Abstract
When we reach toward objects, we easily avoid potential obstacles located in the workspace. Previous studies suggest that obstacle avoidance relies on mechanisms in the dorsal visual stream in the posterior parietal cortex. One fundamental question that remains unanswered is where the visual inputs to these dorsal-stream mechanisms are coming from. Here, we provide compelling evidence that these mechanisms can operate in “real-time” without direct input from primary visual cortex (V1). In our first experiment, we used a reaching task to demonstrate that an individual with a dense left visual field hemianopia after damage to V1 remained strikingly sensitive to the position of unseen static obstacles placed in his blind field. Importantly, in a second experiment, we showed that his sensitivity to the same obstacles in his blind field was abolished when a short 2-s delay (without vision) was introduced before reach onset. These findings have far-reaching implications, not only for our understanding of the time constraints under which different visual pathways operate, but also in relation to how these seemingly “primitive” subcortical visual pathways can control complex everyday behavior without recourse to conscious vision.
Keywords: blindsight, dorsal stream, visuomotor control, visual pathways, consciousness
In everyday life, we rarely reach for objects in presented in isolation. Rather, we typically reach out and grasp objects that are located within cluttered environments, where the need to avoid colliding with other nontarget objects (i.e., potential obstacles) is critical. Although the ability to avoid obstacles seems effortless, it requires a complex interplay between incoming visual information, which is needed to code the position of potential obstacles, and the visuomotor system controlling the execution of the reach (1). Within this context, previous work by Milner, Goodale, and colleagues (2, 3) has argued that there are two separate but interacting visual processing streams within the primate brain that mediate vision for action and vision for perception, respectively. Specifically, a dorsal visual pathway extending from the primary visual cortex (V1) to the superior parietal lobe and intraparietal sulcus is thought to be important for controlling visually guided actions (e.g., reaching, eye movements). In contrast, a ventral visual pathway extending from V1 to the inferior temporal cortex is thought to be important for object recognition and conscious visual perception.
Previous studies examining obstacle avoidance in neurological patients have indicated that lesions to the dorsal stream severely disrupt the ability to avoid obstacles (4, 5), whereas lesions to the ventral stream do not impair obstacle avoidance (6). That is, patients with damage to the dorsal stream who have no trouble recognizing objects nevertheless have difficulty taking into account the position of obstacles while reaching. In contrast, patients with ventral stream damage can avoid obstacles even though they are severely impaired at visual object recognition. This suggests that the visual pathways mediating obstacle avoidance are separate from those that enable recognition of the obstacles themselves. Additional studies in neurological patients have demonstrated that obstacle avoidance can operate even in the absence of visual awareness. Specifically, McIntosh and colleagues demonstrated spared obstacle avoidance in patients with left visual neglect (7) and visual extinction (8) following right temporoparietal damage. Taken together, these data suggest that obstacle avoidance is controlled by circuits within the dorsal stream that can code position of obstacles “implicitly” (i.e., unconsciously). Critically, one important question that has not yet been addressed concerns the visual inputs that are necessary for the dorsal stream to code the position of potential obstacles. All of the previous studies in which neurological patients have demonstrated spared obstacle avoidance have had V1 largely spared. But it is unclear whether or not V1 is necessary for successful obstacle avoidance
It has been known for quite some time that there are multiple routes whereby visual information can reach the primate cerebral cortex. Some of these pathways bypass V1 entirely and instead project directly to extrastriate visual areas (for reviews, see references 3, 9–11). In fact, existence of these secondary visual pathways have been used to explain blindsight—a phenomenon in which patients who lose conscious vision after damage to V1 nevertheless retain the ability to respond at above-chance levels to visual information presented within their blind field (9, 12, 13). Earlier studies have demonstrated that patients with complete cortical blindness after bilateral V1 damage can navigate around obstacles while walking (14, 15). During walking, however, avoidance behavior could be driven by self-generated motion cues (15) and/or optic flow (16–18). It is well-known that patients with V1 lesions often retain sensitivity to motion cues (19), even though they may be unaware of the movement itself (12, 20).
To determine whether or not inputs from V1 to the dorsal stream are necessary for obstacle avoidance we tested patient CB, a 75-year-old male who suffered a right occipital stroke resulting in a dense left visual field hemianopia (Fig. 1 A and B; see Methods for details), on a reaching task in which he had to avoid obstacles. Specifically, we compared his ability to avoid obstacles placed in his sighted (right) visual field and his blind (left) visual field. Testing a patient with hemianopia (as opposed to complete cortical blindness) afforded us the unique advantage of examining how obstacles placed in CB's blind field influenced reaching in his sighted field. Importantly, by controlling fixation, and by stabilizing CB's head, and removing visual feedback during the reach, we eliminated the possibility that the patient was using self-generated motion cues to compute the position of obstacles while reaching toward the target. In addition to testing patient CB, we also tested two healthy elderly controls (EC1 and EC2; both were right-handed males, one aged 83 years [EC1], and one aged 70 [EC2]) and a group of six young healthy controls [mean age = 26.5 years range (22–29); all were right-handed males] under the same testing conditions as the patient. Data from patient CB and EC1 are reported in the main manuscript. Data from the additional elderly control (EC2) and the group of young controls (n = 6) are reported in the SI Text.
