Abstract
An efficient and flexible synthesis of poison-frog alkaloids 251O and trans-223B has been achieved using for both alkaloids an enantiodivergent process starting from the common lactam 1. The relative stereochemistry of 251O and trans-223B was determined to be 7 (R = n- C7H15, R’ = n-Pr) and 14 by the present enantioselective synthesis.
Keywords: poison-frog alkaloids, 251O, trans-223B
Introduction
A variety of lipophilic alkaloids have been detected in skin extracts of neotropical poison-frogs and over 800 alkaloids from 24 classes have been detected to date.1 These alkaloids serve as a chemical defense against predation and some of them also exhibit significant inhibitory effects on nicotinic acetylcholine receptors (nAChRs).2
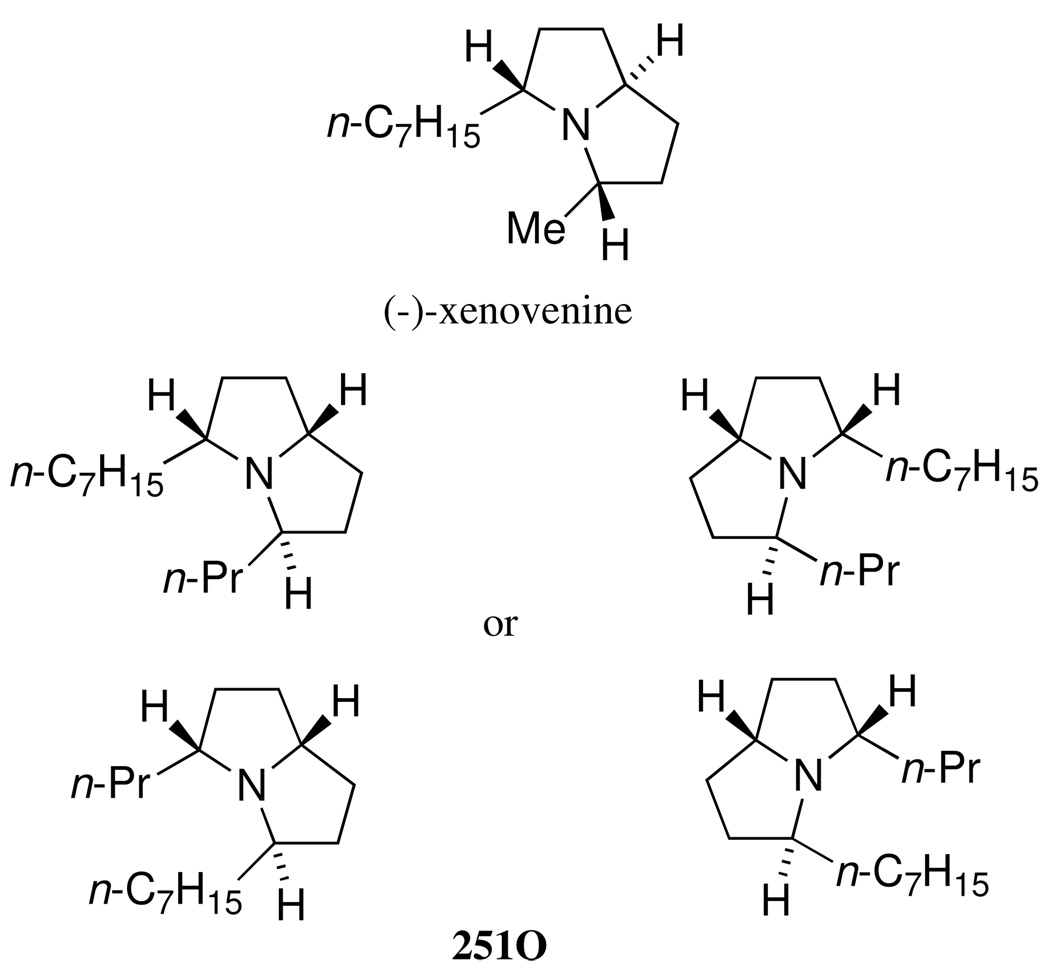
Very interestingly, most of these alkaloids appear to be sequestered from dietary arthropods.3 Thus, the 3,5-disubstituted pyrrolizidine 251O, detected in the poison-frogs Mantella haraldmeieri, M. bernhardi and M. baroni of Madagascar, has also been found in Malagasy ants of the genus Anochetus grandidieri.4 So far, several syntheses of trans, trans-type of 3,5-disubstituted pyrrolizidines such as xenovenine have been reported,5 whereas no synthesis of the trans, cis-type of 3,5-disubstituted pyrrolizidine alkaloids has been reported. Natural xenovenine is found in frogs as cis-223H. It has the 3R, 5S, 8S absolute configuration as shown in Figure 1 (unpublished work, HMG, TFS and JWD). Alkaloid 251O has a trans, cis-type of pyrrolizidine ring system; however, both the relative and absolute stereochemistry are still unknown (Figure 1). As part of a program directed at studying the synthesis of biologically active alkaloids,6 we report herein, the efficient enantio- and diastereo-divergent synthesis of 251O starting from a common lactam, 1.7
Figure 1.
Structure of xenovenine and 251O
Results and Discussion
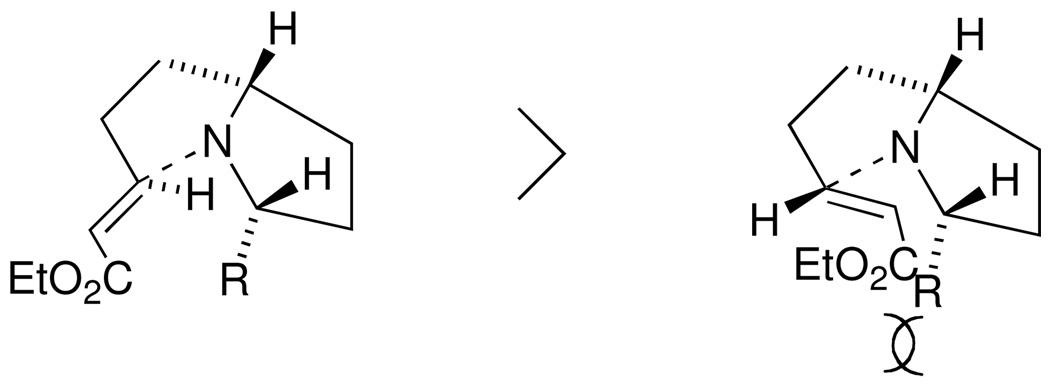
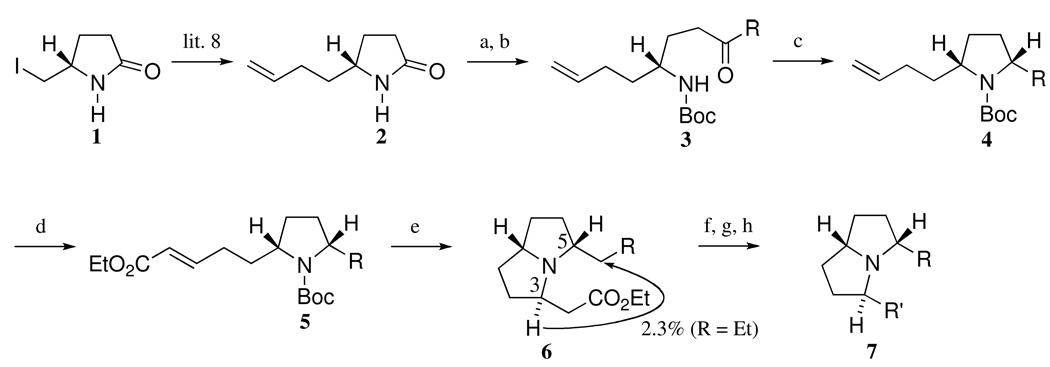
The known olefin 2,8 derived from 1, was converted to a Boc-imide, which was treated with n-Pr or n-C7H15 magnesium bromide according to Martin’s reaction conditions9 to provide the ketones 3 (R = n-Pr or n-C7H15) in high yield. Both ketones 3 were subjected to cyclization and subsequent reduction of the resulting iminium ion with Ph3SiH9 to give rise to the cis-substituted pyrrolidines 4 (R = n-Pr or n-C7H15). The cross-metathesis reaction of 4 (R = n-Pr or n-C7H15) with ethyl acrylate using Grubbs’ second-generation catalyst10 afforded the unsaturated esters 5 (R = n-Pr or n-C7H15). The key pyrrolizidine ring-closure reaction of 5 (R = n-Pr or n-C7H15) resulted after treatment with 2 equivalents of AlCl3 in CH2Cl2 followed by K2CO3 in the desired pyrrolizidines 6 (R = Et or n-C6H13) in excellent yield with high diastereoselectivity (>30:1). The stereochemistry of 6 (R = Et) was determined to be as shown based on an NOE experiment. In a difference NOE experiment, an NOE enhancement (ca. 2.3%) was observed on one of the methylene protons of the side chain at C-5 upon irradiation of the methine proton at C-3.
The highly selective formation of the desired 6 (R = Et or n-C6H13) is explained by invoking kinetic control as shown in Figure 2.11 Finally, half-reduction of the ester moiety with diisobutylaluminum hydride, Wittig olefination of the resulting aldehyde followed by hydrogenation of the corresponding olefin furnished 7 (R = n-Pr, R’ = n-C7H15 or R = n-C7H15, R’ = n-Pr). (Scheme 1)
Figure 2.
Kinetic control on pyrrolizidine formation
Scheme 1.
