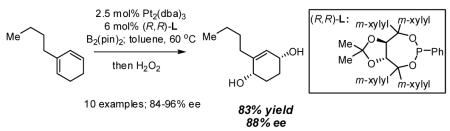
Abstract
The asymmetric 1,4-dihydroxylation of 1,3-dienes, and other transformations, are initiated by the Pt-catalyzed enantioselective addition of bis(pinacolato)diboron (B2(pin)2) to conjugated dienes. The studies reported in this communication suggest that both cyclic and acyclic substrates will participate in this reaction, however, dienes which are unable to adopt the S-cis conformation are unreactive. For most substrates, 1,4-addition is the predominant pathway. In addition to oxidation to the derived 2-buten-1,4-diol, stereoselective carbonyl allylation with the intermediate bis(boronate) ester is also described.
Catalytic enantioselective oxidation reactions provide an important strategy for the conversion of simple unsaturated hydrocarbon substrates to synthetically useful organic compounds. While highly selective asymmetric oxidation of hydrocarbons may be accomplished in many ways, the 1,4-dihydroxylation of 1,3-dienes (Scheme 1), a synthetically valuable process, remains a largely unsolved problem. To accomplish this transformation, [4+2] cycloaddition of dienes with 1O2 is often employed;1 however, this reaction has not been accomplished under the influence of a chiral catalyst.2 Likewise, the Pd(II)-catalyzed 1,4-diacetoxylation and related reactions,3 very promising transformations for asymmetric synthesis, have not yet been accomplished with useful levels of enantioselectivity.4 Our research program has focused on the enantioselective diboration of unsaturated hydrocarbons5 and in this communication we describe an enantioselective 1,4-diboration of 1,3-dienes,6,7 a transformation that furnishes the above-described 1,4-dihydroxylation products in an economical fashion, with good yields, and often with high levels of enantiomeric purity.8
Scheme 1.
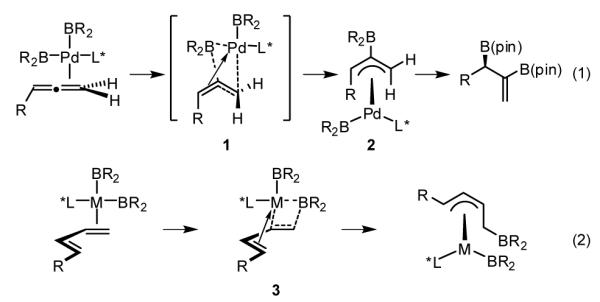
We recently developed a Pd-catalyzed enantioselective diboration of allenes, a process that benefits from accelerated catalysis when done in the presence of monodentate phosphine ligands. Detailed mechanistic studies suggest this reaction proceeds by oxidative addition of the diboron to palladium, a step which is followed by migratory insertion of the terminal alkene into a Pd-B bond (Scheme 2, eq. 1).9 DFT studies revealed that the insertion step proceeds by an unusual elementary reaction that directly provides a stable, coordinatively-saturated η3-allyl palladium complex 2 (see structure 1; rotation of the terminal alkene occurs concomitantly with insertion, allowing π-bonding with the adjacent alkene to develop in the transition state). Considering that olefin insertion involving a 1,3-diene likely benefits from a similar incipient π bond (3), it was of interest to determine whether catalysts developed for the asymmetric allene diboration, would be effective in catalytic diene diboration.
Scheme 2.
