Abstract
Purpose
To determine the effects of age on global and sectoral peripapillary retinal nerve fiber layer (RNFL), macular thicknesses and optic nerve head (ONH) parameters in healthy subjects using optical coherence tomography (OCT).
Design
Retrospective, cross-sectional observational study.
Participants
226 eyes from 124 healthy subjects were included.
Methods
Healthy subjects were scanned using the Fast RNFL, Fast Macula, and Fast ONH scan patterns on a Stratus OCT. All global and sectoral RNFL and macular parameters and global ONH parameters were modeled in terms of age using linear mixed effects models. Normalized slopes were also calculated by dividing the slopes by the mean value of the OCT parameter for inter-parameter comparison.
Main Outcome Measures
Slope of each OCT parameter across age.
Results
All global and sectoral RNFL thickness parameters statistically significantly decreased with increasing age, except for the temporal quadrant and clock hours 8-10, which were not statistically different from a slope of zero. Highest absolute slopes were in the inferior and superior quadrant RNFL and clock hour 1 (superior nasal). Normalized slopes showed similar rate in all sectors except for the temporal clock hours (8-10). All macular thickness parameters statistically significantly decreased with increasing age, except for the central fovea sector, which had a slight positive slope that was not statistically significant. The nasal outer sector had the greatest absolute slope. Normalized macular slope in the outer ring was similar to the normalized slopes in the RNFL. Normalized inner ring had shallower slope than the outer ring with similar rate in all quadrants. Disc area remained nearly constant across the ages, but cup area increased and rim area decreased with age, both of which were statistically significant.
Conclusions
Global and regional changes due to the effects of age on RNFL, macula and ONH OCT measurements should be considered when assessing eyes over time.
Optical coherence tomography (OCT) is a noninvasive technique for cross-sectional tomographic imaging of the eye. OCT has demonstrated good reproducibility for ocular measurement1-5 and has become an important diagnostic tool in glaucoma assessment.6-10 The device is able to measure retinal thickness in the macular region, retinal nerve fiber layer (RNFL) thickness around the optic nerve head (ONH), and optic nerve head parameters such as cup and disc size. Quantitative assessment of ocular structures is clinically valuable because it allows comparisons between scans over time. However, in order to detect changes in OCT measurements over time that are related to glaucoma, the normal aging effect on structural measurements must be considered, since only changes beyond the normal aging effect can be attributed to the glaucomatous disease. However, separation between these two causes of tissue loss can be difficult.
Several previous reports have published rates of circumpapillary RNFL loss measured by OCT, however the rates were substantially different among researchers and the studies were not designed to examine aging effects on sectoral RNFL measurements.11-14 Furthermore, the influence of age on macular and ONH parameters, which are also affected by tissue loss and could be measured by commercially available OCT, has not been previously published. The valuable role of macular and ONH parameters of OCT in glaucoma practice has been shown in several studies.15-17
The purpose of this study was to evaluate age related change of RNFL, macula and ONH parameters in healthy eyes measured by OCT. We evaluated the rates of change both globally and in different sectors to test the hypothesis that normal aging decay could occur at different rates in different locations in the posterior segment.
Methods
Subjects
Healthy subjects from the University of Pittsburgh Medical Center (UPMC) Eye Center were enrolled in the study. The study was approved by the Institutional Review Board ethics committee of the University of Pittsburgh and adhered to the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations. Informed consent was obtained from all participants.
Inclusion criteria were best corrected visual acuity of 20/40 or better, refractive error within ± 6.0 Diopters, no media opacities which interfere with fundus imaging, normal clinical ocular examination with no evidence of retinal or optic nerve head (ONH) pathologies and normal 24-2 standard Swedish interactive thresholding algorithm (SITA) perimetry with less than 30% fixation losses, false-positive and false-negative responses. Normal visual field was defined as glaucoma hemifield test (GHT) within normal limits and pattern standard deviation (PSD) probability greater than 5%. Subjects were excluded if they were using medications that are known to affect retinal thickness or if they had systemic diseases that might affect the retina or visual field. Subjects were also excluded if they had any previous intra-ocular operation other than uneventful cataract extraction. Both eyes were included for analysis if they were eligible.