Synthesis of 7 (R = n-Pr, R' = n-C7H15 or R = n-C7H15, R' = n-Pr)
Reagents and conditions
(a) Boc2O, DMAP, MeCN, rt (95%); (b) n-C3H7MgBr or n-C7H15MgBr, TMEDA, THF, −78 °C (88% for R = n-Pr, 78% for R = n-C7H15); (c) B(C6F5)3, Ph3SiH, CH2Cl2, −78 ° C to rt (85% for R = n-Pr, 89% for R = n-C7H15); (d) ethyl acrylate, Grubbs' 2nd catalyst, CH2Cl2, reflux (96% for R = n-C3H7, 96% for R = n-C7H15); (e) AlCl3, CH2Cl2, rt then K2CO3, CH2Cl2, rt (93% for R = Et, 88% for R = n-C6H13); (f) DIBAL, CH2Cl2, −78 °C; (g) n-C5H11P+Ph3Br− , n-BuLi or MeP+Ph3I− , n-BuLi, THF, 0 °C to rt (46% for R = n-Pr, R' = C7H13, 49% for R = n-C7H15, R' = allyl); (h) 10% Pd/C, H2, EtOAc, 1 atm (quant for R = n-Pr, R' = n-C7H15, 95% for R = n-C7H15, R' = n-Pr)
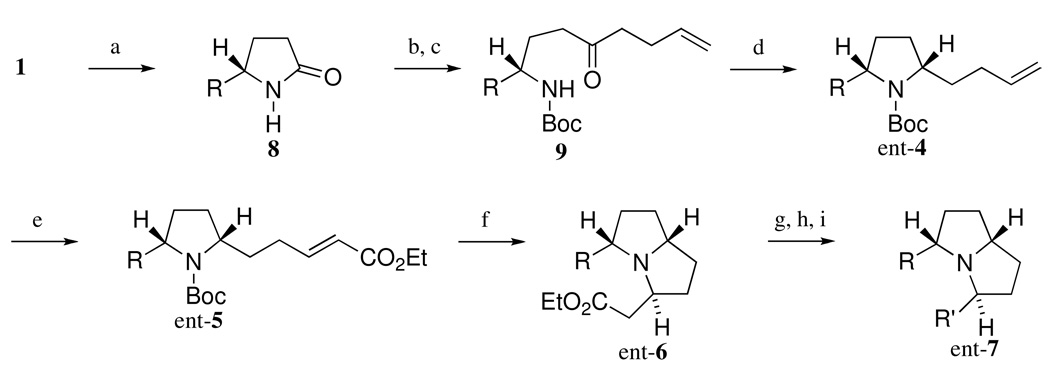
Ent-7 (R = n-Pr, R’ = n-C7H15 or R = n-C7H15, R’ = n-Pr) was synthesized from 1 in a similar way as shown in Scheme 2.
Scheme 2.
Synthesis of ent-7 (R = n-Pr, R' = n-C7H15 or R = n-C7H15, R' = n-Pr)
Reagents and conditions
(a) EtMgBr or n-C6H13MgBr, THF, −35 °C (52% for R = n-Pr, 50% for R = n-C7H15); (b) Boc2O, DMAP, MeCN, rt (94% for R = n-Pr, 93% for R = n-C7H15); (c) 4-butenylMgBr, TMEDA, THF, −78 °C (95% for R = n-Pr, 98% for R = n-C7H15); (d) B(C6F5)3, Ph3SiH, CH2Cl2, −78 ° C to rt (73% for R = n-Pr, 86% for R = n-C7H15); (e) ethyl acrylate, Grubbs' 2nd catalyst, CH2Cl2, reflux (96% for R = n-Pr, 95% for R = n-C7H15); (f) AlCl3, CH2Cl2, rt then K2CO3, CH2Cl2, rt (93% for R = n-Pr, 91% for R = n-C7H15); (g) DIBAL, CH2Cl2, −50 °C; (h) n-C5H11P+Ph3Br− , n-BuLi or MeP+Ph3I− , n-BuLi, THF, 0 °C to rt (48% for R = n-Pr, R' = C7H13, 52% for R = n-C7H15, R' = allyl); (h) 10% Pd/C, H2, EtOAc, 1 atm (96% for R = n-Pr, R' = n-C7H15, quant for R = n-C7H15, R' = n-Pr).
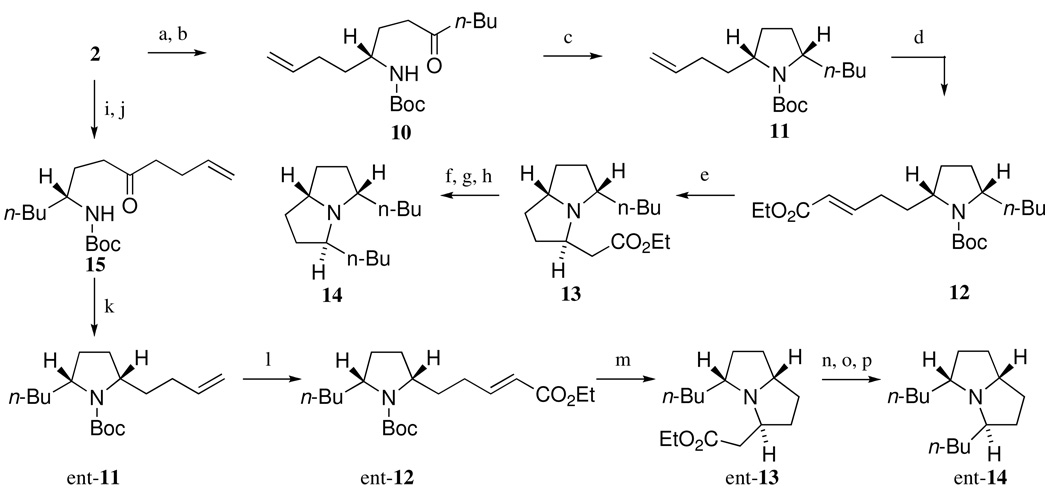
The enantiodivergent synthesis of trans-223B was also achieved starting from common lactam 2 as shown in Scheme 3
Scheme 3.
Synthesis of both enantiomers of trans-223B
Reagents and conditions
(a) Boc2O, DMAP, MeCN, rt (95%); (b) n-BuMgBr, TMEDA, THF, −78 °C (98%); (c) B(C6F5)3, Ph3SiH, CH2Cl2, −78 °C to rt (73%); (d) ethyl acrylate, Grubbs' 2nd catalyst, CH2Cl2, reflux (96%); (e) AlCl3, CH2Cl2, rt then K2CO3, CH2Cl2, rt (92%); (f) DIBAL, CH2Cl2, −50 °C; (g) EtP+Ph3Br− , n-BuLi, THF, 0 °C to rt (50%); (h) 10% Pd/C, H2, EtOAc, 1 atm (quant); (i) Boc2O, DMAP, MeCN, rt (95%); (j) 10% Pd/C, H2, EtOAc, 1 atm, then 4-butenylMgBr, TMEDA, THF, −78 °C (96%); (k) B(C6F5)3, Ph3SiH, CH2Cl2, −78 °C to rt (72%); (l) ethyl acrylate, Grubbs' 2nd catalyst, CH2Cl2, reflux (84%); (m) AlCl3, CH2Cl2, rt then K2CO3, CH2Cl2, rt (92%); (n) DIBAL, CH2Cl2, −50 °C; (o) EtP+Ph3Br− , n-BuLi, THF, 0 °C to rt (53%); (p) 10% Pd/C, H2, EtOAc, 1 atm (quant).
The GC-MS and GC-FTIR spectra of synthetic 7 (R = n- C7H15, R’ = n-Pr) were identical with those for natural 251O present in skin extracts of three Madagascan frogs (Mantella haraldmeieri collected in 2004, M. bernhardi collected in 1997, and M. baroni collected in 2004, unpublished results; see supporting information). Furthermore, the GC-MS and GC-FTIR spectra of synthetic 14 were also identical with those for natural trans-223B present in a skin extract of the Argentinean frog Melanophryniscus stelzneri.12 The mass spec evidence, with a strong loss of propyl from the molecular ion at m/z 208 (251-43) indicates a pseudo-axial position for the propyl group: a group that is trans-antiparallel to the radical ion on the N atom should cleave preferentially, all other things being equal. The heptyl group, being larger cleaves better, but the comparison of the MS on the Supplemental Information, between trans-stereoisomers with a Pr-group pseudo-axial or a Pr-group pseudo-equatorial, clearly indicates a bigger fragmentation of the propyl group for the one in the pseudo-axial position. This MS evidence indicates that natural 251O is (3S, 5S, 8R) or the enantiomer (3R, 5R, 8S) same as 7 (R = n- C7H15, R’ = n-Pr) or ent-7 (R = n-C7H15, R’ = n-Pr). Unfortunately, we could not achieve the separation of both enantiomers of synthetic 7 by GC analysis using two different columns. For the enantiomers of synthetic 14, we anticipate even more difficulty in the separation since the two side-chains are identical; however, this separation was not attempted.
In conclusion, we have achieved the first synthesis of alkaloids 251O and trans-223B in enantio- and diastereo-divergent fashion in 8 or 9 steps in 25~29% overall yield from the known lactam 1, respectively. Furthermore, the relative stereochemistries of natural 251O and trans-223B were determined to be 7 (R = n- C7H15 R’ = n-Pr) and 14, respectively, by the present efficient syntheses and comparisons with the natural compounds. It is difficult to separate the enantiomers of 7 and 14, but further work on the GC separation of the enantiomers of 7 and 14 for the determination of the absolute stereochemistry of natural 251O and trans-223B is in progress.13
Experimental Section
(2R)-(−)-2-(But-3-enyl)-5-oxopyrrolidine-1-carboxylic acid tert-butyl ester
To a stirred solution of 28 (337 mg, 2.42 mmol) in MeCN (20 mL) was added DMAP (326 mg, 2.67 mmol) at 0 °C, and the reaction mixture was stirred at 0 °C for 30 min. To the reaction mixture was added Boc2O (633 mg, 2.90 mmol) at 0 °C, and the resulting mixture was stirred at room temperature for 45 h. The volatiles were evaporated, and the residue was chromatographed on silica gel (20 g, hexane/acetone=15:1) to give the Boc-imide (550 mg, 95%) as a colorless oil.
