Abstract
CD1d-restricted natural killer (NKT) cells are important contributors to antigen-specific antibody responses. There is, therefore, considerable interest in the design and implementation of strategies to appropriately activate NKT cells and boost vaccine-induced protective antibody responses. In order to achieve these goals, investigators are examining the mechanisms by which NKT cells enhance antibody responses. Although information is limited, it is now appreciated that both cognate and noncognate interactions between CD1d-expressing B cells and NKT cells drive enhanced antibody responses. NKT cells may provide B-cell help in the form of direct receptor-mediated interactions as well as by secretion of soluble effectors, including cytokines. In this article, we review the evidence in support of these mechanisms and discuss how they likely take place in the context of interactions of NKT cells with other cell types, such as dendritic cells and helper T cells. We also discuss the evidence that NKT cells affect discrete differentiation events in the multistep process by which a naive B cell experiences antigen and develops into a memory B cell or an antibody-secreting plasma cell. Since most information on NKT cells and humoral immunity has been derived from murine studies, we discuss what is known about human NKT cells and humoral immunity. We offer thoughts on whether the findings in murine systems will translate to humans.
Keywords: antibody, B lymphocyte, cognate interaction, NKT cell, plasma cell
CD1d & NKT cells
CD1d is an MHC class I-related molecule (referred to as class I throughout this article) with limited polymorphism and is expressed primarily by professional antigen-presenting cells (APCs) [1–3]. CD1d contains a large hydrophobic groove [4], which facilitates loading with glycolipid antigens (Ags) and related structures orienting a carbohydrate headgroup for recognition by the T-cell Ag receptor (TCR) on natural killer (NKT) cells [5–8]. CD1d can be loaded with self-glycolipid Ags, such as lysosomal isoglobotrihexosylceramide [9] and the myelin-derived glycolipid sulfatide [10]. Cd1d binds foreign glycolipid Ags including α-galactosylceramide (α-GC) [11], and several bacterial glycolipid and diacylgylcerol molecules [12–14]. The α-GC molecule was originally discovered in the marine sponge Agelas mauritianus but is now synthesized in the laboratory [15]. It consists of an acyl chain and a sphingosine chain coupled to a galactose headgroup. α-GC is recognized by all type I NKT cells and has been a critical reagent Ag used for understanding type I NKT-cell function. The α-GC designation and other terms used throughout this article are described in Table 1.
Table 1.
The terminology specific to the B cell and natural killer T cell fields of research that are used in this article.
| Term | Definition |
|---|---|
| Cells | |
| B-1 B cell | Innate-like B cell found in peritoneal cavity, pleural cavity and spleen |
| Memory B cell | Defines B cells that have experienced Ag and undergone germinal center reactions Can self-renew for maintenance of memory or differentiate into plasma cells upon restimulation with Ags |
| Dendritic cell | A group of professional APCs that express class I, class II and CD1d Potent activators of naive T cells and of resting NKT cells |
| Follicular B cell | Major class of B lymphocyte resident in secondary lymphoid organs and responds to T-cell-dependent Ags |
| Marginal zone B cell | Found in splenic marginal zone of mice Responds to blood-borne T-cell-independent Ags Results in characteristic IgM and IgG3 response |
| Plasma cell | Antibody-secreting cell found in periphery, secondary lymphoid organs or bone marrow |
| Short-lived plasma cell | Can differentiate from Ag-experienced B cells without undergoing germinal center reactions |
| Long-lived plasma cell | Competes for survival niches in bone marrow and maintains serum antibody titers |
| NKT | A lymphocyte with T-cell lineage and shared properties of T cells and natural killer cells |
| – Type I NKT | CD1d-restricted NKT expressing Vα14 invariant TCR (mouse) and Vα24 TCR (human) Reactive to CD1d–α-GC complexes |
| – Type II NKT | CD1d-restricted NKT cells with variable TCR usage Reactive to CD1d–sulfatide complexes |
| – Type III NKT | Diverse group of T cells expressing natural killer markers, with variable TCR usage Not CD1d restricted |
| Helper T cell | CD4+, class II-restricted T cell that following appropriate stimulation by dendritic cells, differentiates into an effector cell that secretes cytokines to influence the immune response |
| Reagents | |
| α-GC | CD1d-binding glycolipid derived from the Agelas Mauritianus marine sponge Stimulates type I NKT cells |
| C-glycoside α-GC | Synthetic α-GC-based molecule that stimulates production of Th1 cytokines by Type I NKT cells |
| NP–α-GC | Synthetic α-GC with nitrophenol hapten (4-hydroxy-3-nitrophenyl-α-GC) |
| OCH | A truncated α-GC-based molecule that stimulates production of Th2 cytokines by Type I NKT cells |
α-GC: α-galactosylceramide; Ag: Antigen; APC: Antigen-presenting cell; NKT: Natural killer T cell; NP: Nitrophenol; TCR: T-cell receptor.
CD1d molecules localize to membrane rafts, recycle from the plasma membrane to early endocytic vesicles and are found in MHC class II-like loading compartments (MIIC) [16–21]. The presence of CD1d at different subcellular locations may allow loading and presentation of structurally diverse Ags following uptake or capture by APCs. Several molecules, including microsomal triglyceride transfer proteins, saposins and the Niemann Pick C2 protein, are important for loading of murine and human CD1d with foreign or self-Ags [22–25]. These significant advances in understanding CD1d cell biology are beyond the scope of the discussion herein and the reader is directed to an excellent review, which discusses trafficking and Ag loading of the major CD1 family members [26].
B cells, the focus of this article, can capture exogenous foreign CD1d Ag in different ways. If present in sufficiently high concentrations, cell-surface CD1d can be loaded with Ag directly [27,28]. In lower concentrations, intracellular trafficking of Ag to MIIC vesicles is generally required for efficient loading and presentation [17–20,29]. The mechanism of Ag capture by B cells is not clear, but may be mediated by Ag bound to serum proteins such as ApoE. It was shown that APCs (dendritic cells [DCs]) could capture and present ApoE/α-GC complexes via the low-density lipoprotein receptor and present them on CD1d to activate NKT cells [30]. CD1d-binding glycolipid can also be captured directly by the B-cell Ag receptor (BCR) and then internalized to CD1d-containing endosomes [28,31,32], a process that appears to make Ag presentation highly efficient [28]. BCR-mediated and non-BCR-mediated capture of CD1d Ag may permit flexibility in the response to CD1d Ags, thus leading to distinct immunological outcomes.
Natural killer T cells represent specialized T-cell subsets that encompass the developmental, phenotypic and functional properties of NK cells and T cells [33,34]. NKT cells make contributions to innate and adaptive immune responses, playing both stimulatory and regulatory roles [33,34]. Consequently, NKT cells have been implicated in autoimmunity, cancer, allergy, asthma and infectious disease. There are different NKT subsets and their basic features are shown in Table 2. Most type I NKT cells express cell surface proteins typical of NK and T cells. These include NK1.1 and an invariant TCR conferred by Vα14 (mouse) TCR gene segments [35]. However, owing to variable expression of NK markers, type I NKT cells are best described as a type of T cell that expresses the Vα14 semi-invariant TCR. Thus, type I NKT cells are routinely tracked using CD1d tetramers loaded with α-GC, which are specifically recognized by the Vα14 TCR [36]. Type I NKT cells are stimulated by CD1d-expressing APCs that present various foreign glycolipid Ags to the Vα14 TCR [11–14]. Importantly, since the initial discovery that NKT cells were CD1d-restricted [37], several laboratories have reported that CD1d/α-GC complexes activate Vα14 NKT cells, leading to the production of cytokines and the stimulation or modulation of immune responses (reviewed in [33,34]).
