Abstract
Maternal licking in rats affects the development of the spinal nucleus of the bulbocavernosus (SNB), a sexually dimorphic motor nucleus that controls penile reflexes involved with copulation. Reduced maternal licking produces decreased motoneuron number, size, and dendritic length in the rostral portion of the adult SNB as well as deficits in adult male copulatory behavior. Previous research suggests that decreases in perineal tactile stimulation may be responsible for these effects. To determine whether the regional effects of maternal licking on SNB morphology are driven by sensory afferent innervation of the lumbosacral spinal cord, we used WGA-HRP to reconstruct the location of sensory afferent fibers from the perineal skin. We found that these fibers are caudally concentrated relative to the area of the SNB dendritic field, with the rostral dendritic arbor receiving little perineal afferent innervation. We also assessed Fos expression following perineal tactile stimulation to determine whether it increased local spinal cord activity in the SNB dendritic field. Sixty seconds of licking-like perineal stimulation produced a transient 115% increase in Fos expression in the area of the SNB dendritic field. This effect was driven by a significant increase in Fos in the caudal portion of the SNB dendritic field, matching the pattern of perineal afferent fiber labeling. Perineal tactile stimulation also produced significantly greater Fos expression in male pups than in female pups. Together, these results suggest that perineal sensory afferent activity mediates the effects of early maternal care on the masculinization of the SNB and resultant male copulatory behavior.
Keywords: maternal care, motoneuron, sexual behavior, development, dendrite
INTRODUCTION
Early social contact between mother and offspring shapes the neural and behavioral development of offspring (Hofer, 1978, 1994). In humans, parental care is a crucial factor in the development of offspring, with parental deprivation or loss predicting future mental health problems (Agid et al., 1999; Carter et al., 1999). Individual differences in maternal care influence the physiological and psychological development of children, for example, with low-quality caregiving predicting greater fearfulness, negative affect, and increased stress reactivity (Hane and Fox, 2006). Other mammals, such as rats and nonhuman primates, also show substantial sensitivity to early maternal influences (e.g., Pryce et al., 2005), and as such serve as excellent model systems to manipulate maternal care. In rats, the maternal care repertoire consists of nursing, licking, grooming, incubating, and retrieving of pups (Stern, 1996). Natural variations in maternal behavior produce offspring that differ on many neural and behavioral dimensions. Pups that receive higher levels of maternal licking, grooming, and arched-back nursing, for example, show greater hippocampal neuron density (Bredy et al., 2003), greater levels of neurotrophin expression throughout the brain (Liu et al., 2000), altered dopamine levels in the prefrontal cortex (Zhang et al., 2005), altered GABA receptor subunit expression in the amygdala (Caldji et al., 1998, 2000), altered hypothalamic-pituitary-adrenal axis development (Liu et al., 1997), altered oxytocin receptor expression (Francis et al., 2000), and consequent changes in the many behaviors mediated by these structures. Cross-fostering shows that these maternal effects are epigenetic, with the level of maternal care received predicting the offspring's phenotype.
Adult sexual behavior is also shaped by early maternal care. For example, natural variations in maternal licking contribute to partner preference, receptivity, and paced mating behavior in female rats (Cameron et al., 2008a,b). In addition to the effects of natural variations in maternal behavior, maternal licking can also be experimentally reduced by interfering with the dam's ability to detect olfactory cues in pup urine that normally drive the licking behavior (Moore, 1981, 1985; Moore et al., 1992). Such experimental reductions in maternal licking also influence sex behavior, specifically producing behavioral deficits in adult male sexual behavior. These deficits include increased latency to ejaculation, increased latency to postejaculatory intromission, and increased interintromission intervals (Moore, 1984).
Reductions in licking influence neural development as well. One of the neural structures that control male copulatory behavior is the spinal nucleus of the bulbocavernosus (SNB). The SNB, also known as the dorsomedial nucleus (Schroder, 1980), is a sexually dimorphic population of motoneurons in the lumbar spinal cord, which innervates the anal sphincter of both males and females and additionally in males, the bulbocavernosus (BC) and levator ani (LA) muscles of the perineum (Breedlove and Arnold, 1980; Schroder, 1980; McKenna and Nadelhaft, 1986). The BC and LA muscles encircle the base of the penis and their fast, robust contractions produce an intense penile erection with flaring of the glans. This “cupping” allows seminal plug formation and removal, essential components of successful copulation and insemination (Sachs, 1982; Hart and Melese-D'Hospital, 1983). The development of SNB motoneurons extends well into the early postnatal period (Nordeen et al., 1985; Goldstein et al., 1990) and, interestingly, is sensitive to maternal care. Specifically, reductions in maternal licking produce decreased motoneuron number, motoneuron size, and dendritic length in the SNB (Moore et al., 1992; Lenz and Sengelaub, 2006). Thus, changes in adult copulatory behavior that result from decreased maternal licking may in part reflect underlying changes in the development of SNB motoneurons.
Previous work has shown that the tactile component of maternal licking behavior, when simulated experimentally, can offset the negative behavioral and physiological effects of maternal deprivation (Suchecki et al., 1993; Levy et al., 2003; Chatterjee et al., 2007). Similarly, supplementing the amount of tactile stimulation that pups receive neonatally alters their HPA axis phenotype to match that of pups that naturally receive higher levels of maternal care (Jutapakdeegul et al., 2003). Tactile stimulation also alters the expression of many proteins throughout the brain which are known to regulate structural plasticity (Chatterjee et al., 2007). These examples suggest that the tactile stimulation component of maternal care plays a crucial role in the neural development of offspring. Indeed, our previous research has used an artificial rearing paradigm to specifically study the role of tactile stimulation on the development of the SNB (Lenz et al., 2008). Pups received low, medium, or high amounts of tactile stimulation during artificial rearing to simulate maternal licking, following which adult penile reflex behavior and SNB morphology were assessed. Animals that had received low levels of tactile stimulation showed several deficits in ex copula penile reflexes as adults relative to maternally reared controls, including an increased latency to first erection, a decreased number of erection clusters, and a lower percentage of intense “cup” erections (Lenz et al., 2008). Animals that had received low or medium levels of tactile stimulation also showed a 27% reduction in dendritic length in the SNB relative to controls, suggesting that the tactile stimulation provided by maternal care influences the development and behavioral output of the SNB.