IR (neat) 3078, 1785, 1750, 1714, 1308, 1153 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.52 (9H, s), 1.59 (1H, m), 1.78 (1H, m), 1.90 (1H, m), 2.03–2.19 (3H, br m), 2.43 (1H, ddd, J = 9.0, 8.5, 2.1 Hz), 2.57 (1H, dd, J = 9.0, 8.5 Hz), 4.12 (1H, m), 5.01 (1H, d, J = 10.7 Hz), 5.04 (1H, d, J = 15.0 Hz), 5.80 (1H, m); 13C NMR (75 MHz, CDCl3) δ 21.9 (t), 27.7 (q), 29.6 (t), 31.0 (t), 32.4 (t), 57.1 (d), 82.2 (s), 115.0 (t), 136.7 (d), 149.3 (s), 173.7 (s); MS 182 (M+-57), 84 (100); HRMS Calcd for C9H12O3N 182.0817, Found 182.0830; [α]D 26 −56.68 (c 2.34, CHCl3).
(1R)-(+)-[1-(3-Oxohexyl)pent-4-enyl]carbamic acid tert-butyl ester (3, R = n-Pr)
To a stirred solution of above Boc-imide (239 mg, 1.00 mmol) in THF (5 mL) was added a solution of n-PrMgBr, prepared from n-PrBr (0.27 mL, 3.00 mmol) and Mg (72 mg, 3.00 mmol) in THF (10 mL) at reflux, and TMEDA (0.48 mL, 3.00 mmol) at −78 °C, and the resulting mixture was stirred at the same temperature for 1.5 h. The reaction was quenched with i-PrOH (5 mL), and the reaction mixture was diluted with Et2O. The organic layer was washed with 10% HCl (aq) solution, dried over MgSO4, and evaporated to give pale yellow oil, which was chromatographed on silica gel (20 g, hexane/acetone=50:1−30:1) to give 3 (R = n-Pr, 250 mg, 88%) as a colorless solid (mp 41~43 °C). IR (KBr) 3349, 3081, 1708, 1689, 1528, 1175 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.90 (3H, t, J = 7.2 Hz), 1.42 (9H, s), 1.48–1.63 (5H, br m), 1.79 (1H, m), 2.08 (2H, q-like, J = 7.2 Hz), 2.37 (2H, t, J = 7.2 Hz), 2.46 (2H, t, J = 7.2 Hz), 3.53 (1H, br), 4.25 (1H, br d, J = 10.2 Hz), 4.95 (1H, d, J = 9.4 Hz), 5.01 (1H, d, J = 14.8 Hz), 5.78 (1H, m); 13C NMR (75 MHz, CDCl3) δ 13.6 (q), 17.1 (t), 28.2 (q), 29.0 (t), 30.1 (t), 35.1 (t), 39.1 (t), 44.6 (t), 49.9 (d), 78.5 (s), 114.5 (t), 137.6 (d), 155.4 (s), 210.3 (s); MS 226 (M+-57), 57 (100); HRMS Calcd for C12H20O3N 226.1443, Found 226.1457; [α]D 26 +1.17 (c 1.21, CHCl3).
(1R)-(−)-[1-(3-Oxodecyl)pent-4-enyl]carbamic acid tert-butyl ester (3, R = n-C7H15)
To a stirred solution of above Boc-imide (239 mg, 1.00 mmol) in THF (5 mL) was added a solution of n-C7H15MgBr, prepared from n-C7H15Br (0.47 mL, 3.00 mmol) and Mg (72 mg, 3.00 mmol) in THF (10 mL) at reflux, and TMEDA (0.48 mL, 3.00 mmol) at −78 °C, and the resulting mixture was stirred at the same temperature for 1.5 h. The reaction was quenched with i-PrOH (5 mL), and the reaction mixture was diluted with Et2O. The organic layer was washed with 10% HCl (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, which was chromatographed on silica gel (20 g, hexane/acetone=50:1−30:1) to give 3 (R = n-C7H15, 265 mg, 78%) as a colorless solid (mp 60~62 °C). IR (KBr) 3347, 3080, 1709, 1684, 1525, 1173 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.85 (3H, t, J = 7.2 Hz), 1.26 (8H, br), 1.43 (9H, s), 1.46–1.61 (5H, br m), 1.81 (1H, m), 2.13 (2H, m), 2.39 (2H, t, J = 7.2 Hz), 2.47 (2H, t, J = 7.2 Hz), 3.53 (1H, br), 4.24 (1H, br d, J = 9.6 Hz), 4.95 (1H, d, J = 9.6 Hz), 5.01 (1H, d, J = 15.0 Hz), 5.78 (1H, m); 13C NMR (75 MHz, CDCl3) δ 14.0 (q), 22.5 (t), 23.7 (t), 28.3 (q), 29.0 (t), 29.1 (t), 30.1 (t), 31.6 (t), 35.2 (t), 39.2 (t), 42.9 (t), 50.0 (d), 78.7 (s), 114.6 (t), 137.7 (d), 155.4 (s), 210.6 (s); MS 282 (M+-57), 57 (100); HRMS Calcd for C16H28O3N 282.2069, Found 282.2091; [α]D 26 −1.56 (c 0.74, CHCl3).
(2R,5S)-(−)-2-But-3-enyl-5-propylpyrrolidine-1-carboxylic acid tert-butyl ester (4, R = n-Pr)
To a stirred solution of 3 (R = n-Pr, 226 mg, 0.80 mmol) in CH2Cl2 (5 mL) was added a solution of (C6F5)3B (82 mg, 0.16 mmol) and Ph3SiH (415 mg, 1.59 mmol) in CH2Cl2 (5 mL) at −78 °C, and the reaction mixture was stirred at −78 °C for 30 min, and then at room temperature for 20 h. The reaction was quenched with Et3N (0.6 mL), and the resulting mixture was stirred at room temperature for 20 min. The mixture was diluted with Et2O, and the organic layer was washed successively with 10% AcOH (aq) solution and satd. NaHCO3 (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, which was chromatographed on silica gel (20 g, hexane/acetone=200:1−150:1) to give 4 (R = n-Pr, 182 mg, 85%) as a colorless oil.
IR (neat) 3073, 1694, 1389 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.92 (3H, t, J = 7.2 Hz), 1.28 (6H, br m), 1.46 (9H, s), 1.61 (2H, m), 1.90 (2H, m), 2.04 (2H, m), 3,76 (2H, br), 4.94 (1H, d, J = 9.6 Hz), 5.03 (1H, d, J = 15.6 Hz), 5.81 (1H, m); 13C NMR (75 MHz, CDCl3) δ 14.2 (q), 19.6 (t), 28.5 (q), 29.4 (t), 30.7 (t), 35.1 (t), 38.2 (t), 57.8 (d), 58.1 (d), 78.7 (s), 114.2 (t), 138.3 (d), 154.7 (s); MS 267 (M+), 57 (100); HRMS Calcd for C16H29O2N 267.2198, Found 267.2215; [α]D 26 −3.23 (c 1.10, CHCl3).
(2R,5S)-(−)-2-But-3-enyl-5-heptylpyrrolidine-1-carboxylic acid tert-butyl ester (4, R = n-C7H15)
To a stirred solution of 3 (R = n-C7H15, 250 mg, 0.77 mmol) in CH2Cl2 (5 mL) was added a solution of (C6F5)3B (78 mg, 0.15 mmol) and Ph3SiH (401 mg, 1.54 mmol) in CH2Cl2 (5 mL) at −78 °C, and the reaction mixture was stirred at −78 °C for 30 min, and then at room temperature for 20 h. The reaction was quenched with Et3N (0.6 mL), and the resulting mixture was stirred at room temperature for 20 min. The mixture was diluted with Et2O, and the organic layer was washed successively with 10% AcOH (aq) solution and satd. NaHCO3 (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, which was chromatographed on silica gel (20 g, hexane/acetone=200:1−150:1) to give 4 (R = n-C7H15, 222 mg, 89%) as a colorless oil.
IR (neat) 3075, 1695, 1390, 1174, 1107 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (3H, t, J = 7.2 Hz), 1.27 (14H, br), 1.46 (9H, s), 1.62 (2H, m), 1.90 (2H, m), 2.07 (2H, br), 3.75 (2H, br), 4.95 (1H, d, J = 10.2 Hz), 5.02 (1H, d, J = 15.8 Hz), 5.84 (1H, m); 13C NMR (75 MHz, CDCl3) δ 14.1 (q), 22.7 (t), 26.4 (t), 28.5 (q), 29.3 (t), 29.6 (t), 30.8 (t), 31.8 (t), 35.1 (t), 35.9 (t), 57.8 (d), 58.4 (d), 78.7 (s), 114.2 (t), 138.3 (d), 154.7 (s); MS 323 (M+), 168 (100); HRMS Calcd for C20H37O2N 323.2824, Found 323.2847; [α]D 26 −0.97 (c 0.55, CHCl3).