Table 2.
Summary of the properties of murine type I, II and III natural killer T cells.
| Type I | Type II | Type III | |
|---|---|---|---|
| Location(s) | Thymus, spleen, liver, lymph node and bone marrow | Mainly spleen and bone marrow | Mainly bone marrow |
| TCR | Invariant Vα (Vα14) Semi-invariant Vβ (Vβ2, Vβ7 and Vβ8) | Diverse | Diverse |
| Reactivity | CD1d restricted | CD1d restricted | Not CD1d restricted |
| Major ligand | α-GC | Sulfatide | NA |
α-GC: α-galactosylceramide; NA: Not available; TCR: T-cell receptor.
Data taken from [33].
Type II NKT cells express diverse non-Vα14 TCRs, are CD1d-restricted and do not react to α-GC [38,39]; however, a subset are stimulated by the lysosulfatide molecule bound to CD1d [10,22]. There is considerably less information on type II NKT cells, although protection against experimental autoimmune encephalitis and the suppression of antitumor immunosurveillance have been ascribed to these cells [10,40].
All NKT cells develop in the thymus and positive selection is governed by cortical thymocyte expression of CD1d [41–43]. Indeed, CD1d−/− mice do not develop any NKT subsets [44–46]. Thus, these models are used by researchers to determine whether specific biological effects are CD1d-dependent. Mice with a deletion in the Jα18 gene segment of the invariant TCR fail to rearrange the Vα14/Jα18 TCR gene and cannot express any functional TCR on type I NKT cells [47]. The Jα18−/− mice therefore develop all T-cell subsets except for type I NKT cells, despite normal CD1d expression. In mice with normal CD1d expression and intact TCR genes, mature NKT cells are exported to the periphery and are detected in the spleen, liver and lymph node, as well as other tissues [33,39]. Further functional subsets appear to exist in the periphery based on differential expression of CD4 and CD8 and on anatomical localization [48,49].
Natural killer T cells have an innate activated/memory phenotype and, unlike CD4+ MHC class II (class II)-restricted T cells, do not require priming for activation by CD1d/glycolipid complexes [50,51]. NKT cells respond immediately to a primary stimulation with CD1d/Ag complexes, leading to production of both Th1 and Th2 cytokines by the same cell (at least in mice) and within a few hours of stimulation (reviewed in [33,34]). By contrast, naive CD4+ class II-restricted T cells require priming by DC class II Ag presentation and engagement of TCR coreceptors. This leads to the development of helper T (Th) cells. These are primarily Th1 or Th2 effector cells that produce large amounts of cytokine upon restimulation with the original Ag but without the requirement for costimulation. Consequently, cytokine production is measured in the order of days rather than hours. This topic is reviewed elsewhere [52]. This suggests that NKT cells function differently from class II-restricted Th cells in regulation of adaptive immunity.
NKT cells in the generation of humoral immunity
Several publications have linked NKT cells to B-cell regulation and to antibody (Ab) responses against allergens and parasitic, bacterial, viral and model Ags. Some of the earliest clues regarding NKT cells and humoral immunity are derived from allergy studies. SJL mice were unable to produce significant IgE upon B-cell IgD cross-linking and also lacked a population of IL-4-producing CD4+ NK1.1+ T cells [53]. This study conceptually linked T cells expressing NK1.1 to the IL-4-dependent production of IgE. By contrast, a CD1d−/− mouse was shown to be deficient in the IL-4-producing NK1.1+ T cells, yet was able to mount an IgE response, suggesting that requirements for NKT cells in Th2-driven Ab responses were not absolute [44]. Other reports have since shown that NKT cells make a measurable contribution to allergic IgE responses [54–56]. Sensitization of mice by subcutaneous administration of ovalbumin (OVA) followed by airway challenge with OVA induces experimental asthma. In this model, NKT-deficient mice had reduced eosinophil recruitment, cytokine production, edema and OVA-specific IgE production as compared with wild-type controls [54,56]. NKT cells contribute to other forms of immune hypersensitivity. In a mouse model of contact-induced hypersensitivity, Ag induced NKT cells to stimulate production of IgM by B-1 B cells in an IL-4- and CD1d-dependent manner. IgM in turn induced complement fixation and T-cell infiltration to the skin [57]. B-1 B cells express CD1d and have also been implicated in the production of autoreactive dsDNA-reactive Abs [58]. In that study, purified NKT cells in vitro were able to stimulate autoantibody production by cultured B-1 B cells from a lupus-prone mouse strain.
The contribution of NKT cells to antiparasite Ab responses has been evaluated with conflicting results. In one study, the production of Abs reactive to glycosylphosphatidylinositol (GPI)-anchored circumsporozoite proteins from Plasmodium falciparum was abrogated in CD1d−/− mice but not class II−/− mice [59]. In another study using a higher dose of Ag, the responses were entirely class II-dependent [60]. It is possible that NKT cells make measurable contributions to the Ab response when the Ag dose is limiting, but are redundant when higher amounts of Ag are administered. In a further study, GPI-anchored mucin-like glycoproteins from Trypanosomes stimulated Ab responses in a CD1d-independent manner [61]. The exact role of NKT cells in antiparasite Ab responses therefore requires clarification.
Natural killer T cells regulate Ab responses to pathogenic bacteria. CD1d−/− mice infected with the spirochetes Borrelia hermsii and Borrelia burgdorferi had impaired production of a specific Ab and a higher pathogen burden than CD1d-expressing controls [62,63]. Production of Streptococcus pneumoniae polysaccharide-reactive Abs in vivo was also shown to be reduced in CD1d−/− mice as compared with the control strain [64].
Natural killer T cells have the potential to improve antiviral Ab responses. α-GC had an adjuvant effect on mucosal IgA production following intranasal coadministration with influenza hemagglutinin Ags [65]. The enhanced anti-hemagglutinin mucosal IgA and serum IgG responses correlated with protection from a lethal challenge with live virus. These findings raise the intriguing possibility that NKT-activating ligands could be incorporated into mucosal vaccine strategies.
The α-GC ligand had an adjuvant effect on humoral responses to T-cell-dependent and T-cell-independent model Ags [66]. Specific anti-OVA or keyhole limpet hemocyanin (KLH) Ab responses were considerably improved by the coadministration of α-GC during vaccination. Anti-nitrophenol (NP) Ab responses induced by NP-ficoll were altered by α-GC. Although no difference in the classical IgM and IgG3 response was detected, there was a selective induction of NP-specific IgG1, suggesting that NKT cells can be harnessed to produce the desired Ab subclasses. This could be of importance for generating suitable Ab responses against carbohydrate Ags, which typically lead to low-affinity short-lived IgM responses unless incorporated into expensive (but effective) glycoconjugate vaccines.