The tactile stimulation provided by maternal licking is likely relayed to the spinal cord via primary sensory afferents from the perineal skin. The development of dendrites is shaped by afferent input (Morest, 1969; Rakic, 1975); therefore, alterations in afferent activity through low tactile stimulation could alter SNB development. SNB dendritic development is activity-dependent, with blockade of NMDA receptors producing decreases in SNB dendritic length (Hebbeler et al., 2002). Conversely, developmental deafferentation negatively affects dendritic arborization, resulting in decreased dendrite length and arbor complexity (Smith, 1974; Bradley and Berry, 1976; Parks, 1981; Mizrahi and Libersat, 2002), and eliminating supraspinal input to the SNB, via thoracic spinal transection, causes localized redistributions of the dendritic arbor (Hebbeler and Sengelaub, 2003).
The reductions in dendritic length observed after low levels of tactile stimulation could reflect the relative lack of local activity of perineal sensory afferents. Perineal sensory afferents terminate in the L5-S1 spinal cord segments (McKenna and Nadelhaft, 1986) where the SNB is also located, thus the regional effects of maternal licking on SNB motoneuron development may result from uneven distributions of sensory afferents in the area of the SNB dendritic arbor and their activation. The purpose of this experiment was to determine the pattern of perineal sensory afferent distribution in the lumbosacral spinal cord, whether licking-like tactile stimulation increases neural activity in the area of the SNB dendritic field, and whether local spinal activation patterns following licking-like stimulation match the pattern of perineal sensory afferent distribution.
METHODS
Perineal Afferent Mapping
Animals
Untimed pregnant Sprague–Dawley rat dams (Harlan, Indianapolis, IN) were maintained on a 12-h light–dark cycle, with unlimited access to food and water. On the day of birth (P1), pups were sexed and culled to litters of eight with even sex ratios where possible. Perineal afferents were mapped in male pups of 7 or 14 days of age and adult males of ∼80–90 days of age.
Histochemistry
Under brief isoflurane anesthesia, a solution of 2% wheat germ agglutinin conjugated HRP in distilled water (WGA-HRP; Sigma) was injected into the perineal skin of male rat pups during the robust maternal licking period [on either postnatal day (P) 5 or P12] or in adulthood (∼P80-90). WGA-HRP anterogradely labels sensory afferent fibers, enabling us to visualize and map their distribution in the spinal cord. For P5 males, 0.5 μL of WGA-HRP was injected, for P12 males, 1 μL was injected, and for adult males 5 μL was injected. To map the cutaneous sensory innervation of the licked perineal region, animals were injected in one of three locations, either immediately rostral, lateral, or caudal to the phallus. Forty-eight (for neonates) or 72 h (for adults) after WGA-HRP injection, a period that ensures optimal labeling of sensory afferent fibers, animals were weighed, overdosed with urethane (∼0.25 g/100 of body weight), and perfused intracardially with saline followed by cold 1% paraformaldehyde/1.25% glutaraldehyde fixative. The lumbar portion of the spinal cord was removed, postfixed in the same fixative for 5 h, and then transferred to sucrose phosphate buffer (10% w/v, pH 7.4) overnight for cryoprotection. The lumbosacral spinal cord was then frozen-sectioned horizontally at 40 μm. For visualization of WGA-HRP, the tissue was immediately reacted using a modified tetramethyl benzidine protocol (Mesulam, 1982), mounted on gelatin-coated slides, counterstained with thionin, and cover-slipped with Permount.
Afferent Label Reconstruction
The distribution of sensory afferent fibers was reconstructed in three dimensions under darkfield illumination using a computer-based morphometry system (Neurolucida, MicroBrightField). For each animal, to determine the area of the SNB dendritic field, the location of SNB motoneuron somata was first mapped under brightfield illumination. The rostrocaudal extent of the dendritic field of SNB motoneurons is broader than that of SNB somata. Thus, the average extent of SNB dendrites beyond the rostral and caudal limits of the soma range were determined under darkfield illumination from pre-existing material (Goldstein et al., 1990; Lenz et al., 2008; unpublished data) from animals at the corresponding ages in which SNB motoneurons had been labeled with HRP (P7 males: n = 7; P14 males: n = 3; adult males: n = 9). These averages were added to the rostrocaudal extent of SNB motoneuron soma range in each afferent-labeled animal to create the rostral and caudal boundaries of the dendritic field for that animal. The distribution of afferent fibers was then mapped relative to the location of the SNB dendritic field in each animal, in an average of 8.57 ± 0.42 sections per animal.
Immediate Early Gene Analysis
Animals
Untimed pregnant Sprague–Dawley rat dams (Harlan, Indianapolis, IN) were maintained on a 12-h light–dark cycle, with unlimited access to food and water. On P1, pups were sexed and culled to litters of eight with even sex ratios where possible. On P10 (during the period when the maternal licking behavior is most robust), pups (both males and females) were removed from the dam and placed on a heating pad in a novel plastic container. Pups were handled individually and stimulated with a moist paintbrush in the anogenital region for 60 s. Pups were then immediately returned to the dam for 30 min, a period that has been previously shown to achieve maximum expression of the immediate early gene, Fos, in the brain following maternal care (Fenoglio et al., 2006). Control groups consisted of an undisturbed control group, which was left with the dam and not stroked, and, additionally for male pups, a handled control group, which was held by the experimenter in the same manner as the stroked animals, but not stroked, for 60 s, and then returned to the dam. A subset of male animals were returned to the dam for longer periods following stroking, for either 60 or 120 min, to map the time course of Fos expression produced by licking-like tactile stimulation. Animals were returned to the dam following the stroking manipulation to avoid the potential confound of producing the known acute physiological effects of brief maternal separation on the pups (e.g., Smythe et al., 1994; Fenoglio et al., 2006).
