Abstract
Stimulus-evoked neural activity is attenuated upon stimulus repetition (‘repetition suppression’), a phenomenon attributed to largely automatic processes in sensory neurons. By manipulating the likelihood of stimulus repetition, we show that repetition suppression in the human brain is reduced when stimulus repetitions are improbable (and thus, unexpected). These data suggest that repetition suppression reflects a relative reduction in top-down perceptual ‘prediction error’ when processing an expected compared to an unexpected stimulus.
Stimulus-specific repetition suppression (RS) – the relative attenuation in neural signal evoked by the repeated occurrence of a stimulus – is among the best-known neural phenomena1–4, and has been widely employed in functional magnetic resonance imaging (fMRI) studies to define functional properties of brain regions5,6 and explore neural substrates of behavioral priming effects2,4. However, the neurocomputational basis for RS remains controversial1. Two influential theories view RS as a relatively automatic consequence of the bottom-up flow of perceptual information through sensory cortex: either neurons tuned to the repeated stimulus fatigue1, or subsequent presentations of a stimulus are encoded more sparsely (and efficiently), leading to a sharpening in the population of neurons recruited4,7. By contrast, a recent model of perceptual inference casts RS as a consequence of top-down perceptual expectations2,8: here, RS reflects a reduction in perceptual ‘prediction error’ (the neural signal evoked by a mismatch between expected and observed percepts) that occurs when sensory evidence conforms to a more probable (previously seen) compared to a less probable (novel) percept. Unlike other theories, the prediction error model holds that RS will vary with contextual factors that affect subjects’ perceptual expectations, and suggests that RS will be reduced under conditions where stimulus repetitions are unexpected.
We created such a situation by presenting subjects (n = 16), who had provided informed written consent, on each trial with either the same face twice, or two different faces, in two experimental contexts – one where repetitions occurred more frequently than alternations, and one where the reverse was the case. Importantly, all face exemplars were trial-unique, such that the probability of a repetition per se, and not the frequency of repetition of a specific face, varied between blocks (Fig. 1a and Supplementary Methods online). Incidental to this manipulation, subjects were required to make a speeded response to occasional inverted faces (‘targets’)9. Limiting our analysis to non-target trials, we measured how face-sensitive visual cortex responded to face repetitions (‘rep trials’) and face alternations (‘alt trials’) that were either expected (in ‘REP BLOCKS’) or unexpected (in ‘ALT BLOCKS’), comparing these estimates in 2 × 2 factorial mixed block/event-related design.
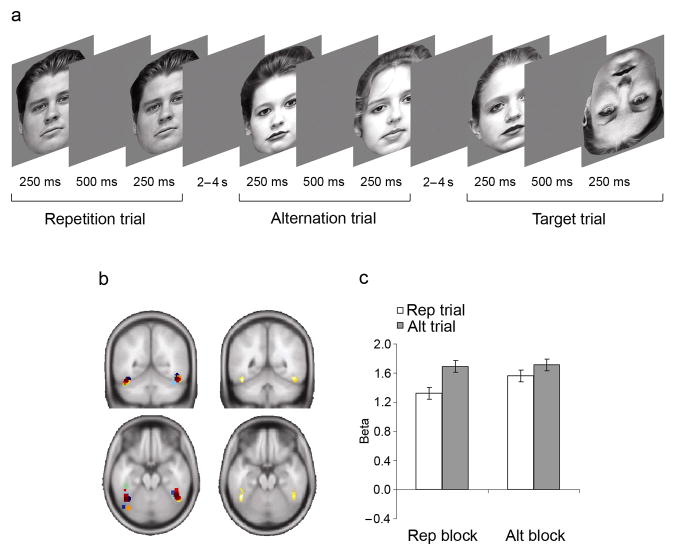
Figure 1.
Main experiment: protocol and results. (a) Faces were presented in successive pairs, with each face presented for 250ms, separated by a blank screen for 500ms, and a jittered interval of 2–4 seconds between pairs. Pairs comprised either the same face (repetition [rep] trials) or two different faces (alternation [alt] trials). Subjects monitored the stimulus stream for occasional inverted faces (target trials), occurring on 20% of all trials. Targets occurred equally often as the first or second stimulus within a pair. Trials were presented within two contexts (blocks of trials), one in which the probability of encountering rep trials was high (75% of non-target trials, REP BLOCKS), and one in which this probability was low (25% of non-target trials, ALT BLOCKS ). (b) In the left panels, center-of-mass locations for individual FFAs (see also Supplementary Table 1 online) are shown in different colors, rendered onto a standard brain (MNI y – 53, z – 21). The right panels display results from a random effects group analyses, thresholded at a whole-brain corrected false discovery rate of P < 0.05 (left FFA peak: MNI x – 44, y – 50, z – 20; right FFA peak: MNI x 46, y – 50, z – 18). (c) Average parameter estimates (± within-subject s.e.m.) obtained from individual FFAs are displayed for non-target rep trials (white bars) and alt trials (grey bars) in REP and ALT BLOCKS.