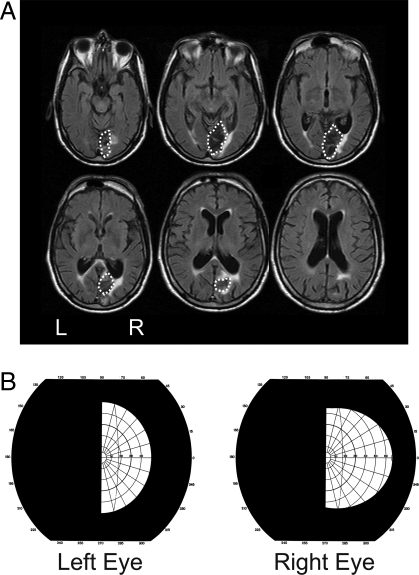
Fig. 1.
Lesion location and visual field testing in patient CB. (A) FLAIR MRI images (5-mm axial slices) of patient CB's lesion acquired 3 years post-stroke. The lesion is largely restricted to the right occipital region and the optic radiations. The dotted lines indicate the boundaries of his lesion. (B) Results of Goldman perimetry conducted at the time of testing (3.5 years post-stroke) by a neuro-ophthalmologist. Testing was conducted using III 4 sized targets, which are commonly used to assess visual field defects after stroke. Importantly, there is no evidence of any macular or temporal crescent sparing in the left (blind) field.
Results
Preliminary behavioral testing indicated that CB demonstrated evidence of implicit visual processing in his blind field (i.e., “blindsight”) (9, 12, 13). Specifically, CB demonstrated a redundant target effect (21), such that when visual targets were presented simultaneously in his sighted and blind fields, he was faster to respond with a button press (431 ms), compared to when only a single target was presented to his sighted field [526 ms; t (24) = 2.29, P = 0.031; see Methods for details]. Importantly, CB never responded when only a single target was presented in his blind field and repeatedly insisted that he never saw anything on the left. These data suggest that CB was able to implicitly process visual stimuli in his blind field even though he was unaware of their presence.
To investigate obstacle avoidance in CB, we adapted a task used previously in healthy individuals and in neurological patients (1, 4, 5, 7, 8). In this task, CB was required to make reaches from a start button (depth 15 cm) to a target strip (5 cm wide at a depth of 55 cm) with his right hand as quickly and accurately as possible (i.e., in “real-time”), while avoiding obstacles (depth 35 cm) that could either be located laterally either 10 cm from the midline (“in”) or 15 cm from midline (“out”) in his right (sighted) or left (blind) visual field or in both visual fields. On some trials, no obstacles were present in either field (Fig. 2A).
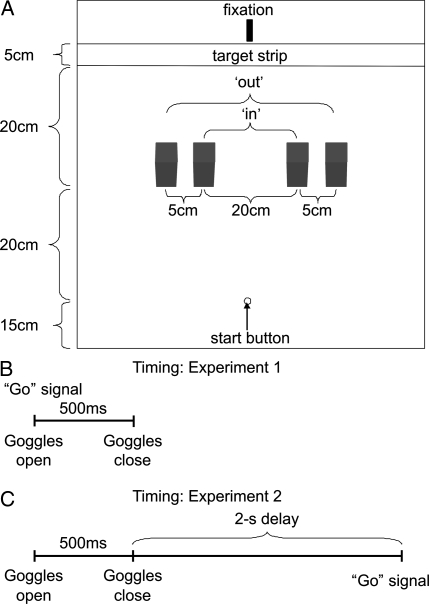
Fig. 2.
Spatial layout and timing of the two experiments. (A) Depicts the spatial layout (overhead view) of the experimental setup. CB and the controls were required to make reaches from at start button (15 cm) to a target strip (55 cm), while avoiding obstacles that could be placed either 10 or 15 cm from midline in the left or right visual field (or in both fields). (B) Depicts the timing and sequence of events for Experiment 1. At the beginning of a trial, the goggles opened for 500 ms and an auditory “Go” signal was presented. (C) Depicts the timing and sequence of events for Experiment 2. At the beginning of a trial, the goggles opened for 500 ms, followed by a 2-s delay period, followed by the auditory “Go” signal.