Following the experimental manipulation, all animals were weighed, overdosed with urethane (∼0.25 g/100 of body weight), and transcardially perfused with saline followed by cold 4% paraformaldehyde. The lumbar portion of the spinal cord was removed, postfixed in the same fixative overnight, and then transferred to sucrose phosphate buffer (30% w/v, pH 7.4) for cryoprotection until tissue sank. The lumbosacral spinal cord was then frozen-sectioned horizontally at 40 μm into PBS into three alternate series. One series was counterstained with thionin to locate the rostrocaudal range of SNB motoneurons. The other two series were processed immunohistochemically to visualize the immediate early gene product, Fos.
Immunohistochemistry
Immunohistochemistry series underwent a procedure similar to Bharati and Goodson (2006). Briefly, sections were rinsed in PBS in free-floating wells, incubated for 1 h in PBS + 5% bovine serum albumin (BSA) + 0.3% Triton-X for blocking, and incubated for 48 h in rabbit anti-Fos (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) in PBS + 2.5% BSA + 0.3% Triton-X + 0.05% sodium azide at 48C. Following primary antibody incubation, sections were rinsed and incubated for 2 h in donkey anti-rabbit secondary antibody conjugated to Alexa Fluor 488 (3 μL/mL; Invitrogen, Eugene, OR) in PBS + 2.5% BSA + 0.3% Triton-X + 0.05% sodium azide at RT. Sections were rinsed in PBS mounted onto gelatin-coated slides and cover-slipped with Vectashield Hard Set (Vector Laboratories, Burlingame, CA).
Immediate Early Gene Mapping
Using the counterstained series, the location of SNB motoneurons was mapped under brightfield illumination using a computer based morphometry system (Stereoinvestigator, MicroBrightField). The location of Fos-positive cells was mapped relative to the area of the SNB dendritic field (determined as described above using pre-existing material from Sengelaub and Arnold, 1986; n = 4) under epifluorescent illumination and reconstructed in three dimensions using a computer-based morphometry system (Stereoinvestigator) in an average of 11.37 ± 0.25 sections per animal. For all conditions and groups, the total number of Fos-positive cells in the area of the SNB dendritic field was determined. Additionally, the total counts were separated into the number of Fos-positive cells in the rostral and caudal halves of the SNB dendritic field.
Data Analysis
Final counts of Fos-positive cells in the rostral and caudal halves of the SNB dendritic field were corrected to control for the nonspecific effects of handling on Fos expression in the SNB dendritic field and isolate the specific effects of licking-like tactile stimulation. The average number of Fos-positive cells in the undisturbed control group was subtracted from the average number of Fos-positive cells in the handled control group to isolate the effects of handling alone on Fos expression. This number was then subtracted from the number of Fos-positive cells for each animal in the stroked group. t-Tests and one-way ANOVAs were performed to compare the number of Fos-positive cells between male and female pups, between male pups across time after stroking, and between the stimulated and undisturbed group in males across the rostral and caudal halves of the SNB dendritic field. Appropriate planned comparisons were made. Digital micrographs were taken under brightfield, darkfield (Kodak MDS 290 digital camera system Eastman Kodak Company, Rochester, NY), or epifluorescent illumination (MicroFire True Color digital CCD camera, Optronics, Goleta, CA).
RESULTS
Afferent Fiber Distribution
Afferent distribution was mapped in 7-day old male pups (n = 8), 14-day old male pups (n = 5), and adult male pups (n = 8). At each age, an approximately equal number of animals were injected rostral to the phallus, lateral to the phallus, and caudal to the phallus. At all ages, as anticipated, afferent fiber labeling was bilateral and largely in the dorsal horn of the spinal cord (see Fig. 1). There were no major differences in afferent labeling patterns depending upon injection location, with the exceptions being that the lateral injections produced heavier ipsilateral label and lighter contralateral label relative to the medial injections rostral and caudal to the phallus and that the rostral injections produced spinal labeling that was somewhat rostrally biased relative to caudal injections (see Fig. 2).
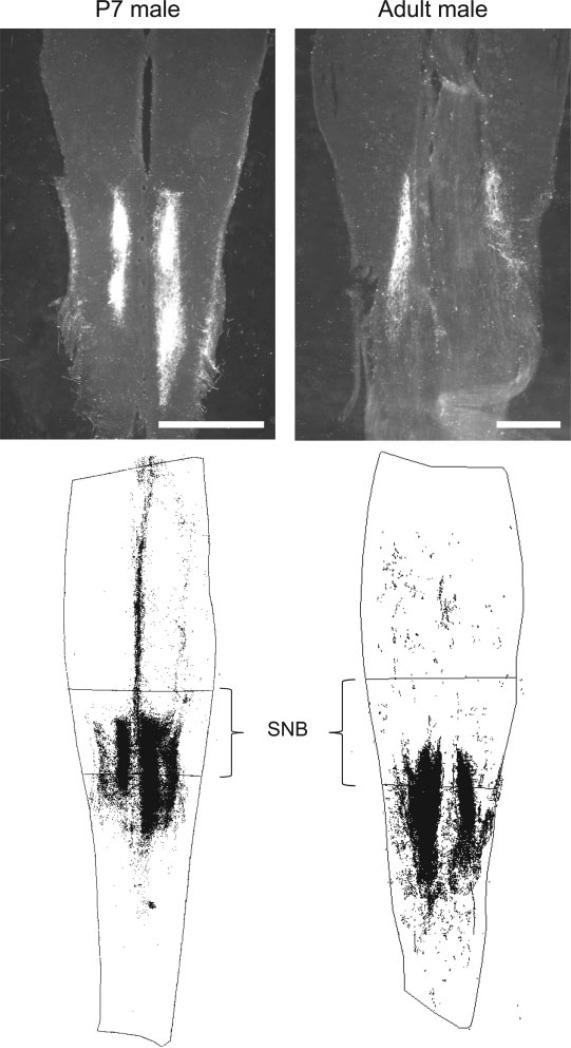
Figure 1.
