Colorectal cancer in Lynch syndrome differs from its sporadic counterpart by virtue of certain clinical-pathology features, namely its poor differentiation with mucoid features and signet cell excess, peritumoral lymphocytic infiltration, Crohn’s-like reaction, and an excess of tumor-infiltrating lymphocytes (1–4). At the molecular level, most Lynch syndrome-associated CRCs are microsatellite instability-high (MSI-H). A central question regarding colorectal cancer patients with Lynch Syndrome that puzzles both clinicians and basic scientists is why these patients do relatively well compared with individuals with sporadic cases of colorectal cancer (CRC), a fact empirically observed prior to the identification of mismatch repair genes in 1993 (5; 6). Anecdotally, patients belonging to Lynch syndrome families were known to have survived two, three, four, or even seven or eight cancers (7). The identification of germline mutations in the mismatch repair genes as being characteristic of Lynch syndrome permitted further refinement in identifying these patients and multiple studies have demonstrated a better prognosis in the Lynch syndrome patients compared to the age- and stage-matched “sporadic” colon cancer patients (8–13). Watson et al. (11) compared Lynch syndrome cases by stage and survival in a retrospective cohort of family members who developed CRC with the same factors in an unselected hospital series of patients with sporadic CRC. Therein, Lynch syndrome cases had lower stage disease (p < 0.001) and fewer distant metastases at diagnosis (p < 0.001 in an analysis stratified by T classification). Importantly, “…In stage-stratified survival analysis, the HNPCC cases had a significant overall survival advantage regardless of adjustment for their younger age. A conservative estimate of the hazard ratio (of HNPCC cases to the unselected series) was 0.67 (P < 0.0012).”(11) It was concluded that the Lynch syndrome patients showed lower stage at diagnosis when compared to the unselected CRC cases, which was mainly attributable to rarer distant metastases at diagnosis. Furthermore, Lynch syndrome patients survived longer in cases with tumors of the same stage. Finally, the estimated death rate for the Lynch syndrome cases, when adjusted for stage and age differences, was at most only two-thirds of the rate for the hospital series. The improved prognosis observed in Lynch syndrome patients was also observed in patients with the subtype of sporadic colorectal cancer characterized by hypermethylation of the MLH1 promoter and associated with microsatellite instability (14–17).
While these studies were interesting from a scientific viewpoint, much interest was aroused by the tentative observation that 5-fluorouracil (5-FU) based chemotherapy might not be beneficial to patients with microsatellite unstable tumors (18; 19). Suddenly the phenomenon of microsatellite instability became of great interest to surgeons, gastroenterologists and oncologists in that microsatellite instability could not only be used as a prognostic marker, but also may be important in guiding better patient treatment. As approximately 15% of all colorectal cancer patients have microsatellite instability, it is critical to conduct large prospective studies to definitively prove whether 5-FU really does not provide a benefit, or might even be harmful, in this subset of CRC patients. To date, the issue regarding the suitability of 5-FU treatment for MSI-H individuals has not yet been resolved.
While the optimal treatments for patients with mismatch repair gene mutations remain undetermined, the ability to easily identify these individuals has become less technologically demanding, thereby simplifying the ability to recruit individuals for large-scale clinical studies. Immunohistochemical staining protocols have been refined and negative staining of tumor tissue indicative of microsatellite instability of mismatch repair genes is highly sensitive (>90%) (20–22). The relatively low level of technology required for immunohistochemical staining compared to the level of expertise and expense to perform molecular studies to detect microsatellite instability has permitted the routine identification in patients wherever there is a pathology laboratory. Consequently, increasing numbers of hospitals have begun to apply immunohistochemistry to identify four mismatch repair proteins (MLH1, MSH2, MSH6, PMS2) to every colorectal cancer specimen. Thus the patients can be triaged into those 15 % with, and those 85% without microstallite instability/mismatch repair deficiency.