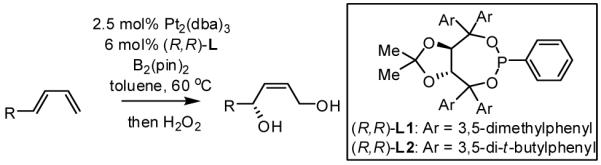
Initial experiments probed the reaction between bis(pinacolato)diboron (B2(pin)2) and 1,3-dienes. While no reaction was observed with a catalyst composed of Pd2(dba)3 and a chiral TADDOL-derived phosphoramidite, the most effective catalyst for enantioselective allene diboration, when the palladium complex was replaced with Pt2(dba)3, a complex that generally exhibits higher reactivity in diboration reactions,10 efficient catalysis and modest levels of enantioselectivity were observed. Several permutations of reaction conditions and ligand structure revealed that a catalyst composed of Pt2(dba)3 and chiral TADDOL-derived phosphonite L1,11 provides good reactivity for a range of substrates and modest to high levels of enantioselection for many (Table 1). As can be observed in Table 1, acyclic dienes bearing aryl or alkyl substitution at the terminus react efficiently and with high selectivity. With ligand L1, butadienes bearing substitution at both C1 and C3 react with lower stereocontrol, however, with L2 high selectivity is obtained (entry 5). Notably, cyclic dienes can be suitable substrates for the asymmetric diboration/oxidation sequence and provide otherwise difficult-to-access products with high levels of asymmetric induction. As noted in entry 10, the 1,2-diol is the predominant product when one terminus of the substrate is disubstituted, an outcome which likely results from enhanced steric congestion. Lastly, the lack of reaction with cis-piperylene (entry 11) suggests that only dienes able to adopt an S-cis conformation will participate in the Pt-catalyzed diene diboration. This observation may reflect the importance of structure 3 (Scheme 2) in the reaction mechanism.
Table 1.
Catalytic Enantioselective Diboration/Oxidation of 1,3-Dienes.(a)
 | ||||
|---|---|---|---|---|
| entry | diene | product | yield (%)(b) | ee(%)(c) |
| 1 | 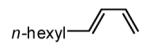 |
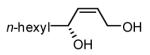 |
83 | 84 |
| 2 | 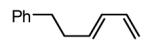 |
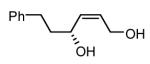 |
81 | 84 |
| 3 | 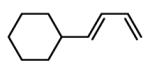 |
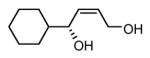 |
83 | 91 |
| 4 | 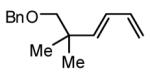 |
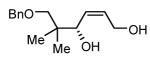 |
48 | 91 |
| 5 | 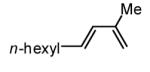 |
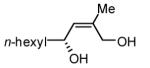 |
92 | 86(d) |
| 6 | 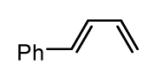 |
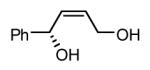 |
77 | 84(91)(e) |
| 7 | 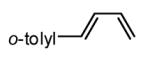 |
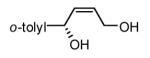 |
89 | 96(f) |
| 8 | 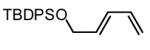 |
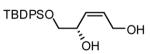 |
70 | 87(g) |
| 9 | 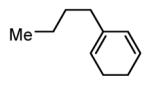 |
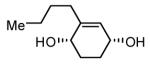 |
83 | 88 |
| 10 | 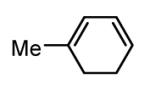 |
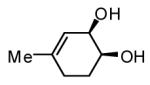 |
83 | 86(h) |
| 11 | 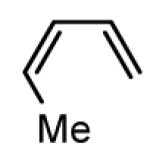 |
No Reaction | 0 | N/A |
Unless otherwise indicated, (R,R)-L1 was employed with reaction at 60 °C for 12 h, followed by oxidation with 30% H2O2 and 3 M NaOH for 3 h.
Percent yield of purified material. Value is an average of two experiments.
Determined by GC or SFC analysis employing a chiral stationary phase.
The product depicted corresponds to that obtained with (S,S)-L2.
Reaction at room temperature; value in parenthesis is after a single recrystallization.
Reaction at room temperature.
3 equivalents of B2(pin)2 employed.
