Abstract
Objective
To examine white matter microstructure, as assessed via diffusion tensor imaging (DTI), in adolescents with bipolar I disorder compared with control volunteers.
Method
Twenty-six (12 male and 14 female subjects) adolescents (mean age, 16.0 years) with bipolar I disorder and 26 (14 male and 12 female subjects) control volunteers (mean age, 15.3 years) completed structural and DTI examinations. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) maps were compared between groups in the brain white matter using a voxelwise analysis after intersubject registration to Talairach space. Exploratory analyses were performed to assess structure–function correlations in a subgroup of 11 patients with available neuropsychological measures.
Results
Compared with the control volunteers, the patients demonstrated abnormalities in white matter regions predicted to differ a priori between groups, including lower FA in the right orbital frontal lobe and higher ADC in the right and left subgenual region (p < .005, uncorrected; cluster size ≥ 100). There were no areas of higher FA or lower ADC in patients compared with control volunteers. Lower FA across regions that differed significantly between groups correlated significantly with slower visuomotor speed among patients with bipolar disorder.
Conclusions
Abnormalities involving the orbital frontal and subgenual white matter in adolescents with bipolar disorder are consistent with neurobiological models that implicate dysregulation of affective systems and impulsivity in the pathophysiology of the disorder. Preliminary findings suggest that white matter abnormalities in pediatric bipolar disorder have functional correlates and may be useful in constructing neurobiological models of the disorder.
Keywords: diffusion tensor imaging, bipolar disorder, white matter
Increasing evidence suggests that bipolar disorder may be characterized by a disruption in white matter integrity as identified from hyperintensities,1,2 structural morphometry,3,4 altered expression of myelin and oligodendrocyte genes,5 and fewer oligodendroglial6 and glial7 cells in patients compared with control volunteers. A potentially more sensitive technique for the assessment of white matter integrity with implications for understanding the anatomic organization of axons and myelin sheath involves the use of diffusion tensor imaging (DTI).8 Diffusion tensor imaging is an in vivo magnetic resonance imaging (MRI) technique that provides quantitative information regarding tissue water mobility. Measures of the coherence of white matter fibers that can be derived from DTI include fractional anisotropy (FA), which is a measure of the tendency for water molecules to move in one direction compared with another, and the apparent diffusion coefficient (ADC), which is a measure of water mobility in brain tissue. This information thus represents a potentially powerful tool for in vivo mapping of anatomic connectivity in humans. Adolescence is a time of enormous structural changes in the brain.9 Because adolescence is a frequent age of onset for bipolar disorder, there may be structural neurodevelopmental deficits that are associated with the expression of bipolar disorder during this period of brain development. In particular, myelination of white matter tracts occurs during this time10 especially in the frontal lobes,11 which are believed to be responsible for emotional regulation, response inhibition, and planning and organization, among other functions. Clinically, these are the very functions that are most disrupted during a manic episode.12
Previous DTI studies in bipolar disorder have yielded inconsistent findings with reports of higher anisotropy,13,14 lower anisotropy,15,16 and no differences in anisotropy17 between patients and controls. The majority of studies, however, used small sample sizes and a region-of-interest approach whose definitions may be unreliable. A potentially important advantage of a voxel-based approach is that abnormalities may be identified across the entire brain and with greater reliability. Moreover, examining patients early in the course of illness may limit potential confounds such as illness duration. In this study, we used DTI to investigate FA and ADC in a cohort of adolescents with bipolar I disorder compared with control volunteers using a voxelwise analysis of brain white matter. We hypothesized that patients would demonstrate abnormal FA and ADC in regions implicated in emotion regulation including orbital frontal and subgenual regions.