(2R,5S)-(−)-2-(4-Ethoxycarbonylbut-3-enyl)-5-propylpyrrolidine-1-carboxylic acid tert-butyl ester (5, R = n-Pr)
To a stirred solution of 4 (R = n-Pr, 117 mg, 0.44 mmol) in CH2Cl2 (8 mL) were added Grubbs 2nd catalyst (15 mg, 0.018 mmol) and ethyl acrylate (0.24 mL, 2.20 mmol), and the resulting mixture was refluxed for 5.5 h. After cooling, the solvent was removed under reduced pressure, and the residue was chromatographed on silica gel (20 g, hexane/acetone=100:1−60:1) to give 5 (R = n-Pr, 143 mg, 96%) as a pale yellow oil.
IR (neat) 1721, 1693, 1390, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.91 (3H, t, J = 7.2 Hz), 1.20–1.30 (8H, br, including at δ 1.27, 3H, t, J = 7.2 Hz), 1.45 (9H, s), 1.61 (2H, m), 1.90 (3H, m), 2.19 (2H, br), 3.76 (2H, br), 4.16 (2H, q, J = 7.2 Hz), 5.85 (1H, d, J = 15.8 Hz), 6.96 (1H, dt, J = 15.8, 6.8 Hz); 13C NMR (75 MHz, CDCl3) δ 14.1 (q), 14.2 (q), 19.5 (t), 28.5 (q), 29.1 (t), 29.5 (t), 34.2 (t), 38.2 (t), 57.6 (d), 58.1 (d), 60.0 (t), 78.8 (s), 121.1 (d), 148.4 (d), 154.6 (s), 166.2 (s); MS 339 (M+), 196 (100); HRMS Calcd for C19H33O4N 339.2410, Found 339.2392; [α]D 26 −6.81 (c 0.66, CHCl3).
(2R,5S)-(−)-2-(4-Ethoxycarbonylbut-3-enyl)-5-heptylpyrrolidine-1-carboxylic acid tert-butyl ester (5, R = n-C7H15)
To a stirred solution of 4 (R = n-C7H15, 134 mg, 0.41 mmol) in CH2Cl2 (7 mL) were added Grubbs 2nd catalyst (14 mg, 0.016 mmol) and ethyl acrylate (0.23 mL, 2.07 mmol), and the resulting mixture was refluxed for 5.5 h. After cooling, the solvent was removed under reduced pressure, and the residue was chromatographed on silica gel (20 g, hexane/acetone=100:1−60:1) to give 5 (R = n-C7H15, 157 mg, 96%) as a pale yellow oil.
IR (neat) 1716, 1696, 1387, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.87 (3H, t, J = 7.2 Hz), 1.17–1.33 (13H, br, including at δ 1.27, 3H, t, J = 7.2 Hz), 1.45 (9H, s), 1.60 (2H, m), 1.92 (2H, m), 2.20 (2H, m), 3.76 (2H, br), 4.16 (2H, q, J = 7.2 Hz), 5.81 (1H, d, J = 15.6 Hz), 6.97 (1H, dt, J = 15.6, 6.8 Hz); 13C NMR (75 MHz, CDCl3) δ 13.9 (q), 14.1 (q), 22.4 (t), 26.2 (t), 28.3 (q), 29.0 (t), 29.1 (t), 29.4 (t), 31.6 (t), 34.1 (t), 35.8 (t), 57.4 (d), 58.2 (d), 59.7 (t), 78.6 (s), 121.0 (d), 148.2 (d), 154.4 (s), 165.9 (s); MS 338 (M+-57), 57 (100); HRMS Calcd for C19H32O4N 338.2331, Found 338.2338; [α]D 26 −1.75 (c 0.88, CHCl3).
(3R,5S,8S)-(+)-(5-Propylhexahydropyrrolizin-3-yl)acetic acid ethyl ester (6, R = Et)
To a stirred solution of 5 (R = n-Pr, 123 mg, 0.36 mmol) in CH2Cl2 (10 mL) was added AlCl3 (106 mg, 0.80 mmol) at 0 °C, and the resulting suspension was stirred at room temperature for 24 h. The reaction was quenched with satd. NaHCO3 (aq) solution, and the organic layer was separated. The aqueous layer was extracted with CHCl3 (10 mL×5), and the organic layer and extracts were combined, dried over K2CO3, and evaporated to give the residue. To a stirred solution of this residue in CH2Cl2 (10 mL) was added K2CO3 (100 mg, 0.72 mmol), and the resulting suspension was stirred at room temperature for 48 h. The insoluble material was filtered off, and washed with CH2Cl2. The filtrate was evaporated to afford the residue, which was chromatographed on silica gel (20 g, hexane/acetone=20:1−12:1) to give 6 (R = Et, 80 mg, 93%) as a pale yellow oil, and the stereoisomer at the 3-position (all cis-pyrrolizidine, 2.5 mg, 3%) also as a pale yellow oil.
IR (neat) 1732, 1178 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.94 (3H, t, J = 7.2 Hz), 1.26 (3H, t, J = 7.2 Hz), 1.31 (1H, m), 1.33–1.44 (4H, br m), 1.52 (1H, m), 1.74–1.81 (4H, br m), 2.03 (2H, m), 2.28 (1H, dd, J = 15.1, 9.8 Hz), 2.57 (1H, dd, J = 15.1, 3.9 Hz), 3.01 (1H, br), 3.40 (1H, m), 3.57 (1H, br), 4.12 (2H, q, J = 7.2 Hz); 13C NMR (75 MHz, CDCl3) δ 14.2 (q), 14.4 (q), 21.4 (t), 30.1 (t), 30.8 (t), 31.3 (t), 32.4 (t), 33.5 (t), 43.2 (t), 54.2 (d), 60.0 (t), 63.5 (d), 65.6 (d), 172.1 (s); MS 239 (M+), 196 (100); HRMS Calcd for C14H25O2N 239.1885, Found 239.1869; [α]D 26 +25.94 (c 1.95, CHCl3).
Stereoisomer at the 3-position: IR (neat) 1736, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.90 (3H, t, J 7.2 Hz), 1.26 (3H, t, J = 7.2 Hz), 1.22–1.37 (2H, m), 1.38–1.52 (5H, m), 1.91–1.98 (2H, m), 2.01–2.04 (2H, m), 2.28 (1H, dd, J = 15.0, 8.6 Hz), 2.53 (1H, dd, J = 15.0, 3.6 Hz), 2.67 (1H, m), 3.15 (1H, m), 3.57 (1H, br), 4.12 (2H, q, J = 7.2 Hz); MS 239 (M+), 83 (100).
(3R,5S,8S)-(+)-(5-Heptylhexahydropyrrolizin-3-yl)acetic acid ethyl ester (6, R = n-C6H13)
To a stirred solution of 5 (R = n-C7H15, 270 mg, 0.68 mmol) in CH2Cl2 (20 mL) was added AlCl3 (200 mg, 1.50 mmol) at 0 °C, and the resulting suspension was stirred at room temperature for 24 h. The reaction was quenched with satd. NaHCO3 (aq) solution, and the organic layer was separated. The aqueous layer was extracted with CHCl3 (20 mL×5), and the organic layer and extracts were combined, dried over K2CO3, and evaporated to give the residue. To a stirred solution of this residue in CH2Cl2 (20 mL) was added K2CO3 (189 mg, 1.37 mmol), and the resulting suspension was stirred at room temperature for 48 h. The insoluble material was filtered off, and washed with CH2Cl2. The filtrate was evaporated to afford the residue, which was chromatographed on silica gel (30 g, hexane/acetone=20:1−12:1) to give 6 (R = n-C6H13, 178 mg, 88%) as a pale yellow oil.
IR (neat) 1731, 1176 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.87 (3H, t, J = 7.2 Hz), 1.25 (3H, t, J = 7.2 Hz), 1.17–2.41 (14H, br m), 1.51 (1H, m), 1.76 (3H, m), 2.01 (2H, m), 2.27 (1H, dd, J = 14.8, 9.6 Hz), 2.56 (1H, dd, J = 14.8, 4.1 Hz), 2.99 (1H, br), 3.38 (1H, m), 3.56 (1H, m), 4.10 (2H, q, J = 7.2 Hz); 13C NMR (75 MHz, CDCl3) δ 13.9 (q), 14.1 (q), 22.5 (t), 28.1 (t), 29.1 (t), 29.7 (t), 30.1 (t), 30.6 (t), 31.1 (t), 31.2 (t), 31.6 (t), 32.2 (t), 43.1 (t), 54.0 (d), 59.8 (t), 63.6 (d), 65.5 (d), 171.8 (s); MS 295 (M+), 196 (100); HRMS Calcd for C18H33O2N 295.2510, Found 295.2501; [α]D 26 +18.82 (c 1.14, CHCl3).
(3S,5S,8S)-(+)-3-Heptyl-5-propylhexahydropyrrolizine (7, R = n-Pr, R’ = n-C7H15)
To a stirred solution of 6 (R = Et, 79 mg, 0.32 mmol) in CH2Cl2 (7 mL) was added a solution of DIBAL (0.98 M in hexane, 0.36 mL, 0.35 mmol) at −50 °C, and the reaction mixture was stirred at −50 °C for 30 min. The reaction was quenched with MeOH, and satd. Rochelle (aq) solution, and the organic layer was separated. The aqueous layer was extracted with CH2Cl2 (5 mL×3), and the organic layer and extracts were combined, dried over K2CO3, and evaporated to give a pale yellow oil, which was used directly in the next step.
To a stirred suspension of n-C5H11P+Ph3Br— (529 mg, 1.28 mmol) in THF (10 mL) was added a solution of n-BuLi (1.6 M in hexane, 0.7 mL, 1.12 mmol) at 0 °C, and the resulting orange suspension was stirred at 0 °C for 10 min. To the suspension was added a solution of the above aldehyde in THF (3 mL) at 0 °C, and the resulting suspension was stirred at room temperature for 22 h. The reaction was quenched with H2O, and the aqueous mixture was extracted with Et2O (15 mL×4). The organic extracts were combined, dried over K2CO3, and evaporated to give a residue, that was chromatographed on silica gel (20 g, hexane/acetone=25:1−10:1) to give the corresponding olefin (37 mg, 46%) as a mixture of E- and Z-isomers.