Top: Representative darkfield digital light micrographs of horizontal sections through the dorsal horn of the lumbosacral spinal cord of a P7 male (left) and an adult male (right), depicting WGA-HRP label of perineal sensory afferent fibers. Bottom: Three-dimensional reconstructions of WGA-HRP labeling in the spinal cord relative to the rostrocaudal range of the SNB dendritic field. In both neonates and adult males, perineal afferent labeling was caudally biased relative to the SNB. Scale bars = 500 μm.
Figure 2.
Three-dimensional reconstructions of WGA-HRP labeling in the spinal cord relative to the rostrocaudal range of the SNB dendritic field following a WGA injection rostral (left), lateral (center), or caudal (right) to the phallus in P7 males. Relative to the SNB, rostral injections produced rostrally biased afferent labeling when compared to caudal injections, and lateral injections produced more ipsilateral labeling.
At P7, the average rostrocaudal range of afferent labeling was 1815.61 ± 163.65 μm (M ± SE), and the average rostrocaudal range of the SNB dendritic field was 1119.85 ± 136.92 μm. There was a caudal bias to afferent labeling relative to the SNB dendritic field, with afferent labeling beginning an average of 160.36 ± 108.65 μm caudal to the rostral boundary of the SNB dendritic field, and extending 842.28 ± 135.67 μm beyond the caudal boundary of the SNB dendritic field (see Fig. 1).
This caudal bias held true for the other ages examined as well. At P14, the average rostrocaudal range of afferent labeling was 1176.02 ± 161.64 μm, and the average rostrocaudal range of the SNB dendritic field was 1314.32 ± 262.07 μm. There was a caudal bias to afferent labeling relative to the SNB dendritic field, with afferent labeling beginning an average of 807.38 ± 240.31 μm caudal to the rostral boundary of the SNB dendritic field, and extending 675.94 ± 150.48 μm beyond the caudal boundary of the SNB dendritic field.
In adult males, the average rostrocaudal range of afferent labeling was 2871.90 ± 175.77 μm, and the average rostrocaudal range of the SNB dendritic field was 2057.14 ± 117.13 μm. There was a caudal bias to afferent labeling relative to the SNB dendritic field, with afferent labeling beginning an average of 720.09 ± 197.55 μm caudal to the rostral boundary of the SNB dendritic field, and afferent labeling extending 1539.44 ± 126.25 μm beyond the caudal boundary of the SNB dendritic field (see Fig. 1).
Time Course of Fos Expression
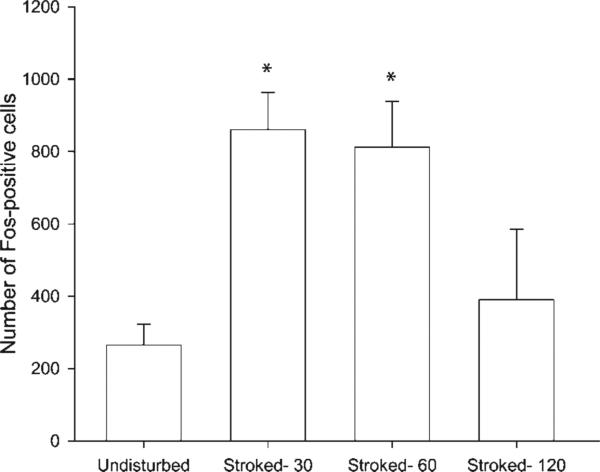
The number of Fos-positive cells in the area of the SNB dendritic field after several latencies following perineal tactile stimulation was documented, either 30 min (n = 4), 60 min (n = 5), or 120 min following stroking (n = 4) and were compared to undisturbed controls (n = 6). There was a significant effect of time on the number of Fos-positive cells, F(3, 15) = 9.10, p < 0.05 (see Fig. 3). The average number of Fos-positive cells in undisturbed controls was 265.5 ± 56.95 cells. Fisher's PLSD comparisons showed that, relative to undisturbed controls, there was a significant increase in the number of Fos-positive cells at 30 min following stroking (789.50 ± 103.22 cells; p < 0.05). Fos expression remained significantly elevated over baseline 60 min following stroking (812.20 ± 126.42 cells, p < 0.05). One hundred and twenty min following stroking, the number of Fos-positive cells was not significantly different from undisturbed controls (390.50 ± 87.95 cells, ns), but was significantly less than in the 30 and 60-min latency stroked groups (ps < 0.05). Because maximum Fos expression appeared at 30 min and was sustained through 60 min, these two groups were collapsed to form the “stroked males” group (n = 9) that was used in the remainder of group comparisons in this experiment.
Figure 3.
The time course of stroking-induced Fos expression in the lumbosacral spinal cord. Thirty or 60 min following stroking, the number of Fos-positive cells was significantly increased relative to undisturbed controls, and by 120 min following stroking, the number of Fos-positive cells was no longer significantly elevated. * denotes p < 0.05.