Optical coherence tomography
All OCT scans were performed by Stratus OCT (Carl Zeiss Meditec, Dublin, CA; software version 4.07) using the Fast Macular, Fast RNFL, and Fast Optic Disc scan patterns. Included scans were required to have signal strength of 6 or greater and were examined visually for presence of overt misalignment of the segmentation lines of no more than 15 consecutive or less than 20 cumulative percent of the scan. OCT fast macula and ONH scans consist of six 6 mm linear scans in a spoke-like radial configuration, with each line containing 128 A-scans, and are centered on the fovea and ONH, respectively. The fast RNFL scan consists of three 3.4 mm diameter circumpapillary scans centered at the ONH, with each scan having 256 A-scans. The RNFL thickness parameters were calculated as the mean of three corresponding parameters measured independently on three individual circular scans.
Analysis
For RNFL scans, overall mean RNFL thickness and RNFL thickness measurements averaged within the 4 quadrants and 12 clock hours were used for analysis. The parameters used for the analysis in the macular region were 9 sectoral retinal thicknesses given automatically by the Stratus OCT macular thickness map, and overall macular thickness, calculated as the weighted average of the sectoral macular thickness measurements using the following formula: 1/36 * center + 1/18 * (sum of inner ring quadrants thickness) + 3/16 * (sum of outer ring quadrants thickness).17 All ONH parameters as appearing in the conventional printout were used for the ONH analysis. Linear mixed effects models were fit to RNFL, Macula, and Optic Nerve Head parameters to assess thicknesses changes across subjects of different ages. The absolute slope over different age groups for the sectoral parameter measurements were examined.
Considering that the rate of change might be influenced by the level of the measurement we normalized the slope values by calculating the slope divided by the average parameter value in-order to asses if the relative rate of change is homogenous throughout the different locations. P < 0.05 was considered as statistically significant.
Results
Two hundreds and twenty six eyes of 124 healthy subjects were enrolled in this study. Of the 124 subjects, 80 were women and 44 were men; 103 were Caucasian, 12 African American, 8 Asian, and 1 Hispanic. The average ± standard deviation (SD) age was 47.5 ± 15.9 years, and ranged from 18 to 85 years. The distribution of ages is presented in Figure 1.
Figure 1.
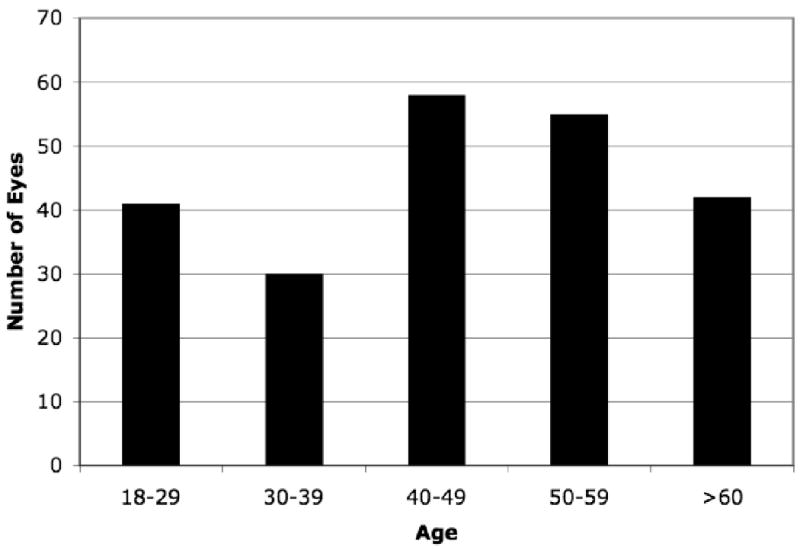
Age distribution by decade.
The population average ± SD of overall RNFL thickness was 100.8 ± 10.5 μm, overall macular thickness was 249.0 ± 11.3 μm and rim area was 1.76 ± 0.42mm2. The number of eyes included with each scan type and the measurements of global and sectoral RNFL, macular and ONH parameters across the different age groups are summarized in Tables 1-3.