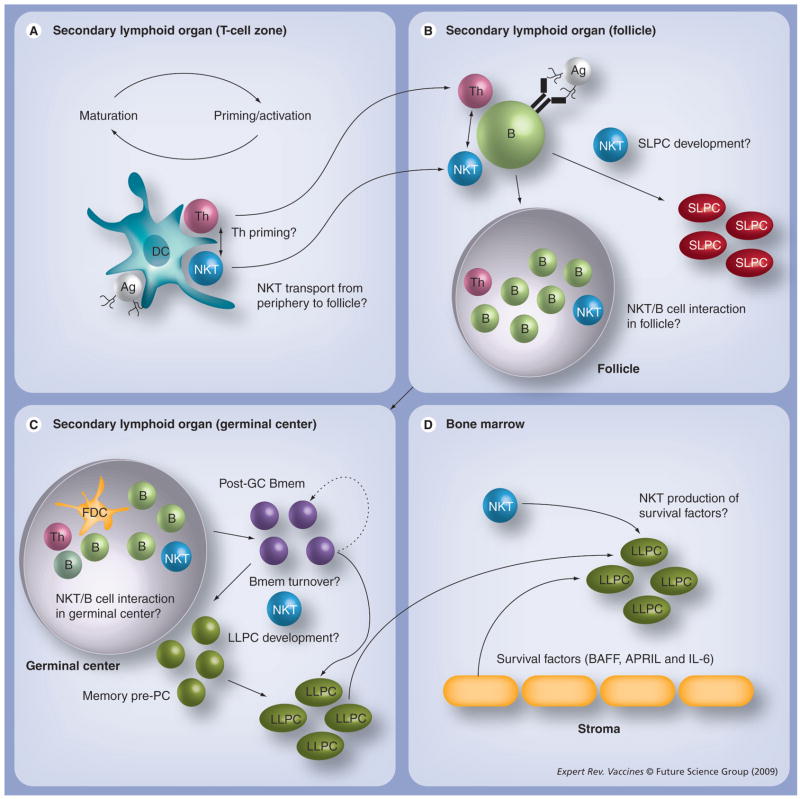
The findings discussed thus far show that NKT cells regulate humoral immune responses. They also highlight the potential value of atypical vaccination strategies that can rely on agents that stimulate cell types not previously targeted by vaccines. However, beyond a demonstration of CD1d dependence, these valuable contributions to the field have not sufficiently elucidated the mechanisms by which NKT cells impact humoral immunity. Therefore, in this article we will discuss the possible points of interaction between NKT cells and the humoral immune apparatus. The reader is referred to Figure 1 throughout the article, which highlights some of the key questions in the field.
Figure 1. Model for regulation of humoral immunity by natural killer T cells.
(A) DCs that have captured T-cell-dependent Ags and CD1d-binding glycolipid Ags in the periphery drain to lymph nodes, process and present the Ags on class II and CD1d to the T-cell receptor of Th and NKT cells, respectively. These interactions are probably reinforced by B7:CD28 and CD40:CD154 interactions, leading to DC maturation and increasing Th priming and NKT interaction. The various cognate interactions between NKT cells and other cell types are shown in Figure 2. NKT cells may also facilitate enhanced Th priming. (B) The primed Th cells and activated NKT cells provide cognate help to B cells, resulting in clonal expansion, further cognate interaction in the secondary follicle and differentiation of some B cells into SLPCs. (C) Germinal center reactions occur, facilitated by FDCs, Th cells and possibly NKT cells, leading to successive rounds of positive and negative deletion, and the emergence of Bmem cells, which then differentiate into LLPC precursors, or self-renew to maintain the Bmem population. (D) LLPCs migrate to the bone marrow and compete for survival niches. Long-term survival is actively supported by stromal cell factors. Some NKT cells (including type 2) are present in BM and could potentially contribute to the survival of LLPCs. Questions indicate events that are not yet understood. Other parts of the model are supported by published work cited throughout the article.
Ag: Antigen; Bmem: Memory B cell; DC: Dendritic cell; FDC: Follicular dendritic cell; GC: Galactosylceramide; LLPC: Long-lived plasma cell; NKT: Natural killer T cell; PC: Plasma cell; SLPC: Short-lived plasma cell; Th: T helper.
Generation of humoral immunity
Foreign T-cell-dependent Ags are captured by DCs, which internalize, process and present the Ag on class II. If the DC has received concomitant adjuvant-derived signals, usually through Toll-like receptors (TLRs), DC maturation is stimulated, leading to more efficient class II presentation and increased expression of costimulatory molecules. Naive T cells are ‘primed’ by DCs presenting Ags and providing co-stimulation, leading to their development into effector cells known as Th cells. The Th cells then provide appropriate help to B cells that have acquired the Ag via the BCR, and presented peptides on class II (Figure 1A & B). Ultimately, many coordinated events lead to the differentiation of B cells into memory B cells or Ig-secreting plasma cells (PCs). Large particulate T-cell-independent Ags with repeating molecular structures stimulate a different response by cross-linking the BCR and stimulating short-lived Ab responses in a manner that is not dependent on T-cell help. The reader is directed to [52] for a thorough discussion of these events.
Importantly, there are distinct fates for Ag-experienced B cells with implications for long-term humoral immunity. Ag-experienced B cells can develop in germinal centers where rounds of clonal expansion, selection and affinity maturation occur. It is not known how (or even if) NKT cells interact with B cells in the germinal center, but previous studies have shown that NKT activation can increase specific Ab affinity [67], suggesting that interaction within the germinal center is possible. At present, it is not known if key cells in the germinal center reaction, such as follicular DCs or CD1d-expressing B cells, present CD1d Ags to NKT cells during this process. Follicular DCs present native T-cell-dependent Ags to the BCR to allow B-cell Ag capture [68], but they have not been examined in the context of CD1d presentation of glycolipids.
Ag-experienced B cells undergoing germinal center reactions emerge largely as postgerminal center B cells with a memory phenotype (Bmem; reviewed in [69]) (Figure 1C). Different populations of Bmem have been described, and are readily detected and distinguished from naive B cells by flow cytometry [70]. Although the mechanisms are not clear, Bmem cells undergo self-renewal to maintain a pool of Ag-experienced Bmem cells that can respond rapidly to secondary exposure to Ags or infection following vaccination (Figure 1C) [69,71]. The survival of Bmem cells appears to be largely independent of persisting Ags after vaccination [72,73]. Polyclonal stimulation of vaccination-induced Bmem with TLR agonists can induce Ab recall responses [74]. Bmem cells are subject to many regulatory processes, which are not fully defined, but researchers agree that they are critical for Ab recall responses and long-term humoral immunity. As we will discuss, NKT cells may impact Bmem development and function.
Bmem cells can differentiate into Ab-producing long-lived PCs (LLPCs) via different developmental pathways [69]. LLPCs are largely bone marrow-resident cells that occupy distinct survival niches and are a major determinant of the duration of a humoral immune response (Figure 1D) [75–79]. Many factors control the development of Bmem and PCs, such as affinity for Ags, cognate interactions in germinal centers, Ag persistence and activation of B-cell survival factors [80–82]. Thus, events at the inductive phase of an immune response, perhaps including NKT-mediated help, govern Bmem and PC development, and therefore long-term humoral immunity. At this time, our understanding of how NKT cells interact with follicles and germinal centers is limited. However, answering several key questions as detailed later will advance our understanding of these events.
Which NKT cells help B cells?
Available evidence strongly supports the idea that type I NKT cells provide B-cell help when an exogenous CD1d Ag is administered. In C57Bl/6 mice with full expression of type I and type II NKT cells, α-GC induces greatly enhanced primary and recall Ab titers [66,67,83], showing that type I NKT cells provide B-cell help when appropriately stimulated. By contrast, in CD1d−/− mice lacking type I and type II cells, or Jα18−/− mice lacking type I NKT cells, α-GC is unable to boost Ab responses.