Stroking-Induced Fos Expression
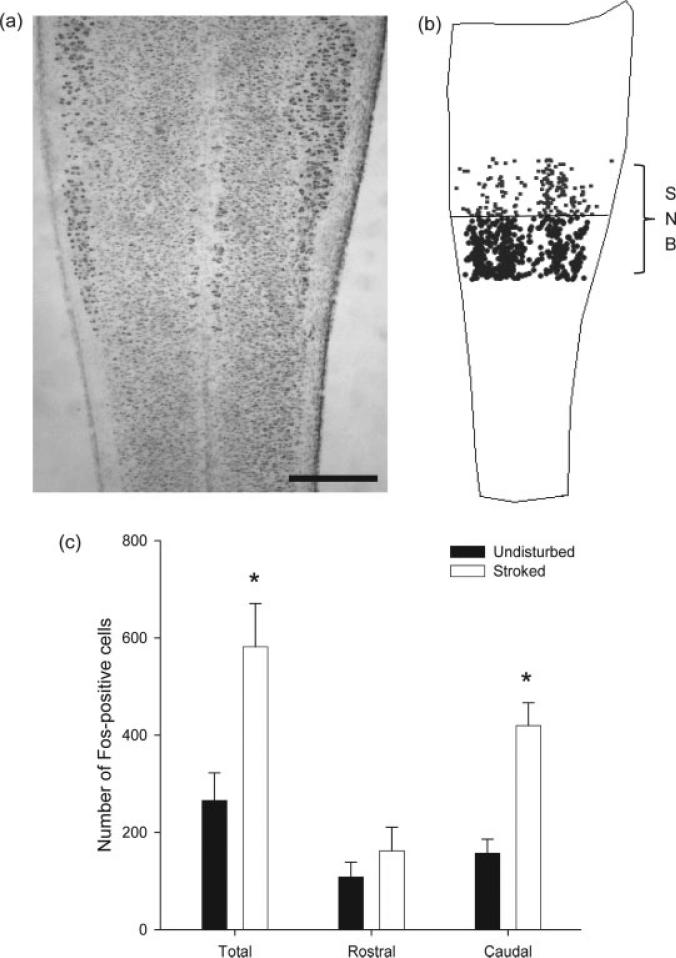
Stroking produced Fos expression in the lumbosacral spinal cord, with a majority of Fos-positive cells being located in the dorsal horn, with a concentration near the lateral margin of the dorsal horn (see Fig. 4). There was a main effect of perineal stroking on Fos expression, with stroking producing a 115% increase in the corrected number of Fos-expressing cells relative to undisturbed control males [undisturbed control males = 265.50 ± 56.95 cells, stroked males = 570.61 ± 78.94 cells; F(1,13) = 7.98, p < 0.05; Figs. 5 and 6]. There was also a main effect of location on the number of Fos-expressing cells, with more cells being located caudally in the area of the SNB dendritic field than rostrally [F(1,13) = 56.78, p < 0.05]. The stroking by location interaction term was also significant [F(1,13) = 26.43, p < 0.05]. Post hoc analyses showed that there was no difference between the number of Fos-positive cells for undisturbed control males and stroked males in the rostral portion of the area of the SNB dendritic field (control = 108.33 ± 30.40 cells; stroked = 156.06 ± 43.12 cells, ns), but a significant 164% increase in the number of Fos-positive cells in the caudal portion (control = 157.17 ± 29.07 cells; stroked = 414.55 ± 41.65 cells; p < 0.05; Fig. 6).
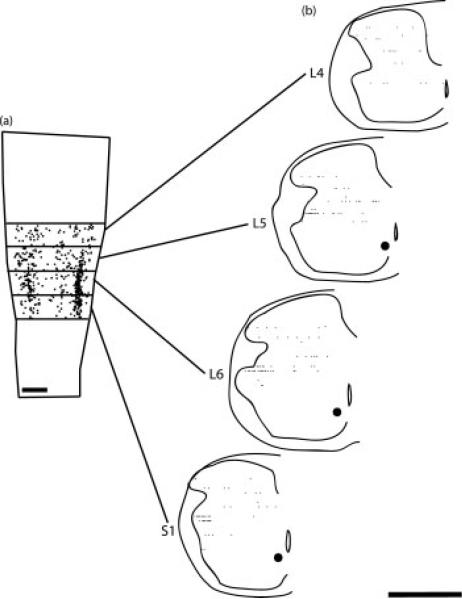
Figure 4.
(a) Computer-generated composite indicating the location of Fos-positive cells in the area of the SNB dendritic field following stroking of the perineum, drawn at 40-μm intervals through the entire dorsoventral extent of the labeling. (b) Reconstructions of spinal cord hemisections in the transverse plane from multiple horizontal sections showing the distribution of Fos-labeled cells in the dorsal horn at the L4 through S1 segment levels. Transverse reconstructions were made at 500-μm intervals as indicated in (a). Scale bars = 500 μm.
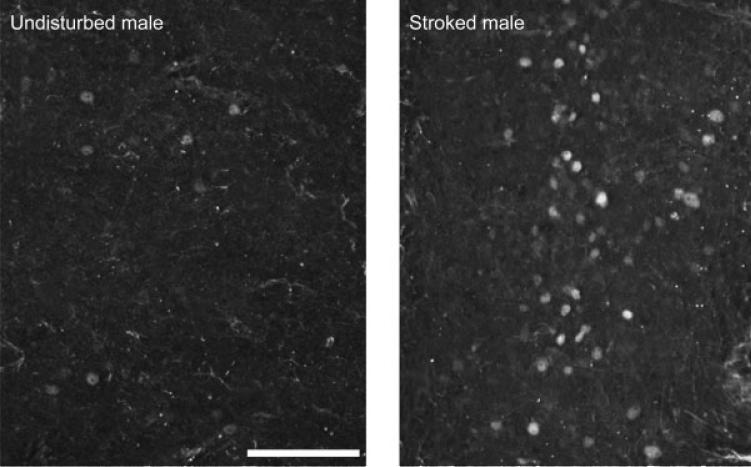
Figure 5.
Digital micrographs under epifluorescent illumination of horizontal sections through the dorsal horn of the lumbosacral spinal cord of an undisturbed control male pup (left) and a stroked male pup (right). Stroking significantly increases the number of Fos-positive cells. Scale bar = 100 μm.
Figure 6.
