Abstract
The outcome of pregnancy in kidney donors has generally been viewed to be favorable. We determined fetal and maternal outcomes in a large cohort of kidney donors.
A total of 2,102 women have donated a kidney at our institution; 1589 donors responded to our pregnancy surveys; 1085 reported 3213 pregnancies and 504 reported none. Fetal and maternal outcomes in post-donation pregnancies were comparable to published rates in the general population. Post-donation (vs. pre-donation) pregnancies, were associated with a lower likelihood of full-term deliveries (73.7% vs. 84.6%, p=0.0004) and a higher likelihood of fetal loss (19.2% vs. 11.3%, p<0.0001). Post-donation pregnancies were also associated with a higher risk of gestational diabetes (2.7% vs. 0.7%, p=0.0001), gestational hypertension (5.7% vs. 0.6%, p<0.0001), proteinuria (4.3% vs. 1.1%, p<0.0001) and preeclampsia (5.5% vs. 0.8%, p<0.0001). Women who had both pre- and post-donation pregnancies were also more likely to have these adverse maternal outcomes in their post-donation pregnancies. In this large survey of previous living donors in a single center, fetal and maternal outcomes, and pregnancy outcomes after kidney donation were similar to those reported in the general population, but inferior to pre-donation pregnancy outcomes.
Keywords: pregnancy, donation, kidney
INTRODUCTION
Chronic kidney disease (CKD) is associated with an increased risk of gestational hypertension, preeclampsia, and intrauterine growth retardation (1–3). Data regarding the impact of reduced glomerular filtration rate (GFR) on pregnancy in women with a single kidney has primarily come from studies in kidney transplant recipients (4,5). In regard to kidney donors, two small series suggested that pregnancy after kidney donation was not associated with an increased risk of adverse fetal or maternal outcomes (6,7). In contrast, a recent analysis of 326 kidney donors from Norway revealed more than a 2.5-fold increase in the adjusted risk of preeclampsia in post-donation pregnancies (8). In total, however, those 3 series reported on only 810 pregnancies. In recognition of the scarcity of available information regarding pregnancy and kidney donation, a recent American Society of Transplantation meeting report on reproduction highlighted the unresolved issue of whether pregnancy in kidney donors is associated with a higher risk of fetal and maternal complications (9). This uncertainty regarding pregnancy outcome after kidney donation and the fact that 59% of all living donors in 2006 were women, the majority of whom were between 18 to 49 years of age, makes this issue a very relevant one (10). Herein, we report on self-reported fetal and maternal outcomes in a large cohort of kidney donors.
MATERIALS AND METHODS
Study population
Between November 1963 and December 21st, 2007, we performed 3,698 donor uninephrectomies. Of those donors, 2,102 were women. None of these women were taking any anti-hypertensive medications and all had normal urinary protein excretion rate as ascertained via 24-hour urine collections, and in the last 5 years via urinary albumin creatinine ratio at the time of donation. We initiated a comprehensive multistep effort to contact all of our donors, by consulting phone and Internet directories and also by asking their recipient. Donors found were asked to submit health status updates and submit urine analysis results, if available. The vital status of all donors was ascertained through the Social Security Death Master File. From June 2003 through December 2007, donors known to be alive and with contact information were sent a simple questionnaire regarding the fetal outcomes of their pregnancies; then, 3–6 months later, they were sent a second questionnaire that focused on maternal outcomes but also contained the same items as in the first questionnaire (Table 1). We sent 2 separate questionnaires because maternal outcomes were not included in the first one; we wanted to test the agreement rate in answers provided by donors regarding fetal outcomes and also in response to the work by Reisater et al. that suggested an increased risk of preeclampsia in kidney donors (8). Questionnaires, similar to ones we used, have previously been shown in non-donors to have high specificity regarding fetal and maternal outcomes but limited sensitivity when compared to the actual medical records up to 30 years after delivery (11). Non-responders were called over the phone and were offered to answer the questions on the phone. All procedures were approved by the University of Minnesota’s Institutional Review Board after obtaining consent from donors.
Table 1.
Questionnaire content
| Questionnaire 1 |
|---|
| For each pregnancy you had, please answer the following questions: Pregnancy Number ___ / Birth date ___________ |
| Outcome of the pregnancy: |
| 1. Live Birth |
| 2. Still Birth |
| 3. Miscarriage |
| 4. Abortion |
| 5. Pre-term delivery (less than 36 weeks) |
| 6. Other problems (list) |
|
|
| Questionnaire 2 |
|
|
| For each pregnancy you had, please answer the following questions: Pregnancy Number ___ / Birth date ___________ |
| Outcome of the pregnancy: |
| 1. Live Birth |
| 2. Still Birth |
| 3. Miscarriage |
| 4. Abortion |
| 5. Pre-term delivery (less than 36 weeks) |
| 6. Other problems (list) |
| Did you have any of the following during your pregnancy? |
| 1. Hypertension requiring medications |
| 2. Preeclampsia / toxemia |
| 3. Gestational diabetes |
| 4. Extra protein in your urine |
Prematurity was defined as delivery ≤ 36 weeks but > 20 weeks gestation. Fetal loss included miscarriage or abortion. Gestational hypertension and gestational diabetes were defined by the need for treatment for these 2 conditions during pregnancy but not before or after. Preeclampsia, typically defined as hypertension associated with new-onset proteinuria and edema, was recorded by the women’s recall of the diagnosis by the primary provider. GFR was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) study equation (12).
