Abstract
Background
Zebrafish is becoming an increasingly attractive model organism for understanding biology and developing therapeutics, because as a vertebrate, it shares considerable similarity with mammals in both genetic compositions and tissue/organ structures, and yet remains accessible to high throughput phenotype-based genetic and small molecule compound screening.
Objective/method
The focus of this review is on the nervous system, which is arguably the most complex organ and known to be afflicted by more than six hundred disorders in humans. I discuss the past, present, and future of using zebrafish to assess the impact of small molecule drugs on neural development and function, in light of understanding and treating neurodevelopmental disorders such as autism, neurodegenerative disorders including Alzheimer’s, Parkinson’s, and Hungtington’s disease, and neural system dysfunctions such as anxiety/depression and addiction.
Conclusion
These studies hold promise to reveal fundamental mechanisms governing nervous system development and function, and to facilitate small molecule drug discovery for the many types of neurological disorders.
Keywords: zebrafish, neural development, neurodegeneration, function, small molecule drug discovery, neurological disorders, autism, Parkinson’s disease, Alzheimer’s disease, Hungtinton’s disease, anxiety/depression, addiction
1. Introduction
Structural similarity of the nervous system emerges at the level of vertebrates, with the central nervous system (CNS) derived from a sheet of dorsally located neuroectoderm that is regionally patterned into the forebrain, midbrain, hindbrain, and spinal cord, while the peripheral nervous system (PNS) is derived from the migratory neural crest cells. During its formation and maturation, the nervous system forms intricate connections within itself and to most if not all tissues and organs in the body, and functions to regulate complex behaviors and vital activities 1. Given such complexity and importance, it is not surprising that more than 600 human disorders afflict the nervous system (sources from National Institute of Health, http://www.ninds.nih.gov/about_ninds/ninds_overview.html). They range from those that affect neural development (e.g. mental retardation, autism, schizophrenia), to those that disrupt neural maintenance (e.g. Alzheimer’s disease, Parkinson’s disease) and neural function (e.g. anxiety/depression, and addictive disorders). Neurological disorders strike an estimated 50 million Americans each year, placing immense burdens to the individuals and family involved, with an annual economic cost of hundreds of billions of dollars in medical expenses and lost productivity (sources from National Institute of Health, http://www.ninds.nih.gov/about_ninds/ninds_overview.html).
Treating neurological disorders is a tremendous challenge, because the CNS is largely inaccessible to intravenously delivered cell- or protein-based therapeutic agents. Small molecule drugs that can penetrate the blood-brain barrier are therefore particularly attractive candidates for treating CNS disorders. Most of the currently available small molecule drugs that alleviate neurological symptoms are discovered in serendipitous ways, and many of them do not represent ideal treatments, either due to inadequate efficacy or troubling side effects. Furthermore, for many neurological disorders, treatments are simply not available. These situations highlight the urgent need to find novel and systematic approaches to facilitate CNS drug discovery.
This review aims to evaluate the feasibility of zebrafish as a system for discovering and studying the impact of drugs on the nervous system and related neurological disorders. First, the use of zebrafish for chemical genetics/genomics and small molecule discovery is briefly reviewed. Second, our current knowledge of the zebrafish nervous system is examined in comparison to that of mammals, at both developmental and adult stages, and in terms of its response to known drugs. Third, neurological disorders, including autism, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, anxiety/depression, and addictive disorders, and overall strategies to model them in zebrafish, are presented. Finally, how these models can be used in small molecule drug discovery is discussed.
2. Use of zebrafish in chemical genetics/genomics and small molecule drug discovery
Chemical genetic approaches have been successfully employed in transparent embryonic and larval zebrafish to probe interesting biological processes as well as to catalyze the discovery of potential therapeutic compounds. Thus far, most studies have been conducted outside of the nervous system, and have been reviewed in detail elsewhere 2–4. Here we take an overview of a few examples and issues generally related to screening and chemical compounds. For instance, small molecules have been identified that suppress mutation-caused cardiovascular defect 5 or cell cycle arrest 6, and those that modulate the embryonic heart rate in wild type 7. In addition, many drugs with known effects in humans have been shown to cause similar effects in zebrafish 8,9. Moreover, a recent study has identified a potent small molecule, prostaglandin E2, through zebrafish-based small molecule screening for chemical regulators of haematopoietic stem cell (HSC) homeostasis. Remarkably, this small molecule also performs similar action in mammalian HSCs, thus validating that zebrafish-based drug discovery can potentially lead to therapeutic compounds for human conditions 10.
Because of the small size of embryonic and larval zebrafish, small molecule compound screens can often be carried out in a high throughput way using 96-well plates. Many small molecule libraries are available for screening. Some contain a collected set of compounds (both natural and synthetic), such as those available from Microsource Discovery systems, whereas others contain compounds synthesized through combinatorial chemistry, such as those available from ChemDiv and Chembridge 2.
3. The zebrafish nervous system: similarities and differences to that of mammals
In order to understand or discover the effects of drugs on the nervous system using zebrafish, it is important to know the organization and overall similarity between zebrafish and mammalian nervous systems. Our understanding of the zebrafish nervous system is still rudimentary, with most of our knowledge gathered on the first 48 hours of the organism’s life. In this section, I provide an overview of the zebrafish nervous system, by discussing the emergence of brain subdivisions and differentiated neural subtypes, the connectivity and systems-level organization in the adult brain, molecular architecture of receptors and signaling molecules expressed in the nervous system, and finally, the effects of known neuro-active drugs on zebrafish. Both the similarities and the differences between zebrafish and mammals are highlighted.
3.1 The emergence of brain subdivisions and differentiated neural subtypes during development
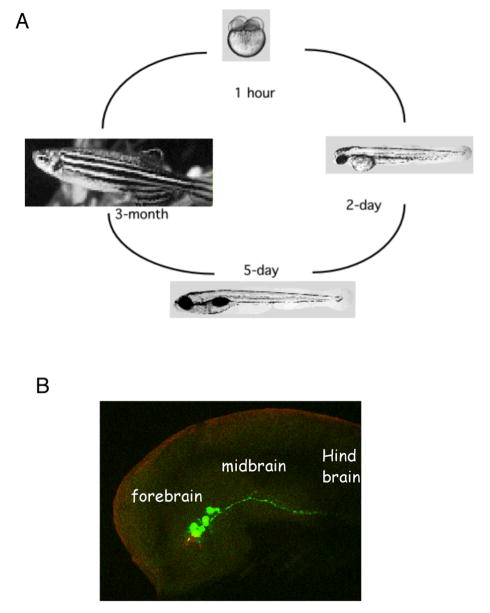
Zebrafish embryos are transparent and develop externally, thereby allowing the entire embryogenesis to be observed with ease (Figure 1). By the end of gastrulation (~10 hours post fertilization, -hpf), the neural plate becomes distinguishable from the rest of the ectoderm, and subsequently forms the neural tube. By 1 day post fertilization (dpf), major brain subdivisions that include the forebrain, midbrain, hindbrain, and spinal cord have formed 11,12. Concomitant with the emergence of brain subdivisions, the earliest clusters of neurons, namely primary neurons, appear in the center of each subdivision, and extend axonal tracts in a discrete and stereotyped pattern 11. These early axonal tracts may serve as a simple scaffold for the axogenesis of later born neurons. By 2–3 dpf, many different neurotransmitter-expressing neurons can be identified. These include the GABAergic 13–15, glutamatergic 13, monoaminergic (dopamine, noradrenaline, serotonin, histamine)16–20, cholinergic 21, and peptidergic 22,23 neurons. Glial subtypes (oligodendrocytes, schwann cells, and astrocytes) are detected beginning at ~4 dpf 24,25.