METHOD
Subjects
Twenty-six adolescents with a diagnosis of bipolar I disorder and 26 control volunteers participated in this study (Table 1). All of the subjects and, separately, a parent or guardian, were interviewed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version.18 None of the comparison subjects met DSM-IV criteria for an Axis I disorder, and none of their first-degree relatives had a diagnosis of bipolar disorder, unipolar disorder, or any psychotic disorder. Additional exclusion criteria for all of the subjects included the following: seizure disorder or other neurological/developmental disorder including mental retardation and autism; history of brain injury/premature birth; cardiovascular disease, hypertension, or diabetes; current/past history of alcohol/substance dependence; history of substance use within 3 months or positive urine toxicology screen; pregnancy as confirmed by urine pregnancy test on day of scan; or contraindications to MRI. The study was approved by the North Shore–Long Island Jewish Health System institutional review board. Parents provided written informed consent, and all of the subjects provided written assent.
TABLE 1.
Sample Characteristics
| Patients (n = 26) | Controls (n = 26) | df | Statistic | p | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 16.0 (1.5) | 15.3 (1.5) | 50 | t = 1.67 | .10 |
| Female, no. (%) | 14 (53.8) | 12 (46.2) | 1 | χ2 = 0.31 | .58 |
| Ethnicity,a no. (%) | 1 | χ2 = 0.69 | .41 | ||
| White | 14 (53.8) | 11 (42.3) | |||
| Hispanic | 3 (11.5) | 4 (15.4) | |||
| African American | 5 (19.2) | 7 (26.9) | |||
| Asian | 3 (11.5) | 3 (11.5) | |||
| Other | 1 (3.8) | 1 (3.8) | |||
| Right-handed, no. (%) | 23 (88.5) | 25 (96.2) | 1 | χ2 = 1.08 | .30 |
| Parental education,b mean (SD), y | 14.2 (3.1) | 15.3 (2.7) | 48 | t = −1.29 | .20 |
Because more than 20% of the categories for race had expected frequencies of less than 5, we combined the latter four groups (i.e., Hispanic, African American, Asian, and other) into a single group for analysis.
There were missing data for two control volunteers for parental education.
Patients met DSM-IV criteria for bipolar I disorder, manic or mixed phase, in at least partial remission. Eleven patients met criteria for additional Axis I disorders, which included a history of a substance use disorder (cannabis [n = 3], alcohol abuse [n = 1], and heroin [n = 1]), attention-deficit/hyperactivity disorder (n = 1), generalized anxiety disorder (n = 2), oppositional defiant disorder (n = 2), obsessive-compulsive disorder (n = 1), panic disorder (n = 2), Tourette’s disorder (n = 1), and eating disorder not otherwise specified (n = 1). The majority of patients (16/26 or 61.5%) were scanned after their first manic or mixed episode. Seventeen (65.3%) had psychotic features, usually grandiose delusions, during their manic episode. At the time of their MRI scan, 21 patients were receiving second-generation antipsychotic medications (olanzapine [n = 9]), risperidone [n = 6], or quetiapine [n = 6]), and 19 patients were receiving lithium. Additional medications at the time of the MRI examination included anticonvulsant mood stabilizers (n = 7), antidepressants (n = 4), stimulants (n = 2), and clonazepam (n = 2). At the time of the scan, the median duration of treatment with a second-generation antipsychotic was 11.3 weeks (range 0–144 weeks), and that with lithium was 8.7 weeks (range 0–107 weeks).
MRI Procedures
Magnetic resonance examinations were conducted on a 1.5-T GE system. Seven DTI volumes were obtained for each subject, including six volumes with diffusion gradients applied along non-collinear directions with b = 860 s/mm2 and number of excitations (NEX) = 4 and a volume without diffusion weighting (b = 0; NEX = 2). Each volume consisted of 18 contiguous axial slices (slice thickness = 5 mm) acquired parallel to the anterior-posterior commissures using a pulsed gradient spin-echo echo-planar imaging method (repetition time [TR] = 6 seconds, time to echo [TE] = 106 ms; 128 × 128 matrix, field of view [FOV] = 22 × 22 cm2). One hundred twenty-four contiguous coronal images (slice thickness = 1.5mm) were also acquired through the whole head using a three-dimensional fast spoiled gradient echo (SPGR) sequence with inversion recovery prep (TR = 14.5 ms, TE = 5.5 ms, FOV = 22 cm, NEX = 1, and inversion time = 600 ms) in a 256 × 256 matrix. Additionally, a double echo-fast spin echo sequence was acquired axially at the same slice positions as the DTI data set (TR = 3000 ms, TE = 17/90 ms, FOV = 22 × 22 cm2, 18 slices, slice thickness = 5.0 mm, gap = 0.0, NEX = 1, and 256 × 256 matrix), providing a pair of T2- and proton density (PD)–weighted volumes that were used to correct distortion on the diffusion tensor images and for segmentation.