Analysis
Continuous variables are presented as mean (standard deviation) and categorical variables are presented as percentages. We classified donors into 3 categories, those with: 1.) pre-donation pregnancies only, 2.) post-donation pregnancies only, and 3.) both pre- and post-donation pregnancies. We used 1-way analysis of variance (ANOVA) to compare continuous baseline variables across these categories. To compare the difference in frequencies of fetal and maternal outcomes between pre- and post-donation pregnancies, the chi-square or the Fischer’s exact test was used. We simplified fetal and maternal outcomes into 3 specific binary outcome measures; 2 fetal and 1 maternal: fetal loss vs. no fetal loss; premature vs. full-term gestation in live birth pregnancies; and gestational hypertension, gestational diabetes, or preeclampsia vs. no event.
To calculate the odds ratio (OR) and 95% confidence interval (CI) for all 3 of the outcomes listed above, we used logistic regression analysis (13). With multiple pregnancies per donor, the marginal regression model accounted for the repeated measures; we used generalized estimating equations (GEEs) to obtain parameter estimates and standard errors. We used an exchangeable working correlation structure. The models included these independent variables: an indicator of post-donation pregnancy, age at pregnancy, parity, serum creatinine at donation, weight at donation, relationship to the recipient, and cause of the kidney disease in the recipient. To examine the effects of time from donation, we constructed models with indicators of time from donation for post-donation pregnancies at 2-year intervals. We used no selection procedure; all covariates were left in the model, regardless of their significance, for adjustment purposes. We used serum creatinine at donation, rather than estimated GFR because estimated GFR already accounts for age and gender so using it would result in overadjustment.
Women with missing serum creatinine or weight at time of donation values were not significantly different from those without. Our transplant program does not accept donors with abnormal serum creatinine (GFR<80 ml/min) or donors who are obese (body mass index BMI >30 kg/m2). To address missing data, we used Markov Chain Monte Carlo (MCMC) methods and produced 5 imputed datasets (14). We ran the marginal logistic regression analysis, using GEEs, on each of the 5 datasets, and then combined the results to produce the final model results. To examine the effect of a post- vs. pre-donation pregnancy in an individual donor, we used a generalized linear mixed-effects model for each of the 3 fetal and maternal binary outcomes. We used SAS 9.1 (SAS Institute Inc., Cary, NC, USA) for all analyses.
RESULTS
To date, 1589 women responded to our pregnancy surveys, 180 did not respond and 333 were not contacted due to lack of contact information or if it was known that they have died. In all, 504 reported no pregnancies and 1085 women reported ≥ 1 pregnancy. Of the 139 donors who are known to be deceased, 73 had responded to our previous surveys regarding pregnancy outcomes. Donors who reported a pregnancy, those who did not and donors who did not respond were comparable on many characteristics ascertained at the time of donation (Table 2). Those who did not report a pregnancy donated more recently and had a higher eGFR (Table 2). Non-responders were less likely to be white, had a higher baseline eGFR and a higher BMI.
Table 2.
General characteristics of responding and non-responding donors at the time of donation.
| Responders | Non-Responders | p-value** | |||
|---|---|---|---|---|---|
| Pregnancy | No Pregnancy | p-value* | |||
| n | 1085 | 504 | 180 | ||
| White | 97.0% | 94.6% | 0.02 | 86.7% | < 0.0001 |
| Age (years) | 39.7(11.7) | 39.4(11.6) | 0.93 | 39.6(11.5) | 0.33 |
| Time from donation (years) | 16.7(11.5) | 14.7(9.8) | < 0.0001 | 14.0(9.5) | 0.44 |
| BMI (kg/m2) | 25.7(4.2) | 26.2(4.7) | 0.06 | 26.6(5.4) | 0.04 |
| Serum creatinine (mg/dL) | 0.84(0.13) | 0.82(0.13) | 0.004 | 0.79(0.13) | 0.0002 |
| eGFR (mL/min/1.73m2) | 83.5(17.9) | 85.7(16.9) | 0.006 | 91.6(20.2) | < 0.0001 |
Pregnant vs. non-pregnant amongst responders.
Responders vs. non-responders.
A total of 1280 donors returned the 2 most recent questionnaires. Responses from both questionnaires, answers given over the phone and from our previous surveys of these donors were used for this analysis.
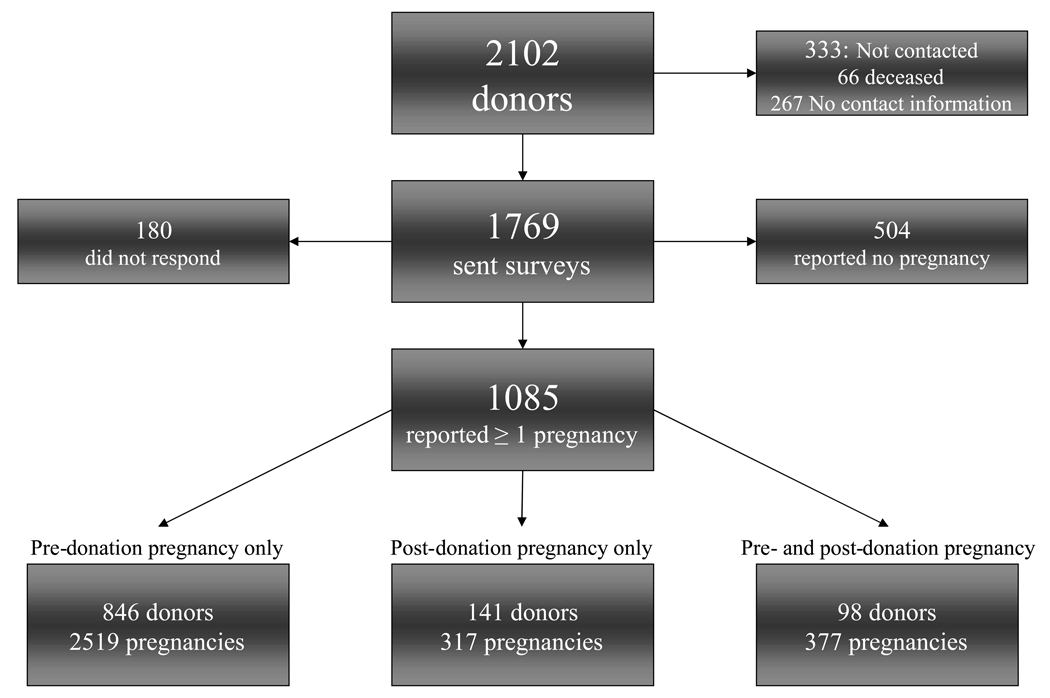
A total of 3213 pregnancies were reported: 846 donors reported 2519 pre-donation pregnancies only, 141 reported 317 post-donation pregnancies only, and 98 reported 204 pre-donation and 173 post-donation pregnancies (Figure 1). 21.7% of the donors who reported becoming pregnancy donated prior to 1980, and 27.7% donated after 2000 (Table 3). The majority of donors were white and had ≥ 3 pregnancies. Donors who reported post-donation pregnancies only were younger at donation, older at the time of their first pregnancy and 5.1±3.8 years elapsed from donation to their first pregnancy after donation (Table 4). Women with pre-donation pregnancy only were less likely to be related to the recipient. eGFR at donation in women who reported post-donation pregnancies only was significantly higher than the two other groups (Table 4).