Figure 1.
The zebrafish life cycle (A) and the developing brain (B, lateral view of ~ 1-day old embryonic head region with the dorsal side up), with the visualization of dopaminergic (anti-TH, green) and serotonergic (anti-serotonin, red) neurons. Images in (A) are form ZFIN.org. Dopaminergic and serotonergic neurons are visualized through antibody labeling of tyrosine hydroxylase and 5HT respectively.
The gross anatomical organization of the CNS and PNS is relatively conserved between zebrafish and mammals. For instance, the counterparts of many brain subdivisions found in the developing mammalian brain are morphologically identifiable in the teleost (zebrafish) brain 26,27. Analyses of gene expression further reveal that many developmentally expressed genes, ranging from pre-pattern to proneural regulators, are orchestrated in a conserved pattern in these brain subdivisions 28–30. Despite these similarities, a clear difference has been observed between the development of mammalian and zebrafish telencephalic hemispheres, with evagination happening in mammals versus eversion occurring in teleosts including zebrafish 31,32. Such difference in the process of telencephalic formation has hampered the recognition and comparative interpretation of zebrafish telencephalic organization. For example, although recent comparative neuroanatomical analyses have postulated the homologous structures in zebrafish to the mammalian cortex, hippocampus, amygdaloid complex, and basal ganglia 15, It remains to be elucidated their neuroanatomical delineations, molecular architecture, functional correlates, and developmental origins.
With regard to differentiated neural subtypes, an astonishing degree of correspondences between GABA cell populations in zebrafish and mice is observed during a critical developmental time window (2–3 dpf in zebrafish versus E12.5-13.5 in mice) 14,15. The development of monoaminergic and peptidergic neurons also follows similar spatial and temporal programs in zebrafish and in mammals. The dopaminergic neurons are found in the retina, olfactory bulb, ventral forebrain, and ventral midbrain in mammals, whereas in zebrafish, they are detected in the retina, olfactory bulb, and ventral forebrain only 16,33. Interestingly, despite the conspicuous absence of dopaminergic neurons in the zebrafish ventral midbrain, groups of dopaminergic neurons located in the diencephalic posterior tubercular area are found to project into the telencephalon, resembling the mammalian mesostriatal and mesolimbic pathways 34. In contrast to the conserved features observed in most parts of the brain including the ventral telencephalon, the development of the teleostean dorsal telencephalon (pallium) appears to be quite different with regard to the region homologous to the mammalian cerebral cortex, which has a highly organized, laminated pattern 30. Although the layered organization of the cortex is known to be a mammalian adaptation 35, it remains to be determined whether major cortical neuronal subtypes identified in the mammalian cortex are present in zebrafish or not.
3.2. Connectivity and circuitry in the adult nervous system
Because of the scarcity of connectivity information in zebrafish, data from the closely related goldfish is used as a reference 36. Hodological studies generally have demonstrated great similarities of teleostean sensory, motor, and integrative central neural circuits in comparison with other vertebrates including mammals. The chain of synaptic relay of teleostean sensory systems ascending to the telencephalon is largely comparable to that in other vertebrates, albeit some differences do exist. For instance, there are two main diencephalic sources of sensory inputs to the teleostean telencephalon: the (periventricular) dorsal thalamus and the preglomerular region. Whereas the dorsal thalamus is the major source for sensory input to the pallial telencephalon in mammals, the preglomerular region predominantly subserves this role in teleosts, and the presence of both sources in teleosts may represent the ancestral vertebrate pattern 37.
3.3. Molecular architecture of the zebrafish nervous system
In addition to neuroanatomical similarities, the zebrafish nervous system also expresses many signaling molecules that show similarities to that of mammals. These include major secreted proteins and their signal transduction pathways such as Sonic Hedgehog, WNTs, BMPs, and FGFs, the neurotransmitter/neuropeptide receptors and transporters including those of GABA, dopamine, glutamate, serotonin, NPY, and opioids 38–42.
3.4. Effects of known drugs on neural development and function in zebrafish
Zebrafish, particularly larval zebrafish, have been used in toxicological studies to analyze the effects of neurotoxins and neuroprotectants on the developing nervous system 43–46. For example, 7 compounds that are well-characterized for their action in mammals have been tested on embryonic and larval zebrafish to examine several parameters of neurotoxicity during development, including teratogenicity, cell death, and selected neuronal subtypes 47. This study finds that Atrazine, dichlorodiphenyltrichloroethane (DDT), and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are primarily teratogenic and not specifically neurotoxic. 2,4-dichlorophenoxyacetic acid (2,4-D), dieldrin, and nonylphenol show specific neurotoxicity; dieldrin and nonylphenol are specifically toxic to catecholaminergic neurons. Malathion, although not teratogenic, shows some nonspecific toxicity. Thus, results from zebrafish show a strong correlation with mammalian data.
Effects of a number of psychoactive compounds have been evaluated in zebrafish. At physiologically relevant concentrations, ethanol has been found to affect locomotor activity, very much like its effect in mammals 48,49. Cocaine 50,51, amphetamine 52, morphine 53,54, and nicotine 55 have been found to produce reward- or anxiety-related effects in zebrafish. The known teratogenic effects of some of these compounds also prompt evaluation of their effects on embryonic development. Indeed, both ethanol 56 and nicotine 57 have been shown to affect nervous system development.
Pharmacological agents that target various neurotransmitter/neuropeptide receptors or transporters have been used to treat either larval or adult zebrafish. Many have predicted effects based on their action on the mammalian nervous system. These include agents that affect the receptors for GABA, glutamate, dopamine, serotonin, acetylcholine, and opioid systems (Table 1).
Table 1.