After eddy current correction, FA and ADC maps were computed for each subject19 and registered to Talairach space using three image registration steps as described in detail previously.20 Briefly, first, we matched all SPGR volumes to a target volume in Talairach space.21 Second, for each subject, the T2- and PD-weighted volumes were registered to the SPGR volume.22 Third, the b = 0 DTI volume was registered to the T2 volume to correct spatial distortion in the FA maps.19 The transformations from these three registration steps were combined numerically and applied to the FA maps using a single interpolation operation. The resulting transformation was then applied to the original FA or ADC map by a single interpolation operation, yielding a voxel size of 1 × 1 × 1 mm3 in common Talairach space. We also created a white matter mask for all of the subjects using FSL software by segmenting the brain based on the combination of the T2, PD, and SPGR volumes. The white matter segmented from each subject was transformed to Talairach space, averaged, and thresholded at 40% to obtain a white matter mask for the group. Both the registered FA and white matter images were smoothed with a three-dimensional isotropic Gaussian kernel with σ = 6 mm.
Neuropsychological Assessment
Neuropsychological data were available for 11 patients with bipolar disorder who also completed MRI examinations. Neuropsychological tests included measures of visuomotor speed (Trail Making Test, Part A),23 set shifting (Trail Making Test, Part B),23 and motor coordination (average Lafayette Grooved Pegboard performance with the dominant and nondominant hand).24 z Scores were computed for each measure based on published norms for reference samples of age-matched control comparison subjects. Full-scale IQ (FSIQ)25,26 was available for 11 patients and 9 control volunteers; of these individuals, FSIQ was estimated for 3 patients and 2 control volunteers using the Vocabulary and Block Design Subtests.27
Clinical Assessment
Nineteen patients received the Hamilton Depression Rating Scale,28 Young Mania Rating Scale,29 and the Clinical Global Assessment Scale.30
Statistical Analyses
Group differences in demographic variables and FSIQ were examined using either χ2 or independent groups t tests. Two-sample t tests were performed at each voxel on the FA values within the brain white matter between the patients and controls, with age as a statistical covariate. We estimated the false discovery rate (FDR) for the applied p value threshold of .005 to be q < 0.035 using the “FDR” program of the FSL package with the conservative option, which estimates the FDR using the method of Benjamini and Hochberg.31 As a further protection against multiple comparisons, significantly different FA values (corresponding to a p value of .005 or less) were required to be part of a spatially contiguous cluster size of 100 voxels or greater.32,33 Maximum t values for regions of FA and ADC that differed significantly between groups were imported into SPSS (version 11.5; SPSS, Chicago, IL) to investigate the potential effects of comorbidity, multiple episodes of illness, and psychosis on the observed findings. These values were also used for the investigation of structure–function relations and to examine in relation to duration of antipsychotic and lithium treatment at the time of the scan. For these analyses, we used Spearman rank order correlations to minimize the influence of any outliers, given the small sample size. Alpha was set to .05. To control type I error in the investigation of structure–function relations and clinical correlates, we computed an average FA measure, and an average ADC measure, across the 5 and 10 regions, respectively, that differed significantly between patients and control volunteers.