While the above developments have occurred in the clinical arena, more limited progress has been made with regard to our understanding of the mechanisms leading to a better patient prognosis in the presence of microsatellite instability. Soon after the phenomenon of deficient mismatch repair was discovered, it was demonstrated that the mutation frequency was perhaps 100–1000 fold higher in microsatellite unstable tumors than in cells proficient in repair (23). This led Bodmer and colleagues to postulate that mutations in surface molecules on the tumor might trigger immune responses that ultimately assist the host in attacking the tumor (24; 25). This hypothesis became even more attractive when it was discovered that one characteristic of these tumors was strong lymphocytic infiltration (12). Smyrk et al. (3) propose that MSI type may be predicted by tumor-infiltrating lymphocytes (TILs), which can be identified through light microscopy and which may be used as a pathology screen for MSI-H status. This was evaluated among 138 carcinomas wherein 67 (48.6%) were MSI-H, 22 (15.9%) were MSI-L, and 49 (35.5%) were MSS. In this cohort, all 25 Lynch syndrome CRCs were MSI-H, and therein TIL counts ranged from 0–300, with a markedly skewed distribution (median, 11; mean, 36). These authors concluded that “The presence of MSI defines a subset of colorectal carcinomas with special molecular etiology and characteristic clinical, pathologic features, inclusive of increased survival… quantification of TILs may provide a simple, single criterion for choosing which colorectal carcinomas are candidates for MSI testing” (3).
The presence of a strong lymphocytic infiltration combined with other characteristics of Lynch syndrome and other microsatellite unstable tumors have been eloquently described, often allowing these tumors to be recognized at routine histological examination by trained pathologists (1; 2). So all in all, an enhancement of the immune response has emerged as an attractive candidate mechanism to explain the better prognosis observed in individuals with microsatellite unstable cancers. Despite this, the discrete events required to impart this protection to Lynch syndrome patients have been incompletely defined.
Phillips et al. (26) began to further define the nature of the infiltrate in patients with MSI-H CRCs. These studies demonstrated increased ratios of CD8:CD3 gene transcripts in MSI-H tumors compared to MSS tumors (p=0.016). These data support the hypothesis that there is increased recruitment of CD8+ T cells into the tumor. As the main function of CD8+ T cells is to lyse target cells (in this case, the tumor cell would be postulated to be the target), these data would suggest that one mechanism of the enhanced survival in Lynch syndrome patients involves direct killing of the tumor cells by the infiltrating CD8+ T cells. Consistent with this hypothesis, the lymphocytes within MSI-H tumors were activated as indicated by significantly higher granzyme B (p = 0.020) and IL-2R (p = 0.017) levels as compared with those observed in MSS tumors. Appropriately, these authors note that these findings support the hypothesis that MSI-H CRCs may be more immunogenic when compared with MSS tumors.