(a) A Brightfield digital light micrograph of a horizontal section through the lumbosacral spinal cord of a P10 pup stained with thionin, depicting the rostrocaudal extent of SNB motoneuron somata. (b) A three-dimensional reconstruction marking the location of Fos-positive cells located in the rostral (gray) and caudal (black) halves of the SNB dendritic field. (c) The number of Fos-positive cells in the SNB dendritic field, both overall and by location. Stroking caused a significant overall increase in the number of Fos-positive cells, which was driven by a significant increase in the number of Fos-positive cells in the caudal, but not rostral, portion of the SNB dendritic field. * denotes p < 0.05; scale bar = 500 μm.
Sex Differences in Fos Expression
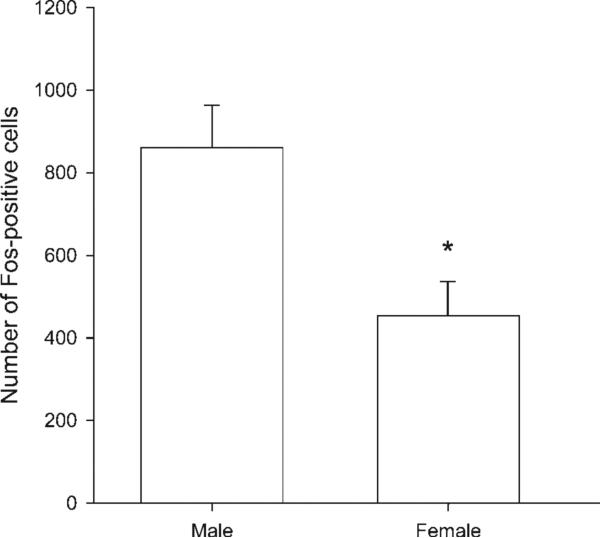
Relative to stroked male pups (n = 9; 802.11 ± 78.94), stroked female pups (n = 4) had 43% fewer Fos-expressing cells in the area of the SNB dendritic field (453.50 ± 82.86 cells; t(11) = 2.64, p < 0.05; Fig. 7).
Figure 7.
Sex differences in stroking-induced Fos expression. Stroked females had significantly fewer Fos-positive cells in the SNB dendritic field following licking-like tactile stimulation than did stroked males. * denotes p < 0.05.
DISCUSSION
Reduced maternal licking has previously been shown to negatively impact adult male copulatory behavior as well as decrease motoneuron number, soma size, and dendritic length in the SNB in a rostrocaudally biased manner (Moore, 1984; Moore et al., 1992; Lenz and Sengelaub, 2006). Our previous work has also shown that the tactile stimulation component of maternal licking seems to be crucial for mediating these effects (Lenz et al., 2008). In this experiment, we found that sensory afferents from the licked perineal skin innervate the spinal cord in a caudally biased manner relative to the SNB, potentially explaining the previously documented regional effects of reduced licking on the SNB. Moreover, we found that tactile stimulation of the perineal skin produces increased Fos expression in the SNB dendritic field in a caudally biased manner. Together, these results implicate local spinal cord activity in mediating the effects of early maternal licking on the development of the SNB and adult male copulatory behavior.
Afferent Labeling
Early maternal care has previously been shown to affect morphological development particularly in the rostral portion of the SNB, suggesting that this portion of the SNB is more vulnerable to reductions in licking. In pups at two ages as well as in adults, cutaneous sensory afferents from the perineal skin innervate the lumbosacral spinal cord in a regionally biased manner relative to the known location of the SNB dendritic field, with a majority of these afferents located in the caudal portion of the dendritic field and extending caudally beyond it. This caudal bias to perineal sensory afferent distribution in the spinal cord suggests that the rostral arbor may be more vulnerable following decreases in maternal licking, because it receives less afferent innervation from the perineal skin than the caudal arbor. As previously mentioned, the development of the SNB is activity-dependent (Hebbeler et al., 2002), and this bias in sensory innervation likely produces a corresponding bias in local activity in the rostral versus caudal portion of the SNB dendritic field. As will be further discussed below, regional differences in Fos expression following licking-like tactile stimulation suggest that this is indeed the case.
Time Course of Fos Expression
Fos is an immediate early gene expressed in the spinal cord following a variety of cutaneous somatosensory stimulation (Hunt et al., 1987). Following acute maternal care manipulations, maximal Fos expression in the brain has been obtained between 30 and 90 min following the manipulation (Caba et al., 2003; Fenoglio et al., 2006), returning to baseline after 120 min (Fenoglio et al., 2006). In the current experiment, maximal Fos expression was obtained 30–60 min following licking-like tactile stimulation of the perineum and was not significantly different from baseline at 120 min following stimulation, in keeping with previous research on Fos expression following maternal care manipulations. The transient nature of Fos expression suggests that the increases in Fos result from the experimental manipulation, and not other incidental sensory stimulation or maternal stimulation occurring before or after the experimental manipulation. If the stroking manipulation caused a subsequent change in maternal behavior following the pups’ return to the dam, for example, we would have expected to see a reflection of that change in the number of Fos-positive cells in the animals sacrificed 60–120 min after their return to the dam. The fact that we observed a transient increase followed by a relatively rapid return to baseline Fos expression indicates that changes in maternal behavior following the stroking manipulation did not likely produce the observed effects. The fact that Fos expression is transient following stroking also suggests that the experimenter-provided tactile stimulation is sufficient to produce a strong Fos signal above that due to other ambient sensory stimulation (e.g., mother, sibling, and bedding contact).
Stroking-Induced Fos Expression
A variety of sensory stimulations evoke Fos expression in the spinal cord, including heat, electrical stimulation of the skin or peripheral nerves, and inflammation (Hunt et al., 1987; Rampin et al., 1997). Specifically, anogenital stroking or stimulation of perineal nerves have been shown to evoke Fos expression in the spinal cord and brain (Rampin et al., 1997; Caba et al., 2003), and Fos expression in several brain regions has been shown to positively correlate with the amount of maternal licking received (Garoflos et al., 2008). In this experiment, we found that 60 s of licking-like tactile stimulation of the perineum of pups produced a significant increase in Fos-expression in the spinal cord, specifically in the area of the SNB dendritic field. Because the number of Fos expressing cells in the area of the SNB dendritic field following tactile stimulation was corrected for the effects of handling, the increase in Fos expression can be attributed to the specific effects of stroking stimulation instead of the nonspecific effects of somatosensory stimulation of handling or handling stress.