1H NMR (500 MHz, CDCl3) δ 0.89 (3H, t, J = 7.2 Hz), 0.94 (3H, t, J = 7.1 Hz), 1.21–1.53 (12H, br m), 1.72–1.88 (3H, m), 1.89–1.94 (1H, m), 1.96–2.07 (3H, m), 2.30–2.41 (1H, m), 2.93–3.00 (1H, m), 3.02–3.16 (1H, m), 3.62 (1H, br), 5.32–5.47 (2H, m).
To a stirred solution of the above olefin (20 mg, 0.08 mmol) in EtOAc (3 mL) was added 10% Pd/C (10 mg), and the resulting suspension was stirred under a hydrogen atmosphere at 1 atm for 40 h. The catalyst was removed by filtration and the filtrate was evaporated to give 7 (R = n-Pr, R’ = n-C7H15, 20 mg, quant) as a pale yellow oil.
IR (neat) 2926, 2869, 1457 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (3H, t, J = 7.1 Hz), 0.94 (3H, t, J = 7.1 Hz), 1.27–1.43 (17H, br m), 1.54 (1H, br), 1.77 (3H, m), 1.97 (3H, m), 2.90 (1H, br), 3.05 (1H, br), 3.57 (1H, br); 13C NMR (75 MHz, CDCl3) δ 14.2 (q), 14.6 (q), 21.6 (t), 22.8 (t), 27.2 (t), 29.4 (t), 29.9 (t), 30.2 (t), 30.9 (t), 31.7 (t), 31.9 (t), 32.1 (t), 33.8 (t), 38.6 (t), 57.7 (d), 63.8 (d), 65.8 (d); MS 251 (M+), 208 (100); HRMS Calcd for C17H33N 251.2613, Found 251.2601; [α]D 26 +36.81 (c0.44, CHCl3).
(3R,5S,8S)-(+)-3-Allyl-5-heptylhexahydropyrrolizine
To a stirred solution of 6 (R = n-C6H13, 91 mg, 0.31 mmol) in CH2Cl2 (7 mL) was added a solution of DIBAL (0.98 M in hexane, 0.35 mL, 0.34 mmol) at −50 °C, and the reaction mixture was stirred at −50 °C for 30 min. The reaction was quenched with MeOH, and then satd. Rochelle (aq) solution, and the organic layer was separated. The aqueous layer was extracted with CH2Cl2 (5 mL×3), and the organic layer and extracts were combined, dried over K2CO3, and evaporated to give a pale yellow oil, that was used directly in the next step.
To a stirred suspension of MeP+Ph3I− (501 mg, 1.24 mmol) in THF (10 mL) was added a solution of n-BuLi (1.6 M in hexane, 0.68 mL, 1.09 mmol) at 0 °C, and the resulting orange suspension was stirred at 0 °C for 10 min. To the suspension was added a solution of the above aldehyde in THF (3 mL) at 0 °C, and the resulting suspension was stirred at room temperature for 27 h. The reaction was quenched with H2O, and the aqueous mixture was extracted with Et2O (15 mL × 4). The organic extracts were combined, dried over K2CO3, and evaporated to give a residue, that was chromatographed on silica gel (20 g, hexane/acetone=20:1−10:1) to give the corresponding olefin (38 mg, 49%) as a pale yellow oil.
IR (neat) 3074, 2953, 2927, 2857, 1465, 909 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.87 (3H, t, J = 7.2 Hz), 1.20–1.41 (14H, br m), 1.50 (1H, m), 1.73–1.83 (3H, m), 1.91 (1H, m), 1.97–2.04 (2H, m), 2.36 (1H, m), 2.95–3.03 (2H, m), 3.57 (1H, m), 4.98 (1H, d, J = 10.2 Hz), 5.02 (1H, d, J = 15.0 Hz), 5.98 (1H, m); 13C NMR (75 MHz, CDCl3) δ 14.2 (q), 22.8 (t), 28.4 (t), 29.3 (t), 30.0 (t), 30.2 (t), 31.0 (t), 31.4 (t), 31.5 (t), 31.6 (t), 31.9 (t), 43.0 (t), 57.1 (d), 64.0 (d), 66.0 (d), 115.8 (t), 136.6 (d); MS 249 (M+), 208 (100); HRMS Calcd for C17H31N 249.2455, Found 249.2473; [α]D 26 +36.81 (c 0.44, CHCl3).
(3S,5S,8R)-(+)-3-Heptyl-5-propylhexahydropyrrolizine (7, R = n-C7H15, R’ = n-Pr)
To a stirred solution of the above olefin (25 mg, 0.10 mmol) in EtOAc (3 mL) was added 10% Pd/C (13 mg), and the resulting suspension was stirred under a hydrogen atmosphere at 1 atm for 44 h. The catalyst was removed by filtration and the filtrate was evaporated to give 7 (R = n-C7H15, R’ = n-Pr, 24 mg, 95%) as a pale yellow oil.
IR (neat) 2966, 2927, 2851, 1458 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.87 (3H, t, J = 7.1 Hz), 0.90 (3H, t, J = 6.9 Hz), 1.40–1.50 (19H, br m), 1.56 (1H, m), 1.79 (2H, m), 1.98 (2H, m), 2.92 (1H, m), 3.08 (1H, br), 3.64 (1H, br); 13C NMR (75 MHz, CDCl3) δ 14.2 (q), 14.4 (q), 20.4 (t), 22.7 (t), 28.3 (t), 29.3 (t), 30.0 (t), 30.1 (t), 30.7 (t), 31.3 (t), 31.7 (t), 31.9 (t), 32.1 (t), 40.4 (t), 57.8 (d), 64.0 (d), 66.0 (d); MS 251 (M+), 208 (100); HRMS Calcd for C17H33N 251.2613, Found 251.2620; [α]D 26 +25.48 (c 0.47, CHCl3).
(5S)-(−)-5-Propyl-2-oxopyrrolidine (8, R = n-Pr)
To a stirred suspension of CuI (3.8 g, 20 mmol) in THF (30 mL) was added a solution of EtMgBr (0.96 M in THF, 42 mL, 40 mmol) at −35 °C, and the resulting suspension was stirred at the same temperature for 30 min. To the suspension was added a solution of 1 (1.8 g, 8 mmol) in THF (15 mL) at −35 °C, and then the reaction mixture was stirred at −35 °C for 18 h. The reaction was quenched with satd. NH4Cl (aq) solution, and the insoluble material was filtered off and washed with CHCl3. The organic layer was separated, and the aqueous layer was extracted with CHCl3 (30 mL × 2). The organic layer and extracts were combined, dried over MgSO4, and evaporated to give a residue, that was chromatographed on silica gel (50 g, hexane/acetone=20:1−2:1) to give 8 (R = n-Pr, 525 mg, 52%) as a pale yellow oil.
IR (neat) 3193, 1699, 1286 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.93 (3H, t, J = 7.2 Hz), 1.36 (2H, m), 1.44 (1H, m), 1.51 (1H, m), 1.69 (1H, m), 2.25 (1H, m), 2.32 (2H, m), 3.63 (1H, quint, J = 6.8 Hz), 6.42 (1H, br); 13C NMR (75 MHz, CDCl3) δ 13.6 (q), 18.7 (t), 26.8 (t), 30.2 (t), 38.6 (t), 54.3 (d), 178.3 (s); MS 127 (M+), 84 (100); HRMS Calcd for C7H13ON 127.0997, Found 127.1001; [α]D 26 −9.20 (c 1.19, CHCl3).
(2S)-(+)-2-Heptyl-5-oxopyrrolidine (8, R = n-C7H15)
To a stirred suspension of CuI (4.52 g, 23.8 mmol) in THF (30 mL) was added a solution of n-C7H15MgBr, prepared from n-C7H15Br (7.34 mL, 52.31 mmol) and Mg (1.26 g, 52.31 mmol) in THF (50 mL) at reflux, at −35 °C, and the resulting suspension was stirred at same temperature for 30 min. To the suspension was added a solution of 1 (2.14 g, 9.51 mmol) in THF (15 mL) at −35 °C, and then the reaction mixture was stirred at −35 °C for 18 h. The reaction was quenched with satd. NH4Cl (aq) solution, and the insoluble material was filtered off and washed with CHCl3. The organic layer was separated, and the aqueous layer was extracted with CHCl3 (30 mL × 2). The organic layer and extracts were combined, dried over MgSO4, and evaporated to give the residue, that was chromatographed on silica gel (50 g, hexane/acetone=20:1−5:1) to give 8 (R = n-C7H15, 870 mg, 50%) as a pale yellow oil.
IR (neat) 3209, 1698, 1284 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (3H, t, J = 7.2 Hz), 1.30 (9H, m), 1.43–1.54 (2H, m), 1.68–1.76 (2H, m), 2.23 (1H, m), 2.33 (2H, m), 3.62 (1H, quint, J = 6.9 Hz), 5.77 (1H, br); 13C NMR (75 MHz, CDCl3) δ 13.84 (q), 22.4 (t), 25.5 (t), 26.9 (t), 28.9 (t), 29.2 (t), 30.3 (t), 31.5 (t), 36.5 (t), 54.6 (d), 178.3 (s); MS 183 (M+), 84 (100); HRMS Calcd for C11H21ON 183.1623, Found 183.1608; [α]D 26 +8.30 (c 0.89, CH2Cl2), Lit. [α]D 26 +9.0 (c 2.0, CH2Cl2).