Table 1.
Average ± standard deviation retinal nerve fiber layer thickness measurements (μm) for mean overall, 4 quadrants and 12 clock hours in different age groups.
| Age (years) | 18-29 | 30-39 | 40-49 | 50-59 | 60-85 |
|---|---|---|---|---|---|
| Number of Eyes | 41 | 30 | 56 | 57 | 42 |
| Overall | 107.4±8.8 | 103.7±9.3 | 102.4±9.0 | 98.3±10.6 | 93.4±9.2 |
| Superior | 132.7±17.2 | 129.8±13.0 | 127.1±14.6 | 123.9±16.2 | 114.0±14.8 |
| Inferior | 136.4±15.4 | 129.4±15.2 | 127.5±15.2 | 123.3±17.8 | 117.6±15.0 |
| Temporal | 74.3±11.2 | 77.6±19.2 | 69.6±9.8 | 64.7±11.6 | 71.3±13.4 |
| Nasal | 86.1±14.4 | 77.9±13.1 | 85.2±15.1 | 81.4±16.3 | 70.7±15.6 |
| 1 o'clock | 126.2±23.8 | 119.2±19.1 | 112.9±20.4 | 114.9±21.0 | 100.8±17.8 |
| 2 o'clock | 103.2±20.3 | 93.4±17.9 | 102.8±17.6 | 97.2±20.6 | 82.1±20.0 |
| 3 o'clock | 70.6±14.1 | 64.4±11.2 | 70.6±14.2 | 66.8±17.8 | 58.3±15.1 |
| 4 o'clock | 84.6±16.9 | 75.8±16.5 | 82.3±19.5 | 80.2±17.8 | 71.9±16.9 |
| 5 o'clock | 117.3±26.1 | 110.9±20.4 | 111.9±22.9 | 108.1±26.4 | 98.2±19.3 |
| 6 o'clock | 147.2±21.4 | 136.2±25.5 | 137.1±21.6 | 137.5±29.5 | 125.5±20.7 |
| 7 o'clock | 144.8±21.5 | 141.3±24.2 | 133.6±17.1 | 124.3±15.2 | 129.3±24.5 |
| 8 o'clock | 76.5±14.6 | 81.8±23.2 | 70.8±12.1 | 66.2±13.1 | 70.9±15.5 |
| 9 o'clock | 58.0±8.5 | 60.0±15.1 | 54.1±7.9 | 50.2±9.3 | 55.9±10.3 |
| 10 o'clock | 88.6±15.6 | 91.0±23.3 | 83.9±14.5 | 78.0±16.6 | 87.3±19.5 |
| 11 o'clock | 138.7±21.7 | 138.4±24.3 | 139.8±20.6 | 126.4±20.2 | 129.6±21.4 |
| 12 o'clock | 133.1±27.1 | 131.9±22.4 | 128.6±22.3 | 130.3±24.3 | 111.4±20.6 |
Table 3.
Average ± standard deviation of optic nerve head parameters in different age groups.
