/JNEUROSCI.20-21-08188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossberg S, Mingolla E. Neural dynamics of form perception: Boundary completion, illusory figures, and neon color spreading. Psychol Rev. 1985;92:173–211. [PubMed] [Google Scholar]
- 26.Mitchison GJ, McKee SP. The resolution of ambiguous stereoscopic matches by interpolation. Vision Res. 1987;27:285–294. doi: 10.1016/0042-6989(87)90191-x. [DOI] [PubMed] [Google Scholar]
- 27.Lamme VAF, Super H, Spekreijse H. Feedforward, horizontal, and feedback processing in the visual cortex. Curr Opin Neurobiol. 1998;8:529–535. doi: 10.1016/s0959-4388(98)80042-1. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama K, He ZJ, Shimojo S. Visual surface representation: A critical link between lower-level and higher-level vision. In: Kosslyn , Oshershon , editors. An Invitation to Cognitive Science: Visual Cognition. Cambridge, MA: MIT Press; 1995. pp. 1–70. [Google Scholar]
- 29.von der Heydt R. Image parsing mechanisms of the visual cortex. In: Werner , Chalupa , editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2003. pp. 1139–1150. [Google Scholar]
- 30.von der Heydt R, Peterhans E, Baumgartner G. Illusory contours and cortical neuron responses. Science. 1984;224:1260–1262. doi: 10.1126/science.6539501. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Friedman HS, von der Heydt R. Coding of border ownership in monkey visual cortex. J Neurosci. 2000;20:6594–6611. doi: 10.1523/JNEUROSCI.20-17-06594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu FT, von der Heydt R. Figure and ground in the visual cortex: V2 combines stereoscopic cues with Gestalt rules. Neuron. 2005;47:155–166. doi: 10.1016/j.neuron.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koffka K. Principles of Gestalt Psychology. Harcourt, Brace and Company: New York; 1935. [Google Scholar]
- 34.Nakayama K, Shimojo S, Silverman GH. Stereoscopic depth: Its relation to image segmentation, grouping, and the recognition of occluded objects. Perception. 1989;18:55–68. doi: 10.1068/p180055. [DOI] [PubMed] [Google Scholar]
- 35.Driver J, Baylis G. Edge-assignment and figure-ground segmentation in short-term visual matching. Cognit Psychol. 1996;31:248–306. doi: 10.1006/cogp.1996.0018. [DOI] [PubMed] [Google Scholar]
- 36.DeYoe EA, Van Essen DC. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988;11:219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- 37.Livingstone MS, Hubel DH. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 38.Ohzawa I, DeAngelis GC, Freeman RD. Stereoscopic depth discrimination in the visual cortex: Neurons ideally suited as disparity detectors. Science. 1990;249:1037–1041. doi: 10.1126/science.2396096. [DOI] [PubMed] [Google Scholar]
- 39.Treisman A, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama K, Shimojo S. Experiencing and perceiving visual surfaces. Science. 1992;257:1357–1363. doi: 10.1126/science.1529336. [DOI] [PubMed] [Google Scholar]
- 41.Purves D, Lotto RB, Williams SM, Nundy S, Yang Z. Why we see things the way we do: Evidence for a wholly empirical strategy of vision. Philos Trans R Soc Lond B. 2001;356:285–297. doi: 10.1098/rstb.2000.0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Helmholtz H. Treatise on Physiological Optics Vol. III. New York, New York: Dover; 1925. [Google Scholar]
- 43.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 44.Pelli DG. The VideoToolBox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]