Abstract
Numerous cross-sectional studies demonstrate an inverse association between plasma 25-hydroxyvitamin D (25[OH]D) and blood pressure or hypertension. Prospective data, however, are limited. Among 1,484 women aged 32-52 years who did not have hypertension at baseline, we prospectively analyzed the association between plasma levels of 25(OH)D and the odds of incident hypertension using a nested case-control study design. We matched cases and controls on age, race, and month of blood collection, and further adjusted for BMI, physical activity, family history of hypertension, oral contraceptive use, and plasma levels of parathyroid hormone, calcium, phosphorous, creatinine, and uric acid. Median plasma 25(OH)D levels were lower in the cases (25.6 ng/mL) than in the controls (27.3 ng/mL, p<0.001). Women in the lowest compared to highest quartile of plasma 25(OH)D had an adjusted OR for incident hypertension of 1.66 (1.11-2.48; p-trend=0.01). Compared to women with sufficient levels, those with vitamin D deficiency (<30 ng/mL, 65.7% of the study population) had a multivariable OR of 1.47 (1.10-1.97). Plasma 25(OH)D levels are inversely and independently associated with risk of developing hypertension.
Keywords: risk factors, hypertension, epidemiology, vitamin D
Introduction
The effect of vitamin D on calcium and phosphate homeostasis is well known.1 However, the intracellular vitamin D receptor is broadly distributed among a variety of tissues, including leukocytes, vascular smooth muscle cells (VSMC), and juxtaglomerular cells, implying other potential effects of vitamin D.2-4 In addition, the 1α-hydroxylase enzyme (which converts 25[OH]D to 1,25[OH]2D) is also widely distributed, expressed in endothelial cells, VSMC, macrophages,5 and various locations in the kidney.6-9 Experimental studies demonstrate that 1,25(OH)2D inhibits renin expression,4, 10 enhances insulin secretion and sensitivity,11, 12 and blocks proliferation of VSMC.13
Although cross-sectional studies demonstrate that 25(OH)D levels, and skin exposure to ultraviolet B radiation (the major source of vitamin D),14, 15 are associated with lower blood pressure,16-22 prospective data are limited. An interventional study conducted in 148 vitamin D deficient elderly women demonstrated a 9% decrease in systolic blood pressure with supplemental vitamin D and calcium compared to calcium alone.23 In a previous prospective study of older women (first Nurses' Health Study) and men (Health Professionals Follow-up Study) we demonstrated an association between deficient levels of 25(OH)D and risk of incident hypertension after adjusting for age, race, BMI, and physical activity. However, that study had limited statistical power (190 incident cases),24 and we did not measure plasma parathyroid hormone (PTH), calcium, or creatinine, factors that are associated with 25(OH)D and which may influence the risk of hypertension.25-28
In order to determine the independent association between plasma 25(OH)D levels and risk of incident hypertension, we conducted a prospective nested case-control study of 1,484 young, healthy women from the second Nurses' Health Study.
Methods
Study Population
The Nurses' Health Study 2 (NHS2) is an ongoing prospective cohort study of 116,671 female registered nurses that began in 1989. Participants are followed via biennial questionnaires that gather information on health-related behaviors and medical events. Follow-up of participants was >90% through 2005. During the years 1997-1999, 29,616 participants contributed blood samples that were stored in liquid nitrogen (-130 C). We conducted a nested case-control study of incident hypertension among those women who contributed blood samples and who did not have prevalent hypertension at the time of blood collection. The institutional review board at Brigham and Women's Hospital reviewed and approved this study.
We selected cases and controls from among those who met the following criteria at the time of blood collection: (1) fasting for at least 8 hours; (2) no diagnosis of hypertension; (3) no use of anti-hypertensive medications; (4) no diagnosis of cancer (except non-melanoma skin cancer); (5) no diagnosis of either coronary heart disease or diabetes; and (6) BMI < 30 kg/m2. This last eligibility criterion is important because high BMI is a powerful predictor of hypertension29, 30 and of 25(OH)D levels.22, 31
Using risk-set sampling, we selected 750 cases who subsequently developed hypertension and 750 controls who did not develop hypertension. Controls were matched to cases on the following factors: age (within 1 year), race, date of blood sample collection (within 1 month), day of menstrual cycle if pre-menopausal (within 2 days), and time of day of the blood collection (within 2 hours). In addition, controls were required to have had at least one clinician examination during the two years prior to being selected as a control. After excluding eight case-control pairs with missing biomarker data, the final study population included 742 case-control pairs (N=1,484).