Figure 1.
Diagram of respondents and non-respondents and the total donor pool.
Table 3.
Distribution of pregnancy and complications by decade of donation, (%)
| Decade | |||||
|---|---|---|---|---|---|
| 1960† | 1970 | 1980 | 1990 | 2000 | |
| Donors | 3.1 | 18.6 | 23.0 | 27.7 | 27.7 |
| Pregnancies | 3.7 | 18.7 | 24.2 | 27.1 | 26.3 |
| Adverse maternal outcomes * | 2.6 | 23.7 | 28.1 | 25.4 | 20.2 |
| Fetal loss | 3.2 | 16.9 | 22.4 | 24.9 | 32.6 |
| Prematurity | 1.4 | 18.6 | 37.9 | 16.6 | 25.5 |
Include gestational hypertension, diabetes and preeclampsia.
Some of the pregnancies in this category took place between 1930–1950.
Table 4.
Pregnancies by time after donation
| All | Pre- donation pregnancies only | Post-donation pregnancies only | Both pre- and post-donation pregnancies | |
|---|---|---|---|---|
| Donors with ≥ 1 pregnancy | 1085 | 846 | 141 | 98 |
| Total number of pregnancies | 3213 | 2519 | 317 | 377 |
| Age at donation, years | 39.7(11.7) | 43.7(9.7) | 23.8(4.7) | 28.2(5.1)* |
| Age at first pregnancy, years | 23.9(5.1) | 23.2(4.6) | 28.8(5.5) | 22.9(4.0)* |
| Time from 1st pregnancy to survey, years | 12.1(8.2) | 16.2(7.1) | 2.5(3.2) | 5.4(4.9) |
| Pregnancies/donor | ||||
| 1 | 5.0% | 4.8% | 12.3% | 0% |
| 2 | 21.8% | 20.9% | 38.5% | 13.8% |
| ≥ 3 | 73.2% | 74.3% | 49.2% | 86.2% |
| Creatinine at donation1 (mg/dL) | 0.84(0.13) | 0.84(0.13) | 0.85(0.13) | 0.81(0.13)† |
| MDRD GFR at donation1 | 83.5(17.9) | 82.1(17.9) | 90.0(16.1) | 92.2(16.3)* |
| Weight at donation2 (kg) | 69.0(12.4) | 69.6(12.3) | 63.9(12.9) | 66.6(12.6)* |
| BMI3 | 25.7(4.2) | 25.9(4.1) | 23.6(4.4) | 24.9(4.5)* |
| White | 97.0% | 96.9% | 98.4% | 96.8% |
| Relationship to recipient | ||||
| Related | 82.8% | 79.6% | 96.2% | 93.1% |
| Cause of recipient kidney disease | ||||
| Diabetes | 35.9% | 37.9% | 28.7% | 28.4% |
| Polycystic kidney disease | 6.0% | 6.9% | 2.5% | 2.9% |
| Glomerular disease | 16.3% | 15.1% | 25.6% | 16.5% |
| Hypertension | 2.9% | 2.9% | 3.5% | 2.7% |
| Other | 38.9% | 37.2% | 39.8% | 49.6% |
p < 0.0001
p= 0.0133
MDRD GFR = Modification of Diet in Renal Disease Glomerular Filtration Rate in mL/min/1.73m2.Missing values:
Serum creatinine and GFR at time of donation – 26.3%
Weight at donation – 52.6%
BMI – 54.3%
Outcomes in pre-donation pregnancies
Of the 2723 pregnancies (includes all pre-donation pregnancies whether they occurred in those who had pre-donation pregnancy only or those that occurred in donors who became pregnant before and after donation) reported by 944 donors, 84.6% resulted in full-term delivery; 4.0%, prematurity; and 11.3% fetal loss (77.9% of fetal losses were due to miscarriage). Gestational diabetes (0.7%), hypertension (0.6%), preeclampsia (0.8%) and proteinuria (1.1%) were exceedingly rare (Table 5).
Table 5.