On each trial, CB was required to fixate on a pole located just behind the center of the target strip. During the experiment, CB wore a pair of PLATO goggles (Translucent Technologies), which were open for 500 ms at the beginning of each trial (Fig. 2B). Given that CB's average reaction time was well >500 ms (547 ms, SE = 1.05), all reaches were completed without terminal visual feedback. During each trial, the patient's eye movements were strictly monitored using a video camera to ensure he was fixating. Trials in which the patient made an eye movement into his blind field were removed from the analyses (this occurred on only two trials). After each trial, CB was asked whether or not an obstacle had been present and where it was located. In between trials, the goggles remained closed, while CB wore headphones that played loud white noise to mask any auditory cues from the placement of the obstacles (this was verified independently before the experiment). Movement trajectories were recorded using infrared emitting diodes (IREDs) placed on the wrist and the tip and base of the index finger of the right hand. The position of these markers was sampled at 150 Hz using two Optotrak 3020 cameras (Northern Digital).
In the current investigation, our analysis focused on those trials in which a single obstacle was present in either the left or the right visual field, since this provides the most direct comparison of obstacle avoidance in CB's blind and sighted fields. Reach trajectories from the two-obstacle and no-obstacle trials are presented in the SI Text and Fig. S1.
Experiment 1: Real-Time Obstacle Avoidance.
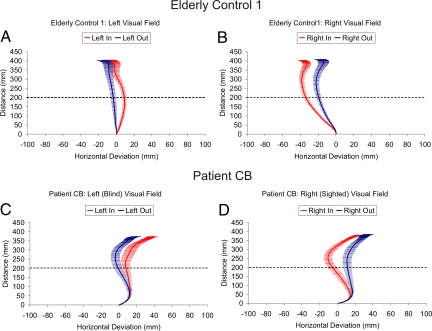
Analysis of the 83-year-old elderly controls data (EC1) revealed that his reach trajectories were significantly influenced by the position of obstacles [F (7, 133) = 32.20, P < 0.0001] in both the left and in the right visual fields (Fig. 3 A and B). Specifically, 20 cm into the reach (i.e., at the distance of the front edge of the obstacle) his trajectories deviated away from an obstacle more when it was in compared to when it was out (left, in = 8.8 mm vs. out = −3.8 mm, P = 0.001; right, in = −34.0 mm vs. out = −19.0 mm, P < 0.0001). For details concerning the additional controls tested, refer to the SI Text and Figs. S2–S5.
Fig. 3.
Averaged spatial trajectories for the real-time obstacle avoidance experiment. Depicts averaged movement trajectories (± standard error) for EC1 (Top) and patient CB (Bottom) as a function of obstacle position (i.e., in vs. out) and left vs. right visual field for the single object trials. The x-axis depicts horizontal deviation in millimeters (mm), and the y-axis depicts reach distance in millimeters. Note that the x-axis has been magnified to illustrate the separation between trajectories. The dotted line represents the depth at which the obstacles were located (35 cm) from the reach start position (15 cm; i.e., 35 cm − 15 cm = 20 cm). Positive values refer to the right of midline, whereas negative values refer to left of midline. Note that patient CB's reaches were always directed to the right side of the target strip, the only part of the strip that he could see. Nevertheless, his reach trajectories were significantly influenced by the position of the obstacles in his blind field (see also Figs. S6 and S7).
Not surprisingly, when the obstacles were placed in CB's sighted (right) field, he behaved in much the same way as the controls (Fig. 3D). Although the initial ANOVA comparing CB's reach trajectories 20 cm into the reach was not significant [F (7, 128) = 1.76, P = 0.10], an independent samples t test (two-tailed) revealed that his reach trajectories were significantly influenced by the position of the obstacles in his sighted (right) visual field (right, in = −7.9 mm vs. out = 7.9 mm; t (32) = 2.30, P = 0.028; Fig. 3D). This resulted from the fact that CB's reach trajectories demonstrated clear spatial separation further into the reach compared to the controls (see Fig. 3 A and B and Figs. S6 and S7). Consistent with this notion, at 25 cm into the reach (i.e., just beyond the back edge of the obstacle), CB's reach trajectories were now more sensitive to the position of obstacles in his sighted (right) visual field [F (7, 128) = 2.01, P = 0.058], showing larger leftward deviations for obstacles placed in (−12.8 mm) compared to out (6.8 mm, P = 0.016)*. Remarkably, however, CB was also sensitive to the position of the obstacles in his blind (left) visual field (see Fig. 3C). Again, although the trajectories took longer to show significant spatial separation (see Fig. S6A), by the end of the movement [F (7, 128) = 2.61, P = 0.015], his reaches were “pushed” significantly rightward when obstacles in his blind field were placed closer to the midline (i.e., at the in position = 38.6 mm) compared to obstacles placed further away (i.e., at the out position = 20.3 mm, P = 0.009). It is worth emphasizing that CB's acute sensitivity to the position of the obstacles in his blind field is clearly indicated not only by the non-overlap of the standard error bars in the average trajectories (Fig. 3C), but also by the fact that the difference scores for the two trajectories (out minus in) are significantly different from zero at the depth of the obstacles and beyond (Fig. S6A). Importantly, CB never reported seeing any of the obstacles placed in his blind field, nor did he collide with any of them. In summary, these data show that CB's reaching movements remain sensitive to the position of obstacles even though he is quite unaware of their presence.