RESULTS
The patients did not differ significantly from control volunteers in distributions of relevant demographics (Table 1). In addition, independent groups t tests did not reveal any significant (p > .05) difference in FSIQ between patients (mean 97.7, SD 17.7) and control volunteers (mean 100.4, SD 16.3) or between groups where FSIQ was not estimated. Bipolar I adolescents demonstrated significantly (p < .005, uncorrected; cluster size ≥ 100) lower FA in the right orbital frontal region compared with control volunteers (Fig. 1). In addition, lower FA was identified in the left and right temporal lobes and left occipital lobe in the patients compared with the control volunteers (Table 2). Higher ADC was observed in both right and left subgenual regions in the patients compared with the control volunteers (Fig. 2). In addition, higher ADC was identified in the postcentral gyrus, precuneus, and temporal and occipital lobes (Table 3). There were no regions of significantly higher FA or lower ADC in the patients compared with the control volunteers at this threshold and α level.
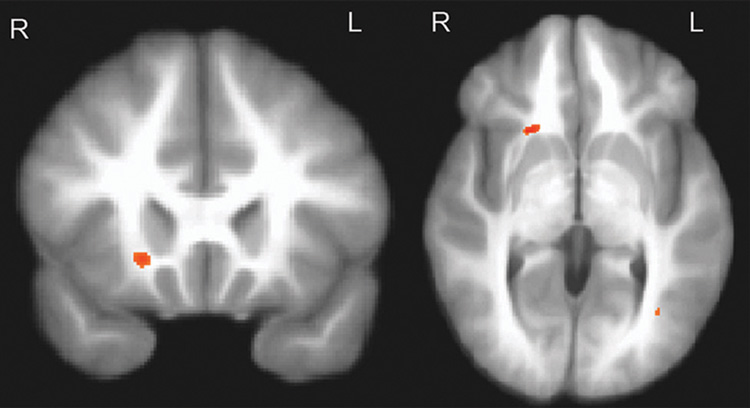
Fig. 1.
Lower fractional anisotropy in the right orbital frontal region in adolescent bipolar patients compared with control volunteers (p < .005; uncorrected, minimum cluster size = 100 voxels). Maximum t value located at x = 20, y = 18, z = −1.
TABLE 2.
Mean Fractional Anisotropy Values for Patients and Healthy Volunteers
| Region | Talairach Coordinates (x, y, z) |
Patients (n = 26), Mean FA (SD)a |
Controls (n = 26), Mean FA (SD)a |
tb,c | Cluster Size |
|---|---|---|---|---|---|
| Right hemisphere | |||||
| Orbital frontal | 20, 18, −1 | 371 (49) | 430 (50) | 4.29 | 112 |
| Temporal | 30, −59, 8 | 521 (50) | 574 (51) | 3.79 | 376 |
| Left hemisphere | |||||
| Temporal | −32, −50, 3 | 534 (49) | 574 (46) | 2.97 | 144 |
| Temporal | −47, −36, −7 | 330 (81) | 399 (69) | 3.30 | 116 |
| Occipital | −36, −59, −7 | 354 (75) | 433 (88) | 3.49 | 251 |
Note: FA = fractional anisotropy.
Values are multiplied by 1,000.
Represents maximum t value for cluster.
p < .005, uncorrected.
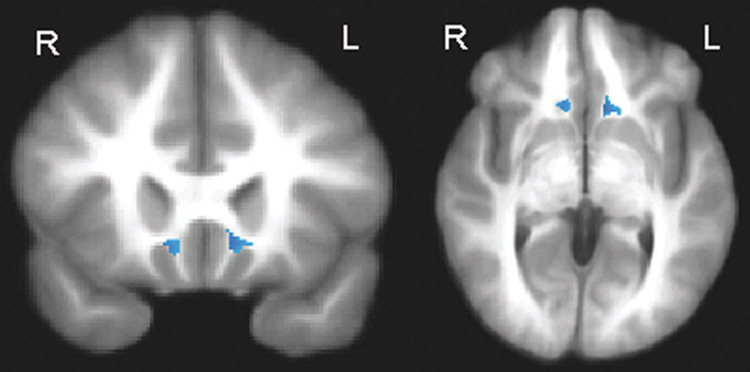
Fig. 2.
Higher apparent diffusion coefficient in the right and left subgenual white matter in adolescent bipolar patients compared with control volunteers (p < .005; uncorrected; minimum cluster size = 100 voxels). Maximum t values located at x = 9, y = 21, z = −4 (right hemisphere) and x = −11, y = 19, z = −2 (left hemisphere).