Fetal and maternal outcomes, n (%)
| Pre-donation pregnancies | Post-donation Pregnancies | Pre- and post-donation pregnancies | Non-Hispanic whites | p-value* | |
|---|---|---|---|---|---|
| Number of donors | 944 | 239 | 98 | ||
| Number of pregnancies | 2,723 | 490 | 377 | ||
| Fetal outcomes | |||||
| Full-term birth | 2,305(84.6%) | 361(73.7%) | 262(69.5%) | 57.3† | 0.0004 |
| Prematurity | 110(4.0%) | 35(7.1%) | 30(8.0%) | 11.7# | 0.0004 |
| Fetal loss | 308(11.3%) | 94(19.2%) | 85(22.6%) | 18%† | < 0.0001 |
| Death | 15(4.9%) | 2(2.1%) | 3(3.5%) | ||
| Miscarriage | 240(77.9%) | 78(83.0%) | 69(81.2%) | ||
| Abortion | 5 (17.2%) | 14(14.9%) | 13(15.3%) | 12%† | |
| Maternal outcomes | |||||
| Gestational diabetes | 20(0.7%) | 13(2.7%) | 2(0.5%) | 3.7# | 0.0001 |
| Gestational hypertension | 17(0.6%) | 28(5.7%) | 7(1.9%) | 4.5# | < 0.0001 |
| Preeclampsia or toxemia | 23(0.8%) | 27(5.5%) | 7(1.9%) | 2–10%** | < 0.0001 |
| Proteinuria | 29(1.1%) | 21(4.3%) | 12(3.2%) | < 0.0001 | |
Outcomes in post-donation pregnancies
As shown in table 5, 73.7% of 490 pregnancies (include all post-donation pregnancies whether it occurred in those who had post-donation pregnancies only or in those who had both pre-and post-donation pregnancies), resulted in full-term delivery (p=0.0004 vs. pre-donation pregnancies); 8.0% premature delivery (p=0.0004 vs. pre-donation); and 19.2% fetal loss (p<0.0001 vs. pre-donation) (Table 5). Of the 239 donors who reported post-donation pregnancies, 13 developed gestational diabetes (p=0.0001 vs. pre-donation); 28 gestational hypertension (p<0.0001 vs. pre-donation); 27 preeclampsia (p<0.0001 vs. pre-donation); and 21 proteinuria (p<0.0001 vs. pre-donation). The rates of adverse outcomes in post-donation pregnancies were highly comparable and in some domains better than what is observed in non-Hispanic whites (15,16).
Outcomes for donors with both pre- and post-donation pregnancies
Ninety-eight donors reported 204 pre- and 173 post-donation pregnancies (Table 5,Table 6). The first pregnancy after donation took place at an average of 2.9±2.5 years following donation. Full-term delivery occurred in 71.6% of pre-donation vs. 67.1% of post-donation pregnancies. Fetal loss occurred in 21.1% vs. 24.3% in post-donation pregnancies. One donor had pre-donation gestational diabetes, 1 had pre-donation gestational hypertension, 1 had preeclampsia, and 4 had proteinuria. In contrast, 1 donor had post-donation gestational diabetes; 6, gestational hypertension; 6, preeclampsia; and 8, proteinuria. Within the same woman, there was no difference in the odds of premature delivery (OR, 1.06; 95% CI, 0.61–1.87) or fetal loss (OR, 1.35; 95% CI 0.58–3.18). However, the odds of adverse maternal outcomes in post-donation pregnancies significantly increased (OR, 5.21; 95% CI, 1.28 –21.22).
Table 6.
Fetal and maternal outcomes in 98 donors with both pre- and post-donation pregnancies
| Pre-donation pregnancies (n=204) | Post-donation pregnancies (n=173) | |
|---|---|---|
| Fetal outcomes | ||
| Full-term birth | 146(71.6%) | 116(67.1%) |
| Prematurity | 15(7.4%) | 15(8.7%) |
| Fetal loss | 43(21.1%) | 42(24.3%) |
| Death | 2(4.7%) | 1(2.4%) |
| Miscarriage | 33(76.7%) | 36(85.7%) |
| Abortion | 8(18.6%) | 5(11.9%) |
| Maternal outcomes | ||
| Gestational diabetes | 1(0.5%) | 1(0.6%) |
| Gestational hypertension | 1(0.5%) | 6(3.5%) |
| Preeclampsia or toxemia | 1(0.5%) | 6(3.5%) |
| Proteinuria | 4(1.5%) | 8(4.6%) |
Risks of prematurity and fetal loss
We first performed Kappa statistic test to address the reliability of this survey in assessing fetal outcomes and found it to be 0.92–0.99 for the first 5 items in the survey.
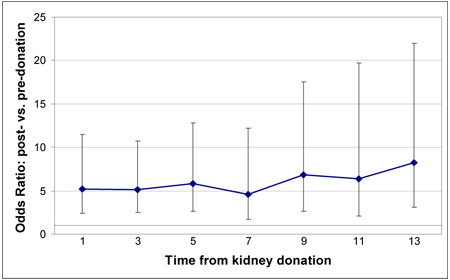
Post-donation pregnancies had a higher odds of a premature delivery (vs. pre-donation pregnancies, OR, 1.90; 95% CI, 1.03–3.48); a risk that was attenuated after the first four years. Donors unrelated to their recipient saw decreased odds (OR, 0.46; 95% CI, 0.24– 0.87) and so did white women (OR, 0.38; 95% CI, 0.18–0.79). History of preeclampsia, gestational diabetes or hypertension in a previous pregnancy increased that risk. Pregnancies after donation were also associated with fetal loss (OR, 1.83; 95% CI, 1.37–2.46); a risk that persisted after the first 4 years. In addition, older maternal age was also associated with fetal loss (OR, 1.04; 95% CI, 1.01–1.06). eGFR at donation was similar in those who reported pregnancies that resulted in full term vs. premature delivery (83.6±18.3 vs. 83.6±15.1 mL/min/1.73m2, p = 0.92) and was also similar in women who reported fetal loss to those who did not (84.4±13.5 vs. 83.5±19.2 mL/min/1.73m2, p = 0.59).