A role for type II cells in providing B-cell help is not clear. In CD1d−/− mice lacking CD1d type I and type II NKT cells, PC induction by T-cell-dependent Ags either administered alone or adsorbed to alum is reduced by 50–70% compared with the control strain [67]. This initially suggests that type I and/or type II NKT cells are important for the response to Ag/Alum. However, we have observed a lower frequency of bone marrow B220lo/CD138+ cells consistent with PC precursors in CD1d−/− mice as compared with C57Bl/6 or Jα18−/− mice [Shah HB, Lang ML; Unpublished Data]. For this reason it is difficult to assign a specific role to type II NKT cells in providing B-cell help. These data suggest the possibility that type II NKT cells are involved in development of the B-cell compartment. Available data indicate as yet undefined roles for type I and type II NKT cells in providing B-cell help, but do suggest the intriguing possibility that they affect B cells differently.
Which B cells receive help from NKT cells?
Follicular (FO), B-1 and marginal zone (MZ) B-cell subsets have varying expressions of CD1d [84–86] but there is little evidence to show that they are differentially responsive to NKT cells. The papers cited earlier in this article show that NKT cells can contribute to T-cell-dependent and -independent Ab responses driven by MZ, B-1 and FO B cells [62,64–66]. Histological analysis supports this contention, since the examination of splenic sections revealed large foci of hen egg lysozyme (HEL)-reactive PCs that developed following immunization with large particulate Ags containing HEL and α-GC [31]. In that study, a T-cell-dependent Ag was administered, but the HEL-specific PCs were extrafollicular and therefore consistent with an expanded MZ population. By contrast, in a lupus-prone mouse strain, some CD1dhi B cells with a MZ phenotype clustered in B-cell follicles [85]. B cells in the context of NKT activation appear to not compartmentalize conveniently according to the FO, MZ or B-1 designation, nor according to low versus high CD1d expression. Furthermore, a recent publication indicates that MZ B cells and DCs can activate NKT cells in a cooperative fashion, reminding researchers not to assume that different B cells and NKT cells interact in isolation [87]. It is, however, tempting to speculate that a B cell instructed by an NKT cell behaves differently from a B cell instructed by a Th cell, and differently again from a B cell instructed cooperatively by Th and NKT cells. Furthermore, the type of B cell, BCR specificity, the Ag, the relative efficiency of class II and CD1d Ag presentation, and the corresponding amount of help from Th cells and NKT cells may subtly change the developmental fate of the B cell and the immunological outcome.
The discovery of previously unidentified CD1d+ B-cell subsets may shed light on these issues. A population of intestinal villous B cells with a IgM+, IgG+, CD19+, CD21lo and CD23lo phenotype was observed at lower frequencies in CD1d−/− and Jα18−/− mice than in C57Bl/6 controls [88]. A similar population of B cells in the gut-associated lymphoid tissue was also able to produce IL-10 and is suggested to have regulatory properties [89]. Whether such NKT-dependent villous B cells receive help from NKT cells to produce Abs is presently unknown. NKT cells are not abundant in the intestine and direct interaction with villous B cells at this location may not be required for their function. Another B-cell population was more recently observed in the spleen, lymph node and peritoneal cavity [90]. These cells are described as B220+, CD19+, CD5+ and CD1dhi, and following activation by lipopolysaccharides are able to produce IL-10 [90]. These ‘B10’ regulatory B cells are speculated to contribute to NKT-induced anti-inflammatory and tolerogenic responses. It therefore appears that multiple B-cell subsets may receive help from NKT cells.
What stimulates a NKT cell to help a B cell?
CD1d expressed by B cells appears necessary for α-GC-enhanced Ab responses [31,32,91]. We transferred CD1d+/+ and CD1d−/− B cells into B-cell-deficient μMT mice before immunization and observed a failure of α-GC to enhance Ab responses, despite CD1d expression on other APCs and NKT responsiveness to α-GC [91]. In an earlier study, we reported that the BCR on CD1d-transfected IIA1.6 cells could capture complexes containing α-GC and present them on CD1d to NKT hybridomas [28]. Two laboratories advanced this work with in vivo experiments to show that BCR-targeted α-GC could enhance Ab responses to coadministered T-cell-dependent Ags, and that Abs could be produced against the NP hapten on a BCR-targeted NP-linked α-GC molecule [31,32]. One recent report also provides evidence that NKT cells can provide B-cell help in the absence of cognate CD1d/TCR interactions between B cells and NKT cells, indicating that other APCs, presumably DCs, could mediate this interaction [92]. In that study, bone marrow chimeras were created in μMT mice, whereby B cells did not express CD1d and α-GC was able to stimulate an NKT-enhanced Ab response. In the same study, a bone marrow chimeric approach revealed that if CD1d and class II were never expressed on the same APC (including B cells and DCs), then the enhancing effect of α-GC was lost [92]. This was consistent with earlier findings that the physical separation of α-GC and Ag by coadministration to different locations during immunization or administration at different times led to diminished Ag-specific responses [66,93]. In light of these data, the concept that NKT cells can provide B-cell help without cognate interactions seems at first counter-intuitive. However, it is possible that in chimeric mice where B cells are CD1d−/− and DCs are CD1d+/+, DC can co-present class II and CD1d Ag, leading to NKT activation and Th priming, such that B cells can still receive help from NKT cells in a noncognate fashion. In chimeric mice where APCs are either class+/+/CD1d−/− or class II−/−/CD1d+/+, the DCs are unable to co-present Ags and invoke NKT cell help. Further research is required to reconcile these apparently differing results from the two studies.
As suggested, it likely that CD1d expressed by cell types other than B cells is important for NKT cells to provide B-cell help. DCs are potent activators of NKT cells, stimulating prolonged IFN-γ responses and leading to induction of tumor-specific cytotoxic T-lymphocyte responses [93–95]. α-GC leads to DC maturation in vivo, suggesting that NKT-derived factors such as CD154 could drive CD40-dependent DC maturation [96]. NKT-matured DCs could therefore have an enhanced capacity to prime Ag-specific, class II-restricted Th cells and improve B-cell help independently of cognate B:NKT-cell interactions. Indeed, NKT-cell activation can lead to priming of Th cells, although a direct role in boosting B-cell help has not been demonstrated [66,93]. DC presentation of class II/Ags is essential for priming of naive Th cells and induction of humoral immunity [97]. The exact role of DC CD1d Ag presentation in Ab production is not known, but it would be very surprising if B-cell-driven help from NKT cells happened without a contribution from CD1d+ DCs. As shown in Figure 1A & B, this raises the question of whether the NKT cells that interact with DCs that encounter Ags in the periphery migrate to secondary lymphoid organs and interact with B cells, or if different pools of NKT cells make distinct contributions to different phases of the humoral immune response.
Which mechanisms do NKT cells employ to help B cells?
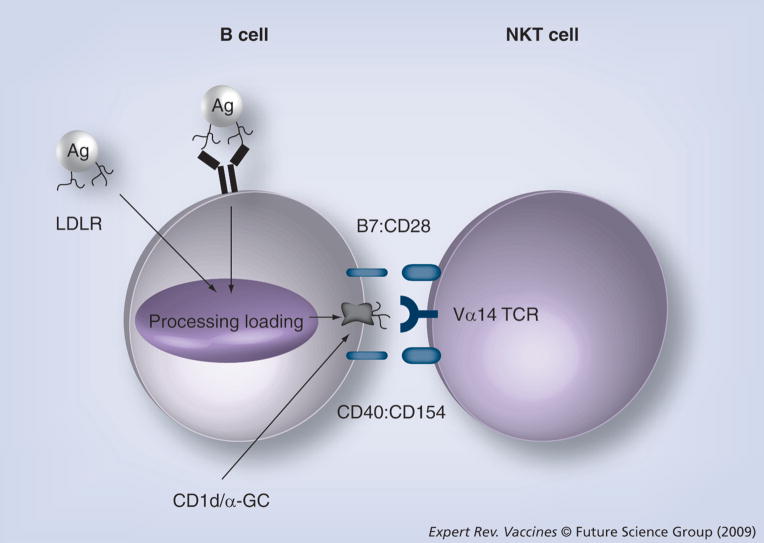
There are likely several mechanisms by which NKT cells help B cells. The findings that both CD1d/TCR cognate interactions [31,32,91] and noncognate interactions [92] lead to B-cell help is consistent with the idea that both NKT cell surface receptors and NKT-derived soluble factors contribute to B-cell help. The current suspected B cell/NKT cell cognate interactions are shown in Figure 2.