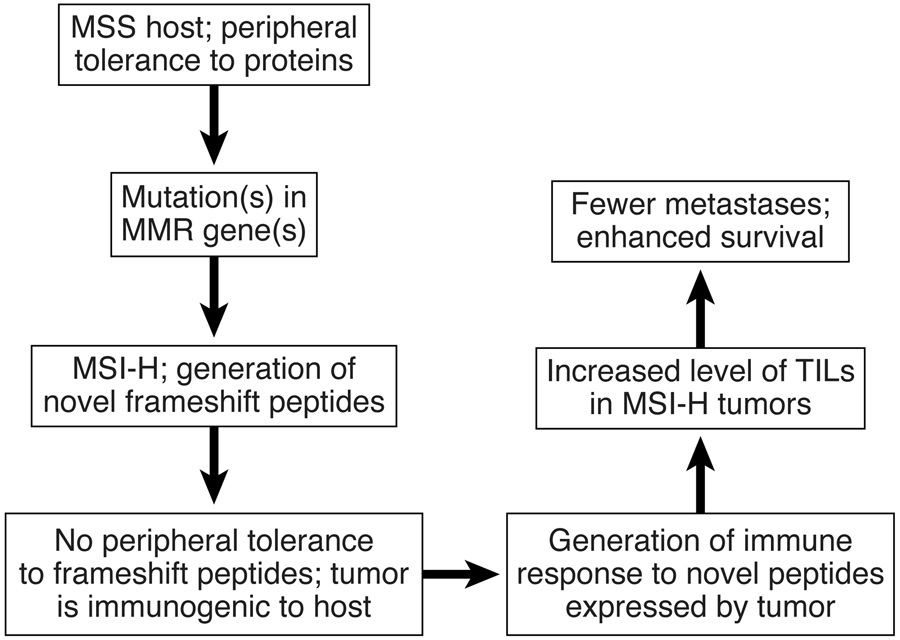
Now on p XXX of this issue, a study is published that provides insight into the possible mechanisms regarding the impact of MMR genes on the immune response. Dr. Magnus von Knebel Doeberitz and his team in Germany postulated that the numerous frameshift mutations resulting from the mismatch repair deficiency induces the production of peptides (pieces of protein) that are structurally altered or truncated (or both) at their carboxyterminal end as compared to the normal product. Because these peptides are ‘altered self’ and immune tolerance to them has not developed, T cells exist in the patient that recognize these peptides as alien and immunogenic. When these peptides are bound to a self MHC molecule on the tumor cell surface, the T cell receptor of an antigen-specific T cell interacts with the peptide/MHC complex resulting in the killing of the tumor cells.
Tumor- infiltrating T cells isolated from 3 tumors played a central role in these experiments. Following isolation and in vitro expansion of these cells, the cells were stimulated with frameshift peptides (FSPs) and assayed for interferon-gamma, a hallmark of T cell activation. The robust production of interferon, paired with the in vitro killing ability of these cells, support a role for these lymphocytes in controlling tumors. The authors demonstrated similar reactivity against truncated peptides in the periphery of most of the 32 patients with microsatellite unstable tumors. Minimal reactivity to the frameshift peptides was observed in 17 patients with microsatellite stable tumors, and in 22 healthy controls when peripheral blood lymphocytes (PBLs) were used in the assays. Of significant interest was the observation that there were statistically increased responses against 7 of the 13 FSPs in healthy HNPCC germline mutation carriers. In particular, peripheral blood lymphocytes from two to three of the individuals in this group had a high level of reactivity against most of the FSPs tested. This provocative finding is counterintuitive as individuals who are heterozygous for a germline mismatch repair gene mutation have no detectable mismatch repair deficiency. This could be interpreted to mean that some carriers of Lynch syndrome mutations have a hitherto undetected mild degree of mismatch repair deficiency. One might speculate that this leads to mildly increased mutation rates in some or all genes, and this in turn might speed up the emergence of the second, somatic hit in the mismatch repair gene that triggers cancer in a colonocyte. In other words, the “second hit” would not be a random event but one triggered by the “first hit”, a novel concept. This might eventually provide diagnostic and even therapeutic openings. There have been reports about the detection of microsatellite instability before any tumor is diagnosed (27; 28). There could be an ongoing low-level degree of somatic mutations, an interesting possibility. Unfortunately, more trivial explanations for the positive T-cell reactivity in the blood of some Lynch syndrome carriers exist. The 2-3/16 T cell-reacting carriers might already have had cancer even though it was not detectable by clinical surveillance. It would be of interest to follow these carrier patients over time to determine if those patients with T cell responses to FSPs develop colorectal cancer at either a faster or higher rate than other patients in the study. Obviously, the small number of patients in this group would require that this question would be examined in a larger series of carriers. It is certainly hoped that the authors have considered these studies.
The totality of these observations support the conclusion that FSPs among MMR-deficient CRC cells can be demonstrated in the patient’s immune system and therein may elucidate the mentioned clinical/pathologic features of Lynch syndrome when compared to sporadic MSI-H CRCs. In this latter setting, it is proposed by these authors that their findings may be highly relevant to the development of FSP-based vaccination approaches given their cancer control potential among MMR mutation affected individuals.