Risk of adverse maternal outcomes
We noted 128 adverse maternal outcomes in all pregnancies reported: 33 gestational diabetes; 45 gestational hypertension; and 50 preeclampsia (Table 4). 80% of these events occurred in donors who donated after 1980 and roughly 50% happened in donors who donated after 1990 (Table 3). A total of 60 events occurred in 2723 pre-donation pregnancies (30 events were in a first pregnancy) and 68 occurred in 490 post-donation pregnancies (44 occurred in a first pregnancy after donation). Six donors developed gestational diabetes in their first pregnancy pre-donation at a mean age of 25.4±6.5 years and 8 developed it in their first pregnancy after donation at a mean age of 33.0±6.2 years. None of these 8 donors had diabetes in any pre-donation pregnancies. Eight donors developed gestational hypertension in their first pregnancy pre-donation at a mean age of 21.0±3.0 years and 17 donors developed it in the pregnancy that followed donation at a mean age of 27.6±6.6 years. None of the latter 17 donors had hypertension in any pregnancy that preceded donation. Sixteen donors developed preeclampsia in their first pre-donation pregnancy at a mean age of 22.1±4.8 years and 18 donors developed it in their first pregnancy after donation at a mean age of 29.5±5.5 years. None of these 19 donors reported preeclampsia prior to donation. In regard to preeclampsia distribution by pregnancy order, 33 occurred in the first pregnancy, 8 in the second, 4 in the third, 3 in the fourth and 1 in the fifth and seventh pregnancies. For the 17 preeclampsia events that occurred in non-first pregnancies; 9 occurred in 7 women who did not have it in their previous pregnancies and 6/9 occurred after kidney donation. Of relevance to this analysis is the observation that 3 donors responded by yes to all domains (preeclampsia, hypertension or diabetes), 3 reported having both preeclampsia and proteinuria, 6 reported both preeclampsia and hypertension and 2 reported concomitant preeclampsia and diabetes.
For the adverse composite maternal outcome of gestational hypertension, diabetes, and preeclampsia, having a post-donation pregnancy at any time after donation was a prominent risk factor (OR, 5.55; 95% CI, 3.42–8.99) (p< 0.0001) (Table 7). Multiparous women had decreased odds of adverse maternal outcomes (OR, 0.51; 95% CI, 0.39–0.67). In addition, a previous history of such outcome was the most prominent factor; (OR, 8.21; 95% CI, 4.45–15.13, p<0.0001), Table 7. Interestingly, eGFR at donation was significantly higher in women with these adverse outcomes (90.9±15.9 vs. 83.3±18.1 mL/min/1.73m2, p = 0.0107).
Table 7.
Multivariable risk of the composite of gestational diabetes, hypertension and preeclampsia
 | ||||
|---|---|---|---|---|
| Parameter | Odds ratio | 95% Confidence Interval | p-value | |
| Intercept | 0.01 | 0.00 | 0.18 | 0.002 |
| Post-donation vs. pre-donation pregnancy | 5.55 | 3.42 | 8.99 | <0.0001 |
| Age at pregnancy (years) | 1.02 | 0.98 | 1.06 | 0.43 |
| White | 1.55 | 0.35 | 6.97 | 0.56 |
| Parity | 0.51 | 0.39 | 0.67 | <0.0001 |
| BMI at donation | 1.05 | 0.98 | 1.13 | 0.16 |
| Serum creatinine at donation (mg/dL) | 0.39 | 0.07 | 2.16 | 0.28 |
| History of preeclampsia, gestational diabetes or hypertension | 8.21 | 4.45 | 15.13 | <0.0001 |
| Unrelated vs. related recipient | 0.76 | 0.38 | 1.51 | 0.43 |
| Cause of renal disease in the recipient* | ||||
| Polycystic disease | 0.69 | 0.24 | 2.01 | 0.49 |
| Glomerular disease | 0.81 | 0.46 | 1.43 | 0.47 |
| Hypertension | 1.08 | 0.36 | 3.26 | 0.89 |
| Other | 0.69 | 0.43 | 1.11 | 0.13 |
Reference group: diabetes
Future maternal risk
We have been asking donors over the years to go to their physicians office or come to the University of Minnesota for serum creatinine measurements, urine analysis and health status updates. Therefore, we are able to use the data that were provided to us in the last 2–3 years to assess long-term kidney function in donors who have reported a pregnancy. The risk of future hypertension and of proteinuria did not differ amongst the donors according to the temporal relationship of the pregnancy to donation. The risk of proteinuria in those with post-donation pregnancies only was, however, almost two fold higher than prevalence observed in those with pre-donation pregnancies only (8.5% vs. 5.8%, p not significant) and this proteinuria appeared earlier (table 8). In contrast, the prevalence of hypertension was numerically lower in those with post-donation pregnancies only (24.5% vs. 29.5% in those with pre-donation pregnancies only) (Table 8).
Table 8.