To account for inter-subject anatomical variation, and to obviate correction for multiple statistical comparisons, each participant’s ‘fusiform face area’10 (FFA) was defined in an independent localizer task (Fig. 1b, Supplementary Table 1 and Supplementary Methods online). Subsequently, modeling each face pair as a composite event, we assessed the degree of FFA activation associated with each trial type in the main task (Fig. 1c). In REP BLOCKS, strong RS effects were observed in the FFA, with rep trials eliciting a decrease in neural signal of ~22% compared to alt trials (t(15) = 4.4, P < 0.001). However, in ALT BLOCKS, RS was reduced to ~9% (t(15) = 2.8, P < 0.05). Formally, a main effect of stimulus (alt > rep, F(1,15) = 20.4, P < 0.001) was superseded by a block × stimulus interaction (F(1,15) = 6.8, P < 0.05), with no main effect of block (F(1,15) = 1.4, P > 0.1). A direct comparison showed that rep trials elicited less FFA activation in REP than in ALT BLOCKS (t(15) = 2.3, P < 0.05). These data clearly demonstrate that RS was modulated by repetition probability.
We dedicated subsequent analyses to ruling out alternative explanations for our findings. RS is known to be modulated by attention11. Although task requirements were identical across blocks, could subjects have paid less attention to the stimuli on ALT BLOCKS? We found reaction times for target detection on REP and ALT BLOCKS to be well-matched (REP: 478ms; ALT 484ms, t(15) = 1.6, P > 0.1; target detection rates were at ceiling), and fMRI responses to target trials did not differ between REP and ALT BLOCKS, either in the FFA (t(15) = 0.3, P > 0.1) or elsewhere in the brain (P < 0.001, uncorrected), further disconfirming an attentional explanation for our data. Moreover, generic ‘oddball’ or trial count effects cannot account for the FFA data, since rare trials were associated with higher activity than frequent trials in REP BLOCKS, but with lower activity in ALT BLOCKS (Fig. 1c). Finally, neither mean activation nor that evoked by alt trials (t(15) = 0.2, P > 0.1) differed between REP and ALT BLOCKS, ruling out a confounding influence of global between-block factors, such as potential differences in the adherence to linearity of the haemodynamic convolution.
Could the FFA data reflect some non-specific consequence of the visual stimulation employed? To ascertain the functional specificity of our results, we conducted a whole-brain search for voxels matching the FFA’s response profile (Supplementary Methods online). Only bilateral clusters in the fusiform gyrus, corresponding to the FFA, and a small region in primary visual cortex passed these criteria (Supplementary Fig. 1). RS in early visual regions is to be expected, given that on rep trials, the two faces were identical in terms of both high- and low-level visual information. Notably, other higher-level visual regions showed neither RS nor RS modulation, even when a parallel ROI approach was applied (Supplementary Fig. 2 and Supplementary Methods online), indicating that modulation of RS by repetition probability was confined to the face processing stream.
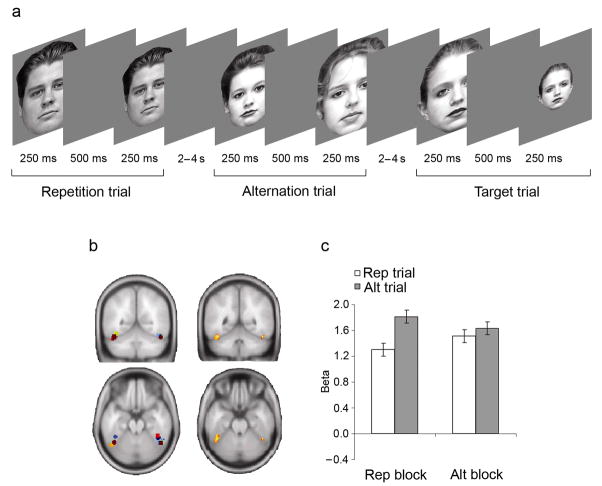
Finally, to eliminate the possibility that our results were specific to our choice of explicit task, we ran a second, control experiment in which a new cohort (n = 8) detected 60% size-deviant faces in a stream of standard faces that differed by only 15% (Fig. 2a and Supplementary Methods online). This task avoided one asymmetry present in the main experiment, namely that a physical stimulus change was common to alt and target trials but not to rep trials. Imaging data from individually defined FFAs (Fig. 2b) were strikingly similar to those from the main experiment, with 26% and 9% reductions in neural signal on rep vs. alt trials for REP and ALT BLOCKS, respectively (Fig. 2c), and no difference in target detection RT between blocks (REP: 486ms; ALT 480ms, t(15) = 0.7, P > 0.1). Formally, a main effect of stimulus on FFA activity (F(1,7) = 17.8, P < 0.005) was again superseded by a stimulus × block interaction (F(1,7) = 6.14, P < 0.05), replicating our results and confirming their independence from the choice of explicit task.