Agonists and antagonists of various neurotransmitter- or neuropeptide-systems that have predicted effects in zebrafish
| Category | Compound name | Known action in mammals | Observed effects in zebrafish |
|---|---|---|---|
| Dopamine | Bupropion | dopamine reuptake transporter blocker | abolished spontaneous fictive swim episodes at 3 dpf 125 |
| L741,626; sulpiride | D2 receptor-specific antagonists | increased the frequency of swim episodes at 3 dpf 125 reduce morphine preference in adult zebrafish 53 |
|
| Apomorphine | Dopamine receptor agonist | suppressed prepulse inhibition of startle in larval zebrafish 126 | |
| Haloperidol | Dopamine receptor antagonist | attenuated the apomorphine induced deficit of PPI 126 | |
| SCH 23390 | D1 receptor-specific antagonist | reduce morphine preference in larval and adult zebrafish 53,54 | |
| SKF38393 | D1 receptor-selective agonist | Decrease K+ current recorded from isolated ON bipolar cells 127 | |
| Serotonin | Fluoxetine (Prozac) | selective serotonin reuptake inhibitor | Causes a transient decrease in spontaneous swimming activity 128 |
| Quipazine | 5-HT receptor agonist | Increase motor output in larval zebrafish129 | |
| Methysergide Ketanserin |
5-HT receptor antagonist | Decrease motor output in larval zebrafish129 | |
| Glutamate | Ketamine AP5 |
NMDA receptor antagonist | Dose dependent effect on PPI 126 Causes complex changes of individual mitral cell responses and spatio-temporal activity patterns 130 |
| NBQX | AMPA/Kainate receptor antagonist | Produces diverse effects on mitral cell activity in an explant of intact brain and nose 130 | |
| GYKI 52466 | AMPAR antagonist | Prevents AMPA-induced increase of acetylcholine release at NMJ 131 | |
| Sym 2801 | KAR antagonist | Prevents KA-induced increase of acetylcholine release at NMJ 131 | |
| GABA | Baclofen | GABAB receptor selective agonist | Attenuates Ca2+ influx in ORN axon terminals during an odor response 132 |
| CGP54626 | GABAB receptor selective antagonist | Increases Ca2+ influx in ORN axon terminals during an odor response 132 | |
| Gabazine | GABAA receptor selective antagonist | Reduces the topological reorganization and the decorrelation of MC activity patterns during an odor response 132 | |
| Diazepam | GABA receptor agonist | Reduces larval locomotor activity 133 | |
| Opioids | Naloxone | Opioid receptor antagonist | Reduce morphine preference in larval and adult zebrafish 53,54 |
In summary, our current knowledge of the teleost fish nervous system suggests that although some differences exist, remarkable similarities with that of mammals have been detected, making it convenient to extrapolate the findings made in zebrafish to mammals. From the standpoint of drug discovery, small molecules that are bioactive in targeting zebrafish gene products are likely to have a similar action on the mammalian orthologues. Given the feasibility of zebrafish for high throughput screening that is much more difficult to carry out in mammalian organisms, it is fair to say that zebrafish offers an excellent opportunity for CNS drug discovery efforts. However, in order to seize upon this opportunity, it is critical to establish relevant models and screening assays in this organism.
4. Strategies for modeling human neurological disorders and identifying neuro-active compounds in zebrafish
In general, several alternative strategies can be employed in animal systems to model human diseases: for the disorders with defined molecular lesions, similar molecular lesions can be created in animal models; for those with defined cellular lesions, similar cellular lesions might be introduced in animal models. The overall similarity of the zebrafish nervous system to that of mammals and the particular amenability of zebrafish for candidate drug evaluation and high throughput drug screening suggests that it is a potentially fruitful system for modeling neurological disorders and moreover for subsequent drug discovery. The available molecular genetic technologies permit inactivation 58–62 or overexpression 63–65 of genes implicated in the human disorders. Tools to introduce defined cellular lesions in specific cell or tissue types 66,67 have also been established in this organism. Because of the scope of this review, the readers are referred to the reviews cited above for details regarding these technologies.
One should also keep in mind, however, while determining the validity of any animal models, that it is impossible to recapitulate all the phenotypes of human disorders in any single given animal model. In this section, I highlight several human neurological disorders including autism spectrum disorders, neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and Hungtinton’s disease, and neuropsychiatric disorders such as anxiety/depression and addiction, and discuss current efforts toward creating zebrafish models for these disorders.
4.1 Autism Spectrum Disorders
Among the different kinds of neurodevelopmental disorders, here we focus on autism spectrum disorders (ASDs). A meta-analysis of ASD prevalence rates suggests that approximately 37 in 10,000 individuals are affected, and currently 1 in 150 children is said to be diagnosed with autism 68. Autism is characterized by three distinctive behaviors: difficulties with social interaction, problems with verbal and nonverbal communication, and repetitive behaviors or narrow, obsessive interests. These behaviors can range in impact from mild to disabling. It is unclear what causes autism, and there is no clear and consistent pathology that has emerged for autism, although multiple regions of the brain including the cerebral cortex, amygdala, brainstem, and cerebellum are involved, and developmental processes including progenitor proliferation, programmed cell death, neuronal migration, axodendritic growth and pruning, synaptogenesis and synaptic transmission may be altered in ASD patients. 69,70. Due to their less ambiguous association with ASDs and interesting biological properties, several types of genetic lesions are worth particular mentioning. 1) The gene encoding methyl-CpG-binding protein 2 (MeCP2), a transcriptional regulator widely expressed throughout the brain, is disrupted in the Rett Syndrome (RTT), a devastating X-linked ASD disorder that affects 1 in 10,000 females 71. Patients with classic RTT suffer from a broad array of phenotypes that affect almost every part of the central and autonomic nervous systems, including impaired social behavior and communication skills, motor abnormalities, and the development of stereotyped movements. Another transcription regulator, Engrailed 2 (EN2), is also associated with ASDs 72,73. 2) Mutations in the fragile X mental retardation (FMR1) gene, which encodes a translational repressor, cause an X-linked form of mental retardation with ~15–30% prevalence of ASD 74. Mutations in Tuberous sclerosis complex (TSC) 75 and the PTEN phosphatase 76 genes, both of which are involved in translational regulation, have also been associated with ASDs, suggesting that defects in translational regulation represent one potential mechanism underlying the development of ASDs. 3) Analyses of de novo chromosomal deletions and duplications have hinted at the involvement of synaptic molecules including neuroligins 3 and 4 (NLGN3 and NLGN4) 77, SHANK3 (a cytoplasmic binding partner of neuroligins) 78, neurexin (NRXN1) 79, contactin associated protein-like 2 (CNTNAP2, a member of neurexin superfamily) 80,81, and CACNA1C (L-type voltage-gated calcium channel) 82. 4) RELN, an extra-cellular matrix protein involved in cell adhesion and migration, is associated with ASD 83. While available therapies and behavioral interventions are designed to remedy specific symptoms, there is currently no cure for autism.
Given the likely developmental origins of ASDs and the suitability of zebrafish for developmental studies, surprisingly little has been done with respect to modeling ASDs in zebrafish. A review discussing the potential of the system has been published 84. This may be in part due to the complex behavioral abnormalities associated with ASDs that include deficits in language, social interaction, and breadth of interests. Deficits in language and breadth of interests are indeed difficult to model perhaps in any animal systems. However, the observed developmental and cellular defects as well as defects in social interaction can certainly be modeled in zebrafish. Indeed, zebrafish homologues of genes implicated in ASDs, including neurexins 85, reelin 35, mecp2 86, and met 87, have been identified. Assays that measure social interaction have been developed 88–90. Thus, it will be interesting to assess whether lesions in ASD-associated genes lead to developmental and cellular defects as well as defects in social interaction similar to what has been observed in mammals. Uncovering developmental and cellular defects could lead to the establishment of potential screening assays, which can be used to identify small molecule compounds that can modify such phenotypes.