| Age (years) | 18-29 | 30-39 | 40-49 | 50-59 | 60-85 |
|---|---|---|---|---|---|
| Number of Eyes | 41 | 30 | 56 | 57 | 41 |
| Disc area (mm2) | 2.30±0.49 | 2.19±0.47 | 2.28±0.36 | 2.21±0.43 | 2.23±0.31 |
| Rim area (mm2) | 2.03±0.51 | 1.79±0.40 | 1.79±0.39 | 1.64±0.34 | 1.55±0.31 |
| Cup area (mm2) | 0.26±0.21 | 0.40±0.30 | 0.49±0.29 | 0.57±0.42 | 0.67±0.35 |
| Cup/disc area ratio | 0.11±0.09 | 0.18±0.12 | 0.21±0.11 | 0.24±0.15 | 0.30±0.13 |
| Vertical cup/disc ratio | 0.29±0.16 | 0.35±0.18 | 0.42±0.14 | 0.44±0.16 | 0.51±0.14 |
| Horizontal cup/disc ratio | 0.31±0.16 | 0.41±0.20 | 0.47±0.16 | 0.49±0.19 | 0.55±0.13 |
| Vertical integrated rim area | 0.80±0.36 | 0.62±0.27 | 0.58±0.29 | 0.53±0.29 | 0.39±0.21 |
| Horizontal integrated rim width | 2.03±0.26 | 1.90±0.21 | 1.83±0.24 | 1.77±0.23 | 1.59±0.21 |
| Cup area topo | 0.22±0.22 | 0.36±0.29 | 0.39±0.27 | 0.46±0.37 | 0.54±0.32 |
| Cup volume topo | 0.02±0.04 | 0.05±0.05 | 0.06±0.07 | 0.08±0.10 | 0.09±0.08 |
The slope change of RNFL over people of different ages was statistically significantly different from a slope of zero for overall, and in superior, inferior and nasal quadrants (p ≤ 0.01) but not statistically significant in the temporal quadrant (p = 0.46) (Table 4). Clock-hour 1 had the most rapid tissue loss with age with slope of -0.45 μm/year (normalized: -0.004), which reached statistical significance, along with clock hours 11-7 (Table 4). The steepest quadrant (inferior) was significantly steeper than the temporal quadrants and clock hours 8-10 (p ≤ 0.01). The steepest clock hour (1 o'clock) showed similar pattern with significantly steeper slope than temporal quadrants and clock hours 3-4 and 8-10 (p ≤ 0.02). Conversely, the temporal quadrant and clock hour 9, the shallowest sectors, were statistically significantly shallower than the inferior and superior quadrants and clock hours 12-2 and 5-7 (p ≤ 0.03 for temporal quadrant, p ≤ 0.02 for 9 o'clock).
Table 4.
The absolute and normalized slopes for retinal nerve fiber layer thickness over ages in different sectors, with absolute slope 95% confidence interval and p value.
| Absolute slope (μm/year) | Confidence Interval | p value* | Normalized Slope | |
|---|---|---|---|---|
| Overall | -0.2552 | -0.4391 - -0.0712 | 0.01 | -0.0025 |
| Superior | -0.3452 | -0.5291 - -0.1612 | <0.001 | -0.0028 |
| Inferior | -0.3606 | -0.5446 - -0.1767 | <0.001 | -0.0029 |
| Temporal | -0.0687 | -0.2527 - 0.1152 | 0.46 | -0.0010 |
| Nasal | -0.2457 | -0.4297 - -0.0617 | 0.01 | -0.0030 |
| 1 o'clock | -0.4464 | -0.6304 - -0.2625 | <0.001 | -0.0039 |
| 2 o'clock | -0.3616 | -0.5455 - -0.1776 | <0.001 | -0.0038 |
| 3 o'clock | -0.1846 | -0.3686 - -0.0007 | 0.05 | -0.0028 |
| 4 o'clock | -0.1866 | -0.3706 - -0.0027 | 0.05 | -0.0024 |
| 5 o'clock | -0.3856 | -0.5696 - -0.2017 | <0.001 | -0.0035 |
| 6 o'clock | -0.3868 | -0.5707 - -0.2028 | <0.001 | -0.0028 |
| 7 o'clock | -0.3108 | -0.4948 - -0.1268 | 0.001 | -0.0023 |
| 8 o'clock | -0.1066 | -0.2905 - 0.0774 | 0.25 | -0.0015 |
| 9 o'clock | -0.0359 | -0.2198 - 0.1481 | 0.70 | -0.0007 |
| 10 o'clock | -0.0662 | -0.2501 - 0.1178 | 0.48 | -0.0008 |
| 11 o'clock | -0.2311 | -0.4151 - -0.0471 | 0.01 | -0.0017 |
| 12 o'clock | -0.3636 | -0.5476 - -0.1797 | <0.001 | -0.0029 |
P value for an absolute slope difference from a zero slope.