Long-term kidney outcomes of pregnant donors
| Pre-donation pregnancies only | Post-donation pregnancies only | Pre- & Post-donation pregnancies | |
|---|---|---|---|
| Number of donors | 846 | 141 | 98 |
| Donors with serum creatinine (Cr) available, n (%) | 743(87.8%) | 101(71.6%) | 81(82.7%) |
| Most recent Cr (mg/dL) | 1.1(0.2) | 1.0(0.2) | 0.9(0.2) |
| Time from donation to Cr | 11.8(11.5) | 21.8(11.0) | 18.7(12.1) |
| Time from last pregnancy to Cr | 27.1(13.4) | 13.5(10.7) | 13.1(13.3) |
| Donors with hypertension, n (%) | 259(30.6) | 35(24.8) | 28(28.6) |
| Time from donation to hypertension | 13.7(11.9) | 23.3(7.8) | 21.0(9.5) |
| Time from last pregnancy to hypertension | 30.1(12.7) | 14.1(9.6) | 15.6(10.8) |
| Donors with proteinuria, n (%) | 49(5.8) | 12(8.5) | 9(9.2) |
| Time from donation to proteinuria | 8.3(16.1) | 11.6(9.2) | 17.2(8.9) |
| Time from last pregnancy to proteinuria | 21.1(17.9) | 3.0(8.5) | 9.1(7.9) |
Time units are years; values are mean (standard deviation)
Lastly, we assessed whether having gestational diabetes, hypertension, or preeclampsia has different long-term consequences if the event occurred before or after donation. Most recent eGFR in women with and without adverse maternal complications was similar (60.6±17.5 vs. 59.7±13.1 mL/min/1.73m2, p = 0.70. Roughly half of the women who had gestational hypertension went on to develop hypertension and 8/45 (17.8%) developed proteinuria (Table 9). Donors with a history of preeclampsia developed proteinuria in 36% of the cases and 58% became hypertensive (Table 9).
Table 9.
Consequences of gestational hypertension, gestational diabetes, and preeclampsia, mean (standard deviation)
| Events | Pre-donation Pregnancies | Post-donation pregnancies | Time from last pregnancy to outcome assessment (years) | |
|---|---|---|---|---|
| Gestational diabetes | ||||
| n | 33 | 20 | 13 | |
| Most recent Cr | 1.1(0.2) | 1.1(0.1) | 15.2(14.1) | |
| Available data | (n = 19) | (n = 10) | ||
| Proteinuria | 3 | 0 | 2.8(16.5) | |
| Hypertension | 7 | 2 | 19.4(17.9) | |
| Gestational hypertension | ||||
| n | 45 | 17 | 28 | |
| Most recent Cr | 1.1(0.3) | 1.1(0.3) | 19.5(13.4) | |
| Available data | (n = 16) | (n = 22) | ||
| Proteinuria | 4 | 4 | 1.0 (13.3) | |
| Hypertension | 13 | 13 | 8.6(14.5) | |
| Preeclampsia | ||||
| n | 50 | 23 | 27 | |
| Most recent Cr | 1.1(0.2) | 1.1(0.4) | 16.1(12.7) | |
| Available data | (n = 18) | (n = 23) | ||
| Proteinuria (n) | 10 | 8 | 3.1(15.9) | |
| Hypertension | 11 | 18 | 9.4(14.4) | |
DISCUSSION
This data demonstrates that having a post-donation pregnancy is associated with a higher likelihood of adverse outcomes whether a donor had a pre-donation pregnancy or not. Nevertheless, the frequency of adverse outcomes continues to be similar and in some domains better than those reported in the general population. Importantly, there is a 7-fold increase in the adjusted risk of preeclampsia in post-donation pregnancies, preeclampsia was seen in women who had prior normal pregnancies and the future risk of hypertension and proteinuria in donors who had preeclampsia were higher than what is typically observed in kidney donors.
In 2004, there were an estimated 6.39 million pregnancies in the United States that resulted in live births in 69% of pregnancies that occurred in non-Hispanic whites (57.3% fullterm and 11.7% preterm); rates that are comparable to what we observed in post-donation pregnancies but not in pre-donation ones (15,16).
Two previous small studies of kidney donors suggested no significant differences in maternal or fetal outcomes as compared with the general population (6,7). Neither study, however, compared post-donation and pre-donation pregnancies in the same woman. A more recent and a larger study from Norway, in contrast, demonstrated that the adjusted risk of preeclampsia was significantly higher in post-donation pregnancies; 5.7% pre- vs. 2.6% post-donation (8).
Preeclampsia occurs in 2–10% of pregnancies and a previous history of preeclampsia, presence of antiphospholipid antibodies, preexisting diabetes, hypertension, kidney disease, twin pregnancies, nulliparity, family history of preeclampsia, overweight, maternal age > 40 years, and proteinuria are associated with its development (17–22). Prospective kidney donors are typically excluded at our center if they have any hypertension, diabetes, proteinuria, a measured or estimated GFR < 80 mL/min/1.73m2 or a BMI > 30 kg/m2. Such careful screening for many of the risk factors associated with preeclampsia and the possibility that women with a history of a complicated pregnancy are less likely to consider donation may explain the observation that fetal outcomes in pre-donation pregnancies are better than those in the general population and that the rates of gestational diabetes, hypertension, and preeclampsia pre-donation were remarkably low. This very careful screening, may also explain the better outcomes (vs. general population) in some domains for post-donation pregnancies, as well.
Factors contributing to the increased risk of preeclampsia in kidney donors after donation are unknown. None of our kidney donors with preeclampsia reported having proteinuria or hypertension going into their pregnancies, as indicated at least by their responses and laboratory testing they have submitted to our request of longitudinal follow-up of all donors. In addition, no donors reported twin pregnancies and preeclampsia occurred in multiparous women who had a previous normal pregnancy. The mean age of our kidney donors at the time of their pregnancy post-donation was higher vs. those with pre-donation pregnancies which could partly explain their increased risk of preeclampsia. More than 95% of our donors with preeclampsia, however, were 35 or younger at the time of their pregnancy post-donation; an age well below the age at which the risk increases considerably. While we adjusted for age in our multivariate analysis, one can not easily separate increasing age from increasing time from donation.