4.2 Neurodegenerative disorders: Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease
Parkinson’s disease
Parkinson’s disease (PD) is the most common movement disorder, manifested with four primary symptoms including tremor, rigidity, bradykinesia, and postural instability. The loss of brain dopamine neurons and formation of proteinaceous aggregates known as Lewy bodies are pathological hallmarks of PD. PD usually affects people over the age of 50, but rare familial forms can strike at a much younger age. Several genes have now been definitively linked to PD. They encode α-synuclein 91, Parkin 92, DJ-1 93, PINK1 94, and LRRK2 95,96, the dysfunctions of which are thought to cause either proteasomal abnormalities or oxidative stress. At present, there is no cure for PD, but a variety of medications provide symptomatic relief. Although levodopa combined with carbidopa helps at least 75% parkinsonian cases, not all symptoms respond equally to the drug. Prolonged use of these drugs unfortunately also has troubling side effects such as the development of dyskinesia. Other drugs, such as bromocriptine, pramipexole, and ropinirole, mimic the role of dopamine in the brain, causing the neurons to react as they would to dopamine. An antiviral drug, amantadine, with possible mechanisms as a nicotinic agonist or NMDA antagonist, also appears to reduce symptoms.
Given the ease of delivering chemical compounds to zebrafish, the potential PD-inducing effects of MPTP, its metabolite MPP+, and the pesticides including rotenone and paraquat, have been evaluated in both larval and adult zebrafish 97–100. One issue relating to the validity of this MPTP neurotoxin-induced PD model is whether the loss of DA neurons represents a selective event as in humans, i.e. other groups of neurons are largely spared. Such selective effect of MPTP has indeed been observed in larval zebrafish. It will be of interest in the future to treat larval zebrafish of a later stage and see if DA neuronal loss can be similarly observed, in order to further determine whether MPTP affects the development or the survival of DA neurons. Moreover, it will be of great interest to know whether a recovery of DA neuronal loss can be observed after the removal of MPTP. In addition to locomotor deficits, MPTP has also elicited remarkable peripheral phenotypes in zebrafish, including a difficulty in respiration and darkened skin pigmentation. It should be further validated whether the change in skin pigmentation might be related to a loss of norepinephrine contents or neuronal terminals in MPTP-treated fish. If so, this easy-to-observe phenotype might serve as a convenient indicator of MPTP toxicity on catecholamine terminals, and could be used as a simple assay for high throughput screens of chemicals that can counteract the neurotoxic effects of MPTP.
Genes involved in PD have also been studied in zebrfish, including the characterization of a zebrafish orthologue of ubiquitin C-terminal hydrolase L1 (UCH-L1), which has been associated with inherited forms of PD 101. While no stable transgenic or mutational lines in PD-associated genes have been reported in zebrafish, two studies have examined the effects of transient knockdown of DJ-1 and PINK1 using morpholino antisense oligonucleotides 102,103. DJ-1 morphants are more sensitive to oxidative stress, as evidenced by a significant reduction of DA neurons in DJ-1 morphants as compared to control embryos upon hydrogen peroxide (H2O2) exposure. DJ-1 morphants also have increased levels of superoxide dismutase (SOD1) mRNA. While the link between DJ-1 and oxidative stress is quite intriguing, it remains unclear whether the loss of DA neurons was selective, and whether it is due to neuronal degeneration or abnormal development. PINK1 konckdown results in the severe developmental phenotype that is rescued by wildtype human PINK1 mRNA> Morphants display a moderate decrease in the numbers of central dopaminergic neurons and laterations of mitochondrial function, including increases in caspase-3 activity and reactive oxygen species (ROS) levels. When the porphants were exposed to several drugs with antioxidant properties, ROS levels were normalized and associated phenotype improved. In addition, GSK-3b-related mechanisms can account for some of the effects of PINK1 knockdown, as morphant fish show elevated GSK3b activity and their phenotype is partially abrogated by GSK3b inhibitors, such as LiCl and SB216763 [3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)1H-pyrrole-2,5-dione].
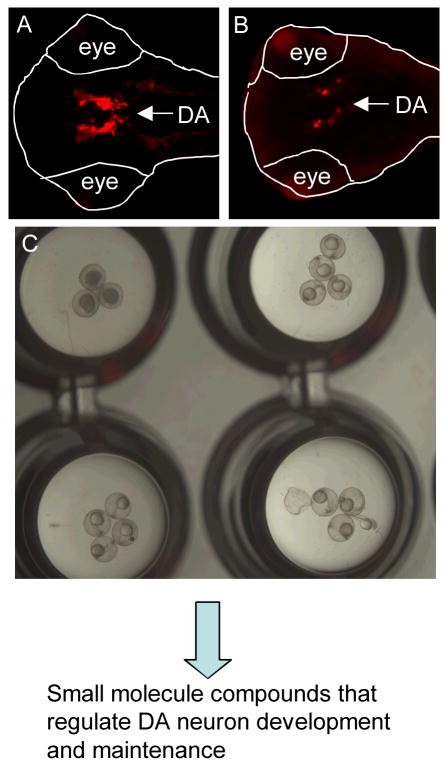
In addition to understanding the mechanism of DA neuron degeneration, another potentially fruitful avenue for treating PD involves understanding the mechanisms of DA neuron development and regeneration. Work from our laboratory has employed zebrafish dopaminergic (DA) neurons to identify genetic factors and small molecule drugs that can regulate the development and maintenance of these neuronal groups 16. Using a TH-GFP transgenic line (Q. Li and S. Guo, unpublished data) and/or immunofluorescent labeling to visualize DA neurons, the effect of the neurotoxin MPTP or MPP+ has been visualized (Fig. 2). A small molecule screening of ~5000 FDA-approved bioactive compounds has been carried out. With our screening assays, ~300 compounds can be easily screened per person per week. A handful of compounds have been identified that can inhibit DA neuron development (S. Chatterjee, H. Khodabakhsh, and S. Guo, unpublished data). However, no compounds that can increase the number of DA neurons have been identified so far. It is possible that the wildtype number of DA neurons represents an optimum, and it is difficult to further improve upon it by addition of a single small molecule. Therefore, screening strategies are currently being devised to create DA-deficient models in order to identify compounds that can rescue DA deficits.
Figure 2.
Identification of small molecule compounds that can regulate DA neuron development and maintenance. DA neurons are visualized through tyrosine hydroxylase immunostaining in normal (A) and MPTP-treated (B) embryos (ventral view with the anterior to the left). (C) Visualization of zebrafish embryos in a 96-well plate, showing the relative size of a zebrafish embryo to the well of the 96-well plate, and allowing one to appreciate the suitability of embryonic and larval zebrafish for high throughput screens. These embryos are first screened for morphological defects, followed by screening for DA neuron phenotypes.