Overall macular thickness statistically significantly decreased by -0.42 μm/year (normalized: -0.002) (p < 0.001), but increased in the foveal center by 0.12 μm/year (normalized: 0.001), which was not significant (p = 0.28) (Table 5). The steepest sector, nasal outer, was statistically significantly steeper than all other sectors except superior outer and inferior outer (p < 0.005). The shallowest sector, the center, was statistically significantly shallower than all other macular parameters (p < 0.0001). Normalized macular slope in the outer ring was similar to the normalized slopes in the RNFL. Normalized inner ring had shallower slope than the outer ring with similar rate in all quadrants.
Table 5.
The absolute and normalized slope for macular thickness measured overall and in different sectors in terms of age, with absolute slope 95% confidence intervals and p value.
| Absolute Slope (μm/year) | Confidence Interval | p value* | Normalized Slope | |
|---|---|---|---|---|
| Overall | -0.4214 | -0.5688 - -0.2741 | <0.001 | -0.0018 |
| Superior outer | -0.4973 | -0.6639 - -0.3307 | <0.001 | -0.0021 |
| Temporal outer | -0.4308 | -0.5966 - -0.2650 | <0.001 | -0.0020 |
| Inferior outer | -0.4886 | -0.6550 - -0.3222 | <0.001 | -0.0022 |
| Nasal outer | -0.5899 | -0.7528 - -0.4270 | <0.001 | -0.0023 |
| Superior inner | -0.4154 | -0.5771 - -0.2538 | <0.001 | -0.0015 |
| Temporal inner | -0.3336 | -0.4961 - -0.1711 | <0.001 | -0.0013 |
| Inferior inner | -0.4295 | -0.5897 - -0.2694 | <0.001 | -0.0016 |
| Nasal inner | -0.3170 | -0.4838 - -0.1501 | <0.001 | -0.0012 |
| Center | 0.1209 | -0.1009 - 0.3427 | 0.28 | 0.0006 |
P value for an absolute slope difference from a zero slope.
Disc area remained stable with a non-significant slope of -0.001 (normalized: -0.0004) (p = 0.69), but rim area decreased by the rate of -0.009 mm2/year (normalized: -0.005) and cup area increased by 0.009 (normalized: 0.017), respectively (both p < 0.0001) (Table 6). Horizontal and vertical C/D showed similar normalized slopes.
Table 6.
The absolute and normalized slope for optic nerve head parameters over ages, with absolute slope 95% confidence interval and p value.
| Absolute Slope (units/yr) | Confidence Interval | p value* | Normalized Slope | |
|---|---|---|---|---|
| Disc area (mm2) | -0.0008 | -0.0049 - 0.0032 | 0.69 | -0.0004 |
| Rim area (mm2) | -0.0092 | -0.0131 - -0.0052 | <0.001 | -0.0052 |
| Cup area (mm2) | 0.0085 | 0.0050 - 0.0121 | <0.001 | 0.0174 |
| Cup/disc area ratio | 0.0039 | -0.0003 - 0.0081 | 0.07 | 0.0183 |
| Vertical cup/disc ratio | 0.0037 | -0.0015 - 0.0088 | 0.17 | 0.0090 |
| Horizontal cup/disc ratio | 0.0047 | -0.0010 - 0.0105 | 0.11 | 0.0104 |
| Vertical integrated rim area | -0.0077 | -0.0113 - -0.0040 | <0.001 | -0.0082 |
| Horizontal integrated rim width | -0.0039 | -0.0117 - -0.0039 | 0.33 | -0.0021 |
| Cup area topo | 0.0080 | -0.0008 - 0.0168 | 0.08 | 0.0200 |
| Cup volume topo | 0.0058 | 0.0009 - 0.0106 | 0.02 | 0.0900 |
P value for an absolute slope difference from a zero slope.
Discussion
There was statistically significant detectable loss of RNFL associated with age in most of the regional thicknesses of the peripapillary RNFL and macula, except for the temporal quadrant RNFL and corresponding 8, 9 and 10 o'clock sectors. The slopes in the sectoral areas varied around the macula and the ONH, both in terms of absolute and normalized values. As would be expected, the overall mean RNFL and macular thickness slopes fall in the middle of the slopes of the sectoral parameters and can be used as a robust indicator of age related retinal tissue loss. Disc size did not show significant change with age, but significant cup and rim area changes likely reflect neural tissue loss.