(S,S) enantiomer of ligand L1 employed for this experiment
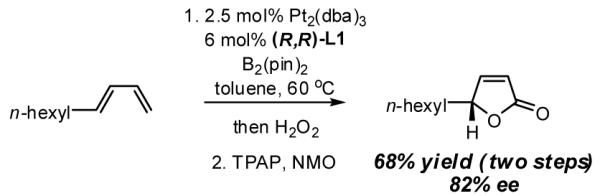
In addition to 2-buten-1,4-diols, other important scaffolds can be easily prepared from simple dienes through the asymmetric diboration reaction. Enantiomerically enriched butenolides and derived butyrolactones are prominent structural elements in natural products and straightforward methods for their preparation are scarce.12 Diene diboration provides a new approach: subsequent to diboration and oxidation, the unpurified material was subjected to perruthenate-catalyzed oxidation13 and furnished the derived butenolide in good yield and without compromising the integrity of the carbinol stereocenter.
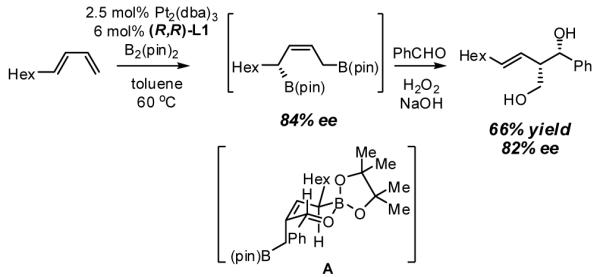
The α-chiral allylboronate functionality embedded in diene diboration products also finds use in stereoselective carbonyl allylation.14 Critical concerns are whether this transformation proceeds with high chirality transfer and whether it is selective for one constitutional isomer. To address this, benzaldehyde was added to an unquenched diboration reaction. After 12 hours of reaction and oxidative work-up, this sequence provided a single diastereomer of a single constitutional isomer, with near-perfect chirality transfer. The product structure suggests that this transformation proceeds through chair-like transition state A that minimizes A(1,3) interactions and with C-C bond formation occurring at the least hindered carbon of the intermediate diboron.
In conclusion, we have described a catalytic enantioselective diboration of 1,3-dienes, a process which generally provides synthetically useful chiral 2-buten-1,4-diols as the reaction product. Further studies of the substrate scope and reaction utility are in progress and will be reported in the near future.
Supplementary Material
Scheme 3.
Scheme 4.
Acknowledgment
Support by the NIGMS (GM-59417) and Merck is gratefully acknowledged, as is the NSF (DBI-0619576) for support of the BC Mass Spectrometry Center. We also thank AllyChem, Co., Ltd. for a generous donation of B2(pin)2.
Footnotes
Supporting Information Available: Characterization and procedures. This information is available free of charge through the internet at http://pubs.acs.org.
References
- (1) (a).For recent applications of the singlet oxygen [4+2] cycloaddition in synthesis, see:Lee JS, Fuchs PL. J. Am. Chem. Soc. 2005;127:13122. doi: 10.1021/ja0531935.Barbarow JE, Miller AK, Trauner D. Org. Lett. 2005;7:2901. doi: 10.1021/ol050831f.Ono K, Nakagawa M, Nishida A. Angew. Chem. Int. Ed. 2004;43:2020. doi: 10.1002/anie.200453673.Kusama H, Hara R, Kawahara S, Nishimori T, Kashima H, Nakamura N, Morihira K, Kuwajima I. J. Am. Chem. Soc. 2000;122:3811.Zhou G, Gao X, Li WZ, Li Y. Tetrahedron Lett. 2001;42:3101.Izzo I, Meduri G, Avallone E, De Riccardis F, Sodano G. Eur. J. Org. Chem. 2000:439.For recent reviews, see:Clennan EL, Pace A. Tetrahedron. 2005;61:6665.
- (2).For the enantioselective desymmetrization of 1O2 cycloadducts, see: Staben ST, Xin L, Toste FD. J. Am. Chem. Soc. 2006;128:12658. doi: 10.1021/ja065464x.
- (3).For a recent review of this process: Bäckvall J-E. Palladium-Catalyzed 1,4-Additions to Conjugated Dienes. In: De Meijere A, Diederich F, editors. Metal Catalyzed Cross-Coupling Reactions. 2nd Edition Weinheim; Wiley-VCH: 2004. pp. 479–529.