Alzheimer’s disease
Alzheimer’s disease (AD) is an age-related, non-reversible brain disorder that develops over a period of years. Initially, people experience memory loss and confusion, gradually leading to behavioral and personality changes, a decline in cognitive abilities such as decision-making and language skills, as well as problems recognizing family and friends. It is the most common cause of dementia among people age 65 and older. Three major hallmarks in the brain are associated with the disease processes of AD. 1) Amyloid plaques, which are composed of fragments of a protein called β-amyloid peptide. 2) Neurofibrillary tangles (NFTs), found inside neurons, are abnormal collections of the protein Tau. 3) Loss of connections between neurons responsible for memory and learning. Among the complex genetic underpinnings of AD, three genes, amyloid precursor protein (APP), presenilin 1(PS1), and presinilin 2 (PS2), have been identified to play important roles in the pathogenesis of AD 104. Currently there are no medicines that can slow the progression of AD. Four FDA-approved medications are used to treat AD symptoms. However, they will not stop or reverse AD and appear to help individuals for only a few months to a few years. Donepezil (Aricept), rivastigmine (Exelon), and galantamine (Reminyl), all of which act as cholinesterase inhibitors, are prescribed to treat mild to moderate AD symptoms. The newest AD medication is memantine (Namenda, a NMDA receptor blocker), which is prescribed to treat moderate to severe AD symptoms.
Zebrafish orthologues of APP 105, PS1 106, and PS2 107 have been identified. These genes are widely expressed beginning at early embryonic stages, suggesting that they may play an important role in regulating development. Thus, creation of zebrafish models of AD will likely require conditional manipulation of the activity of these genes. On the other hand, a transient transgenic model of tauopathy has been analyzed 108. In this model, a FTDP-17 mutant form of human tau expressed in zebrafish neurons produces a cytoskeletal disruption that closely resembles the NFT in human disease. However, since no stable transgenic lines have been reported, it is difficult to know whether neurodegenerative and behavioral phenotypes are obtained.
As mentioned above, enhancement of cholinergic transmission has been used as a symptomatic treatment for AD. It is interesting to mention that nicotine is found to improve memory in a learning paradigm in zebrafish 109.
Huntington’s disease
Huntington’s disease (HD) is an autosomal dominantly inherited, trinucleotide repeat disorder. The mutant protein huntingtin (Htt) inhabits an expanded polyglutamine (polyQ) repeat at its N terminal region. Both gain-of-function and haploinsufficiency of the HD gene (also referred to as IT15) seem to contribute to the development of this disorder, but the precise mechanisms leading to HD are still poorly understood 110,111. Apart from symptomatic treatment to manage the movement abnormalities, no drugs are available that can slow down the progression of HD 112.
The zebrafish orthologue of HTT has been identified 113. Its requirement for zebrafish development and iron utilization has been revealed through morpholino knockdown 114. Although loss-of-function of HTT did not lead to selective neurodegeneration, mis-expression of poly-Q expanded HTT fragment does cause protein aggregation and neuronal death. Interestingly, molecular chaperones can suppress aggregate formation and neuronal death, and several classes of small molecule compounds including anti-prion compounds have been validated to inhibit poly-Q aggregate formation in zebrafish 115,116. These studies show that zebrafish is a promising system for modeling HD and for finding potential therapeutic treatment of HD.
4.3 Neuropsychiatric disorders: anxiety/depression and addictive disorders
Six forms of anxiety disorders have been described in humans 117: 1) Panic disorder is characterized by unpredictable, sudden attacks of intense anxiety; 2) Generalized anxiety disorder is marked by exaggerated worry and tension, even though there is little or nothing to provoke it. 3) Social phobia, also called social anxiety disorder, involves overwhelming anxiety and excessive self-consciousness in daily social situations. 4) Specific phobias are intense fears of some specific objects that in reality pose little or no danger. 5) Obsessive-compulsive disorder involves anxious thoughts and repetitive rituals, which, when performed, help reduce anxiety. 6) Post-traumatic stress disorder (PTSD) is characterized by persistent frightening thoughts and intrusive memories of a terrifying event. Currently two types of treatment are available for anxiety disorders 118: they are medications (many are classical examples of serendipity in drug discovery) and specific types of psychotherapy. Although these treatments are effective in some people, many patients are left with residual symptoms or experience side effects that limit the use of these currently available mediations.
Abuse and addiction to alcohol, nicotine, and illegal substances manifest as the inability to break free from these substances, despite a full awareness of their adverse effects by the addicted individuals. Although still poorly understood, addiction is believed to be a result of long-term interactions between addictive substances and multiple brain neural systems, leading to maladaptation of brain signaling pathways. Medications used to treat drug addiction include drugs that antagonize the action of addictive substances. For example, Naltrexone helps prevent relapse to alcohol and heroin abuse, although many side effects exist. In addition to drug therapy, behavioral treatments are also used but with limited success. Because of the complexity and environmental attributions to neuropsychiatric disorders, despite the strong underlying genetic basis, definitive genetic factors remain to be identified in most cases.
Several behavioral paradigms have been exploited to model fear/anxiety in zebrafish, which includes phototaxis 48,119, thigmotaxis 49, behavioral responses to alarm substance 120,121, anti-predator behavior 122, and novelty-elicited diving response 55. While the novelty induced diving is shown to be affected by nicotine and nicotinic antagonists, implicating the involvement of both alpha7 and alpha4beta2 receptors 123, rigorous pharmacological validations of other behavioral paradigms remain to be carried out.
The psycho-stimulatory 48,49 and reinforcing 50,52–54 effects of alcohol and drugs of abuse have been examined in zebrafish. These studies show that zebrafish readily respond to addictive substances, and neural substrates such as dopamine, serotonin, opioid systems, and acetylcholine systems are involved.
Behavior-based approaches to neuroactive drug discovery have a long history with much serendipity. For example, many psychoactive substances such as ethanol, caffeine, and opium have been discovered due to their rapid perceptual and behavioraleffects on humans and other animals. However, is it feasible to use behavior as a read out in a small molecule based drug screening? Behavioral assays are often more complex than cell based assays and subject to more variability from both genetic and environmental influences. A recent review by Kokel and Peterson argues that these problems can be overcome, and such screening represents a necessity for systematic drug discovery 124.
5. Conclusions
The zebrafish nervous system is similar to that of mammals, and is accessible to small molecule drugs. These features make zebrafish a potentially suitable organism to evaluate the impact of drugs on neuronal development and function. However, before such potential can be fully realized, a better understanding of its nervous system and further establishment of appropriate models for human conditions are needed. For the purpose of drug discovery, the small size and relative abundance of zebrafish embryos allow for 96- well format chemical screening. However, such phenotype-based screens still provide much lower throughput than in vitro assay-based screening. Thus, additional technological breakthroughs in animal handling, imaging, and quantification are necessary to help further automate the screening process as much as possible.