Histological studies have demonstrated that the number of retinal ganglion cell axons in the human eye decreases as one ages.18-20 This age related axonal loss leads to thinning of the RNFL that can be detected with ocular imaging technologies, including scanning laser polarimetry21-24 and OCT.11-14, 25-26 However, the focus has been primarily on the effects of age on mean RNFL thickness, and no studies have examined macular and ONH data over a variety of ages. Our data agree with previous publications indicating that older individuals have a thinner RNFL than younger people by 0.16 – 0.44 μm per year of age.12,13,22 Better understanding of the age effect on regional measurements as well as other locations in the posterior segment can further enhance our ability to detect glaucomatous related changes which exceeds the normal rate of tissue alteration.
Because the eyeball is a closed chamber it can be expected that the rate of tissue loss will be similar after normalizing or accounting for mean value of each individual parameter. Localized variation might indicate selective areas that are more sensitive to neural tissue loss. We observed that the rate of change related to age varied among parameters but was mostly similar between sectors after normalizing the measurements. The superior, inferior and nasal sectors have shown a very similar normalized rate of RNFL decline while the rate was three fold slower in the temporal sector. Likewise, the slope was similar in clock hours 12 - 2 and 5 - 7. Clock hours 3, 4 showed similar slopes but with marginal statistical significance, and clock hours 8 - 10 showed a shallower rate of decline with increasing age. This difference in rate may be due to the concentration of thinner nerve fibers in the papillomacular bundle at the temporal aspect of the ONH as has been reported in histology sections.27 Identical number of axonal loss will cause a shallower decline in locations predominantly composed by thinner fibers. Alternatively, one might speculate preferential neural loss in the retina would maintain vision with relative preservation of the fovea reflected by lower rate of RNFL decline in the temporal quadrant and the total retina thickness in the inner ring of the macula.
The sectoral RNFL rate of thinning with age in our study were substantially different than those reported by Parikh et al.13 While we observed the steepest slopes in the superior, inferior and nasal quadrants and shallowest in the temporal they reported on the superior and temporal as the steepest quadrants and the inferior as the shallowest. Similarly, clock hours 8-10 did not showed statistically significant slope in our study but were highly significant in the other study. The reason for these discrepancies is unclear and might be due to the inclusion of a substantial number of subjects younger than 18 years old in the other study while our study included subjects above this age with a larger sample in elder ages.
Local differences in thickness decrease were also found within the macular region. The inner sectors lost less thickness with age than the outer sectors, both when judged absolutely and when normalized. The rate of loss in the outer ring corresponded with the rate of loss in the superior, inferior and nasal quadrants of the RNFL thickness. The rate of loss in the inner ring corresponded with the rate in the temporal sector of the RNFL. These results are in agreement with the known distribution of the nerve bundles in the eye.
The central foveal area, which is devoid of the RNFL remains stable throughout life with a slight tendency of thickening (although not statistically significant). Since the majority of the tissue thinning seen in the macula is most likely occurring due to ganglion cell and RNFL these changes are not reflected in the central foveal measurements. The slight increase observed in the model in the older subjects may be also related to sub-clinical macular change, too small to be detected through fundus visualization or OCT, but causing a slight increase in the retinal thickness.
Optic nerve head analysis showed that while the disc size remained stable there was a significant decrease in rim area and increase cup area. The rate of change varied substantially among the various parameters even after normalization and differs from the rate in other locations. This difference might be due to fundamental discrepancy between thickness measurements obtained in the macula and RNFL scans and parameters such as the cup/disc area ratio that combine two areas measurements. Alternatively this might indicate preferential loss at the optic nerve head level that is less pronounced in the retina itself. Finally, ONH measurements have been shown to have the worst reproducibility among all scanned areas3 or the least reliable which might confound our findings.