TABLE 3.
Mean ADC Values for Patients and Healthy Volunteers
| Region | Talairach Coordinates (x, y, z) |
Patient (n = 26), Mean ADC (SD) |
Controls (n = 26), Mean ADC (SD) |
ta,b | Cluster Size |
|---|---|---|---|---|---|
| Right hemisphere | |||||
| Subgenual | 9, 21, −4 | 860 (193) | 733 (67) | 3.14 | 102 |
| Precuneus | 14, −59, 44 | 690 (33) | 663 (23) | 3.12 | 166 |
| Precuneus | 12, −54, 52 | 750 (50) | 710 (33) | 3.37 | 128 |
| Sublobar extranuclear | 29, −12, 16 | 667 (20) | 653 (20) | 2.70 | 118 |
| Occipital | 32, −72, 22 | 717 (73) | 657 (40) | 3.59 | 160 |
| Left hemisphere | |||||
| Subgenual | −11, 19, −2 | 773 (83) | 723 (40) | 2.74 | 139 |
| Postcentral gyrus | −51, −26, 21 | 750 (73) | 707 (30) | 3.00 | 129 |
| Temporal | −31, −61, 26 | 693 (53) | 650 (33) | 3.49 | 156 |
| Precuneus | −13, −62, 42 | 707 (40) | 667 (37) | 3.90 | 167 |
| Postcentral gyrus | −40, −29, 44 | 743 (87) | 693 (40) | 2.70 | 111 |
Note: ADC = apparent diffusion coefficient.
Represents maximum t value for cluster.
p < .005, uncorrected.
Ancillary analyses confirmed significantly (p < .05) lower FA in the right orbital frontal region and higher ADC in the right subgenual region in the patients without a comorbid diagnosis (n = 15), those experiencing their first episode of mania (n = 16), and those without psychosis (n = 9) compared with the control volunteers. In the left subgenual region, the group of patients without a comorbid diagnosis (n = 15) and the group experiencing their first episode of mania (n = 16) had significantly (p < .05) lower ADC compared with the control volunteers. In addition, duration of antipsychotic and lithium treatment at the time of the scan was not significantly correlated with FA in the right orbital frontal lobe or ADC in the right or left subgenual regions.
The patients with neuropsychological data (n = 11) did not differ significantly from the patients without neuropsychological data (n = 15) in distributions of age, sex, number of patients with a comorbid diagnosis, number of patients experiencing a first episode of illness, or number of patients with psychosis. Investigation of structure–function relations revealed a significant positive correlation between average FA across the five regions that differed significantly between groups and worse functioning on the Trail Making Test, Part A (r = −0.64, df = 11, p = .035). Trails A performance was not correlated significantly, however, with any of the five regions of FA that differed significantly between groups. Average ADC across the 10 regions that differed significantly between groups did not correlate significantly with any of the neuropsychological or clinical measures.
DISCUSSION
The main findings of this study support our a priori hypothesis that adolescents with bipolar I disorder have abnormalities in the ventral prefrontal white matter, including orbital frontal and subgenual regions. Specifically, patients demonstrated lower FA in the right orbital frontal white matter and higher ADC bilaterally in the subgenual white matter compared with the control volunteers. Regions of lower FA and higher ADC were also observed in nonhypothesized regions in patients compared with the control volunteers, including the temporal and occipital lobes, postcentral gyrus, and precuneus. Strengths of the present study include the relatively large sample size and the assessment of white matter integrity early in the course of illness before extensive pharmacological intervention.