It has been argued that visuomotor networks in the dorsal stream work optimally in real-time, but when action is driven by memory (rather than by direct input from the retina), the ventral perception stream is engaged (3, 22). This hypothesis has received compelling support from studies of patients with ventral-stream damage whose performance deteriorates profoundly when even a short delay of a few seconds is introduced between seeing the goal and initiating the action (22, 23). Remarkably, patients who have damage restricted to the dorsal stream, who typically do poorly in real-time, actually show an paradoxical improvement in visuomotor performance after a short delay (5, 24–28). Given these data, we surmised that if CB's sensitivity to the position of obstacles in his blind field is mediated by the dorsal stream, then the effect should disappear if a short delay were introduced before reach onset. To test this, we ran a second experiment that was identical to the first, except that now a short 2-s delay was inserted after the PLATO goggles closed (Fig. 2C). CB was instructed not to initiate his reach until he heard an auditory “Go” signal following the 2-s delay.
Experiment 2: Delayed Obstacle Avoidance.
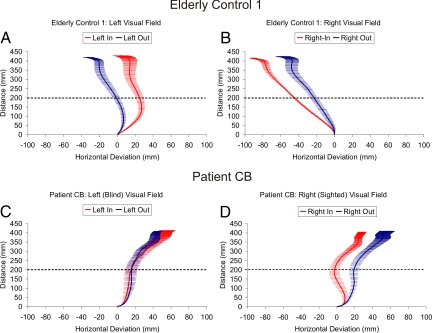
In the delay task, the elderly control subject (EC1) remained highly sensitive to the position of the obstacles 20 cm into the reach [F (7, 64) = 32.57, P < 0.0001] in both his left (in = 23.1 mm vs. out = −1.3 mm, P < 0.0001) and right (in = −45.3 mm vs. out = −29.9 mm, P = 0.007) visual fields (Fig. 4 A and B). In addition, at 20 cm into the reach, CB's reach trajectories also remained sensitive to the position of the obstacles [F (7, 61) = 2.63, P = 0.019] in his sighted visual field (right, in = −3.7 mm vs. out = 18.7 mm, P = 0.006; Fig. 4D). Consistent with our hypothesis, however, at 20 cm into the reach (left, in = 15.3 mm vs. out = 15.7 mm, P = 0.96), and at the reach end points [F (7, 61) = 3.62, P = 0.003], CB was no longer sensitive to the position of the obstacles in his blind field (left, in = 55.9 mm vs. out = 45.8 mm, P = 0.25; observed power = 0.96; Fig. 4C). Note the almost complete overlap of the standard error bars depicted on the trajectory traces for CB's blind field (Fig. 4C) and the fact that the 95% confidence intervals for the difference scores overlapped zero throughout the reach (Fig. S6C). This failure to take into account the position of the obstacles in his blind field in the delay condition stands in striking contrast to his remarkable sensitivity to the position of the very same obstacles in the real-time task.
Fig. 4.
Averaged spatial trajectories for the delayed obstacle avoidance experiment. Depicts averaged movement trajectories (± standard error) for EC1 (Top) and patient CB (Bottom) as a function of obstacle position (i.e., in vs. out) and left versus right visual field for the single object trials. The x-axis depicts horizontal deviation in millimeters (mm), and the y-axis depicts reach distance in millimeters. Note that the x-axis has been magnified to illustrate the separation between trajectories. The dotted line represents the depth at which the obstacles were located (35 cm) from the reach start position (15 cm; i.e., 35 cm − 15 cm = 20 cm). Positive values refer to the right of midline, whereas negative values refer to left of midline. Patient CB's reaches were always directed to the right side of the target strip, the only part of the strip that he could see. Note that CB's reach end points are no longer sensitive to the position of obstacles in his blind (left) visual field (i.e., the error bars are completely overlapping; see also Figs. S6 and S7).
Discussion
Previous research suggests that the dorsal stream can mediate obstacle avoidance (4, 5) even in the absence of visual awareness (7, 8). Importantly, however, the visual inputs that are necessary for obstacle avoidance to operate remain unknown. In the current study, CB, a patient with a dense left visual field hemianopia after damage to V1, remained strikingly sensitive to the position of static obstacles placed in his blind field. That is, he was able to code the position of the obstacles despite being unaware of their presence. Although it took longer for the reach trajectories in CB's his blind field to show clear separation (see Fig. S6), they were significantly different by the end of the reach (Fig. 3C). While this result clearly indicates that CB remains sensitive to the position of obstacles in his blind field, the reason his trajectories took more time to show significant spatial separation may reflect the fact that CB is no longer as sensitive to the depth of obstacles in his blind field. This is consistent with previous work that indicates that retinal disparity signals in V1 (which is clearly damaged in CB's right hemisphere) are critical for depth perception (see refs. 29 and 30).