(2R)-(−)-5-Propyl-2-oxopyrrolidine-1-carboxylic acid tert-butyl ester
To a stirred solution of 8 (R = n-Pr, 247 mg, 1.94 mmol) in MeCN (10 mL) was added DMAP (261 mg, 2.14 mmol) at 0 °C, and the reaction mixture was stirred at 0° C for 30 min. To the reaction mixture was added Boc2O (508 mg, 2.33 mmol) at 0 (C, and then the resulting mixture was stirred at room temperature for 43 h. The solvent was removed under reduced pressure, and the residue was chromatographed on silica gel (20 g, hexane/acetone=30:1−15:1) to give the title Boc-imide (414 mg, 94%) as a pale yellow oil.
IR (neat) 1786, 1749, 1714, 1306, 1153 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.95 (3H, t, J = 7.2 Hz), 1.34 (1H, m), 1.39 (1H, m), 1.52 (9H, s), 1.69–1.78 (2H, m), 2.42 (1H, m), 2.57 (1H, m), 4.10 (1H, m); 13C NMR (75 MHz, CDCl3) δ 13.6 (q), 18.5 (t), 22.1 (t), 27.6 (q), 31.0 (t), 31.5 (t), 35.4 (t), 57.4 (d), 82.0 (s), 149.3 (s), 173.8 (s); MS 227 (M+), 84 (100); HRMS Calcd for C12H21O3N 227.1522, Found 227.1527; [α]D 26 −62.86 (c 0.87, CHCl3).
(2R)-(−)-2-Heptyl-5-oxopyrrolidine-1-carboxylic acid tert-butyl ester
To a stirred solution of 8 (R = n-C7H15, 602 mg, 3.29 mmol) in MeCN (20 mL) was added DMAP (442 mg, 3.62 mmol) at 0 °C, and the reaction mixture was stirred at 0 °C for 30 min. To the reaction mixture was added Boc2O (862 mg, 3.95 mmol) at 0 °C, and then the resulting mixture was stirred at room temperature for 48 h. The solvent was removed under reduced pressure, and the residue was chromatographed on silica gel (40 g, hexane/acetone=30:1−15:1) to give the desired Boc-imide (866 mg, 93%) as a pale yellow oil.
IR (neat) 1788, 1750, 1714, 1308, 1153 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (3H, t, J = 7.2 Hz), 1.28 (10H, m), 1.46 (1H, br), 1.52 (9H, s), 1.76 (2H, m), 2.08 (1H, m), 2.41 (1H, m), 2.53 (1H, m), 4.09 (1H, m); 13C NMR (75 MHz, CDCl3) δ 13.9 (q), 22.3 (t), 22.4 (t), 25.4 (t), 27.8 (q), 29.0 (t), 31.2 (t), 31.5 (t), 33.5 (t), 57.8 (d), 82.2 (s), 149.5 (s), 174.0 (s); MS 283 (M+), 57 (100); HRMS Calcd for C16H29O3N 283.2148, Found 283.2128; [α]D 26 −57.34 (c 1.21, CHCl3).
(1R)-(+)-[1-(3-Oxohept-7-enyl)butyl]carbamic acid tert-butyl ester (9, R = n-Pr)
To a stirred solution of the above Boc-imide (1.08 g, 4.75 mmol) in THF (15 mL) was added a solution of 4-butenylMgBr, prepared from 1-bromo-4-butene (1.45 mL, 14.27 mmol) and Mg (342 mg, 14.27 mmol) in THF (60 mL) at reflux, and TMEDA (2.27 mL, 14.27 mmol) at −78 °C, and the resulting mixture was stirred at the same temperature for 1.5 h. The reaction was quenched with i-PrOH (5 mL), and the reaction mixture was diluted with Et2O. The organic layer was washed with 10% HCl (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, that was chromatographed on silica gel (40 g, hexane/acetone=50:1−40:1) to give 9 (R = n-Pr, 1.28 g, 95%) as a colorless solid (mp 64~66 °C).
IR (KBr) 3349, 3083, 1707, 1685, 1528, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.89 (3H, t, J = 7.2 Hz), 1.26–1.38 (5H, br m), 1.42 (9H, s), 1.50 1H, m), 1.72 (1H, m), 2.30 (2H, q, J = 7.2 Hz), 2.48 (3H, q, J = 7.2 Hz), 3.51 (1H, br), 4.23 (1H, br d, J = 9.1 Hz), 4.94 (1H, d, J = 9.5 Hz), 5.01 (1H, d, J = 15.0 Hz), 5.79 (1H, m); 13C NMR (75 MHz, CDCl3) δ 13.7 (q), 18.9 (t), 27.5 (t), 28.1 (q), 29.0 (t), 37.9 (t), 39.1 (t), 41.6 (t), 49.8 (d), 78.3 (s), 114.7 (t), 136.6 (d), 155.4 (s), 209.2 (s); MS 283 (M+), 57 (100); HRMS Calcd for C16H29O3N 283.2148, Found 283.2142; [α]D 26 +5.10 (c 1.15, CHCl3).
(1R)-(−)-[1-(3- Oxohept-7-enyl)octyl]carbamic acid tert-butyl ester (9, R = n-C7H15)
To a stirred solution of the above Boc-imide (724 mg, 2.56 mmol) in THF (10 mL) was added a solution of 4-butenylMgBr, prepared from 1-bromo-4-butene (0.78 mL, 7.68 mmol) and Mg (185 mg, 7.68 mmol) in THF (40 mL) at reflux, and TMEDA (1.16 mL, 7.68 mmol) at −78 °C, and the resulting mixture was stirred at the same temperature for 1.5 h. The reaction was quenched with i-PrOH (5 mL), and the reaction mixture was diluted with Et2O. The organic layer was washed with 10% HCl (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, that was chromatographed on silica gel (30 g, hexane/acetone=50:1−30:1) to give 9 (R = n-C7H15, 850 mg, 98%) as a colorless solid (mp 48~50 °C).
IR (KBr) 3348, 3080, 1707, 1685, 1531, 1173 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.87 (3H, t, J = 7.2 Hz), 1.25 (12H, br), 1.43 (9H, s), 1.49 (1H, br), 1.77 (1H, m), 2.31 (2H, q-like, J = 7.2 Hz), 2.49 (4H, m), 3.50 (1H, br), 4.20 (1H, br d, J = 9.4 Hz), 4.95 (1H, d, J = 9.6 Hz), 5.01 (1H, d, J = 15.0 Hz), 5.79 (1H, m); 13C NMR (75 MHz, CDCl3) δ 14.0 (q), 22.5 (t), 25.8 (t), 27.6 (t), 28.3 (q), 29.1 (t), 29.2 (t), 29.3 (t), 31.6 (t), 35.9 (t), 39.3 (t), 41.8 (t), 50.3 (d), 78.6 (s), 114.8 (t), 136.8 (d), 155.5 (s), 209.5 (s); MS 339 (M+), 57 (100); HRMS Calcd for C16H28O3N 282.2068, Found 282.2091; [α]D 26 −1.04 (c 1.05, CHCl3).
(1R)-(−)-[1-(3-Oxoheptyl)pent-4-enyl]carbamic acid tert-butyl ester (10)
To a stirred solution of the Boc-imide (239 mg, 1.00 mmol), prepared from 2 (as described in the preparation of 3), in THF (10 mL) was added a solution of n-PrMgBr, prepared from n-BuBr (0.32 mL, 3.00 mmol) and Mg (72 mg, 3.00 mmol) in THF (10 mL) at reflux, and TMEDA (0.48 mL, 3.00 mmol) at −78 °C, and the resulting mixture was stirred at the same temperature for 1.5 h. The reaction was quenched with i-PrOH (5 mL), and the reaction mixture was diluted with Et2O. The organic layer was washed with 10% HCl (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, that was chromatographed on silica gel (20 g, hexane/acetone=50:1−30:1) to give 10 (292 mg, 98%) as a colorless solid (mp 42~43 °C).
IR (KBr) 3351, 3080, 1688, 1530, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (3H, t, J = 7.2 Hz), 1.28 (2H, sext, J = 7.2 Hz), 1.42 (9H, s), 1.47–1.61 (5H, br m), 1.77 (1H, m), 2.08 (2H, q-like, J = 7.2 Hz), 2.38 (2H, t, J = 7.2 Hz), 2.46 (2H, t, J = 7.2 Hz), 3.50 (1H, br), 4.26 (1H, br d, J = 9.1 Hz), 4.94 (1H, d, J = 10.2 Hz), 5.00 (1H, d, J = 15.0 Hz), 5.78 (1H, m); 13C NMR (75 MHz, CDCl3) δ 13.7 (q), 22.1 (t), 25.7 (t), 28.2 (q), 29.0 (t), 30.0 (t), 35.0 (t), 39.1 (t), 42.4 (t), 49.8 (d), 78.4 (s), 114.5 (t), 137.6 (d), 155.4 (s), 210.4 (s); MS 240 (M+-57), 57 (100); HRMS Calcd for C13H22O3N 240.1600, Found 240.1616; [α]D 26 −0.47 (c 1.20, CHCl3).
(2R,5S)-(−)-2-But-3-enyl-5-butylpyrrolidine-1-carboxylic acid tert-butyl ester (11)
To a stirred solution of 10 (279 mg, 0.94 mmol) in CH2Cl2 (7 mL) was added a solution of (C6F5)3B (96 mg, 0.19 mmol) and Ph3SiH (490 mg, 1.88 mmol) in CH2Cl2 (10 mL) at −78 °C, and the reaction mixture was stirred at −78 °C for 30 min, and then at room temperature for 24 h. The reaction was quenched with Et3N (1.0 mL), and the resulting mixture was stirred at room temperature for 20 min. The mixture was diluted with Et2O, and the organic layer was washed successively with 10% AcOH (aq) solution and satd. NaHCO3 (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, that was chromatographed on silica gel (20 g, hexane/acetone=200:1−150:1) to give 11 (194 mg, 73%) as a colorless oil.