Figure 2.
Control experiment: protocol and results. (a) Task parameters were identical to the main experiment (Fig. 1a), except that face images in each pair were subject to a variation in size. In standard alt and rep trials, either the first (in 50% of trials) or the second image in a pair of faces was reduced in size by 15%. Subjects monitored the stimulus stream for occasional targets, consisting of a face reduced by 60% in size, which occurred on 20% of all trials. The target face occurred equally often as the first or second stimulus within a pair, and half of the target trials showed the same face twice, while the other half showed two different faces. Trials were presented in blocks where the probability of encountering rep trials was either high (75% of non-target trials, REP BLOCKS), or low (25% of non-target trials, ALT BLOCKS). (b) In the left panels, center-of-mass locations for individual FFAs (see also Supplementary Table 1 online) are shown in different colors, rendered onto a standard brain (MNI y – 56, z – 21). The right panels display results from a random effects group analyses, thresholded at an uncorrected P < 0.005 (left FFA peak: MNI x – 44, y – 44, z – 20; right FFA peak: MNI × 46, y – 50, z – 24). (c) Average parameter estimates (± within-subject s.e.m.) obtained from individual FFAs are displayed for non-target rep trials (white bars) and alt trials (grey bars) in REP and ALT BLOCKS.
This modulation of RS by perceptual expectations cannot be explained by the fatigue or sharpening models, according to which RS is an inevitable consequence of stimulus repetition, independent of the probability associated with a repetition per se. It is, however, consistent with a Bayesian model of perceptual inference proposing that RS indexes a decrease in computational demand occurring when expected and observed sensory information coincide (lower ‘prediction error’)8,12. We argue that RS modulation occurs because on REP BLOCKS the brain ‘predicts’ the reoccurrence of the first face (or formally, that a higher weight is assigned to its prior probability), leading to reduced processing demands, and consequently less fMRI signal, for the second face on rep trials. Predictive influences on visual processing can thus accrue from statistical regularities in the flow of incoming sensory information, even when they are divorced from the frequencies of individual stimulus exemplars (and thus constitute higher-order or ‘meta-predictive’ information). Keeping track of the probability of a visual event, conditioned on some short-term state, may play an important role in mitigating the exponential computational demands associated with visual object recognition, and contribute to well-described effects of local environmental context13 and statistical regularities in the natural images14 on perceptual processing.
Although RS was greatly reduced on ALT BLOCKS, it was not abolished, suggesting that even when repetitions were relatively unlikely, the repetition of a given face exemplar was still more ‘expected’ than the occurrence of a specific novel face. Although RS on ALT BLOCKS may reflect residual contributions from fatigue or sharpening processes, it could also feasibly reflect the fact that, in real life, perceptual context tends to be highly stable across short time-scales, with the mere occurrence of a particular percept being a strong predictor of its recurrence in the near future15. In other words, the perceptual apparatus may generally expect stimulation to be relatively consistent from moment-to-moment, and expectation-based processes may therefore be an important contributor to RS even when there is no preponderance of stimulus repetitions6,9.
Supplementary Material
Acknowledgments
We are grateful to Etienne Koechlin and Sid Kouider for helpful comments on an earlier version of this manuscript. This work was in part supported by NINDS NS30863 (MMM).
Footnotes
AUTHOR CONTRIBUTIONS
C.S, T.E., E.H.T., and M.M.M. conceived of the experiment, C.S. and T.E. designed the experiment, T.E., E.H.T. and J.M. collected the data, T.E. analyzed the data, and C.S. and T.E. wrote the paper.
References
- 1.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 3.Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 4.Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- 5.Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 6.Kourtzi Z, Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- 7.Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- 10.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray SO, Wojciulik E. Attention increases neural selectivity in the human lateral occipital complex. Nat Neurosci. 2004;7:70–74. doi: 10.1038/nn1161. [DOI] [PubMed] [Google Scholar]
- 12.Friston K, Kilner J, Harrison L. A free energy principle for the brain. J Physiol (Paris) 2006;100:70–87. doi: 10.1016/j.jphysparis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 14.Kersten D, Mamassian P, Yuille A. Object perception as Bayesian inference. Annu Rev Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- 15.Summerfield C, Koechlin E. A neural representation of prior information during perceptual inference. Neuron. doi: 10.1016/j.neuron.2008.05.021. (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.