Critically, in a second experiment, CB's sensitivity to the position of the same obstacles in his blind field was abolished after a short 2-s delay. In contrast, CB's reaches remained highly sensitive to the position of the obstacles in his sighted field in the delay condition. This is consistent with previous work demonstrating that the introduction of a short delay before reach onset can severely disrupt visuomotor performance in patients with damage to V1 and the ventral stream (22, 23). Although one could argue that response variability would have increased after a delay (thus leading to a null-effect), it is important to note that, when tested under the exact same conditions, the elderly controls (EC1, Fig. 4 A and B; and EC2, Fig. S2 C and D), and a group of younger controls (Fig. S3 C and D) demonstrated a significant sensitivity to the position of the obstacles in both visual fields in the delay condition. This makes the absence of any avoidance effect in CB's blind field in the delay condition even more striking, especially given that we had sufficient power to detect any differences if they existed (1 − β = 0.96). Taken together, these two experiments demonstrate that obstacle avoidance can operate in real-time in the absence of V1 input and that this ability does not depend on conscious perception of the location of potential obstacles.
It is important to emphasize that the obstacles in CB's blind field influenced reaching in his sighted field. Although previous work has demonstrated that stimuli presented in the blind field can influence both perceptual performance (21, 31) and eye movements (32, 33) in the sighted field, our study demonstrates such an effect in a reaching task. More importantly, given that CB completed all of his reaches without visual feedback, our data suggest that the visuomotor networks mediating reaching do not require V1 input to integrate the location of potential obstacles when planning these complex movements. Furthermore, given that CB was fixating throughout the experiment, was reaching without visual feedback, and that auditory cues from the placement of the obstacles were masked, our results cannot be due to the use of motion cues or auditory cues that may have been present in previous experiments (14, 15).
Although the current findings do not allow us to directly identify the precise neural structures that may underlie CB's remarkable sensitivity to obstacles in his blind field, recent neurophysiological, neuropsychological, and functional brain imaging data offer some clues. In the current study, CB demonstrated both a sensitivity to the position of obstacles in his blind field, as well as an implicit redundant target effect (RTE). Recent data from patients with blindsight after V1 damage (34, 35) or hemispherectomy (36, 37) implicate the retino-tectal-pulvinar pathway in the implicit RTE. Given that patient CB also demonstrates a similar implicit RTE, we have every reason to believe that his residual capacity relies on the same neural pathway identified in previous studies.
Unfortunately, the neural pathways that are responsible for spared visuomotor abilities in blindsight are not as well characterized. Recently, Danckert and Rossetti (see ref. 9) have suggested that “action blindsight” may be related to a similar retino-tectal-pulvinar pathway that sends projections to areas in dorsal parietal cortex that are known to be involved in visuomotor control (3). These projections may arrive in parietal cortex via the motion sensitive area MT+ (10). In addition, previous work suggests that an area in the ventral intraparietal sulcus (VIP) in the primate responds to objects looming near the body (38, 39). Electrical stimulation of this area evokes defensive avoidance-like behaviors (for a review, see reference 17). Finally, VIP receives direct inputs from area MT+, which in turn receives direct projections from the retino-tectal-pulvinar pathway (10, 17). Based on these data, Graziano and colleagues (17) have suggested that area VIP might be a region that is responsible for coding the position of objects in the region of space near the body. In turn, this information could be used for avoiding obstacles located near the body during movement.
In conclusion, although it is likely that these remarkably intact visuomotor abilities are mediated by the dorsal stream (3, 9), further research is needed to determine the specific cortical structures and input pathways that are involved. Future studies employing neuroimaging techniques such as functional MRI and diffusion-tensor imaging (DTI) may be able to shed light on this important issue. In any case, the results of the current study clearly indicate that we have to rethink the role of what are often considered primitive visual pathways in the mediation of complex motor behavior.
Methods
Patient CB.
CB is a 75-year-old male who suffered a right posterior cerebral artery stroke in 2005, which resulted in a lesion to the right occipital cortex (Fig. 1A). Goldman perimetry conducted at the time of current testing (3.5 years post-stroke) indicated a dense left visual field hemianopia with no evidence of macular or temporal crescent sparing (Fig. 1B). CB was born in England but immigrated to Canada after the Second World War where he worked as a pipe-fitter. In addition to having a hemianopia, CB also has Charles Bonnet Syndrome—a strange condition in which patients with sudden visual field loss experience vivid visual hallucinations in their blind field. Although CB's hallucinations have largely subsided, he still occasionally describes “seeing” trees or buildings in his blind field that are not actually present in the real world.