IR (neat) 3080, 1696, 1388 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.90 (3H, t, J = 7.2 Hz), 1.24–1.40 (6H, br m), 1.46 (9H, s), 1.61 (2H, br), 1.93 (4H, br m), 2.05 (2H, br m), 3,75 (2H, br), 4.80 (1H, d, J = 10.5 Hz), 5.03 (1H, d, J = 16.0 Hz), 5.82 (1H, m); 13C NMR (75 MHz, CDCl3) δ 14.1 (q), 22.7 (t), 28.5 (q), 28.6 (t), 29.4 (t), 30.7 (t), 35.1 (t), 35.6 (t), 57.8 (d), 58.3 (d), 78.6 (s), 114.2 (t), 138.2 (d), 154.6 (s); MS 281 (M+), 170 (100); HRMS Calcd for C17H31O2N 281.2355, Found 281.2355; [α]D 26 −2.72 (c 1.15, CHCl3).
(2R,5S)-(−)-2-(4-Ethoxycarbonylbut-3-enyl)-5-butylpyrrolidine-1-carboxylic acid tert-butyl ester (12)
To a stirred solution of 11 (155 mg, 0.55 mmol) in CH2Cl2 (10 mL) were added Grubbs 2nd catalyst (19 mg, 0.022 mmol) and ethyl acrylate (0.30 mL, 2.76 mmol), and the resulting mixture was refluxed for 5 h. After cooling, the solvent was removed under reduced pressure, and the residue was chromatographed on silica gel (20 g, hexane/acetone=100:1−60:1) to give 12 (186 mg, 96%) as a pale yellow oil.
IR (neat) 1718, 1696, 1389, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.89 (3H, t, J = 7.2 Hz), 1.20–1.37 (10H, br, including at δ 1.28, 3H, t, J = 7.2 Hz), 1.45 (9H, s), 1.60 (2H, br), 1.91 (3H, br), 2.20 (2H, br), 3.77 (2H, br), 4.17 (2H, q, J = 7.2 Hz), 5.82 (1H, d, J = 15.7 Hz), 6.96 (1H, dt, J = 15.7, 6.8 Hz); 13C NMR (75 MHz, CDCl3) δ 14.0 (q), 14.1 (q), 22.6 (t), 28.4 (q), 28.5 (t), 29.1 (t), 29.4 (t), 34.2 (t), 35.6 (t), 57.6 (d), 58.3 (d), 59.9 (t), 78.8 (s), 121.1 (d), 148.3 (d), 154.6 (s), 166.1 (s); MS 353 (M+), 252 (100); HRMS Calcd for C20H35O4N 353.2564, Found 353.2544; [α]D 26 −2.89 (c 0.75, CHCl3).
(3R,5S,8S)-(+)-(5-Butylhexahydropyrrolizin-3-yl)acetic acid ethyl ester (13)
To a stirred solution of 12 (100 mg, 0.28 mmol) in CH2Cl2 (7 mL) was added AlCl3 (83 mg, 0.62 mmol) at 0 °C, and the resulting suspension was stirred at room temperature for 24 h. The reaction was quenched with satd. NaHCO3 (aq) solution, and the organic layer was separated. The aqueous layer was extracted with CHCl3 (10 mL×5), and the organic layer and extracts were combined, dried over K2CO3, and evaporated to give a residue. To a stirred solution of this residue in CH2Cl2 (7 mL) was added K2CO3 (78 mg, 0.57 mmol), and the resulting suspension was stirred at room temperature for 48 h. The insoluble material was filtered off, and washed with CH2Cl2. The filtrate was evaporated to afford a residue, that was chromatographed on silica gel (20 g, hexane/acetone=20:1−8:1) to give 13 (66 mg, 92%) as a pale yellow oil.
IR (neat) 1733, 1181 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.90 (3H, t, J = 7.2 Hz), 1.26 (3H, t, J = 7.2 Hz), 1.28–1.43 (7H, br m), 1.52 (1H, m), 1.69 (1H, br), 1.78 (3H, m), 2.28 (1H, dd, J = 15.0, 9.8 Hz), 2.57 (1H, dd, J = 15.0, 4.3 Hz), 3.00 (1H, br), 3.40 (1H, m), 3.57 (1H, br), 4.11 (2H, q, J = 7.2 Hz); 13C NMR (75 MHz, CDCl3) δ 14.1 (q), 14.2 (q), 22.9 (t), 30.2 (t), 30.4 (t), 30.8 (t), 31.0 (t), 31.3 (t), 32.4 (t), 43.2 (t), 54.2 (d), 60.0 (t), 63.7 (d), 65.6 (d), 172.1 (s); MS 253 (M+), 196 (100); HRMS Calcd for C15H27O2N 253.2042, Found 253.2058; [α]D 26 +21.06 (c 0.83, CHCl3).
(3S,5S,8S)-(+)-3,5-Dibutylhexahydropyrrolizine (14)
To a stirred solution of 13 (70 mg, 0.28 mmol) in CH2Cl2 (7 mL) was added a solution of DIBAL (0.98 M in hexane, 0.31 mL, 0.31 mmol) at −50 °C, and the reaction mixture was stirred at −50 °C for 30 min. The reaction was quenched with MeOH, and satd. Rochelle (aq) solution, and the organic layer was separated. The aqueous layer was extracted with CH2Cl2 (5 mL × 3), and the organic layer and extracts were combined, dried over K2CO3, and evaporated to give a pale yellow oil, that was used directly in the next step.
To a stirred suspension of EtP+Ph3Br− (410 mg, 1.12 mmol) in THF (7 mL) was added a solution of n-BuLi (1.6 M in hexane, 0.6 mL, 0.98 mmol) at 0 °C, and the resulting orange suspension was stirred at 0 °C for 10 min. To the suspension was added a solution of the above aldehyde in THF (3 mL) at 0 °C, and the resulting suspension was stirred at room temperature for 27 h. The reaction was quenched with H2O, and the aqueous mixture was extracted with Et2O (15 mL × 4). The organic extracts were combined, dried over K2CO3, and evaporated to give a residue, that was chromatographed on silica gel (20 g, hexane/acetone=25:1−10:1) to give the corresponding olefin (31 mg, 50%) as a mixture of E- and Z-isomers.
1H NMR (500 MHz, CDCl3) δ 0.90 (3H, t, J = 6.8 Hz), 1.24–1.41 (8H, br m), 1.48 (1H, m), 1.62 (3H, d, J = 6.8 Hz), 1.72–1.93 (4H, br m), 1.95–2.08 (2H, m), 2.31 (1H, br), 2.96 (1H, m), 3.04 (1H, br), 3.60 (1H, br), 5.36–5.51 (2H, br m).
To a stirred solution of the above olefin (20 mg, 0.09 mmol) in EtOAc (3 mL) was added 10% Pd/C (10 mg), and the resulting suspension was stirred under a hydrogen atmosphere at 1 atm for 45 h. The catalyst was removed by filtration and the filtrate was evaporated to give 14 (20 mg, quant) as a pale yellow oil.
IR (neat) 2928, 2858, 1457, 1099 cm−1; 1H NMR (300 MHz, CDCl3) δ 0.89 (3H, t, J = 7.2 Hz), 0.90 (3H, t, J = 6.8 Hz), 1.20–1.51 (15H, br m), 1.60 (1H, m), 1.81 (2H, m), 1.98 (2H, m), 2.91 (1H, m), 3.10 (1H, br), 3.68 (1H, br); 13C NMR (75 MHz, CDCl3) δ 14.1 (q), 14.2 (q), 22.9 (t), 23.0 (t), 29.4 (t), 30.1 (t), 30.4 (t), 30.7 (t), 30.9 (t), 31.6 (t), 32.1 (t), 37.6 (t), 58.2 (d), 64.0 (d), 66.0 (d); MS 223 (M+), 55 (100); HRMS Calcd for C15H29N 223.2300, Found 223.2312; [α]D 26 +29.77 (c 0.40, CHCl3).
(1R)-(+)-[1-(3-Oxohept-7-enyl)pentyl]carbamic acid tert-butyl ester (15)
To a stirred solution of 28(337 mg, 2.42 mmol) in MeCN (20 mL) was added DMAP (326 mg, 2.67 mmol) at 0 °C, and the reaction mixture was stirred at 0 °C for 30 min. To the reaction mixture was added Boc2O (633 mg, 2.90 mmol) at 0 °C, and the resulting mixture was stirred at room temperature for 45 h. The volatiles were evaporated, and the residue was chromatographed on silica gel (20 g, hexane/acetone=15:1) to give the Boc-imide (550 mg, 95%) as a colorless oil.
IR (neat) 3078, 1785, 1750, 1714, 1308, 1153 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.52 (9H, s), 1.59 (1H, m), 1.78 (1H, m), 1.90 (1H, m), 2.03–2.19 (3H, br m), 2.43 (1H, ddd, J = 9.0, 8.5, 2.1 Hz), 2.57 (1H, dd, J = 9.0, 8.5 Hz), 4.12 (1H, m), 5.01 (1H, d, J = 10.7 Hz), 5.04 (1H, d, J = 15.0 Hz), 5.80 (1H, m); 13C NMR (75 MHz, CDCl3) δ 21.9 (t), 27.7 (q), 29.6 (t), 31.0 (t), 32.4 (t), 57.1 (d), 82.2 (s), 115.0 (t), 136.7 (d), 149.3 (s), 173.7 (s); MS 182 (M+-57), 84 (100); HRMS Calcd for C13H21O3N 239.1520, Found 239.1534; [α]D 26 −56.68 (c 2.34, CHCl3).