- (4) (a).Thorarensen A, Palmgren A, Itami K, Bäckvall J-E. Tetrahedron Lett. 1997;38:8541. [Google Scholar]; (b) Itami K, Palmgren A, Thorarensen A, Bäckvall J-E. J. Org. Chem. 1998;63:6466. [Google Scholar]; (c) Verboom RC, Plietker BJ, Bäckvall J-E. J. Organomet. Chem. 2003;687:508. [Google Scholar]; (d) El-Qisairi AK, Qaseer HA, Fennelly JP, Chiarelli MP, Henry PM. Catalysis Commun. 2008;9:1661. [Google Scholar]
- (5) (a).Recent reviews: Burks HE, Morken JP. Chem. Commun. 2007:4717. doi: 10.1039/b707779c.Ramirez J, Lillo V, Segarra AM, Fernandez E. Comp. Rend. Chim. 2007;10:138.Beletskaya I, Moberg C. Chem. Rev. 2006;106:2320. doi: 10.1021/cr050530j.Ishiyama T, Miyaura N. Chem. Record. 2004;3:271. doi: 10.1002/tcr.10068.
- (6) (a).Non-enantioselective diene diboration: Ishiyama T, Yamamoto M, Miyaura N. Chem. Commun. 1996:2073.Ishiyama T, Yamamoto M, Miyaura N. Chem. Commun. 1997:689.Clegg W, Thorsten J, Marder TB, Norman NC, Orpen AG, Peakman TM, Quayle MJ, Rice CR, Scott AJ. J. Chem. Soc., Dalton Trans. 1998:1431.Morgan JB, Morken JP. Org. Lett. 2003;5:2573. doi: 10.1021/ol034936z.
- (7) (a).For asymmetric silylboration of dienes: Gerdin M, Moberg C. Adv. Synth. Catal. 2005;347:749.
- (8). B2(pin)2 is a readily available inexpensive reagent ($800/kg, AllyChem, Co., Ltd. ( www.allychem.com)
- (9) (a).Burks HE, Liu S, Morken JP. J. Am. Chem. Soc. 2007;129:8766. doi: 10.1021/ja070572k. [DOI] [PubMed] [Google Scholar]; (b) Pelz NF, Woodward AR, Burks HE, Sieber JD. J. Am. Chem. Soc. 2004;126:16328. doi: 10.1021/ja044167u. [DOI] [PubMed] [Google Scholar]
- (10) (a).Ishiyama T, Matusuda N, Miyaura N, Suzuki A. J. Am. Chem. Soc. 1993;115:11018.Ishiyama T, Matsuda N, Murata M, Ozawa F, Suzuki A, Miyaura N. Organometallics. 1996;15:713. For Computational studies comparing Pd and Pt in diboration, see:Cui Q, Musaev DG, Morokuma K. Organometallics. 1998;17:742.Sakaki S, Kikuno T. Inorg. Chem. 1997;36:226.
- (11) (a).Sakaki J, Schweizer WB, Seebach D. Helv. Chem. Acta. 1993;76:2654. [Google Scholar]; (b) Seebach D, Hayakawa M, Sakaki J, Schweizer WB. Tetrahedron. 1993;49:1711. [Google Scholar]; (c) Alexakis A, Burton J, Vastra J, Benhaim C, Fournioux X, van den Heuvel A, Leveque J-M, Maze F, Rosset S. European Journal of Organic Chemistry. 2000:4011. [Google Scholar]; (d) Bee C, Han SB, Hassan A, Iida H, Krische MJ. J. Am. Chem. Soc. 2008;130:2746. doi: 10.1021/ja710862u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Seitz M, Reiser O. Curr. Opinion in Chem. Biol. 2005;9:285. doi: 10.1016/j.cbpa.2005.03.005. [DOI] [PubMed] [Google Scholar]
- (13).Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994:639. [Google Scholar]
- (14) (a).Hoffmann RW. Pure Appl. Chem. 1988;60:123. [Google Scholar]; (b) Hoffmann RW, Neil G, Schlapbach A. Pure. Appl. Chem. 1990;62:1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.