With regard to the location of Fos expressing cells relative to the SNB dendritic field, we found that stroking-induced increases in Fos expression were driven by a significant increase in Fos expression in the caudal portion of the SNB dendritic field, with no increase in rostral Fos expression. This result corresponds well with the caudal bias we found in perineal afferent labeling and suggests that the caudal concentration of perineal sensory afferents in the SNB dendritic field is responsible for the caudally biased increases in Fos expression following stroking stimulation. In our previous research, we found that reducing maternal licking detrimentally and specifically affects the rostral portion of the SNB (Lenz and Sengelaub, 2006). Our working model based on our current results is that the caudal concentration of perineal sensory afferents produces a caudal bias to local spinal cord activity in the SNB dendritic field in response to maternal licking. When maternal licking levels are normal, the perineal afferent activation is sufficient to provide adequate local spinal cord activation to support the normal growth and development of SNB dendrites, both rostrally and caudally. However, when maternal licking levels are reduced, the relative lack of perineal sensory afferents innervating the rostral dendritic arbor render it more vulnerable to resulting decreases in local spinal cord activation.
Sex Differences in Fos Expression
Even though females have few SNB motoneurons, the SNB in females is also sensitive to reductions in early maternal care (Moore et al., 1992). However, females have been shown to receive less maternal licking than males (Moore and Morelli, 1979). We tested whether there was a sex difference in the number of Fos expressing cells in the area of the SNB dendritic field of each animal following licking-like tactile stimulation. We found that females have significantly fewer Fos-expressing cells in the area of their SNB dendritic fields than males following tactile stimulation. Interestingly, there are sex differences in the diameter of the sensory branch of the pudendal nerve (Moore and White, 1996) as well as in the number of dorsal root ganglion cells (McKenna and Nadelhaft, 1986; Mills and Sengelaub, 1993), with males having a significantly larger sensory sensory pudendal nerve, more myelinated axons in the sensory pudendal nerve, and the corresponding dorsal root ganglia containing significantly more cells than females. Thus, the lumbosacral spinal cord of females receives sparser sensory innervation from these sources than do males. Together, these factors suggest that females show less activation in the area of the SNB dendritic field following licking-like stimulation because they receive less licking than males, but also because each bout of licking produces less sensory drive to the area of the SNB dendritic field than in males.
Mechanisms
The current afferent labeling and Fos expression results implicate primary sensory afferents as a mediator of the effects of early maternal licking on the development of the SNB. As previously mentioned, these afferents innervate the L5-S1 spinal segments where SNB motoneurons are also located; however, SNB motoneurons do not receive any monosynaptic input from primary sensory afferents (McKenna and Nadelhaft, 1986; Collins et al., 1991). Therefore, the effects of perineal sensory afferent activation on SNB development must be polysynaptically mediated. Sensory afferent labeling was restricted to the dorsal horn, and Fos-positive cells were distributed throughout lamina I-V of the dorsal horn, with a concentration at the lateral margin, potentially corresponding to the location of preganglionic neurons (see Fig. 4).
Many interneurons and most motoneurons themselves receive modulatory input from various supraspinal afferent populations, including noradrenergic, serotonergic, dopaminergic, and oxytocinergic afferents, which regulate their excitability (Rekling et al., 2000). We know, for example, that oxytocin modulates glutamatergic signaling of dorsal horn neurons in the spinal cord (Jo et al., 1998), therefore oxytocin afferents could modulate primary sensory afferent activation of the spinal cord by increasing local excitability. It should also be noted that propriospinal afferents from the perineum also innervate the lumbosacral spinal cord (Mills and Sengelaub, 1993), and, in addition, SNB motoneurons have been shown to be dependent upon trophic support from the BC/LA target musculature to maintain normal dendritic morphology (e.g., Rand and Breedlove, 1995; Yang et al., 2004). Either or both of these sources of feedback from the target musculature could conceivably be altered by changes in licking-like tactile stimulation and thereby influence SNB motoneuron morphology. Thus, though the current results suggest that primary sensory activation of the spinal cord mediates the effects of maternal licking on the development of the SNB, these effects could well be modulated by supraspinal input, feedback from the target musculature, or hormonal signals converging with changes in primary afferent activity.
CONCLUSIONS
Previous research has shown that maternal care during the early postnatal period influences sexual differentiation of the nervous system, including the developmental masculinization of the SNB and resultant adult male sexual behavior (Moore, 1984; Moore et al., 1992; Lenz and Sengelaub, 2006). We have previously shown that the tactile stimulation component of maternal licking is a critical component of the maternal care stimulus (Lenz et al., 2008), and the current experiment focused on the role that perineal sensory afferents play in mediating these effects. We found that the distribution of perineal sensory afferent fibers in the spinal cord relative to the SNB dendritic field corresponds well with the regional specificity of maternal licking effects on the SNB dendritic arbor and that this afferent distribution pattern matches the pattern of Fos expression in the spinal cord produced by licking-like tactile stimulation of the perineum. We also found that licking-like stimulation causes less Fos expression in the spinal cords of females than in males, further implicating licking as a potent modulator of sexual differentiation. Together, these results suggest that maternal licking regulates SNB motoneuron development via a sensory afferent, activity-dependent mechanism. Overall, the current results are further evidence that early maternal care acts as an important regulator of neural and behavioral development of offspring.
Acknowledgments
We are grateful to Dr. James Goodson for instruction on the immunolabeling and measurement of Fos. We also wish to thank our anonymous reviewers for their helpful comments on the manuscript.
Contract grant sponsor: NIH; contract grant numbers: 5T32HD049336 and 5R01NS047264.