Although CB shows clear evidence of obstacle avoidance and a redundant target effect, he does not show any obvious evidence of Riddoch phenomenon (i.e., a residual sensitivity to motion in his blind field). Specifically, he was not able to detect moving targets in his blind field during perimetry testing conducted at the time of the present study. In addition, he describes no subjective ability to perceive the sensation of motion in his blind field. However, with more rigorous testing and enough training it may be the case that CB might be able to demonstrate some sensitivity to motion signals in his blind field (see reference 40). Finally, CB is not able to accurately localize targets presented in his blind field by pointing (we presented circular black targets 1.6 cm in diameter on a gray background at eccentricities of 15°, 25°, 35°, and 45°). While this might seem to imply that CB does not demonstrate visuomotor blindsight, we suggest that the demonstration of intact obstacle avoidance in his blind field suggests strongly that CB does have spared visuomotor abilities for stimuli presented in his blind field. It may be the case that the obstacle avoidance task we used in the current experiment may simply be a more sensitive measure of visuomotor blindsight than methods in which the patient is forced to guess the location of targets they cannot see.
Apparatus and Procedure.
Redundant target effect.
Before testing CB in the obstacle avoidance experiments, we also assessed his ability to implicitly process visual information in his blind field. To investigate this, we used the redundant target effect (a.k.a. spatial summation) developed by Marzi and colleagues (21). CB sat with his head in a chin rest 30 cm away from a 17″ CRT monitor (refresh rate 100 Hz). In this task, CB was simply asked to fixate on a central cross and to respond as quickly as possible with a button press whenever he saw a target appear on the screen. Targets were black circles (1.6 cm in diameter) presented on a uniform dark gray background that appeared 10° from fixation for 150 ms in either the right (sighted) or left (blind) visual field, or in both fields simultaneously.
Obstacle avoidance task.
In the obstacle avoidance task (see Fig. 2A) participants were required to make reaches from at start button (15 cm) to a target strip (5 cm wide at a distance of 55 cm) while avoiding obstacles that could be placed either 10 cm (or 15 cm from midline in the left or right visual field (or in both fields). During the task the participant was required to maintain fixation on an elevated fixation point located just beyond the target strip (distance = 60 cm). At this fixation distance, obstacles at the in position were located ≈10° from midline, and obstacles in the out position were located ≈15° from midline (i.e., both obstacles were well within his blind field). In Experiment 1, at the beginning of a trial, the goggles opened for 500 ms and an auditory “Go” signal was presented. In Experiment 2, at the beginning of a trial, the goggles opened for 500 ms, followed by a 2-s delay period, followed by the auditory “Go” signal.
In total there were eight different conditions tested in the experiment. (i) left in; (ii) left out; (iii) right in; (iv) right out; (v) both in; (vi) left in, right out; (vii) left out, right in; and (viii) no obstacles. The obstacles were tall wooden rectangular objects (4 × 4 × 25 cm) that were painted dark-gray. The bottoms of the obstacles were covered with felt pads to eliminate sound and vibration when the objects were placed on the table top. Both CB and the two elderly controls completed 16 repetitions of each condition in the real-time experiment (Experiment 1) and 10 repetitions of each condition in the delay experiment (Experiment 2). The younger controls completed 10 repetitions of each condition in the real-time and the delay experiments. All trials were presented in a pseudorandom sequence.
During the experiment, CB (and controls) sat at a white table in a comfortable chair with his head fixed in a chin rest with his midline aligned to an elevated fixation point positioned just beyond the target area. The experiment was completed with full overhead illumination. Fixation was strictly monitored on each trial using a video camera that was zoomed in on the patient's eyes and projected on a video monitor for inspection. Any trials in which an eye movement occurred into the patient's blind field while the PLATO goggles were open were discarded from the analysis (this occurred on only two trials). There were two experimenters in the room throughout the study. One experimenter placed the obstacles, and the second continuously monitored CB's fixation.
Data Analysis.
For details on how the kinematic data were processed, see reference 1. To analyze our data statistically, we computed separate ANOVAs for the mean horizontal deviation (i.e., X-position) at 20 cm into the reach (i.e., the distance at which the front edge of the obstacle was located) and for the reach end points for CB and the each of the elderly controls. Initially we computed one-way ANOVAs before conducting planned comparisons (2-tailed). To examine obstacle avoidance in the sighted and blind visual fields in CB and the controls, we computed three planned comparisons using Fisher's LSD tests and a Bonferroni correction [thus P = 0.016 (0.05/3)]. Specifically, we compared horizontal deviation between the two different obstacle positions in the left and the right visual fields (in vs. out). In addition, we compared horizontal deviation on trials in which two obstacles were present (i.e., left in, right out vs. left out, right in. For cases in which the overall ANOVA was not significant, we carried out these same planned comparisons using independent-samples t tests (two-tailed) with a Bonferroni correction [i.e., P = 0.016 (0.05/3)].