6. Expert opinion
At present, the drug discovery process is either largely serendipitous or based on in vitro screens. Serendipity is not a reliable and rational approach, whereas in vitro assay-based screening often leads to compounds that have limited in vivo efficacy or unforeseeable toxicity. The pre-clinical drug testing process almost exclusively uses mammalian animal models, with a low throughput and high cost. The use of zebrafish could potentially facilitate both drug screening and the processes of evaluating candidate drugs. Investment into this organism, to further understand its biology and disease modeling capability, and to facilitate technological build-ups, has the possibility of revolutionizing drug discovery for CNS disorders.
Acknowledgments
I apologize to authors whose work cannot be cited in this review due to space limitations. I thank S. Chatterjee, E. Hurlock, T. Mueller, and Y. Sun for helpful comments on the review.
Our research on zebrafish neural development and function is supported by NIH (DA023904, AA016021, and NS042626).
References
- 1.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill Health Professions Division; 2000. [Google Scholar]
- 2.Peterson RT, Fishman MC. Discovery and use of small molecules for probing biological processes in zebrafish. Methods Cell Biol. 2004;76:569–591. doi: 10.1016/s0091-679x(04)76026-4. [DOI] [PubMed] [Google Scholar]
- 3.Zon LI, Peterson RT. In vivo drug discovery in zebrafish. Nat Rev Drug Discovery. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 4.Lan C, Laurenson S, Copp BR, Cattin PM, Love DR. Whole organism approaches to chemical genomics: the promising role of zebrafish (Danio rerio) Expert Opin Drug Discov. 2007;2:1–13. doi: 10.1517/17460441.2.10.1389. [DOI] [PubMed] [Google Scholar]
- 5.Peterson RT, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotech. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 6.Stern HM, et al. Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol. 2005;1:366–370. doi: 10.1038/nchembio749. [DOI] [PubMed] [Google Scholar]
- 7.Burns CG, et al. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- 8.Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- 9.Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 10.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel CB. Patterning the brain of the zebrafish embryo. Annu Rev Neurosci. 1993;16:707–732. doi: 10.1146/annurev.ne.16.030193.003423. [DOI] [PubMed] [Google Scholar]
- 12.Wilson SW, Brand M, Eisen JS. Patterning the zebrafish central nervous system. Results Probl Cell Differ. 2002;40:181–215. doi: 10.1007/978-3-540-46041-1_10. [DOI] [PubMed] [Google Scholar]
- 13.Higashijima S, Mandel G, Fetcho JR. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J Comp Neurol. 2004;480:1–18. doi: 10.1002/cne.20278. [DOI] [PubMed] [Google Scholar]
- 14.Mueller T, Vernier P, Wullimann MF. A phylotypic stage in vertebrate brain development: GABA cell patterns in zebrafish compared to mouse. J Comp Neurol. 2006;494:620–34. doi: 10.1002/cne.20824. [DOI] [PubMed] [Google Scholar]
- 15.Mueller T, Wullimann MF, Guo S. Early teleostean basal ganglia development visualized by zebrafish Dlx2a, Lhx6, Lhx7, Tbr2 (eomesa), and GAD67 gene expression. J Comp Neurol. 2008;507:245–57. doi: 10.1002/cne.21604. [DOI] [PubMed] [Google Scholar]
- 16.Guo S, et al. Mutations in the zebrafish unmask shared regulatory pathways controlling the development of catecholaminergic neurons. Dev Biol. 1999;208:473–487. doi: 10.1006/dbio.1999.9204. [DOI] [PubMed] [Google Scholar]
- 17.Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 2001;101:237–243. doi: 10.1016/s0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- 18.Bellipanni G, Rink E, Bally-Cuif L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Gene Expr Patterns. 2002;2:251–256. doi: 10.1016/s1567-133x(02)00056-x. [DOI] [PubMed] [Google Scholar]
- 19.McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- 20.Kaslin J, Panula P. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio) J Comp Neurol. 2001;440:342–77. doi: 10.1002/cne.1390. [DOI] [PubMed] [Google Scholar]
- 21.Arenzana FJ, et al. Development of the cholinergic system in the brain and retina of the zebrafish. Brain Res Bull. 2005;66:421–425. doi: 10.1016/j.brainresbull.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J Neurosci. 2004;24:2678–89. doi: 10.1523/JNEUROSCI.4908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrasekar G, Lauter G, Hauptmann G. Distribution of corticotropin-releasing hormone in the developing zebrafish brain. J Comp Neurol. 2007;505:337–51. doi: 10.1002/cne.21496. [DOI] [PubMed] [Google Scholar]
- 24.Brosamle C, Halpern ME. Characterization of myelination in the developing zebrafish. Glia. 2002;39:47–57. doi: 10.1002/glia.10088. [DOI] [PubMed] [Google Scholar]
- 25.Kawai H, Arata N, Nakayasu H. Three-dimensional distribution of astrocytes in zebrafish spinal cord. Glia. 2001;36:406–13. doi: 10.1002/glia.1126. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein JL, Martinex S, Shimaura K, Puelles L. The embryonic vertebrate forebrain: the prosomeric model. Science. 1994;266:576–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- 27.Mueller T, Wullimann MF. Atlas of early zebrafish brain development. Elsevier B.V.; Amsterdam, the Netherlands: 2005. [Google Scholar]
- 28.Hauptmann G, Soll I, Gerster T. The early embryonic zebrafish forebrain is subdivided into molecularly distinct transverse and longitudinal domains. Brain Res Bull. 2002;57:371–75. doi: 10.1016/s0361-9230(01)00691-8. [DOI] [PubMed] [Google Scholar]
- 29.Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 30.Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neurol. 2004;475:143–62. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- 31.Northcutt RG, Braford MRJ. New observations on the organization and evolution of the telencephalon of actinopterygian fishes. In: Ebbesson SOE, editor. Comparative neurology of the telencephalon. Plenum Press; New York: 1980. pp. 41–98. [Google Scholar]
- 32.Meek J, Nieuwenhuys R. In: The central nervous system of vertebrates. Nieuwenhuys R, Donkelaar HJ, Nicholson C, editors. Berlin: Springer; 1998. pp. 759–937. [Google Scholar]
- 33.Smeets WJAJ, Reiner A. Phylogeny and development of catecholamine systems in the CNS of vertebrates. Cambridge University Press; Cambridge, England: 1994. [Google Scholar]
- 34.Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- 35.Costagli A, Kapsimali M, Wilson SW, Mione M. Conserved and divergent patterns of Reelin expression in the zebrafish central nervous system. J Comp Neurol. 2002;450:73–93. doi: 10.1002/cne.10292. [DOI] [PubMed] [Google Scholar]
- 36.Rupp B, Wullimann MF, Reichert H. The zebrafish brain: a neuroanatomical comparison with the goldfish. Anat Embryol. 1996;194:187–203. doi: 10.1007/BF00195012. [DOI] [PubMed] [Google Scholar]
- 37.Wullimann MF. The central nervous system. In: Evans DH, editor. The Physiology of Fishes. CRC Press LLC; New York: 1997. pp. 245–281. [Google Scholar]
- 38.Bjarnadottir TK, Fredriksson R, Schioth HB. The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste(1) and other related G protein-coupled receptors. Gene. 2005;362:70–84. doi: 10.1016/j.gene.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 39.Boehmler W, et al. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev Dyn. 2004;230:481–493. doi: 10.1002/dvdy.20075. [DOI] [PubMed] [Google Scholar]
- 40.Norton WH, Folchert A, Bally-Cuif L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J Comp Neurol. 2008;511:521–42. doi: 10.1002/cne.21831. [DOI] [PubMed] [Google Scholar]