Despite the increase in adverse maternal outcomes after donation they remain comparable to published rates and certainly more favorable than outcomes in women with polycystic kidney disease and normal serum creatinine, for example (23). In 428 such pregnancies, 35% developed maternal complications (mostly new hypertension and edema).
The higher incidence of adverse fetal and maternal outcomes in post-donation pregnancies raises 2 possibilities. First, it is conceivable that women with a history of gestational hypertension, gestational diabetes, or preeclampsia are generally excluded from becoming kidney donors and are less likely to even come forward to be evaluated as donors thus explaining the extremely low rates of these complications prior to donation. Our data is somewhat supportive of the lack of routine screening for these conditions as 5 of our donors reported gestational diabetes prior to donation, 8 reported having gestational hypertension and 12 reported having had preeclampsia prior to donation. Second, it is also possible that the woman’s post-donation reduction in renal function is the culprit for their increased risk during subsequent pregnancies. In our previous studies of kidney donors, roughly 15% of donors had a measured GFR < 60 mL/min/1.73m2 and therefore it is possible that it is this group that is most likely to have maternal complications (24,25). Interestingly, adverse maternal events were most clustered in those with the highest eGFR at donation; i.e. the group that had post-donation pregnancies only, whose eGFR at donation was 92.2±16.3 mL/min/1.73m2. Moreover, there was no difference in baseline eGFR in women with or without these complications. Data from uninephrectomized pregnant rats exposed to high protein diet resulted in no worsening in proteinuria suggesting that reduction in renal mass at the uninephrectomy level may not be sufficient to cause renal damage (26). The increased risk for adverse maternal complications may still however be due to the burden of pregnancy imposed on a single kidney analogous to the burden multi-fetal pregnancy would have on a woman with two kidneys. There has been a substantial increase in knowledge regarding the aetiology of preeclampsia particularly regarding the pathogenetic role of angiotensin II agonistic antibodies and circulating angiogenic factors; aspects that cannot be tested in this cohort (27,28). In aggregate, our data cannot single out reduction in GFR as the main reason behind the observed associations but larger numbers, formal measurement of kidney function and a design that is superior to the retrospective design employed here would be needed to really address this critical question.
Preeclampsia may have long-term renal consequences. Vikse et al. found that women who had preeclampsia during their first pregnancy were at increased risk of undergoing a future kidney biopsy and are also more likely to develop ESRD (29,30). In our study, we found an increased prevalence of proteinuria and hypertension in donors who had preeclampsia. A proteinuria prevalence of 36% in women who had hypertension or preeclampsia is very similar to the 20–40% reported rate of microalbuminuria 3–5 years after pregnancies that were associated with preeclampsia or gestational hypertension in women with two kidneys (31,32). These rates of hypertension and proteinuria in donors with complicated pregnancies are significantly higher that what we and others have reported in kidney donors in general (24,25,33–36). One has to seriously consider the possibility that these higher rates are, of course, a result of ascertainment bias; i.e., they were monitored more closely by their physicians.
This analysis has many limitations. Our results suffer not only from response bias but also survival bias as we have no information from those who have died. The responses to the items in the questionnaire were not verified against hospital or clinic records and therefore this data should be interpreted with great caution. In regard to the validity of this survey, Tomeo et al. has demonstrated that maternal recall of many events including fetal (sensitivity 68% and specificity 92%) and pregnancy complications such as hypertension, proteinuria and preeclampsia (sensitivity 23% and specificity 89%) are both reproducible on repeat questioning and reasonably accurate up to thirty or more years after delivery (11). Whether this is true in women who donated a kidney is not known. The concordance rate between our 2 questionnaires for all fetal outcomes was over 99%, suggesting a good reliability, but definitely does not indicate validity in maternal recall regarding maternal outcomes. Notably, the rates of maternal complications observed in our kidney donors are almost identical to the rates reported in the study from Norway that utilized National databases to confirm diagnosis of adverse maternal outcomes in former kidney donors (8). Nevertheless, this instrument was not validated in kidney donors and its use constitutes a major limitation of this analysis.
Conditions such as gestational hypertension and preeclampsia may have been difficult for our respondents and/or their physicians to differentiate from chronic conditions such as hypertension and glomerular diseases. Estimating GFR at time of donation with the MDRD study equation has major limitations since this equation was developed in those with GFR < 60 mL/min/1.73m2. Another critical shortcoming of this analysis is the lack of an internal control group. Lastly, our study summarizes the donors' self-reports of outcomes after pregnancy. The experience covers 4 decades. Definitions of some of the entities (e.g. preeclampsia) may have changed over time. Therefore, it is possible that era affected both the definitions used in self reporting and in our donor selection criteria.
Our data shows that post-donation pregnancies appear to be associated with fetal and maternal outcomes that are similar to what is observed in the general population but are less favorable than pre-donation pregnancies. Given that women of childbearing age are the largest group of kidney donors, the effects of donation on maternal and fetal outcomes should be part of the routine discussion of risk and prospective national registries should be instituted to follow all kidney donors.
Acknowledgments
Funding Source:
NIH: PO1DK13083 (HNI and AJM).
Footnotes
CONFLICT OF INTEREST
None of the authors, or the institutions for which they work, declare any conflict of interest regarding the subject matter discussed in this manuscript.