Utilizing the findings of this study we can estimate that an individual 65 years old will lose, by age 85, 0.26*20=5.2 μm at the RNFL adjacent to the disc. This loss represents 5% of the total tissue thickness, which needs to be compared to the loss that would be associated with glaucoma. At the present, we know that about 50% loss is necessary to yield a functional change as measured by perimetry.28
The main limitation of our study was that it was based on cross sectional data rather than longitudinal data. It would be ideal if we could follow the change of retinal tissue in each individual longitudinally but for obvious reasons this is not feasible at this stage of the technology. Therefore, we acknowledge that we are not measuring true thickness changes but rather looking at differences among a large, broad population. This can cause some artifacts as can be observed in age group 30-39 years that had thinner RNFL thickness as compared to the 40-49 year old (Table 1).
In conclusion, global and regional changes due to the effects of age on RNFL, macula and ONH OCT measurements should be considered when assessing eyes over time.
Table 2.
Average ± standard deviation macular retinal thickness measurements (μm) overall and in 9 sectors across different age groups.
| Age (years) | 18-29 | 30-39 | 40-49 | 50-59 | 60-85 |
|---|---|---|---|---|---|
| Number of Eyes | 41 | 28 | 58 | 55 | 41 |
| Overall | 249.0±11.3 | 248.5±11.4 | 241.1±12.8 | 237.9±14.4 | 232.2±12.5 |
| Superior outer | 248.8±15.3 | 244.2±14.3 | 238.9±13.5 | 234.9±14.3 | 229.6±15.8 |
| Inferior outer | 236.7±12.2 | 232.7±13.3 | 227.7±14.8 | 222.0±16.0 | 218.0±15.5 |
| Temporal outer | 225.4±14.3 | 227.1±14.2 | 219.6±14.4 | 214.0±15.4 | 210.2±11.9 |
| Nasal outer | 265.3±12.7 | 261.3±11.1 | 253.6±15.4 | 250.5±16.0 | 241.0±16.1 |
| Superior inner | 277.6±14.8 | 281.0±13.2 | 270.5±13.3 | 270.2±16.9 | 263.6±14.8 |
| Inferior outer | 276.4±13.0 | 280.9±16.0 | 270.5±14.6 | 268.7±17.7 | 262.1±14.1 |
| Temporal inner | 262.3±15.0 | 268.6±15.2 | 257.6±15.2 | 257.6±17.2 | 252.5±11.8 |
| Nasal inner | 274.4±13.6 | 282.0±16.6 | 270.3±15.7 | 272.3±18.4 | 265.4±14.7 |
| Center | 192.9±19.4 | 205.5±21.8 | 198.2±23.4 | 207.1±28.3 | 203.4±18.9 |
Acknowledgments
Support: National Institutes of Health R01-EY13178-09, R01-EY11289-23, P30-EY008098, The Eye and Ear Foundation (Pittsburgh, PA) and unrestricted grant from Research to Prevent Blindness.
Footnotes
Meeting Presentation: The study was partially presented at the American Glaucoma Society annual meeting, March 2008, Washington, DC.