- 41.Salaneck E, Larsson TA, Larson ET, Larhammar D. Birth and death of neuropeptide Y receptor genes in relation to the teleost fish tetraploidization. Gene. 2008;409:61–71. doi: 10.1016/j.gene.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Simon FM, Rodriguez RE. Developmental expression and distribution of opioid receptors in zebrafish. Neuroscience. 2008;151:129–37. doi: 10.1016/j.neuroscience.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 43.Scalzo FM, Levin ED. The use of zebrafish (Danio rerio) as a model system in neurobehavioral toxicology. Neurotoxicol Teratol. 2004;26:708–8. doi: 10.1016/j.ntt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicol Teratol. 2004;26:709–18. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Parng C, Ton C, Lin YX, Roy NM, PM A zebrafish assay for identifying neuroprotectants in vivo. Neurotoxicol Teratol. 2006;28:509–16. doi: 10.1016/j.ntt.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Parng C, Roy NM, Ton C, Lin Y, McGrath P. Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods. 2007;55:103–12. doi: 10.1016/j.vascn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Ton C, Lin Y, Willett C. Zebrafish as a model for developmental neurotoxicity testing. Birth Defects Res A Clin Mol Teratol. 2006;76:553–67. doi: 10.1002/bdra.20281. [DOI] [PubMed] [Google Scholar]
- 48.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: zebrafish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharm Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 49.Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharm Biochem Behav. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad USA. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Patino MA, Yu L, Cabral H, Zhdanova IV. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol Behav. 2008;93:160–71. doi: 10.1016/j.physbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Ninkovic J, et al. Genetic identification of AChE as a positive modulator of addiction to the psychostimulant D-amphetamine in zebrafish. J Neurobiol. 2006;66:463–475. doi: 10.1002/neu.20231. [DOI] [PubMed] [Google Scholar]
- 53.Lau B, Bretaud S, Huang Y, Lin E, Guo S. Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav. 2006;5:497–505. doi: 10.1111/j.1601-183X.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 54.Bretaud S, et al. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 55.Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–8. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 56.Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol. 2004;26:737–43. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Menelaou E, Svoboda KR. Secondary motoneurons in juvenile and adult zebrafish: axonal pathfinding errors caused by embryonic nicotine exposure. J Comp Neurol. 2009;512:305–22. doi: 10.1002/cne.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasevicius A, Ekker SC. Effective targeted gene “knockdown” in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 59.Wienholds E, Schulte-Merker S, Walderich B, Plasterk RHA. Target-selected inactivation of the zebrafish rag1 gene. Science. 2002;297:99–102. doi: 10.1126/science.1071762. [DOI] [PubMed] [Google Scholar]
- 60.Wang D, et al. Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proc Natl Acad Sci. 2007;104:12428–33. doi: 10.1073/pnas.0705502104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doyon Y, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–8. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thermes V, et al. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002 doi: 10.1016/s0925-4773(02)00218-6. in press. [DOI] [PubMed] [Google Scholar]
- 64.Shin J, Park HC, Topczewska JM, Mawdsley DJ, Appel B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 2003;25:7–14. doi: 10.1023/B:MICS.0000006847.09037.3a. [DOI] [PubMed] [Google Scholar]
- 65.Kawakami K. Tol2: a versatile gene transfer vector in vertebrates. Genome Biol. 2007;8:S7. doi: 10.1186/gb-2007-8-s1-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curado S, et al. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–35. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 68.Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66:3–8. [PubMed] [Google Scholar]
- 69.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Schmitz C, Rezaie P. The neuropathology of autism: where do we stand? Neuropathol Appl Neurobiol. 2008;34:4–11. doi: 10.1111/j.1365-2990.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 71.Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr Opin Genet Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 72.Gharani N, Benayed R, Mancuso V, Brzustowicz LM, Millonig JH. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Mol Psychiatry. 2004;9:474–84. doi: 10.1038/sj.mp.4001498. [DOI] [PubMed] [Google Scholar]
- 73.Benayed R, et al. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77:851–68. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–87. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- 75.Wiznitzer M. Autism and tuberous sclerosis. J Child Neurol. 2004;19:675–9. doi: 10.1177/08830738040190090701. [DOI] [PubMed] [Google Scholar]
- 76.Butler MG, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jamain S, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;10:329–32. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Durand CM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–7. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HG, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arking DE, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:7–9. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alarcon M, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–9. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Splawski I, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Persico AM, et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry. 2001;6:150–9. doi: 10.1038/sj.mp.4000850. [DOI] [PubMed] [Google Scholar]
- 84.Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–81. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 85.Rissone A, et al. Comparative genome analysis of the neurexin gene family in Danio rerio: insights into their functions and evolution. Mol Biol Evol. 2007;24:236–52. doi: 10.1093/molbev/msl147. [DOI] [PubMed] [Google Scholar]
- 86.Coverdale LE, Martyniuk CJ, Trudeau VL, Martin CC. Differential expression of the methyl-cytosine binding protein 2 gene in embryonic and adult brain of zebrafish. Brain Res Dev Brain Res. 2004;153:281–7. doi: 10.1016/j.devbrainres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 87.Tallafuss A, Eisen JS. The Met receptor tyrosine kinase prevents zebrafish primary motoneurons from expressing an incorrect neurotransmitter. Neural Dev. 2008;3:18. doi: 10.1186/1749-8104-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colman JR, Baldwin D, Johnson LL, Scholz NL. Effects of the synthetic estrogen, 17alpha-ethinylestradiol, on aggression and courtship behavior in male zebrafish (Danio rerio) Aquat Toxicol. 2009;91:346–54. doi: 10.1016/j.aquatox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Engeszer RE, Wang G, Ryan MJ, Parichy DM. Sex-specific perceptual spaces for a vertebrate basal social aggregative behavior. Proc Natl Acad Sci. 2008;105:929–33. doi: 10.1073/pnas.0708778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Larson ET, O’Malley DM, Melloni RHJ. Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav Brain Res. 2006;167:94–102. doi: 10.1016/j.bbr.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 91.Polymeropoulos MH, et al. Mutations in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 92.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 93.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 94.Valente EM, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–60. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 95.Paisan-Ruiz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 96.Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–7. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 97.Anichtchik OV, Kaslin J, Peitsaro N, Scheinin M, Panula P. Neurochemical and behavioral changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem. 2004;88:443–453. doi: 10.1111/j.1471-4159.2004.02190.x. [DOI] [PubMed] [Google Scholar]