Figure 2. Possible cognate interactions between B cells and natural killer T cells during antibody responses.
B cell to NKT cell CD1d:TCR interactions were shown to be important for α-GC-enhanced Ab responses against T-cell-dependent Ag [31,91] and for antibody responses against nitrophenol-α-GC [32]. Specific interactions between NKT cell CD154 and B cell CD40 or NKT cell CD28 and B cell B7 have not yet been demonstrated in the context of antibody responses but evidence cited herein suggests that these molecules are important. In this model, intracellular CD1d is loaded with glycolipid Ag captured via the BCR or LDLR and presented on the cell surface as CD1d/Ag complexes. CD1d/Ag and B7 engage the NKT TCR and CD28, respectively, leading to NKT activation and upregulation of CD154 expression. The increased CD154 expression leads to heightened engagement of CD40 on the B cell, providing important help for development and differentiation into memory B cells. It is likely that numerous as yet unidentified interactions take place between B cells and NKT cells. Not shown are interactions with Th cells, which will utilize similar cognate interactions, while presenting T-cell-dependent Ag-derived peptide Ag on class II.
Ag: Antigen; GC: Galactosylceramide; LDLR: Low-density lipoprotein receptor;
NKT: Natural killer T cell; TCR: T-cell antigen receptor.
Class II-null mice lack all class II-restricted T cells, including all Th cells and are typically unable to mount an Ab response against T-cell-dependent Ag. We reported that a combination of OVA, α-GC and an agonistic anti-CD40 mAb could induce an OVA-specific IgG response [66], showing that NKT cells can provide limited B-cell help independently of class II:Th TCR interactions. Galli and colleagues confirmed our findings using tetanus toxoid as the Ag, but did not require anti-CD40 mAbs for an Ab response [83]. The reason for this disparity is unclear, but the data suggest that NKT-derived CD154 could be important for NKT-enhanced Ab production, since the signal could not have come from class II-restricted OVA-specific Th cells. In order to address these issues, experimental systems are required in which CD154 is expressed normally on all cells except for NKT cells.
There has been significant progress in understanding the role of B7 costimulation via CD28 pathways in NKT cell development and activation, but there is little direct evidence regarding NKT-enhanced Ab responses. CD28 and B7-blocking mAbs have revealed that α-GC-stimulated expression of IFN-γ, but not IL-4, by NKT cells is CD28 dependent [11,98]. B7−/− mice are also unable to mount an anti-NP Ab response against NP–α-GC molecules [32], suggesting, but not demonstrating, that CD28 expressed by NKT cells could be important. Unfortunately, a chimeric system where only NKT cells lack CD28 is not feasible at this time. CD28−/− and B7−/− mice have a decreased number of thymic and therefore peripheral NKT cells [98–100].
Some information is available on the NKT-derived cytokines that regulate humoral immunity [32,83]. IFN-γ−/− mice but not IL-4−/− mice were unable to mount Ab responses against NP–α-GC molecules [32]. This demonstrated that IL-4 from any source is redundant for this specific Ab response but does not preclude IL-4 as playing some modulatory role. IFN-γ is undoubtedly critical for the anti-NP–α-GC Ab response, but since it could not be produced by any cell in IFN-γ−/− mice it remains unknown whether NKT-derived IFN-γ is important. In another study, IL-4 and IFN-γ were dispensable for α-GC-enhanced Ab responses against tetanus toxin using similar methods [83]. Although both IL-4−/− and IFN-γR−/− mice were able to mount an Ab response against tetanus toxoid when α-GC was coadministered, the titers were noticeably lower in the IL-4−/− mice.
Several structural variants of the α-GC molecule have been designed and tested for their ability to polarize Th1 or Th2 cytokine production by NKT cells. Specifically, a Th1-polarizing molecule described and known as C-glycoside α-GC was able to induce higher amounts of IFN-γ production by type I NKT cells than α-GC, and led to improved rejection of B16 melanoma in C-glycoside-treated mice [101]. Other variants such as OCH, a truncated form of α-GC, are Th2-polarizing, resulting in the production of IL-4 but little IFN-γ [102,103]. Our laboratory tested the C-glycoside and OCH molecules as adjuvants for anti-NP–KLH Ab responses, and observed that the Th1-polarizing molecule was the more potent adjuvant, resulting in higher end point-specific Ab titers and induction of a greater number of persistent Ag-specific bone marrow PCs [67]. In that study, the C-glycoside molecule induced a greater IgG1 response, with no detectable skewing towards other Ab subclasses. This was surprising since IgG1 is a prototypical Th2-driven Ab response. The reasons for this are presently unclear, but suggest that NKT-derived IFN-γ is unimportant for determining the Ab class produced, and has another positive effect on the humoral immune response. Further investigation of this issue is warranted. Whether NKT-derived IFN-γ and IL-4 are necessary components of B-cell help is not known. Experimental systems where IL-4 and IFN-γ are expressed by all relevant cells except NKT cells will be required to delineate the role of the cytokines in NKT-enhanced Ab responses.
Thus far, it appears that NKT cells may employ similar mechanisms to Th cells (CD154, CD28, IL-4 and IFN-γ) to provide B-cell help, although the mode of activation is very different. This raises an important question for vaccinologists as to whether NKT cells and Th cells employ the same mechanisms to help B cells, but, more importantly, whether the outcome for the B cell is different. Clearly more research on this topic is a priority.
When do NKT cells help B cells?
Natural killer T cell activation with exogenous glycolipid typically leads to a rapid production of cytokines within a few hours and spanning a 24-h period. During this time and within 4–6 h, the TCR is downregulated on NKT cells, rendering them nonresponsive to further stimulation and is followed by re-expression of the TCR within 24–48 h [104,105]. However, newer findings show that glycolipid-activated NKT cells remain anergic (in the mouse) for a period of several weeks [106–109]. At first glance, it would seem that NKT cells could only contribute to humoral immunity at the very earliest stages of humoral immunity. However, this concept warrants closer examination. First, not all CD1d ligands induce NKT cell anergy. For example, the threitolceramide agent, a nonglycosidic lipid, binds CD1d, activates NKT cells but is followed by a rapid recovery of NKT cell function inconsistent with anergy [110]. Experiments in which NKT anergy is demonstrated are typically performed by intraperitoneal administration of the α-GC ligand in phosphate-buffered saline containing a polysorbate vehicle. This method results in a rapid and potent release of NKT-derived cytokines in the sera and is followed by a state of anergy [106–109]. By contrast, when α-GC is administered subcutaneously in the absence of vehicle, this massive release of cytokine is not detected and splenic NKT cells remain responsive to in vitro restimulation [66,67,83]. It is possible that methods of α-GC administration that are most likely to be used for vaccinations do not lead to systemic or widespread NKT cell anergy. However, it is equally possible that subcutaneous and intranasal immunizations lead to at least a localized or partial anergy. Following immunization (without polysorbate vehicle), local draining lymph node NKT cells may be rendered anergic, whereas distal lymph nodes may not. Alternatively, subcutaneous and intranasal immunization without vehicle may lead to a partial anergy in several secondary lymphoid organs. More investigation is required to assess the NKT cell anergy (if any) following immunization without polysorbate vehicle. NKT cell anergy thus far has been considered in the context of TCR-stimulated cytokine release. Such models do not preclude the ability of anergized NKT cells to regulate humoral immunity through other mechanisms not dependent on the TCR. Further work is required to fully understand the range of functions that are diminished during NKT anergy in order to understand when they help B cells during the course of a humoral immune response.