To a stirred solution of the above Boc-imide (239 mg, 1 mmol) in EtOAc (10 mL) was added 10% Pd/C (50 mg), and the resulting suspension was stirred under a hydrogen atmosphere at 1 atm for 48 h. The catalyst was removed by filtration and the filtrate was evaporated to give the corresponding imide as a pale yellow oil, that was used directly in the next step.
To a stirred solution of the imide prepared above in THF (5 mL) was added a solution of 4-butenylMgBr, prepared from 4-bromo-1-butene (0.30 mL, 3.00 mmol) and Mg (72 mg, 3.00 mmol) in THF (8 mL) at reflux, and TMEDA (0.48 mL, 3.00 mmol) at −78 °C, and the resulting mixture was stirred at the same temperature for 1.5 h. The reaction was quenched with i-PrOH (5 mL), and the reaction mixture was diluted with Et2O. The organic layer was washed with 10% HCl (aq) solution, dried over MgSO4, and evaporated to give a pale yellow oil, that was chromatographed on silica gel (20 g, hexane/acetone=50:1−30:1) to give 15 (284 mg, 96%) as a colorless solid (mp 38~39 °C).
IR (KBr) 3355, 3070, 1709, 1685, 1530, 1174 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.88 (3H, t, J = 7.2 Hz), 1.30 (6H, br m), 1.43 (9H, s), 1.51 (1H, br), 1.78 (1H, m), 2.31 (2H, q-like, J = 6.9 Hz), 2.49 (4H, m), 3.50 (1H, br), 4.21 (1H, br d, J = 9.0 Hz), 4.96 (1H, d, J = 10.2 Hz), 5.00 (1H, d, J = 16.0 Hz), 5.82 (1H, m); 13C NMR (75 MHz, CDCl3) δ 13.6 (q), 22.2 (t), 27.3 (t), 27.7 (t), 28.0 (q), 28.9 (t), 35.2 (t), 39.0 (t), 41.4 (t), 49.9 (d), 78.0 (s), 114.5 (t), 136.5 (d), 155.3 (s), 209.0 (s); MS 297 (M+), 57 (100); HRMS Calcd for C17H31O3N 297.2304, Found 297.2315; [α]D 26 +2.76 (c 1.04, CHCl3).
Supplementary Material
Acknowledgment
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science. Work at NIH was supported by intramural funds of NIDDK.
Footnotes
Supporting Information Available
Experimental details for compounds ent-4, -5, -6, -7, -11, -12, -13, and -14, characterization data for all new synthetic compounds, including 1H and 13C NMR spectra, the GC-MS of coinjection data, and FTIR spectra of synthetic stereoisomers of 251O and natural 251O are available free of charge via the internet at http://pubs.acs.org.
REFERENCES and NOTES
- 1.(a) Daly JW. J. Med. Chem. 2003;46:445–452. doi: 10.1021/jm0204845. [DOI] [PubMed] [Google Scholar]; (b) Daly JW, Spande TF, Garraffo HM. J. Nat. Prod. 2005;68:1556–1575. doi: 10.1021/np0580560. [DOI] [PubMed] [Google Scholar]
- 2.(a) Tsuneki H, You Y, Toyooka N, Kagawa S, Kobayashi S, Sasaoka T, Nemoto H, Kimura I, Dani JA. Mol. Pharmacol. 2004;66:1061–1069. doi: 10.1124/mol.104.000729. [DOI] [PubMed] [Google Scholar]; (b) Toyooka N, Kobayashi S, Zhou D, Tsuneki H, Wada T, Sakai H, Nemoto H, Sasaoka T, Garraffo HM, Spande TF, Daly JW. Bioorg. Med. Chem. Lett. 2007;17:5872–5875. doi: 10.1016/j.bmcl.2007.08.045. [DOI] [PubMed] [Google Scholar]; (c) Kobayashi S, Toyooka N, Zhou D, Tsuneki H, Wada T, Sasaoka T, Sakai H, Nemoto H, Garraffo HM, Spande TF, Daly JW. Beilstein J. Org. Chem. 2007;3:30. doi: 10.1186/1860-5397-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Toyooka N, Zhou D, Kobayashi S, Tsuneki H, Wada T, Sakai H, Nemoto H, Sasaoka T, Tezuka Y, Subehan, Kadota S, Garraffo HM, Spande TF, Daly JW. Synlett. 2008:61–64. [Google Scholar]
- 3.(a) Dumbacher JP, Beehler BM, Spande TF, Garraffo HM, Daly JW. Science. 1992;258:799–801. doi: 10.1126/science.1439786. [DOI] [PubMed] [Google Scholar]; (b) Dumbacher JP, Spande TF, Daly JW. Proc. Natl. Acad. Sci. USA. 2000;97:12970–12975. doi: 10.1073/pnas.200346897. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dumbacher JP, Wako A, Derrickson SR, Samuelson A, Spande TF, Daly JW. Proc. Natl. Acad. Sci. USA. 2004;101:15857–15860. doi: 10.1073/pnas.0407197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark VC, Raxworthy CJ, Rakotomalala V, Sierwald P, Fisher BL. Proc. Natl. Acad. Sci. USA. 2005;102:11617–11622. doi: 10.1073/pnas.0503502102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Takano S, Otaki S, Ogasawara K. J. Chem. Soc. Chem. Commun. 1983:1172–1174. [Google Scholar]; (b) Arseniyadis S, Huang PQ, Husson HP. Tetrahedron Lett. 1988;29:1391–1394. [Google Scholar]; (c) Takahata H, Bandoh H, Momose T. Tetrahedron: Asymmetry. 1991;2:351–352. [Google Scholar]; (d) Takahata H, Bandoh H, Momose T. J. Org. Chem. 1992;57:4401–4404. [Google Scholar]; (e) Grandjean C, Rosset S, Celerier JP, Lhommet G. Tetrahedron Lett. 1993;34:4517–4518. [Google Scholar]; (f) Oppolzer W, Bochet CG, Merifield E. Tetrahedron Lett. 1994;35:7015–7018. [Google Scholar]; (g) Cuny GD, Buchwald SL. Synlett. 1995:519–522. [Google Scholar]; (h) Dhimane H, Vanucci-Bacque C, Hamon L, Lhommet G. Eur. J. Org. Chem. 1998:1955–1963. [Google Scholar]; (i) Arredondo VM, Tian S, McDonald FE, Marks TJ. J. Am. Chem. Soc. 1999;121:3633–3639. [Google Scholar]; (j) Takahata H, Takahashi S, Azer N, Eldefrawi AT, Eldefrawi ME. Bioorg. Med. Chem. Lett. 2000;10:1293–1295. doi: 10.1016/s0960-894x(00)00221-3. [DOI] [PubMed] [Google Scholar]
- 6.(a) Toyooka N, Zhou D, Nemoto H. J. Org. Chem. 2008;73:4575–4577. doi: 10.1021/jo800593n. [DOI] [PubMed] [Google Scholar]; (b) Toyooka N, Zhou D, Nemoto H, Tezuka Y, Kadota S, Jones TH, Garraffo HM, Spande TF, Daly JW. Synlett. 2008:1894–1896. [Google Scholar]; (c) Toyooka N, Tsuneki H, Kobayashi S, Zhou D, Kawasaki M, Kimura I, Sasaoka T, Nemoto Curr. Chem. Biol. 2007;1:97–114. [Google Scholar]; (d) Toyooka N, Tsuneki H, Nemoto H. Yuki Gosei Kagaku Kyokaishi. 2006;64:49–60. [Google Scholar]; (e) Toyooka N, Nemoto H. In: New Methods for the Asymmetric Synthesis of Nitrogen Heterocycles. Vicario JL, editor. India: Research Signpost; 2005. pp. 149–163. [Google Scholar]; (f) Toyooka N, Nemoto H. In: Recent Research Developments in Organic Chemistry. Pandalai SG, editor. Vol. 6. India: TRANSWORLD RESEARCH NETWORK; 2002. pp. 611–624. [Google Scholar]
- 7.Kamimura A, Nagata Y, Kadowaki A, Uchida K, Uno H. Tetrahedron. 2007;63:11856–11861. [Google Scholar]
- 8.Hjelmgaard T, Søtofte I, Tanner D. J. Org. Chem. 2005;70:5688–5697. doi: 10.1021/jo0506682. [DOI] [PubMed] [Google Scholar]
- 9.Brenneman JB, Machauer R, Martin SF. Tetrahedron. 2004;60:7301–7314. [Google Scholar]
- 10.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 11.Exposure of the stereoisomer at the 3-position (all cis-pyrrolizidine) of 6 (R = Et) to the same reaction conditions (K2CO3 in CH2Cl2 at room temperature for 48 h) recovered the starting material, and no formation of 6 (R = Et) was observed.
- 12.Garraffo HM, Spande TF, Daly JW, Baldessari A, Gros EG. J. Nat. Prod. 1993;56:357–373. doi: 10.1021/np50093a008. [DOI] [PubMed] [Google Scholar]
- 13.No separation of a mixture of 7 and ent-7 (ca. 1:1) was observed with either of two beta cyclodextrin chiral columns. One column was a 30 m × 0.25 mm i.d. (0.25 um film thickness) beta Dex 120 column (Supelco); the other column was a 25 m × 0.22 mm i.d. (0.25 um film thickness) permethylated beta cyclodextrin column (SGE). Both were operated at a head pressure of 20 psi and used a gas chromatograph with flame ionization detection. A 3390A recorder integrator was used and a temperature program of 100 °C to 150 °C at 1 or 2 °C/min. A separation of a mixture of 14 and ent-14 was not attempted.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.