Biomarker Measurement
The plasma concentration of 25(OH)D was determined by an enzyme immunoassay from Immunodiagnostic Systems Inc. (Fountain Hills, AZ). This assay is sensitive down to a 25(OH)D concentration of 2.0 ng/mL. The coefficient of variation (CV) using quality control samples was 3.2%. Intact PTH was measured by an electrochemiluminescence immunoassay on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN); the CV for this assay was 13.4%. Other plasma factors measured in participants of this study included: calcium (colorimetric assay, CV=3.6%); phosphorous (photometric assay, CV=9.5%), uric acid (oxidization with uricase to form allantoin and H2O2, CV=3.4%); and creatinine (modified Jaffe method, CV=6.5%).
Ascertainment of Other Covariates
Age and BMI (weight in kilograms divided by the height in meters squared) were obtained from the supplemental questionnaire that accompanied the submitted blood samples. Smoking status (never, past, current), physical activity (in metabolic equivalent task scores; METS), current oral contraceptive use (yes, no), and alcohol intake (g/d) were ascertained from the biennial questionnaire that immediately followed submission of the blood sample (typically the 1999 biennial questionnaire). Family history of hypertension was obtained from the 1989 questionnaire; race was self-classified.
Ascertainment of Hypertension
Clinician-diagnosed hypertension was self-reported by these health professionals on biennial questionnaires. To validate hypertension self-report in these nurses, we obtained relevant medical records from a sub-set of randomly selected NHS2 participants who self-reported a new diagnosis of hypertension on the 2005 biennial questionnaire, as well as randomly selected participants who denied this diagnosis in 2005 and in every prior year. The sensitivity of self-reported hypertension was 94%. The specificity of a nurse reporting no diagnosis of hypertension was 85%.
Women were considered to have prevalent hypertension at the time of blood collection if they reported hypertension on the biennial questionnaire immediately following their blood collection or on any prior questionnaire. This study was prospective; women with prevalent hypertension or women who reported taking anti-hypertensive medications on the questionnaire immediately following blood collection were not considered for selection as a case or control.
Statistical Analyses
Because the continuous baseline variables, including the levels of 25(OH)D and PTH, were not normally distributed, differences between these variables among cases and controls were analyzed using the Wilcoxon rank-sum test. Differences in categorical variables between cases and controls were compared using the chi square test.
The association between plasma 25(OH)D levels and incident hypertension was analyzed using conditional logistic regression conditioning on the matching factors to generate odds ratios (OR) and 95% confidence intervals (95% CI). Multivariable models included the following a priori potential confounders plus those factors with univariate associations with hypertension at baseline: BMI, physical activity, oral contraceptive use, family history of hypertension, and levels of PTH, calcium, phosphorous, creatinine, and uric acid. Plasma levels of 25(OH)D were initially examined in quartiles, with the highest quartile defined as the reference group. In an additional analysis, we examined the odds of hypertension among women whose 25(OH)D level was <30 ng/mL (the definition of vitamin D deficiency)26 compared to women with sufficient levels.
Based upon our prior analysis of this association in other cohorts,24 and because the biologic relationship between 25(OH)D and PTH levels are non-linear (with a 25[OH]D inflection point of approximately 30 ng/mL),26 we decided a priori to analyze plasma 25(OH)D levels categorically as just described. Nevertheless, we performed a secondary analysis of plasma 25(OH)D levels as a continuous variable and computed adjusted ORs for a 5 ng/mL decrease in 25(OH)D. We also tested whether the association between a 5 ng/mL decrease in 25(OH)D level and odds of hypertension varied significantly by vitamin D status (deficient vs. sufficient).