Data are emerging to suggest that NKT cells do not directly impact all of the major steps in the initiation, development and maintenance of a humoral immune response. Preliminary studies in our laboratory indicate that induction of Bmem cells is comparable in C57Bl/6 and CD1d−/− mice immunized with Alum-adsorbed Ags [Lang ML; Unpublished Data]. Furthermore, the proportion of high-affinity Abs in sera of Ag/Alum-immunized C57Bl/6 and CD1d−/− mice appears similar, indicating that while α-GC-activated NKT cells may be able to boost the germinal center reaction, they may not be required for it [67]. By contrast, the induction of both short-lived PCs (SLPCs) and LLPCs is greatly diminished in CD1d−/− mice immunized with Ag/Alum, although, as we mentioned, this could be attributable to deficiencies in subsets of PC precursors [Lang ML, Shah HB, Unpublished Data]. We showed that although C57Bl/6 mice induced more PC in response to Ag/Alum than CD1d−/− mice, both populations appeared equally persistent [67]. When Galli and colleagues compared C57Bl/6 and Jα18−/− mice, the initial post-booster Ab titers were similar but decayed faster in the Jα18−/− mice, suggesting a role for type I NKT cells in PC maintenance [83]. Taken together, available data indicate that in mice immunized with Ag/Alum, NKT cells perhaps have a minor effect on Bmem and PC induction and maintenance.
Assigning a specific role for α-GC-activated type I NKT cells may prove challenging. Thus far, all aspects of humoral immunity that have been assessed show a positive increase when type I NKT cells are activated with α-GC. This indicates that either type I NKT cells provide help at several points along the differentiation process or that there is a discrete initiating event at an early stage. Thus far, Bmem induction [83], affinity maturation [67], and induction of SLPCs and LLPCs [Lang ML, Lang, GA, Shah HB; Unpublished Data] are increased by α-GC, suggesting that type I NKT cells provide B-cell help at an early stage, perhaps prior to, or during follicle formation and germinal center reactions. Since SLPCs often do not go through a germinal center reaction [79,111], it is possible that B-cell help can also occur distal to germinal centers.
Will studies in mice translate to humans?
Clinical trials are now needed to determine if vaccines that incorporate NKT cell activation with exogenous ligand will be efficacious and induce protective immunity. In general, murine B cells serve as a fairly good model for human B cells and murine CD1d-restricted NKT cells share many properties with human CD1d-restricted NKT cells. However, there are important differences between the two species to consider that could impact vaccine development (box 1). These include differences in the numbers of circulating Bmem and the BCR repertoire of MZ B cells [112–115], suggesting that mice and humans may respond differentially to T-cell-dependent and -independent Ags when receiving help from activated NKT cells. Furthermore, mouse and human NKT cells differ with regard to frequencies at anatomical locations [116] and which subsets produce Th1 and Th2 cytokines [117]. There is also a widely varying frequency of NKT cells in human peripheral blood and variable responses to NKT-cell stimulation in vivo [118–121]. It is evident that prediction of efficacy in humans cannot readily be achieved by examining data from murine studies and there is a paucity of studies examining human NKT cells in the context of Ab responses. Galli and colleagues performed experiments whereby peripheral blood B cells (CD27− naive and CD27+ memory) were cocultured with autologous peripheral blood-derived NKT cell clones (CD4+ and DN) [122]. In this study, they observed the CD1d-dependent proliferation of naive and memory B cells upon stimulation with NKT cell clones. The CD4+ NKT cells provided more efficient help, leading to higher Ab titers. Although Ag-specific Ab responses were not the focus of the study, B-cell help in vitro was clearly demonstrated. Further studies on Ag-specific Ab responses in humans are much needed.
Box 1. Key differences between human and mouse B cells and natural killer T cells
B cells
Larger pool of peripheral blood Bmem and MZ B cells in humans than in mice [112–114]
Primarily germline BCRs in mouse MZ B cells [115]
Somatic hypermutated BCR in human MZ B cells [115]
CD1d/NKT cells
Mice have single CD1 molecule, CD1d [1]
Humans have CD1a, b, c, d and e, and T cells restricted by various CD1 family members [1]
Mouse NKT cells produce Th1 and Th2 cytokines Human CD4+ NKT cells produce Th2 and Th1 cytokines [117]
Human DN NKT cells produce Th1 but not Th2 cytokines [117]
Humans have highly variable frequencies of peripheral blood NKT cells and variable responses to NKT-stimulating tumor immunotherapies [118,119,121,123]
Lower frequency of NKT cells in human liver than murine liver [116]
Human hepatic NKT cells are Th1 polarized in response to α-GC [116]
This box summarizes some differences between murine and human B cells, and NKT cells that could impact vaccine efficacy. Papers cited concern studies of human NKT cells.
α-GC: α-galactosylceramide; BCR: B-cell receptor; Bmem: Memory B cell; DN: Double negative (CD4−/CD8−); MZ: Marginal zone; NKT: Natural killer T cell.
Expert commentary & five-year view
In the next 5 years, we will likely improve our understanding of the mechanisms by which NKT cells affect humoral immunity. It is likely, given the good safety record of adjuvants such as α-GC in clinical trials with cancer patients [118–120,123,124], that researchers will embark on testing of prophylactic vaccines designed to harness NKT cells. This may include α-GC or a structural derivative that stimulates the most appropriate immune response. Vaccinologists will also have to consider issues of NKT cell anergy. If a vaccine causes NKT cell anergy, then a patient could be rendered temporarily less responsive to boosters, other vaccines or opportunistic pathogens. Developing an appropriate NKT cell-activating ligand that does not induce anergy may be an impediment to harnessing NKT cells for Ab-inducing vaccines.
A reliable model of how NKT cells impact humoral immunity may be required to develop practical NKT cell-based vaccines. This will require the determination of which NKT cell-expressing APCs stimulate NKT cells, and then understanding which NKT-derived signals influence B cells. In turn, the NKT-driven differentiation of the Ag-experienced B cell to Bmem, SLPCs and LLPCs will have to be examined closely. Finally, we must ask the question of whether a Th/NKT cell-influenced B cell is differentially programmed from a Th-influenced B cell, and what this means for long-term protective humoral immunity.
Key issues
Type I natural killer (NKT)-cell activation is important for antibody responses against allergens, parasites, bacteria, viruses, T-cell-dependent and T-cell-independent antigens (Ags).
CD1d Ag presentation by B cells stimulates Type I NKT cell activation.
Type I NKT cells provide B-cell help driven by cognate and noncognate interactions.
Type I NKT-derived soluble factors such as cytokines may contribute to B-cell help.
Memory B-cell responses and the longevity of antibody-secreting plasma cells are boosted by type I NKT cells.
A careful analysis of when and where type I and type II NKT cells interact with B cells will assist in understanding how NKT cells achieve their effects on humoral immune responses.