A question left unanswered by the authors’ results relates to the specificity of the T cell reactivity against altered peptides. The response by peripheral T cells was measured specifically against predicted abnormal peptides from 13 chosen genes. Reactivity was seen in most of the 32 patients with microsatellite-unstable tumors, and it was peptide-specific. One might expect there be a correlation in any given patient between the presence of T-cell reactivity against a peptide, and a frameshift mutation in the corresponding gene. Such correlations were searched for but not observed, and the data were not shown. This clearly calls for further studies.
The data described in the studies by Schwitalle et al. (29) are extremely interesting, both from a clinical and basic scientist’s standpoint. Hopefully, this report will stimulate further studies focused on the precise immune response of Lynch syndrome patients and carriers of the disease with the goal of identifying these individuals at the earliest stages of disease to better improve their prognosis.
Figure 1.
Contributor Information
Henry T. Lynch, Department of Preventive Medicine and Public Health, Creighton University School of Medicine, Omaha, Nebraska
Kristen M. Drescher, Department of Medical Microbiology and Immunology, Creighton University School of Medicine, Omaha, Nebraska.
Albert de la Chapelle, Human Cancer Genetics Program, Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio
References
- 1.Jass JR, Do K-A, Simms LA. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jass JR, Biden KG, Cummings MC. Characterisation of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J Clin Pathol. 1999;52:455–460. doi: 10.1136/jcp.52.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyrk TC, Watson P, Kaul K, Lynch HL. Tumor infiltrating lymphocytes are a marker for microsatellite instability in colorectal cancer. Cancer. 2001;91:2417–2422. [PubMed] [Google Scholar]
- 4.Jass JR. HNPCC and sporadic MSI-H colorectal cancer: a review of morphologic similarities and differences. Fam Cancer. 2004;3:93–100. doi: 10.1023/B:FAME.0000039849.86008.b7. [DOI] [PubMed] [Google Scholar]
- 5.Lynch HT, Bardawil WA, Harris RE, Lynch PM, Guirgis HA, Lynch JF. Multiple primary cancers and prolonged survival: familial colonic and endometrial cancers. Dis Colon Rectum. 1978;21:165–168. doi: 10.1007/BF02586560. [DOI] [PubMed] [Google Scholar]
- 6.Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Parsons R, Peltomaki P, Sistonen P, Aaltonen LA, Nystrom-Lahti M, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 7.Albano WA, Recabaren JA, Lynch HT, Campbell AS, Mailliard JA, Organ CH, Lynch JF, Kimberling WJ. Natural history of hereditary cancer of the breast and colon. Cancer. 1982;50:360–363. doi: 10.1002/1097-0142(19820715)50:2<360::aid-cncr2820500233>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Sankila R, Aaltonen LA, Jarvinen HJ, Mecklin JP. Better survival rates in patients with MLH-1 associated hereditary colorectal cancer. Gastroenterol. 1996;110:682–687. doi: 10.1053/gast.1996.v110.pm8608876. [DOI] [PubMed] [Google Scholar]
- 9.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Eng J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 10.Hemminki A, Mecklin JP, Jarvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterol. 2000;119:921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 11.Watson P, Lin K, Rodriguez-Bigas MA, Smyrk T, Lemon S, Shashidharan M, Franklin B, Karr B, Thorson A, Lynch HT. Colorectal carcinoma survival among hereditary nonpolyposis colorectal cancer family members. Cancer. 1998;83:259–266. [PubMed] [Google Scholar]
- 12.Lothe RA, Peltomaki P, Meling GI. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–5852. [PubMed] [Google Scholar]