The population attributable risk was calculated for vitamin D deficiency using the adjusted OR from the multivariable model and with the sufficient group defined as the “unexposed” group. A baseline incidence rate of 14.6 cases per 1000 women annually (1.46% of the population per year) for the unexposed group was determined using the incidence rate for the parent cohort (NHS2).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written
Results
Baseline Characteristics
The baseline characteristics of the entire study population by case status are shown in Table 1. The median age of the population was 43 years, and because this was a matching factor, did not differ. The median BMI was higher among the cases (25.1 kg/m2) compared to controls (23.2 kg/m2). Cases were also less physically active, were more likely to have a family history of hypertension, and to have used of oral contraceptives.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | All (n=1,484) | Cases (n=742) | Controls (n=742) | P-value |
|---|---|---|---|---|
| 25-hydroxyvitamin D (ng/mL) | 26.6 (21.0-32.3) | 25.6 (20.3-31.0) | 27.3 (21.7-33.3) | <0.001 |
| Demographic variables | ||||
| Age (y) | 43 (40-46) | 43 (40-46) | 43 (40-46) | matching factor |
| BMI (kg/m2) | 24.1 (22.0-26.6) | 25.1 (22.8-27.5) | 23.2 (21.1-25.4) | <0.001 |
| Physical activity (METS) | 12.3 (5.0-25.9) | 11.2 (4.6-23.7) | 13.3 (5.4-27.2) | 0.006 |
| Current smoker (%) | 5.1 | 5.8 | 4.5 | 0.24 |
| Past smoker (%) | 22.6 | 23.3 | 22.0 | 0.53 |
| Alcohol intake (g/d) | 1.6 (0-5.5) | 1.5 (0-5.8) | 1.8 (0-5.1) | 0.94 |
| Family history of hypertension (%) | 55.2 | 62.8 | 47.6 | <0.001 |
| Oral contraceptive use, ever (%) | 85.1 | 86.8 | 83.3 | 0.06 |
| Current oral contraceptive use (%) | 5.1 | 6.4 | 3.8 | 0.03 |
| Physiologic variables | ||||
| Parathyroid hormone (pg/mL) | 30.9 (23.6-39.3) | 31.7 (24.1-40.4) | 30.1 (23.1-38.0) | 0.02 |
| Calcium (mg/dL) | 9.3 (9.0-9.6) | 9.3 (9.0-9.6) | 9.3 (9.0-9.6) | 0.88 |
| Phosphorous (mg/dL) | 4.4 (4.1-4.8) | 4.4 (4.1-4.8) | 4.5 (4.1-4.8) | 0.08 |
| Uric acid (mg/dL) | 3.9 (3.4-4.6) | 4.1 (3.5-4.7) | 3.7 (3.2-4.4) | <0.001 |
| Creatinine (mg/dL) | 0.79 (0.72-0.86) | 0.78 (0.71-0.86) | 0.80 (0.73-0.87) | 0.14 |
Continuous variables are expressed as median (interquartile range). Categorical variables are expressed as percent.
Continuous variables were analyzed using the Wicoxon rank-sum test, and categorical variables with the chi square test.
The median plasma level of 25(OH)D was lower among cases (25.6 ng/mL) compared to controls (27.3 ng/mL; p<0.001). The median plasma levels of PTH (31.7 pg/mL among cases and 30.1 pg/mL among controls; p=0.02) and uric acid (4.1 mg/dL among cases and 3.7 mg/dL among controls; p<0.001) were higher among cases. Calcium, phosphorous, and creatinine levels were not significantly different between cases and controls.
Plasma 25(OH)D and PTH levels were correlated. However, this correlation was present only among the subgroup of women with vitamin D deficiency. The spearman correlation coefficient was -0.22 (p<0.0001) among women whose plasma 25(OH)D level was <30 ng/mL, and was -0.08 (p=0.06) among women whose level was ≥30 ng/mL.