Acknowledgments
The author would like to thank Loren Erickson (University of Virginia, VA, USA) for helpful comments on the manuscript, and also thanks the various members of his laboratory for their experimental work that has contributed to answering the questions discussed herein.
Financial & competing interests disclosure
Research contributing to the ideas discussed in this article is supported by: NIH grant 1RO1AI078993 (to Mark L Lang) and 5P20RR015564 from the National Center for Research Resources. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 2.Mandal M, Chen XR, Alegre ML, et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35(9):525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 3.Zimmer MI, Nguyen HP, Wang B, et al. Polymorphisms in CD1d affect antigen presentation and the activation of CD1d-restricted T cells. Proc Natl Acad Sci USA. 2009;106(6):1909–1914. doi: 10.1073/pnas.0808476106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277(5324):339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 5.Koch M, Stronge VS, Shepherd D, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;6(8):819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 6.Moody DB, Zajonc DM, Wilson IA. Anatomy of CD1–lipid antigen complexes. Nat Rev. 2005;5(5):387–399. doi: 10.1038/nri1605. [DOI] [PubMed] [Google Scholar]
- 7.Zajonc DM, Maricic I, Wu D, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202(11):1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d–iGb3 complex and its cognate Vα14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377(4):1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou D, Mattner J, Cantu C, 3rd, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 10.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 13.Wu D, Xing GW, Poles MA, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102(5):1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 15.Natori T, Akimoto K, Motoki K, Koezuka Y, Higa T. Development of KRN7000, derived from agelasphin produced by Okinawan sponge. Nippon Yakurigaku Zasshi – Folia Pharmacologica Japonica. 1997;110(Suppl 1):63P–68P. doi: 10.1254/fpj.110.supplement_63. [DOI] [PubMed] [Google Scholar]
- 16.Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of α-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology. 2004;112(3):386–396. doi: 10.1111/j.1365-2567.2004.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15(6):897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 18.Roberts TJ, Sriram V, Spence PM, et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168(11):5409–5414. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- 19.Cernadas M, Sugita M, van der Wel N, et al. Lysosomal localization of murine CD1d mediated by AP-3 is necessary for NK T cell development. J Immunol. 2003;171(8):4149–4155. doi: 10.4049/jimmunol.171.8.4149. [DOI] [PubMed] [Google Scholar]
- 20.Lawton AP, Prigozy TI, Brossay L, et al. The mouse CD1d cytoplasmic tail mediates CD1d trafficking and antigen presentation by adaptor protein 3-dependent and -independent mechanisms. J Immunol. 2005;174(6):3179–3186. doi: 10.4049/jimmunol.174.6.3179. [DOI] [PubMed] [Google Scholar]
- 21.Park YK, Lee JW, Ko YG, Hong S, Park SH. Lipid rafts are required for efficient signal transduction by CD1d. Biochem Biophys Res Comm. 2005;327(4):1143–1154. doi: 10.1016/j.bbrc.2004.12.121. [DOI] [PubMed] [Google Scholar]
- 22.Roy KC, Maricic I, Khurana A, Smith TR, Halder RC, Kumar V. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J Immunol. 2008;180(5):2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 23.Schrantz N, Sagiv Y, Liu Y, Savage PB, Bendelac A, Teyton L. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J Exp Med. 2007;204(4):841–852. doi: 10.1084/jem.20061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan W, Qi X, Tsang P, et al. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci USA. 2007;104(13):5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dougan SK, Salas A, Rava P, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202(4):529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumperz JE. The ins and outs of CD1 molecules: bringing lipids under immunological surveillance. Traffic (Copenhagen, Denmark) 2006;7(1):2–13. doi: 10.1111/j.1600-0854.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 27.Burdin N, Brossay L, Koezuka Y, et al. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates V α 14+ NK T lymphocytes. J Immunol. 1998;161(7):3271–3281. [PubMed] [Google Scholar]
- 28••.Lang GA, Illarionov PA, Glatman-Freedman A, Besra GS, Lang ML. BCR targeting of biotin-{α}-galactosylceramide leads to enhanced presentation on CD1d and requires transport of BCR to CD1d-containing endocytic compartments. Int Immunol. 2005;17(7):899–908. doi: 10.1093/intimm/dxh269. First paper showing B-cell receptor (BCR)-mediated internalization and presentation of CD1d-binding glycolipid antigen (Ag) [DOI] [PubMed] [Google Scholar]
- 29.Prigozy TI, Naidenko O, Qasba P, et al. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291(5504):664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 30.van den Elzen P, Garg S, Leon L, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437(7060):906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 31••.Barral P, Eckl-Dorna J, Harwood NE, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0802968105. Demonstrated that BCR capture of CD1d-binding glycolipid Ag led to specific antibody (Ab) responses in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Leadbetter EA, Brigl M, Illarionov P, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0801375105. Demonstrated that BCR capture of CD1d-binding glycolipid Ag led to specific Ab responses in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 34.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 35.Ballas ZK, Rasmussen W. NK1.1+ thymocytes. Adult murine CD4−, CD8− thymocytes contain an NK1.1+, CD3+, CD5hi, CD44hi, TCR-V β8+ subset. J Immunol. 1990;145(4):1039–1045. [PubMed] [Google Scholar]
- 36.Matsuda JL, Naidenko OV, Gapin L, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192(5):741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268(5212):863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 38.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202(12):1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21(11):573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 40.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202(12):1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei DG, Lee H, Park SH, et al. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202(2):239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griewank K, Borowski C, Rietdijk S, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev. 2007;7(7):505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 44.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275(5302):977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 45.Exley MA, Bigley NJ, Cheng O, et al. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110(4):519–526. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6(4):469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 47.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Wang X, Besra GS, Gumperz JE. Modulation of CD1d-restricted NKT cell responses by CD4. J Leuk Biol. 2007;82(6):1455–1465. doi: 10.1189/jlb.0307163. [DOI] [PubMed] [Google Scholar]
- 49.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202(9):1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Der Vliet HJ, Nishi N, de Gruijl TD, et al. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95(7):2440–2442. [PubMed] [Google Scholar]
- 51.Park SH, Benlagha K, Lee D, Balish E, Bendelac A. Unaltered phenotype, tissue distribution and function of Vα14+NKT cells in germ-free mice. Eur J Immunol. 2000;30(2):620–625. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 52.Paul WE. Fundamental Immunology. 6. Lippincott Williams and Wilkins; PA, USA: 2008. [Google Scholar]
- 53.Yoshimoto T, Bendelac A, Hu-Li J, Paul WE. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc Natl Acad Sci USA. 1995;92(25):11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lisbonne M, Diem S, de Castro Keller A, et al. Cutting edge: invariant V α 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171(4):1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimoto T, Min B, Sugimoto T, et al. Nonredundant roles for CD1d-restricted natural killer T cells and conventional CD4+ T cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J Exp Med. 2003;197(8):997–1005. doi: 10.1084/jem.20021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 57.Campos RA, Szczepanik M, Itakura A, et al. Cutaneous immunization rapidly activates liver invariant V{α}14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198(12):1785–1796. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38(1):156–165. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schofield L, McConville MJ, Hansen D, et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283(5399):225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 60.Molano A, Park SH, Chiu YH, Nosseir S, Bendelac A, Tsuji M. Cutting edge: the IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NK T cell activation and antimalarial responses. J Immunol. 2000;164(10):5005–5009. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- 61.Procopio DO, Almeida IC, Torrecilhas AC, et al. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J Immunol. 2002;169(7):3926–3933. doi: 10.4049/jimmunol.169.7.3926. [DOI] [PubMed] [Google Scholar]
- 62•.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174(9):5681–5686. doi: 10.4049/jimmunol.174.9.5681. Along with [63] linked natural killer (NK), T cells and CD1dhimarginal zone (MZ) B cells to production of Abs that could protect against bacterial pathogens. [DOI] [PubMed] [Google Scholar]