Supplementary Material
Acknowledgments.
The authors thank David Nicolle for performing the visual field testing with CB, James Danckert for referring patient CB to us, Gavin Buckingham for helpful comments on an earlier draft of this manuscript, and Haitao Yang for his technical assistance with this project. This work was funded through a Heart and Stroke Foundation of Canada Postdoctoral Fellowship (to C.L.S.), a Natural Sciences and Engineering Research Council (NSERC) Ph.D. award (to C.S.C.), and a Canadian Institutes of Health Research (CIHR) operating grant (to M.A.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905549106/DCSupplemental.
Although the overall ANOVA was marginally significant (P = 0.058), an independent samples t test confirmed that there was a significant difference between the reach trajectories for the two obstacle positions 25 cm into the reach in the right visual field [t(32) = 2.67, P = 0.012].
References
- 1.Chapman CS, Goodale MA. Missing in action: The effect of obstacle position and size on avoidance while reaching. Exp Brain Res. 2008;191:83–97. doi: 10.1007/s00221-008-1499-1. [DOI] [PubMed] [Google Scholar]
- 2.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 3.Milner AD, Goodale MA. The Visual Brain in Action. New York, NY: Oxford Univ Press; 2006. [Google Scholar]
- 4.Schindler I, et al. Automatic avoidance of obstacles is a dorsal stream function: Evidence from optic ataxia. Nat Neurosci. 2004;7:779–784. doi: 10.1038/nn1273. [DOI] [PubMed] [Google Scholar]
- 5.Rice NJ, et al. Delay abolishes the obstacle avoidance deficit in unilateral optic ataxia. Neuropsychologia. 2008;46:1549–1557. doi: 10.1016/j.neuropsychologia.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Rice NJ, et al. Intact automatic avoidance of obstacles in patients with visual form agnosia. Exp Brain Res. 2006;174:176–188. doi: 10.1007/s00221-006-0435-5. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh RD, McClements KI, Dijkerman HC, Birchall D, Milner AD. Preserved obstacle avoidance during reaching in patients with left visual neglect. Neuropsychologia. 2004;42:1107–1117. doi: 10.1016/j.neuropsychologia.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh RD, et al. Avoidance of obstacles in the absence of visual awareness. Proc Biol Sci. 2004;271:15–20. doi: 10.1098/rspb.2003.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danckert J, Rossetti Y. Blindsight in action: What can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci Biobehav Rev. 2005;29:1035–1046. doi: 10.1016/j.neubiorev.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vakalopoulos C. A theory of blindsight—the anatomy of the unconscious: A proposal for the koniocellular projections and intralaminar thalamus. Med Hypotheses. 2005;65:1183–1190. doi: 10.1016/j.mehy.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Weiskrantz L. Blindsight: A Case Study and Implications. Toronto, Canada: Oxford Univ Press; 1986. [Google Scholar]
- 13.Weiskrantz L, Warrington EK, Sanders MD, Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97:709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- 14.de Gelder B, et al. Intact navigation skills after bilateral loss of striate cortex. Curr Biol. 2008;18:R1128–R1129. doi: 10.1016/j.cub.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Dutton GN. Cognitive vision, its disorders and differential diagnosis in adults and children: Knowing where and what things are. Eye. 2003;17:289–304. doi: 10.1038/sj.eye.6700344. [DOI] [PubMed] [Google Scholar]
- 16.Warren WH, Jr, Kay BA, Zosh WD, Duchon AP, Sahuc S. Optic flow is used to control human walking. Nat Neurosci. 2001;4:213–216. doi: 10.1038/84054. [DOI] [PubMed] [Google Scholar]
- 17.Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:845–859. doi: 10.1016/j.neuropsychologia.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Mestre DR, Brouchon M, Ceccaldi M, Poncet M. Perception of optical flow in cortical blindness: A case report. Neuropsychologia. 1992;30:783–795. doi: 10.1016/0028-3932(92)90082-w. [DOI] [PubMed] [Google Scholar]
- 19.Riddoch G. Dissociation of visual perceptions due to occipital injuries, with especial reference to appreciation of movement. Brain. 1917;40:15–57. [Google Scholar]
- 20.Zeki S, Ffytche DH. The Riddoch syndrome: Insights into the neurobiology of conscious vision. Brain. 1998;121:25–45. doi: 10.1093/brain/121.1.25. [DOI] [PubMed] [Google Scholar]