25-Hydroxyvitamin D Level and Risk of Incident Hypertension
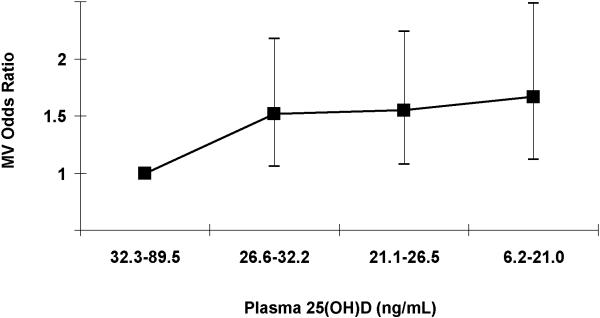
In logistic regression models conditioned on matching factors (age, race, month of blood sample collection, day of menstrual cycle if pre-menopausal, and hour of blood collection), women in the lowest quartile (6.2-21.0 ng/mL) compared to highest (32.3-89.5 ng/mL) of 25(OH)D had an OR for incident hypertension of 2.21 (95% CI, 1.57-3.12, p-trend<0.001; Table 2). After also adjusting for BMI, physical activity, family history of hypertension, oral contraceptive use, and plasma levels of PTH, calcium, phosphorous, creatinine, and uric acid, the OR comparing the lowest to highest quartile of 25(OH)D was 1.66 (95% CI, 1.11-2.48, p-trend=0.01; Table 2 and Figure 1). PTH was not independently associated with hypertension.
Table 2.
Quartiles of Plasma 25-Hydroxyvitmain D and Odds of Incident Hypertension
| Median (range), ng/mL | 37.9 (32.3-89.5) | 29.2 (26.6-32.2) | 23.8 (21.1-26.5) | 16.7 (6.2-21.0) | P-trend |
|---|---|---|---|---|---|
| No. of participants | 369 | 374 | 370 | 371 | |
| No. of cases | 151 | 195 | 188 | 208 | |
| Model 1 | 1.0 (ref) | 1.74 (1.27-2.37) | 1.74 (1.27-2.39) | 2.21 (1.57-3.12) | <0.001 |
| Model 2 | 1.0 (ref) | 1.52 (1.06-2.18) | 1.55 (1.07-2.23) | 1.66 (1.11-2.48) | 0.01 |
Results are OR (95% CI).
Model 1: conditioned on matching factors (age, race, month of blood sample collection, day of menstrual cycle if premenopausal, and hour of the blood collection).
Model 2: conditioned on matching factors, and adjusted for BMI, physical activity, family history of hypertension, oral contraceptive use (past and current), creatinine, PTH, calcium, phosphorous, and uric acid.
Figure 1. Quartiles of Plasma 25-Hydroxyvitmain D and Adjusted Odds of Incident Hypertension.
Quartiles arranged from highest to lowest. MV, multivariable. P-trend = 0.01.
The prevalence of vitamin D deficiency (<30 ng/mL) in our study population was 65.7%. We analyzed the odds of developing hypertension among women who were vitamin D deficient compared to those with sufficient levels (Table 3). Vitamin D deficient women had a 47% increased odds (OR=1.47; 95% CI, 1.10-1.97) after multivariable adjustment.
Table 3.
Plasma 25-Hydroxyvitamin D Deficiency and Odds of Incident Hypertension
| 25(OH)D level, ng/mL | ≥ 30.0 | < 30.0 |
|---|---|---|
| No. of participants | 509 | 975 |
| No. of cases | 222 | 520 |
| Model 1 | 1.0 (ref) | 1.64 (1.29-2.10) |
| Model 2 | 1.0 (ref) | 1.47 (1.10-1.97) |
Results are OR (95% CI).
Model 1: conditioned on matching factors (age, race, month of blood sample collection, day of menstrual cycle if premenopausal, and hour of the blood collection).
Model 2: conditioned on matching factors, and adjusted for BMI, physical activity, family history of hypertension, oral contraceptive use (past and current), creatinine, PTH, calcium, phosphorous, and uric acid.
We also analyzed plasma 25(OH)D levels as a continuous variable. Every 5 ng/mL lower 25(OH)D level was associated with an adjusted OR for incident hypertension of 1.08 (95% CI, 1.01-1.15). However, in the subgroup of women with vitamin D deficiency, every 5 ng/mL lower 25(OH)D level was associated with an adjusted OR of 1.11; the OR among women with sufficient vitamin D status was 0.97 for every 5 ng/mL lower 25(OH)D level (p-interaction = 0.12).
Population Attributable Risk
Assuming an incidence rate for hypertension among young women of 14.6 cases per 1000 women annually (derived from the parent cohort), the estimated incidence rate among young women with vitamin D deficiency is 21.5 cases per 1000 women annually. Given that 65.7% of women were vitamin D deficient, the population risk attributable to vitamin D deficiency is 4.53 new cases of hypertension per 1000 young women annually. If this association were causal, then vitamin D deficiency may account for 23.7% of all new cases of hypertension developing among young women every year.