- 63•.Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol. 2000;165(9):4797–4801. doi: 10.4049/jimmunol.165.9.4797. Along with [62] linked NKT cells and, CD1dhiMZ B cells to the production of specific Abs that could protect against bacterial pathogens. [DOI] [PubMed] [Google Scholar]
- 64.Kobrynski LJ, Sousa AO, Nahmias AJ, Lee FK. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J Immunol. 2005;174(4):1787–1790. doi: 10.4049/jimmunol.174.4.1787. [DOI] [PubMed] [Google Scholar]
- 65••.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. α-galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175(5):3309–3317. doi: 10.4049/jimmunol.175.5.3309. Shows that α-galactosylceramide can be used as an adjuvant for inducing protective Ab at mucosal surfaces. [DOI] [PubMed] [Google Scholar]
- 66.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid α-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119(1):116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur J Immunol. 2008;38(4):1001–1011. doi: 10.1002/eji.200738000. Demonstrated that NKT activation led to induction of persistent plasma cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20(1):14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 70.Shinall SM, Gonzalez-Fernandez M, Noelle RJ, Waldschmidt TJ. Identification of murine germinal center B cell subsets defined by the expression of surface isotypes and differentiation antigens. J Immunol. 2000;164(11):5729–5738. doi: 10.4049/jimmunol.164.11.5729. [DOI] [PubMed] [Google Scholar]
- 71.McHeyzer-Williams LJ, Cool M, McHeyzer-Williams MG. Antigen-specific B cell memory: expression and replenishment of a novel b220(−) memory B cell compartment. J Exp Med. 2000;191(7):1149–1166. doi: 10.1084/jem.191.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gray D, Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- 73.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407(6804):636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 74.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 75.Moser K, Tokoyoda K, Radbruch A, MacLennan I, Manz RA. Stromal niches, plasma cell differentiation and survival. Curr Opin Immunol. 2006;18(3):265–270. doi: 10.1016/j.coi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev. 2006;6(10):741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 77.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev. 2005;5(3):230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 78.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 79.McHeyzer-Williams MG, Ahmed R. B cell memory and the long-lived plasma cell. Curr Opin Immunol. 1999;11(2):172–179. doi: 10.1016/s0952-7915(99)80029-6. [DOI] [PubMed] [Google Scholar]
- 80.Noelle RJ, Erickson LD. Determinations of B cell fate in immunity and autoimmunity. Curr Dir Autoimmun. 2005;8:1–24. doi: 10.1159/000082084. [DOI] [PubMed] [Google Scholar]
- 81.Zinkernagel RM. On differences between immunity and immunological memory. Curr Opin Immunol. 2002;14(4):523–536. doi: 10.1016/s0952-7915(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 82.Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007;19(3):275–280. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83•.Galli G, Pittoni P, Tonti E, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104(10):3984–3989. doi: 10.1073/pnas.0700191104. Demonstrated that Ab recall responses were improved by initial NKT activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amano M, Baumgarth N, Dick MD, et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β 2-microglobulin-dependent and independent forms. J Immunol. 1998;161(4):1710–1717. [PubMed] [Google Scholar]
- 85.Duan B, Niu H, Xu Z, et al. Intrafollicular location of marginal zone/CD1dhi B cells is associated with autoimmune pathology in a mouse model of lupus. Lab Invest. 2008;88(9):1008–1020. doi: 10.1038/labinvest.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makowska A, Faizunnessa NN, Anderson P, Midtvedt T, Cardell S. CD1high B cells: a population of mixed origin. Eur J Immunol. 1999;29(10):3285–3294. doi: 10.1002/(SICI)1521-4141(199910)29:10<3285::AID-IMMU3285>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 87.Bialecki E, Paget C, Fontaine J, Capron M, Trottein F, Faveeuw C. Role of marginal zone B lymphocytes in invariant NKT cell activation. J Immunol. 2009;182(10):6105–6113. doi: 10.4049/jimmunol.0802273. [DOI] [PubMed] [Google Scholar]
- 88.Velazquez P, Wei B, McPherson M, et al. Villous B cells of the small intestine are specialized for invariant NK T cell dependence. J Immunol. 2008;180(7):4629–4638. doi: 10.4049/jimmunol.180.7.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 90.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 91••.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111(4):2158–2162. doi: 10.1182/blood-2007-10-117309. First report that B cell CD1d Ag presentation drives NKT-enhanced Ab responses in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P. NKT cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2008;113(2):370–376. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 93.Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4 + and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171(10):5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 94.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat Immunol. 2002;3(9):867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 95.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202(2):203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198(2):267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166(10):6012–6018. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 99.Williams JA, Lumsden JM, Yu X, et al. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway. J Immunol. 2008;181(2):907–917. doi: 10.4049/jimmunol.181.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng X, Zhang H, Yin L, Wang CR, Liu Y, Zheng P. Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation. PLoS ONE. 2008;3(7):e2703. doi: 10.1371/journal.pone.0002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J Exp Med. 2003;198(11):1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mizuno M, Masumura M, Tomi C, et al. Synthetic glycolipid OCH prevents insulitis and diabetes in NOD mice. J Autoimmun. 2004;23(4):293–300. doi: 10.1016/j.jaut.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Oki S, Tomi C, Yamamura T, Miyake S. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int Immunol. 2005;17(12):1619–1629. doi: 10.1093/intimm/dxh342. [DOI] [PubMed] [Google Scholar]
- 104.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171(8):4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 105.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100(19):10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uldrich AP, Crowe NY, Kyparissoudis K, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175(5):3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parekh VV, Wilson MT, Olivares-Villagomez D, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115(9):2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang WS, Kim JY, Kim YJ, et al. Cutting edge: programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol. 2008;181(10):6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 109.Sullivan BA, Kronenberg M. Activation or anergy: NKT cells are stunned by α-galactosylceramide. J Clin Invest. 2005;115(9):2328–2329. doi: 10.1172/JCI26297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silk JD, Salio M, Reddy BG, et al. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180(10):6452–6456. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]
- 111.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10(3):252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 112.Klein U, Kuppers R, Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 1997;89(4):1288–1298. [PubMed] [Google Scholar]
- 113.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188(9):1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Es JH, Meyling FH, Logtenberg T. High frequency of somatically mutated IgM molecules in the human adult blood B cell repertoire. Eur J Immunol. 1992;22(10):2761–2764. doi: 10.1002/eji.1830221046. [DOI] [PubMed] [Google Scholar]
- 115.Weller S, Braun MC, Tan BK, et al. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kenna T, Golden-Mason L, Porcelli SA, et al. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171(4):1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 117.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(α)24 natural killer T cells. J Exp Med. 2002;195(5):637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosylceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201(9):1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giaccone G, Punt CJ, Ando Y, et al. A Phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702–3709. [PubMed] [Google Scholar]
- 120.Ishikawa A, Motohashi S, Ishikawa E, et al. A Phase I study of α-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11(5):1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 121.Uchida T, Horiguchi S, Tanaka Y, et al. Phase I study of α-galactosylceramide-pulsed antigen-presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57(3):337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Galli G, Nuti S, Tavarini S, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197(8):1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Motohashi S, Ishikawa A, Ishikawa E, et al. A Phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12(20 Pt 1):6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 124.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97(9):807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]