The few studies that have investigated FA or ADC in pediatric bipolar samples have used smaller sample sizes and younger patients, and thus, it is difficult to compare our findings with previous results. Our findings are generally consistent with a report of lower FA in the brain white matter in medication-naïve adolescents (mean age, 14 years) experiencing their first episode of mania.16 However, Adler et al.16 restricted their analysis to prefrontal white matter regions-of-interest above the anterior commissure and thus did not investigate the ventral prefrontal white matter. Our data converge more specifically with Frazier et al.,34 who reported lower FA in the orbital frontal white matter in a cohort of very young (mean age, 9.2 years) medicated patients with bipolar disorder compared with controls. Findings of lower FA or higher ADC in pediatric bipolar cohorts could conceivably represent microstructural abnormalities in the brain white matter involving the myelin sheath and/or a disruption in axonal organization. Any abnormalities may become evident during adolescence when these white matter pathways are developing.
Previous DTI studies in adults with bipolar disorder have yielded mixed findings but may be informative regarding neurobiological mechanisms that could play a role in adolescent forms of the disorder. In this regard, our data are consistent with the results of Beyer et al.,17 who reported significantly higher ADC in the orbital frontal white matter in 14 adult patients with bipolar disorder compared with 21 nonpsychiatrically ill control volunteers. Other studies investigating adult patients with bipolar disorder, however, have yielded findings of higher anisotropy in the internal capsule adjacent to the striatum and thalamus,13 higher FA in the genu of the corpus callosum,14 and a significantly increased number of reconstructed fibers between the left subgenual cingulate and left amygdalo-hippocampal complex35 compared with control volunteers. Methodological differences, including the use of voxel-based, region-of-interest, and tractography approaches; the lack of statistical power in studies with small sample sizes; inconsistencies in regions examined; potentially differing neurobiologies for pediatric and adult samples; and illness duration may contribute to discrepant findings between our pediatric study findings and those observed in adult studies.
Our data provide converging evidence, using both FA and ADC, for abnormalities in the ventral prefrontal white matter in pediatric bipolar disorder, which has been hypothesized to play an important role in neurobiological models of the disorder.36 For example, some data suggest that patients with bipolar disorder engage emotional brain areas, including the orbital frontal lobe, more often than do control volunteers while performing neuropsychological tasks.37 In addition, impairments in neuropsychological measures with purported sensitivity to ventral prefrontal functioning have been reported in pediatric patients with bipolar disorder.12 Moreover, functional neuroimaging studies report ventral prefrontal cortical activation abnormalities in patients compared with control volunteers while viewing positive and negative stimuli38 and during performance of the Stroop39 and emotional go/no-go tasks.40 Our findings are thus consistent with the hypothesis that certain aspects of phenomenology including impulsivity41 response inhibition39 and decision making42 in bipolar disorder may be linked to a defect involving ventral prefrontal abnormalities,42 making it a potentially useful endophenotypic marker for bipolar disorder.42
It is conceivable that manic states may result from the episodic failure of the ventral prefrontal cortex to inhibit the expression of exaggerated mood states such as elation and irritability and the manifestation of behaviors associated with mania such as impulsivity, talkativeness, and flight of ideas. Structural abnormalities within inhibitory tracts, such as those we report in our pediatric sample, may increase vulnerability to such failures of inhibition,43 thereby allowing pathological behaviors that are usually suppressed to be expressed during manic episodes. The inclusion of neuropsychological or clinical measures specifically tapping the integrity of ventral prefrontal regions would be an important consideration for future studies investigating structure–function relations to directly test this hypothesis.
It may be noteworthy that, in our study, abnormal FA in the orbital frontal white matter was evident in the right hemisphere. Some neuropsychological studies indicate that patients with bipolar disorder have a differential right versus left hemisphere deficit with respect to neurocognitive tasks.44 Moreover, Blumberg et al.45 indicated that mania was associated with decreased right orbital frontal activation during word generation, and Leibenluft et al.46 reported that children with bipolar disorder may have deficits in their ability to engage the right ventral prefrontal cortex during unsuccessful inhibition. Taken together with the present findings, these studies support the possibility that right hemispheric abnormalities may play an important role in modulating mood in bipolar disorder.