Discussion
Among 1,484 non-obese young women without hypertension, diabetes, or coronary disease at baseline, lower levels of plasma 25(OH)D levels independently predicted clinically important differences in the odds of subsequently developing hypertension. Furthermore, almost two thirds of the population had vitamin D deficiency; if this association were causal, then a substantial proportion of hypertension incidence among young women could be attributed to sub-optimal levels of 25(OH)D.
Cross-sectional observations support a relation between vitamin D and blood pressure. Older studies examined UVB radiation as a surrogate marker of vitamin D synthesis in the skin, which declines with increasing distance from the equator and is lower in the winter compared to summer.14 In the INTERSALT study, which examined over 10,000 participants from around the world, systolic and diastolic blood pressure were significantly and positively associated with distance from the equator.16, 17 Further evidence comes from geographical differences in blood pressure among individuals of African origin, with those residing in northern regions having higher blood pressure than those residing closer to the equator.18 Several studies have shown seasonal variations within the same population, with blood pressure peaking in winter and falling in summer.19, 20 More recently, in an analysis of the third National Health and Nutrition Examination Survey, the prevalence of hypertension was 30% higher among individuals in the lowest compared to highest quartile of 25(OH)D.22
Prospective studies of this association are few and small in size. For example, Kruse et al. randomized eighteen patients with mild hypertension to receive UVB exposure or UVA (ultraviolet A does not produce vitamin D) three times weekly for six weeks.21 Along with a 162% rise in plasma 25(OH)D in the UVB group, both systolic and diastolic blood pressure fell by 6 mmHg. No change was observed with UVA exposure.21 Another small interventional trial conducted in 148 vitamin D deficient elderly women demonstrated that 800 IU/d of oral vitamin D for six weeks lowered systolic blood pressure by 9.3%.23 In a prospective cohort study of older men and women we previously demonstrated that vitamin D deficient individuals had a 3.2-fold higher risk of hypertension incidence compared to those with optimal levels after adjusting for age, race, BMI, and physical activity; however, that study had limited statistical power (only 190 cases), and lacked measurement of other potential confounders such as PTH, renal function, and other circulating biomarkers.24
Several potential mechanisms for an association between 25(OH)D and hypertension have been suggested. First, Li et al. showed that 1,25(OH)2D, the result of 1-hydroxylation of 25(OH)D, inhibits renin expression in mice.4 Second, lower levels of 25(OH)D are associated with insulin resistance,25 and vitamin D therapy may enhance insulin secretion and insulin sensitivity.11, 12 Insulin resistance has been proposed to be involved in the pathogenesis of hypertension.32 Third, 1,25(OH)2D inhibits growth of cultured VSMC in vitro.13 Thus the vitamin D - hypertension association may be mediated the renin-angiotensin system, insulin resistance, and vascular function.
Furthermore, the 1α-hydroxylase enzyme that converts 25(OH)D to 1,25(OH)2D is expressed in a variety of tissues, including human endothelial cells, human VSMC, macrophages,5 and throughout the kidney.6-9 Therefore, 25(OH)D may have biologic effects that are independent of measurable circulating 1,25(OH)2D levels; this challenges the traditional notion that biologic activity of vitamin D is primarily dependent upon conversion in the renal proximal tubule.
Our study has limitations that deserve mention. First, we relied on self-reported hypertension and did not directly measure the blood pressure of our participants; however, all participants are registered nurses, and we demonstrated that hypertension reporting by participants of this cohort is highly sensitive. Second, the specificity of hypertension reporting may have resulted in the misclassification of a few truly hypertensive individuals as being non-hypertensive controls; however, such misclassification would tend to diminish the magnitude of the odds ratio. Therefore, our findings may indeed be an underestimate of the true association. Third, we purposefully restricted our sample to women with BMI values < 30 kg/m2. Although this limits the generalizability of our findings to non-obese women, obesity is such a powerful risk factor for both hypertension and reduced 25(OH)D levels, inclusion of a large number of obese women may have obscured a true association.30, 31 Fourth, we had a single measurement of 25(OH)D; because levels may fluctuate over time, longer periods of follow-up may result in more random misclassification. However, as with misclassification of hypertension status, this would tend to diminish the magnitude of the odds ratio, thereby underestimating the true association. Fifth, our study population was almost entirely white and, therefore, not necessarily generalizable to non-whites. Finally, we had insufficient power to demonstrate that the association between plasma 25(OH)D levels and incident hypertension differed according to vitamin D status (deficient vs. sufficient). Nevertheless, the shape of the association depicted in Figure 1, as well as the lack of any association among women with sufficient vitamin D levels suggests a non-linear association.