Conflict of Interest: Dr. Wollstein received research funding from Carl Zeiss Meditec and Optovue. Drs. Wollstein, Ishikawa and Schuman receive royalties for intellectual property licensed by the University of Pittsburgh to Biopigen. Drs. Schuman and Fujimoto receive royalties for intellectual property licensed by Massachusetts Institute of Technology to Carl Zeiss Meditec. Dr. Fujimoto is a scientific advisor and has stock options with Optovue.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Villain MA, Greenfield DS. Peripapillary nerve fiber layer thickness measurement reproducibility using optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2003;34:33–7. [PubMed] [Google Scholar]
- 2.Schuman JS, Pedut-Kloizman T, Hertzmark E, et al. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996;103:1889–98. doi: 10.1016/s0161-6420(96)30410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci. 2004;45:1716–24. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budenz DL, Chang RT, Huang X, et al. Reproducibility of retinal nerve fiber thickness measurements using the Stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–3. doi: 10.1167/iovs.04-1174. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal EZ, Williams JM, Weinreb RN, et al. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000;107:2278–82. doi: 10.1016/s0161-6420(00)00341-9. [DOI] [PubMed] [Google Scholar]
- 6.Hoh ST, Greenfield DS, Mistlberger A, et al. Optical coherence tomography and scanning laser polarimetry in normal, ocular hypertensive, and glaucomatous eyes. Am J Ophthalmol. 2000;129:129–35. doi: 10.1016/s0002-9394(99)00294-9. [DOI] [PubMed] [Google Scholar]
- 7.Bowd C, Zangwill LM, Berry CC, et al. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42:1993–2003. [PubMed] [Google Scholar]
- 8.Guedes V, Schuman JS, Hertzmark E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology. 2003;110:177–89. doi: 10.1016/s0161-6420(02)01564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanamori A, Nakamura M, Escano MF, et al. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135:513–20. doi: 10.1016/s0002-9394(02)02003-2. [DOI] [PubMed] [Google Scholar]
- 10.Budenz DL, Michael A, Chang RT, et al. Sensitivity and specificity of the StratusOCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 12.Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003;87:899–901. doi: 10.1136/bjo.87.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh RS, Parikh SR, Sekhar GC, et al. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007;114:921–6. doi: 10.1016/j.ophtha.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114:1046–52. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeimer R, Asrani S, Zou S, et al. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping: a pilot study. Ophthalmology. 1998;105:224–31. doi: 10.1016/s0161-6420(98)92743-9. [DOI] [PubMed] [Google Scholar]
- 16.Greenfield DS, Bagga H, Knighton RW. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol. 2003;121:41–6. doi: 10.1001/archopht.121.1.41. [DOI] [PubMed] [Google Scholar]
- 17.Wollstein G, Ishikawa H, Wang J, et al. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol. 2005;139:39–43. doi: 10.1016/j.ajo.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Dolman CL, McCormick AQ, Drance SM. Aging of the optic nerve. Arch Ophthalmol. 1980;98:2053–8. doi: 10.1001/archopht.1980.01020040905024. [DOI] [PubMed] [Google Scholar]
- 19.Balazsi AG, Rootman J, Drance SM, et al. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–6. doi: 10.1016/0002-9394(84)90509-9. [DOI] [PubMed] [Google Scholar]
- 20.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 21.Weinreb RN, Shakiba S, Zangwill L. Scanning laser polarimetry to measure the nerve fiber layer of normal and glaucomatous eyes. Am J Ophthalmol. 1995;119:627–36. doi: 10.1016/s0002-9394(14)70221-1. [DOI] [PubMed] [Google Scholar]
- 22.Poinoosawmy D, Fontana L, Wu JX, et al. Variation of nerve fibre layer thickness measurements with age and ethnicity by scanning laser polarimetry. Br J Ophthalmol. 1997;81:350–4. doi: 10.1136/bjo.81.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Da Pozzo S, Iacono P, Marchesan R, et al. The effect of ageing on retinal nerve fibre layer thickness: an evaluation by scanning laser polarimetry with variable corneal compensation. Acta Ophthalmol Scand. 2006;84:375–9. doi: 10.1111/j.1600-0420.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 24.Toprak AB, Yilmaz OF. Relation of optic disc topography and age to thickness of retinal nerve fibre layer as measured using scanning laser polarimetry, in normal subjects. Br J Ophthalmol. 2000;84:473–8. doi: 10.1136/bjo.84.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok KH, Lee VW, So KF. Retinal nerve fiber layer measurement of the Hong Kong Chinese population by optical coherence tomography. J Glaucoma. 2002;11:481–3. doi: 10.1097/00061198-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal Latinos. Invest Ophthalmol Vis Sci. 2003;44:3369–73. doi: 10.1167/iovs.02-0975. [DOI] [PubMed] [Google Scholar]
- 27.Mikelberg FS, Drance SM, Schultzer M, et al. The normal human optic nerve: axon count and axon diameter distribution. Ophthalmology. 1989;96:1325–8. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- 28.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–46. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]