- 13.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 14.Alemayehu A, Tomkova K, Zavodna K, Ventusova K, Krivulcik T, Bujalkova M, Bartosova Z, Fridrichova I. The role of clinical criteria, genetic and epigenetic alterations in Lynch-syndrome diagnosis. Neoplasma. 2007;54:391–401. [PubMed] [Google Scholar]
- 15.Bettstetter M, Dechant S, Ruemmele P, Grabowski M, Keller G, Holinski-Feder E, Hartmann A, Hofstaedter F, Dietmaier W. Distinction of heritary nonpolyposis colorectal cancer and sporadic microsatellite-unstable colorectal cancer through quantification of MLH1 methylation by real-time PCR. Clin Can Res. 2007;13:3221–3228. doi: 10.1158/1078-0432.CCR-06-3064. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler JM, Loukola A, Aaltonen LA, Mortensen NJ, Bodmer WF. The role of hypermethylation of the hMLH1 promoter region in HNPCC versus MSI+ sporadic colorectal cancers. J Med Genet. 2000;37:588–592. doi: 10.1136/jmg.37.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potocnik U, Glavac D, Golouh R, Ravnik-Glavac M. Causes of microsatellite instability in colorectal tumors: implications for non-polyposis colorectal cancer screening. Cancer Genet Cytogenet. 2001;126:85–96. doi: 10.1016/s0165-4608(00)00399-x. [DOI] [PubMed] [Google Scholar]
- 18.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluoruracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, Cabrera BL, Goel A, Arnold CA, Miyai K, Boland CR. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterol. 2004;126:394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Eng J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 21.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, Jass JR, Hopper J, Gallinger S, Bapat B, Redston M, Thibodeau SN. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncology. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 22.Casey G, Lindor NM, Papadopoulos N, Thibodeau SN, Moskow J, Steelman S, Buzin CH, Sommer SS, Collins CE, Butz M, Aronson M, Gallinger S, Barker MA, Young JP, Jass JR, Hopper JL, Diep A, Bapat B, Salem M, Seminara D, Haile R. Conversion analysis for mutation detection in MLH1 and MSH2 in patients with colorectal cancer. JAMA. 2008;293:799–809. doi: 10.1001/jama.293.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons R, Li GM, Longley MJ, Fang WH, Papadopoulos N, Jen J, de la Chapelle A, Kinzler KW, Vogelstein B, Modrich P. Hypermutability and mismatch deficiency in RER+ tumor cells. Cell. 1993;75:1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 24.Branch P, Bicknell DC, Rowan A, Bodmer WF, Karran P. Immune surveillance in colorectal carcinoma. Nat Genet. 1995;9:231–232. doi: 10.1038/ng0395-231. [DOI] [PubMed] [Google Scholar]
- 25.Bodmer W, Bishop T, Karran P. Genetic steps in colorectal cancer. Nat Genet. 1994;6:217–219. doi: 10.1038/ng0394-217. [DOI] [PubMed] [Google Scholar]
- 26.Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surgery. 2004;91:469–475. doi: 10.1002/bjs.4472. [DOI] [PubMed] [Google Scholar]
- 27.Alazzouzi H, Domingo E, Gonzalez S, Blanco I, Armengol M, Espin E, Plaja A, Schwartz S, Capella G, Schwartz S., Jr Low levels of microsatellite instability characterize MLH1 and MSH2 HNPCC carriers before tumor diagnosis. Hum Mol Genet. 2008;14:235–239. doi: 10.1093/hmg/ddi021. [DOI] [PubMed] [Google Scholar]
- 28.Parsons R, Li GM, Longley MJ, Modrich P, Liu B, Berk T, Hamilton SR, Kinzler KW, Vogelstein B. Mismatch repair deficiency in phenotypically normal human cells. Science. 1995;268:738–740. doi: 10.1126/science.7632227. [DOI] [PubMed] [Google Scholar]
- 29.Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune responses against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterol. 2008;134 doi: 10.1053/j.gastro.2008.01.015. in press. [DOI] [PubMed] [Google Scholar]