Abnormalities in ADC were evident among pediatric patients with bipolar disorder in the subgenual white matter. Morphological alterations in this region have been linked to both depressive47 and bipolar48 disorders. A recent meta-analysis, however, identified subgenual cingulate volume reductions in patients with unipolar but not bipolar disorder.49 This raises the possibility that DTI may be a more sensitive technique for assessing the integrity of this region compared with regional volumetry, although it should be acknowledged that the majority of studies examining this region have focused solely on the gray matter. In this regard, it may be particularly noteworthy that both animal50 and human51 studies have identified a pattern of strong connectivity between the subgenual region and amygdala, which has been demonstrated to be abnormal in bipolar disorder35 and thus may contribute to aspects of bipolar phenomenology.
Investigation of structure–function relations among the subset of patients with DTI and neuropsychological data available revealed that lower FA across the five regions that differed significantly between groups was associated with worse overall neuropsychological functioning. More specifically, lower FA across these regions was associated with slower performance on a task of visual–motor speed. Although neuropsychological deficits on measures of visual–motor functioning have been reported in patients with bipolar disorder,43 the neurobiology underlying these deficits is not well understood. Our findings suggest that a distributed network of regions encompassing the white matter in the orbital frontal, temporal, and occipital lobes may contribute to deficits in motor processing speed. These analyses should be considered preliminary, however, because of the small sample size and number of correlations performed.
There were a number of limitations in our study that should be acknowledged. Although there is the risk for type I error with the use of any voxelwise analysis, we limited this possibility by restricting the analysis to the white matter and by using a large extent threshold. Moreover, we believe it is noteworthy that, despite the potential bias for a type I error, we did not observe any regions of higher FA or lower ADC in the patients compared with the control volunteers, thus supporting the specificity of the observed findings. Another potential study limitation is that the patients were receiving antipsychotics and/or mood stabilizers at the time of the scan, and the potential effects of these medications on white matter integrity are not well understood.52 Thus, these findings need to be replicated in medication-naïve samples.
In sum, our data provide support for ventral prefrontal white matter abnormalities in the pathogenesis of bipolar disorder. Future work could focus on the use of tractography to investigate these abnormalities in bipolar disorder as well as their functional significance.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health to Dr. Kafantaris (R03 MH064554 and R01 MH60845), Dr. Lencz (K01 MH65580), Dr. Szeszko (K01 MH01990), and the Feinstein Research Institute of the North Shore–Long Island Jewish Health System General Clinical Research Center (M01 RR018535).
Preliminary findings from this study were presented at the Sixth International Conference on Bipolar Disorder, Pittsburgh, PA, June 2005.
The authors thank Joshua Vogel, B.A., Kelly Dillon, B.A., Alison Berest, B.S., Ellen Leigh, Ph.D., and Kim Gallelli, Ph.D., for support in subject recruitment, assessment, and image processing.
Footnotes
Disclosure: The authors report no conflicts of interest.
Contributor Information
Vivian Kafantaris, Feinstein Institute for Medical Research, North Shore–Long Island Jewish Health System
Peter Kingsley, Department of Radiology/MRI, North Shore University Hospital, North Shore–Long Island Jewish Health System
Babak Ardekani, Nathan S. Kline Institute for Psychiatric Research
Ema Saito, Zucker Hillside Hospital
Todd Lencz, Feinstein Institute for Medical Research, North Shore–Long Island Jewish Health System
Kelvin Lim, Department of Psychiatry, University of Minnesota
Philip Szeszko, Feinstein Institute for Medical Research, North Shore–Long Island Jewish Health System
REFERENCES
- 1.Aylward EH, Roberts-Twillie JV, Barta PE, et al. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- 2.Ahn KH, Lyoo IK, Lee HK, et al. White matter hyperintensities in subjects with bipolar disorder. Psychiatry Clin Neurosci. 2004;58:516–521. doi: 10.1111/j.1440-1819.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- 3.Kieseppä T, van Erp TG, Haukka J, et al. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol Psychiatry. 2003;54:896–905. doi: 10.1016/s0006-3223(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 4.Strakowski SM, Wilson DR, Tohen M, et al. Structural brain abnormalities in first-episode mania. Biol Psychiatry. 1993;33:602–609. doi: 10.1016/0006-3223(93)90098-x. [DOI] [PubMed] [Google Scholar]
- 5.Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dys-function in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 6.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 7.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 8.Kingsley PB. Introduction to diffusion tensor imaging mathematics. Part I. Tensors, rotations, and eigenvectors. Concepts Magn Reson. 2006;28A:101–122. [Google Scholar]