REFERENCES
- 1.Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33(2):235–252. doi: 10.1016/s0272-6386(99)70296-9. [DOI] [PubMed] [Google Scholar]
- 2.Jones DC, Hayslett JP. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med. 1999;335(4):226–232. doi: 10.1056/NEJM199607253350402. [DOI] [PubMed] [Google Scholar]
- 3.Katz AI, Davison JM, Hayslett JP, Singson E, Lindheimer MD. Pregnancy in women with kidney disease. Kidney Int. 1980;18(2):192–206. doi: 10.1038/ki.1980.128. [DOI] [PubMed] [Google Scholar]
- 4.Armenti VT, Radomski JS, Moritz MJ, Gaughan WJ, Hecker WP, Lavelanet A, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2004:103–114. [PubMed] [Google Scholar]
- 5.Davison JM, Bailey DJ. Pregnancy following renal transplantation. J Obstet Gynaecol Res. 2003;29(4):227–233. doi: 10.1046/j.1341-8076.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 6.Buszta C, Steinmuller DR, Novick AC, Schreiber MJ, Cunningham R, popowniak KL, et al. Pregnancy after donor nephrectomy. Transplantation. 1985;40(6):651–654. doi: 10.1097/00007890-198512000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Wrenshall LE, McHugh L, Felton P, Dunn DL, Matas AJ. Pregnancy after donor nephrectomy. Transplantation. 1996;62(12):1934–1936. doi: 10.1097/00007890-199612270-00044. [DOI] [PubMed] [Google Scholar]
- 8.Reisaeter AV, Roislien J, Henriksen T, Irgens LM, Hartmann A. Pregnancy and birth after kidney donation: The Norwegian experience. Am J Transplant. 2008;8:1–5. doi: 10.1111/j.1600-6143.2008.02427.x. [DOI] [PubMed] [Google Scholar]
- 9.McKay DB, Josephson MA, Armenti VT, August P, Coscia LA, Davis CL, et al. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant. 2005;5(7):1592–1599. doi: 10.1111/j.1600-6143.2005.00969.x. [DOI] [PubMed] [Google Scholar]
- 10.Organ Procurement and Transplantation Network. Accessed at www.optn.org on April 28, 2008. [Google Scholar]
- 11.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774–777. [PubMed] [Google Scholar]
- 12.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [abstract] J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 13.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Boston, MA: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 14.Robert CP, Casella G. Monte Carlo statistical methods. 2nd ed. New York, NY: Springer; 2004. [Google Scholar]
- 15.Ventura SJ, Abma JC, Mosher WD, Henshaw S. Estimated pregnancy rates for the United States, 1990–2000: An update. Natl Vital Stat Rep. 2004;52(23):1–9. [PubMed] [Google Scholar]
- 16.Martin JA, Kung HC, Mathews TJ, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 17.Eskenazi B, Fenster L, Sidney S. A multivariate analysis of risk factors for preeclampsia. JAMA. 1991;266(2):237–241. [PubMed] [Google Scholar]
- 18.Pipkin FB. Risk Factors for Preeclampsia. N Engl J Med. 2001;344(12):925–926. doi: 10.1056/NEJM200103223441209. [DOI] [PubMed] [Google Scholar]
- 19.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas JS, Fuentes-Afflick E, Stewart AL, et al. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159(1):58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 21.Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;339(10):667–671. doi: 10.1056/NEJM199809033391004. [DOI] [PubMed] [Google Scholar]
- 22.Franceschini N, Savitz DA, Kaufman JS, Thorp JM. Maternal urine albumin excretion and pregnancy outcome. Am J Kidney Dis. 2005;45(6):1010–1018. doi: 10.1053/j.ajkd.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. JASN. 1994;5:1178–1185. doi: 10.1681/ASN.V551178. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim HN, Rogers T, Tello A, Matas A. The performance of three serum creatinine-based formulas in estimating GFR in former kidney donors. Am J Transplant. 2006;6(6):1479–1485. doi: 10.1111/j.1600-6143.2006.01335.x. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim HN, Rogers TB, Matas AJ. Predictors of reduced measured GFR, albuminuria and hypertension in former kidney donors [abstract] Am J Transplant. 2007;7(s2):217. [Google Scholar]
- 26.Baylis C. Renal disease in gravid animal models. Am J Kidney Dis. 1987;9(4):350–353. doi: 10.1016/s0272-6386(87)80135-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C, Zhang Y, Irani R, Zhang H, Mi T, Popek E, Hicks MN, Ramin S, Kellems R, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nature Medicine. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hladunewich M, Karumanchi A, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2:543–549. doi: 10.2215/CJN.03761106. [DOI] [PubMed] [Google Scholar]
- 29.Vikse BE, Irgens LM, Bostad L, Iversen BM. Adverse perinatal outcome and later kidney biopsy in the mother. J Am Soc Nephrol. 2006;17(3):837–845. doi: 10.1681/ASN.2005050492. [DOI] [PubMed] [Google Scholar]
- 30.Vikse BE, Irgens LM, Bostad L, Iverson BM. Preeclampsia and risk of end stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 31.Bar J, Kaplan B, Wittenberg C, et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol Dial Transplant. 1999;14:1129–1132. doi: 10.1093/ndt/14.5.1129. [DOI] [PubMed] [Google Scholar]
- 32.Nisell H, Lintu H, Lunell NO, Möllerström G, Pettersson E. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol. 1995;102:876–881. doi: 10.1111/j.1471-0528.1995.tb10874.x. [DOI] [PubMed] [Google Scholar]
- 33.Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145(3):185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 34.Ramcharan T, Matas AJ. Long-term (20–37 years) follow-up of living kidney donors. Am J Transplant. 2002;2(10):959–964. doi: 10.1034/j.1600-6143.2002.21013.x. [DOI] [PubMed] [Google Scholar]
- 35.Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 years or more of follow-up of living kidney donors. Lancet. 1992;340(8823):807–810. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 36.Garg AX, Muirhead N, Knoll G, Yang RC, Prasad GV, Thiessen-Philbrook H, et al. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70(10):1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]