Perspectives
Our prospective analysis suggests that lower plasma 25(OH)D levels are independently associated with a higher risk of incident hypertension. Given the high prevalence of vitamin D deficiency plus the availability of relatively cheap and effective interventions to raise 25(OH)D levels, these results could have substantial public health implications. Our findings should be tested in randomized trials to determine whether vitamin D supplementation could be effective in reducing blood pressure.
Acknowledgments
SOURCES OF FUNDING This study was funded by the American Heart Association grant 0535401T, and NIH grants HL079929-01A2 and CA50385.
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES None.
References
- 1.Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- 2.Henry HL, Norman AW. Vitamin D: metabolism and biological actions. Annu Rev Nutr. 1984;4:493–520. doi: 10.1146/annurev.nu.04.070184.002425. [DOI] [PubMed] [Google Scholar]
- 3.Minghetti PP, Norman AW. 1,25(OH)2-vitamin D3 receptors: gene regulation and genetic circuitry. Faseb J. 1988;2:3043–3053. doi: 10.1096/fasebj.2.15.2847948. [DOI] [PubMed] [Google Scholar]
- 4.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richart T, Li Y, Staessen JA. Renal versus extrarenal activation of vitamin D in relation to atherosclerosis, arterial stiffening, and hypertension. Am J Hypertens. 2007;20:1007–1015. doi: 10.1016/j.amjhyper.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ, Haussler MR, Rauterberg EW, Ritz E. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 8.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 9.Zehnder D, Bland R, Walker EA, Bradwell AR, Howie AJ, Hewison M, Stewart PM. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in the human kidney. J Am Soc Nephrol. 1999;10:2465–2473. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]
- 10.Qiao G, Kong J, Uskokovic M, Li YC. Analogs of 1alpha,25-dihydroxyvitamin D(3) as novel inhibitors of renin biosynthesis. J Steroid Biochem Mol Biol. 2005;96:59–66. doi: 10.1016/j.jsbmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57:258–261. [PubMed] [Google Scholar]
- 12.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 13.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 14.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 15.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–189. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 16.Intersalt Cooperative Research Group Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Bmj. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 18.Cooper R, Rotimi C. Hypertension in populations of West African origin: is there a genetic predisposition? J Hypertens. 1994;12:215–227. [PubMed] [Google Scholar]
- 19.Kunes J, Tremblay J, Bellavance F, Hamet P. Influence of environmental temperature on the blood pressure of hypertensive patients in Montreal. Am J Hypertens. 1991;4:422–426. doi: 10.1093/ajh/4.5.422. [DOI] [PubMed] [Google Scholar]
- 20.Woodhouse PR, Khaw KT, Plummer M. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J Hypertens. 1993;11:1267–1274. [PubMed] [Google Scholar]
- 21.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 22.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 24.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 25.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007;71:134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 27.Jorde R, Svartberg J, Sundsfjord J. Serum parathyroid hormone as a predictor of increase in systolic blood pressure in men. J Hypertens. 2005;23:1639–1644. doi: 10.1097/01.hjh.0000179764.40701.36. [DOI] [PubMed] [Google Scholar]
- 28.St John A, Dick I, Hoad K, Retallack R, Welborn T, Prince R. Relationship between calcitrophic hormones and blood pressure in elderly subjects. Eur J Endocrinol. 1994;130:446–450. doi: 10.1530/eje.0.1300446. [DOI] [PubMed] [Google Scholar]
- 29.Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A prospective study of body mass index and the risk of developing hypertension in men. Am J Hypertens. 2007;20:370–377. doi: 10.1016/j.amjhyper.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 32.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]