- 98.Bretaud S, Lee S, Guo S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotox Teratol. 2004;26:857–864. doi: 10.1016/j.ntt.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 99.Lam CS, Korzh V, Strahle U. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. Eur J Neurosci. 2005;21:1758–1762. doi: 10.1111/j.1460-9568.2005.03988.x. [DOI] [PubMed] [Google Scholar]
- 100.McKinley ET, et al. Neuroprotection of MPTP-induced toxicity in zebrafish dopaminergic neurons. Brain Res Mol Brain Res. 2005;141:128–137. doi: 10.1016/j.molbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 101.Son OL, et al. Cloning and expression analysis of a Parkinson’s disease gene, uch-L1, and its promoter in zebrafish. Biochem Biophys Res Commun. 2003;312:601–7. doi: 10.1016/j.bbrc.2003.10.163. [DOI] [PubMed] [Google Scholar]
- 102.Bretaud S, Allen C, Ingham PW, Bandmann O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson’s disease. J Neurochem. 2007;100:1626–1635. doi: 10.1111/j.1471-4159.2006.04291.x. [DOI] [PubMed] [Google Scholar]
- 103.Anichtchik O, et al. Loss of PINK1 function affects development and results in neurodegeneration in zebrafish. J Neurosci. 2008;28:8199–207. doi: 10.1523/JNEUROSCI.0979-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–78. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 105.Musa A, Lehrach H, Russo VA. Distinct expression patterns of two zebrafish homologues of the human APP gene during embryonic development. Dev Genes Evol. 2001;211:563–7. doi: 10.1007/s00427-001-0189-9. [DOI] [PubMed] [Google Scholar]
- 106.Leimer U, et al. Zebrafish (Danio rerio) presenilin promotes aberrant amyloid beta-peptide production and requires a critical aspartate residue for its function in amyloidogenesis. Biochemistry. 1999;38:3602–9. doi: 10.1021/bi991453n. [DOI] [PubMed] [Google Scholar]
- 107.Groth C, Nornes S, McCarty R, Tamme R, Lardelli M. Identification of a second presenilin gene in zebrafish with similarity to the human Alzheimer’s disease gene presenilin2. Dev Genes Evol. 2002;212:486–90. doi: 10.1007/s00427-002-0269-5. [DOI] [PubMed] [Google Scholar]
- 108.Tomasiewicz HG, Flaherty DB, Soria JP, Wood JG. Transgenic zebrafish model of neurodegeneration. J Neurosci Res. 2002;70:734–45. doi: 10.1002/jnr.10451. [DOI] [PubMed] [Google Scholar]
- 109.Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2005 doi: 10.1007/s00213-005-0162-9. in press. [DOI] [PubMed] [Google Scholar]
- 110.Rubinsztein DC. Lessons from animal models of Huntington’s disease. Trends Genet. 2002;18:202–9. doi: 10.1016/s0168-9525(01)02625-7. [DOI] [PubMed] [Google Scholar]
- 111.Ross CA. Polyglutamine pathogenesis; emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron. 2002;35:819–22. doi: 10.1016/s0896-6273(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 112.Bonelli RM, Hofmann P. A systematic review of the treatment studies in Huntington’s disease since 1990. Expert Opin Pharmacother. 2007;8:141–53. doi: 10.1517/14656566.8.2.141. [DOI] [PubMed] [Google Scholar]
- 113.Karlovich CA, John RM, Ramirez LSD, Myers RM. Characterization of the Huntington’s disease (HD) gene homologue in the zebrafish Danio rerio. Gene. 1998;217:117–25. doi: 10.1016/s0378-1119(98)00342-4. [DOI] [PubMed] [Google Scholar]
- 114.Lumsden AL, Henshall TL, Dayan S, Lardelli MT, Richards RI. Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum Mol Genet. 2007;16:1905–20. doi: 10.1093/hmg/ddm138. [DOI] [PubMed] [Google Scholar]
- 115.Schiffer NW, et al. Identification of anti-prion compounds as efficient inhibitors of polyglutamine protein aggregation in a zebrafish model. J Biol Chem. 2007;282:9195–203. doi: 10.1074/jbc.M607865200. [DOI] [PubMed] [Google Scholar]
- 116.Miller VM, et al. CHIP suppresses polyglutamine aggregation and toxicity in vitro and in vivo. J Neurosci. 2005;25:9152–61. doi: 10.1523/JNEUROSCI.3001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kessler RC, Greenberg PE. The economic burden of anxiety and stress disorders. In: Davis KL, Charney DS, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Lippincott Williams and Wilkins; Philadelphia: 2002. [Google Scholar]
- 118.Gorman JM, Kent JM, Coplan JD. Current and emerging therapeutics of anxiety and stress disorders. In: Davis KL, Charney DS, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Lippincott Williams and Wilkins; Philadelphia: 2002. [Google Scholar]
- 119.Guo S. Linking genes to brain, behavior, and neurological diseases: what can we learn from zebrafish? Genes, Brain & Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 120.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.10.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jesuthasan SJ, Mathuru AS. The alarm response in zebrafish: innate fear in a vertebrate genetic model. J Neurogenet. 2008;22:211–28. doi: 10.1080/01677060802298475. [DOI] [PubMed] [Google Scholar]
- 122.Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–17. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 123.Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol Behav. 2008;95:408–12. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kokel D, Peterson RT. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Brief Funct Genomic Proteomic. 2008;7:483–90. doi: 10.1093/bfgp/eln040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thirumalai V, Cline HT. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J Neurophysiol. 2008;100:1635–48. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27:4884–94. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu CJ, Li L. Dopamine modulates voltage-activated potassium currents in zebrafish retinal on bipolar cells. J Neurosci Res. 2005;82:368–76. doi: 10.1002/jnr.20637. [DOI] [PubMed] [Google Scholar]
- 128.Airhart MJ, et al. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–64. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 129.Brustein E, Chong M, Holmqvist B, PD Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J Neurobiol. 57:303–22. doi: 10.1002/neu.10292. [DOI] [PubMed] [Google Scholar]
- 130.Tabo R, Friedrich RW. Pharmacological analysis of ionotropic glutamate receptor function in neuronal circuits of the zebrafish olfactory bulb. PLoS ONE. 2008;3:e1416. doi: 10.1371/journal.pone.0001416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Todd KJ, Slatter CA, Ali DW. Activation of ionotropic glutamate receptors on peripheral axons of primary motoneurons mediates transmitter release at the zebrafish NMJ. J Neurophysiol. 2004;91:828–40. doi: 10.1152/jn.00599.2003. [DOI] [PubMed] [Google Scholar]
- 132.Tabor R, Yaksi E, Friedrich RW. Multiple functions of GABA A and GABA B receptors during pattern processing in the zebrafish olfactory bulb. Eur J Neurosci. 2008;28:117–27. doi: 10.1111/j.1460-9568.2008.06316.x. [DOI] [PubMed] [Google Scholar]
- 133.Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–8. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]