- 21.Marzi CA, Tassinari G, Aglioti S, Lutzemberger L. Spatial summation across the vertical meridian in hemianopics: A test of blindsight. Neuropsychologia. 1986;24:749–758. doi: 10.1016/0028-3932(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 22.Goodale MA, Jakobson LS, Keillor JM. Differences in the visual control of pantomimed and natural grasping movements. Neuropsychologia. 1994;32:1159–1178. doi: 10.1016/0028-3932(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Rossetti Y. Implicit short-lived motor representations of space in brain damaged and healthy subjects. Conscious Cogn. 1998;7:520–558. doi: 10.1006/ccog.1998.0370. [DOI] [PubMed] [Google Scholar]
- 24.Milner AD, Dijkerman HC, McIntosh RD, Rossetti Y, Pisella L. Delayed reaching and grasping in patients with optic ataxia. Prog Brain Res. 2003;142:225–242. doi: 10.1016/S0079-6123(03)42016-5. [DOI] [PubMed] [Google Scholar]
- 25.Milner AD, et al. Grasping the past. Delay can improve visuomotor performance. Curr Biol. 2001;11:1896–1901. doi: 10.1016/s0960-9822(01)00591-7. [DOI] [PubMed] [Google Scholar]
- 26.Rossetti Y, et al. Visually guided reaching: Bilateral posterior parietal lesions cause a switch from fast visuomotor to slow cognitive control. Neuropsychologia. 2005;43:162–177. doi: 10.1016/j.neuropsychologia.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Revol P, et al. Pointing errors in immediate and delayed conditions in unilateral optic ataxia. Spat Vis. 2003;16:347–364. doi: 10.1163/156856803322467572. [DOI] [PubMed] [Google Scholar]
- 28.Himmelbach M, Karnath HO. Dorsal and ventral stream interaction: Contributions from optic ataxia. J Cognit Neurosci. 2005;17:632–640. doi: 10.1162/0898929053467514. [DOI] [PubMed] [Google Scholar]
- 29.Cowey A, Wilkinson F. The role of the corpus callosum and extra striate visual areas in stereoacuity in macaque monkeys. Neuropsychologia. 1991;29:465–479. doi: 10.1016/0028-3932(91)90005-s. [DOI] [PubMed] [Google Scholar]
- 30.Parker AJ. Binocular depth perception and the cerebral cortex. Nat Rev Neurosci. 2007;8:379–391. doi: 10.1038/nrn2131. [DOI] [PubMed] [Google Scholar]
- 31.Danckert J, Maruff P, Kinsella G, de Graaff S, Currie J. Investigating form and colour perception in blindsight using an interference task. Neuroreport. 1998;9:2919–2925. doi: 10.1097/00001756-199809140-00001. [DOI] [PubMed] [Google Scholar]
- 32.Van der Stigchel S, van Zoest W, Theeuwes J, Barton JJ. The influence of “blind” distractors on eye movement trajectories in visual hemifield defects. J Cognit Neurosci. 2008;20:2025–2036. doi: 10.1162/jocn.2008.20145. [DOI] [PubMed] [Google Scholar]
- 33.Rafal R, Smith J, Krantz J, Cohen A, Brennan C. Extrageniculate vision in hemianopic humans: Saccade inhibition by signals in the blind field. Science. 1990;250:118–121. doi: 10.1126/science.2218503. [DOI] [PubMed] [Google Scholar]
- 34.Marzi CA, Mancini F, Metitieri T, Savazzi S. Blindsight following visual cortex deafferentation disappears with purple and red stimuli: A case study. Neuropsychologia. 2009;47:1382–1385. doi: 10.1016/j.neuropsychologia.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Tamietto M, et al. Collicular vision guides nonconscious behavior. J Cognit Neurosci. 2009 doi: 10.1162/jocn.2009.21225. Mar 25 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Leh SE, Mullen KT, Ptito A. Absence of S-cone input in human blindsight following hemispherectomy. Eur J Neurosci. 2006;24:2954–2960. doi: 10.1111/j.1460-9568.2006.05178.x. [DOI] [PubMed] [Google Scholar]
- 37.Leh SE, Ptito A, Schonwiesner M, Chakravarty MM, Mullen KT. Blindsight mediated by an S-cone-independent collicular pathway: An fMRI study in hemispherectomized subjects. J Cognit Neurosci. 2009 doi: 10.1162/jocn.2009.21217. Mar 23 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Colby CL, Duhamel JR. Spatial representations for action in parietal cortex. Brain Res Cogn Brain Res. 1996;5:105–115. doi: 10.1016/s0926-6410(96)00046-8. [DOI] [PubMed] [Google Scholar]
- 39.Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: Anatomic location and visual response properties. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- 40.Huxlin KR, et al. Perceptual relearning of complex visual motion after V1 damage in humans. J Neurosci. 2009;29:3981–3991. doi: 10.1523/JNEUROSCI.4882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.