REFERENCES
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P, Berry M. The effects of reduced climbing and parallel fibre input on Purkinje cell dendritic growth. Brain Res. 1976;109:133–151. doi: 10.1016/0006-8993(76)90384-x. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Brain Res Dev Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE. 2008a;3:e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm Behav. 2008b;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Carter JD, Joyce PR, Mulder RT, Luty SE, Sullivan PF. Early deficient parenting in depressed outpatients is associated with personality dysfunction and not with depression subtypes. J Affect Disord. 1999;54:29–37. doi: 10.1016/s0165-0327(98)00132-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Res. 2007;1158:11–27. doi: 10.1016/j.brainres.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Collins WF, III, Erichsen JT, Rose RD. Pudendal motor and premotor neurons in the male rat: A WGA transneuronal study. J Comp Neurol. 1991;308:28–41. doi: 10.1002/cne.903080104. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci. 2006;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Garoflos E, Stamatakis A, Rafrogianni A, Pondiki S, Stylianopoulou F. Neonatal handling on the first postnatal day leads to increased maternal behavior and Fos levels in the brain of the newborn rat. Dev Psychobiol. 2008;50:704–713. doi: 10.1002/dev.20332. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychol Sci. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hart BL, Melese-D'Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:807–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Sengelaub DR. Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: Morphologic changes and implications for estrogen sites of action. J Comp Neurol. 2003;467:80–96. doi: 10.1002/cne.10911. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. N-methyl-d-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Hidden regulatory processes in early social relationships. In: Bateson PPG, Klopfer PH, editors. Perspectives in Ethology. Plenum Press; New York, NY: 1978. pp. 135–166. [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Jo YH, Stoeckel ME, Freund-Mercier MJ, Schlichter R. Oxytocin modulates glutamatergic synaptic transmission between cultured neonatal spinal cord dorsal horn neurons. J Neurosci. 1998;18:2377–2386. doi: 10.1523/JNEUROSCI.18-07-02377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutapakdeegul N, Casalotti SO, Govitrapong P, Kotchabhakdi N. Postnatal touch stimulation acutely alters corticosterone levels and glucocorticoid receptor gene expression in the neonatal rat. Dev Neurosci. 2003;25:26–33. doi: 10.1159/000071465. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Graham MD, Parada M, Fleming AS, Sengelaub DR, Monks DA. Tactile stimulation during artificial rearing influences adult function and morphology in a sexually dimorphic neuromuscular system. Dev Neurobiol. 2008;68:542–557. doi: 10.1002/dneu.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal licking influences dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Brain Res. 2006;1092:87–99. doi: 10.1016/j.brainres.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Levy F, Melo AI, Galef BG, Jr., Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Dev Psychobiol. 2003;43:177–191. doi: 10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tracing neural connections with horseradish peroxidase. Wiley; Chichester: 1982. [Google Scholar]
- Mills AC, Sengelaub DR. Sexually dimorphic neuron number in lumbosacral dorsal root ganglia of the rat: Development and steroid regulation. J Neurobiol. 1993;24:1543–1553. doi: 10.1002/neu.480241108. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Libersat F. Afferent input regulates the formation of distal dendritic branches. J Comp Neurol. 2002;452:1–10. doi: 10.1002/cne.10275. [DOI] [PubMed] [Google Scholar]
- Moore CL. An olfactory basis for maternal discrimination of sex of offspring in rats (Rattus norvegicus). Anim Behav. 1981;29:383–386. [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL. Sex differences in urinary odors produced by young laboratory rats (Rattus norvegicus). J Comp Psychol. 1985;99:336–341. [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Moore CL, White RH. Sex differences in sensory and motor branches of the pudendal nerve of the rat. Horm Behav. 1996;30:590–599. doi: 10.1006/hbeh.1996.0062. [DOI] [PubMed] [Google Scholar]
- Moore CL, Dou H, Juraska JM. Maternal stimulation affects the number of motor neurons in a sexually dimorphic nucleus of the lumbar spinal cord. Brain Res. 1992;572:52–56. doi: 10.1016/0006-8993(92)90449-j. [DOI] [PubMed] [Google Scholar]
- Morest DK. The growth of dendrites in the mammalian brain. Z Anat Entwicklungsgesch. 1969;128:290–317. doi: 10.1007/BF00522529. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Parks TN. Changes in the length and organization of nucleus laminaris dendrites after unilateral otocyst ablation in chick embryos. J Comp Neurol. 1981;202:47–57. doi: 10.1002/cne.902020105. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rakic P. Role of cell interaction in development of dendritic patterns. Adv Neurol. 1975;12:117–134. [PubMed] [Google Scholar]
- Rampin O, Gougis S, Giuliano F, Rousseau JP. Spinal Fos labeling and penile erection elicited by stimulation of dorsal nerve of the rat penis. Am J Physiol. 1997;272:R1425–R1431. doi: 10.1152/ajpregu.1997.272.5.R1425. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;20:771–782. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Schroder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Arnold AP. Development and loss of early projections in a sexually dimorphic rat spinal nucleus. J Neurosci. 1986;6:1613–1630. doi: 10.1523/JNEUROSCI.06-06-01613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE. The effect of deafferentation on the postnatal development of Clarke's nucleus in the kitten—A Golgi study. Brain Res. 1974;74:119–130. doi: 10.1016/0006-8993(74)90115-2. [DOI] [PubMed] [Google Scholar]
- Smythe JW, Rowe WB, Meaney MJ. Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: Relationship to the handling effect on glucocorticoid receptor expression. Brain Res Dev Brain Res. 1994;80:183–189. doi: 10.1016/0165-3806(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Stern JM. Somatosensation and maternal care in Norway rats. In: Slater PJB, Rosenblatt JS, Snowdon CT, Milinski M, editors. Advances in the Study of Behavior. Academic Press; San Diego: 1996. pp. 243–294. [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: The roles of feeding and stroking. Brain Res Dev Brain Res. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci. 2005;25:1493–1502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]