- 9.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 10.Ashtari M, Cervellione KL, Hasan KM, et al. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 11.Giorgio A, Watkins KE, Douaud G, et al. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 12.Bearden CE, Glahn DC, Caetano S, et al. Evidence for disruption in prefrontal cortical functions in juvenile bipolar disorder. Bipolar Disord. 2007;9:145–159. doi: 10.1111/j.1399-5618.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- 13.Haznedar MM, Roversi F, Pallanti S, et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Yurgelun-Todd DA, Silveri MM, Gruber SA, et al. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9:504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 15.Adler CM, Holland SK, Schmithorst V, et al. Abnormal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disorders. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 16.Adler CM, Adams J, DelBello MP, et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging stuzdy. Am J Psychiatry. 2006;163:322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 17.Beyer J, Taylor WD, MacFall JR, et al. Cortical white matter micro-structural abnormalities in bipolar disorder. Neuropsychopharmacol. 2005;30:2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman J, Birmaher B, Brent D, et al. The schedule for affective disorders and schizophrenia for school-age children–present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 20.Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- 21.Ardekani BA, Guckemus S, Bachman A, et al. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Ardekani BA, Braun M, Hutton BF, et al. A fully automatic multi-modality image registration algorithm. J Comput Assist Tomogr. 1995;19:615–623. doi: 10.1097/00004728-199507000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Tucson: Reitan Neuropsychology Laboratories; 1979. [Google Scholar]
- 24.Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. Madison: University of Wisconsin Medical School; 1964. [Google Scholar]
- 25.Wechsler D. Wechsler Intelligence Scale for Children–Third Edition. San Antonio: The Psychological Corporation, Harcourt Brace Jovanovich; 1991. [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 27.Kaufman AS. Assessing Adolescent and Adult Intelligence. Boston: Allyn & Bacon; 1990. [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 30.Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. Washington: U.S. Department of Health, Education and Welfare publication (Publication ADM 76-338); 1976. [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 32.Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 33.Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 34.Frazier JA, Breeze JL, Papadimitriou G, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9:799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 35.Houenou J, Wessa M, Douaud G, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12:1001–1110. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 36.Blumberg HP, Kaufman J, Martin A, et al. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci. 2004;1021:376–383. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh AM, Whalley HC, McKirdy J, et al. Prefrontal function and activation in bipolar disorder and schizophrenia. Am J Psychiatry. 2008;165:378–384. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Kronhaus DM, Lawrence NS, Williams AM, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 40.Wessa M, Houenou J, Paillère-Martinot ML, et al. Fronto-striatal over-activation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- 41.Christodoulou T, Lewis M, Ploubidis GB, Frangou S. The relationship of impulsivity to response inhibition and decision-making in remitted patients with bipolar disorder. Eur Psychiatry. 2006;21:270–273. doi: 10.1016/j.eurpsy.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol Psychiatry. 2005;58:838–839. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Haldane M, Cunningham G, Androutsos C, Frangou S. Structural brain correlates of response inhibition in bipolar disorder I. J Psychopharmacol. 2008;22:138–143. doi: 10.1177/0269881107082955. [DOI] [PubMed] [Google Scholar]
- 44.Frantom LV, Allen DN, Cross CL. Neurocognitive endophenotypes for bipolar disorder. Bipolar Disord. 2008;10:387–399. doi: 10.1111/j.1399-5618.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 45.Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–1999. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 46.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 47.Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. 2005;162:1706–1712. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- 48.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 49.Hajek T, Kozeny J, Kopecek M, Alda M, Höschl C. Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. J Psychiatry Neurosci. 2008;33:91–99. [PMC free article] [PubMed] [Google Scholar]
- 50.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- 51.Johansen-Berg H, Gutman DA, Behrens TE, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]