Abstract
T cells can be engineered to express the genes of chimeric antigen receptors (CARs) that recognize tumor-associated antigens. We constructed and compared two CARs that contained a single chain variable region moiety (scFv) that recognized CD19. One CAR contained the signaling moiety of the 4-1BB molecule and the other did not. We selected the CAR that did not contain the 4-1BB moiety for further pre-clinical development. We demonstrated that gammaretroviruses encoding this receptor could transduce human T cells. Anti-CD19-CAR-transduced CD8+ and CD4+ T cells produced interferon-γ and interleukin-2 specifically in response to CD19+ target cells. The transduced T cells specifically killed primary chronic lymphocytic leukemia (CLL) cells. We transduced T cells from CLL patients that had been previously treated with chemotherapy. We induced these T cells to proliferate sufficiently to provide enough cells for clinical adoptive T cell transfer with a protocol consisting of an initial stimulation with an anti-CD3 monoclonal antibody (OKT3) prior to transduction followed by a second OKT3 stimulation seven days after transduction. This protocol was successfully adapted for use in CLL patients with high peripheral blood leukemia cell counts by depleting CD19+ cells prior to the initial OKT3 stimulation. In preparation for a clinical trial that will enroll patients with advanced B cell malignancies, we generated a producer cell clone that produces retroviruses encoding the anti-CD19 CAR, and we produced sufficient retroviral supernatant for the proposed clinical trial under good manufacturing practice (GMP) conditions.
Keywords: Chimeric antigen receptor, gene therapy, CD19, T cell, gammaretrovirus, adoptive T cell therapy
Introduction
Approximately twenty-two thousand people die of B cell malignancies each year in the United States 1. Patients with some B cell malignancies including mantle cell lymphoma and chronic lympocytic leukemia (CLL) cannot be cured by therapies such as conventional chemotherapy and monoclonal antibodies 2,3, but some patients with these diseases can achieve prolonged disease-free survival after allogeneic stem cell transplantation 4-6. Unfortunately, allogeneic stem cell transplantation is limited by significant transplant-related mortality and a shortage of suitable donors 2,6,7. In patients with B cell malignancies that relapse after allogeneic stem cell transplantation, infusion of allogeneic donor lymphocytes can induce remissions 8-10. The effectiveness of these lymphocyte infusions provides a rationale for attempts to develop other cellular immunotherapies for B cell malignancies.
Adoptive transfer of autologous T cells that are cultured from tumor infiltrating lympohocytes can cause regressions of advanced melanoma in humans 11,12. Because tumor-reactive T cells cannot be reliably cultured from most human tumors, methods have been developed to engineer T cells to express genes encoding tumor-antigen-specific T cell receptors 13,14. Adoptive transfer of these genetically-modified T cells is a promising approach to cancer immunotherapy 15. Another approach to adoptive T cell therapy is to engineer T cells to express chimeric antigen receptors (CARs) 16,17. CARs are made up of an antigen-recognizing receptor coupled to signaling molecules that can activate T cells expressing the CAR 18-20. The antigen-receptors most commonly incorporated into CARs are single chain variable region moieties (scFv) that consist of the light chain and heavy chain variable regions of a monoclonal antibody joined by a peptide linker. Murine models have shown that T cells transduced with retroviruses encoding CARs can protect mice from tumor challenges in vivo 21,22.
Our group has completed a phase I clinical trial in which patients with ovarian carcinoma were treated with T cells that were transduced with a CAR that was specific for the ovarian-carcinoma-associated-antigen α-folate receptor 23. No objective tumor regressions were seen 23. The CAR used in this clinical trial incorporated the Fc receptor-γ cytoplasmic signaling chain without any costimulatory molecules such as CD28 or 4-1BB. More recent work in mice has demonstrated that CARs containing the TCR-ζ cytoplasmic signaling chain had superior in vitro function and in vivo anti-tumor efficacy than CARs containing the Fc receptor-γ cytoplasmic signaling chain 24. In addition, in vitro studies with human cells and murine in vivo studies have shown that incorporating the signaling domain of CD28 into CARs enhances function and in vivo anti-tumor efficacy 22,25-27. Signaling of the 4-1BB costimulatory molecule has been shown to enhance T cell proliferation and persistence 28,29, and 4-1BB signaling enhanced the function of CARs in vitro 30,31. Thus, significant advances in CAR design have occurred since our last clinical trial using CAR-transduced T cells.
CD19 is a promising target for antigen-specific T cell therapies because CD19 is expressed on most malignant B cells 32,33, and the only normal cells that express CD19 are B cells and perhaps follicular dendritic cells 33,34. Importantly, CD19 is not expressed on pluripotent hematopoietic stem cells 35. While destruction of normal B cells is a drawback to targeting CD19, destruction of normal B cells is well tolerated. In most patients that receive the widely-used anti-CD20 monoclonal antibody rituximab, the number of normal peripheral blood B cells is severely depressed for several months 36, yet patients that receive chemotherapy plus rituximab do not have an increased rate of infections when compared to patients that receive chemotherapy alone 37. Finally, patients can be treated with intravenous infusions of IgG if necessary to increase IgG levels 38.
We constructed two CARs that target CD19 and selected the one with the best in vitro function for further testing in preparation for a clinical trial. T cells that were transduced with gammaretroviruses encoding this CAR recognized CD19-expressing cells in an antigen-specific manner and killed primary B cell chronic lymphocytic leukemia (CLL) cells. We have optimized methods of T cell culture and transduction to generate highly active anti-CD19-CAR-expressing T cells from the blood of patients with CLL.
Materials and Methods
Construction of the MSGV-FMC63-28Z and MSGV-FMC63-CD828BBZ Recombinant Retroviral Vectors
The MSGV-FMC63-28Z recombinant retroviral vector encodes the MSGV (mouse stem cell virus-based splice-gag vector) retroviral backbone and the FMC63-28Z CAR. The FMC63-28Z CAR consists of an anti-CD19 scFv that was derived from the FMC63 mouse hybridoma 39, a portion of the human CD28 molecule, and the intracellular component of the human TCR-ζ molecule. The exact sequence of the CD28 molecule included in the FMC63-28Z CAR corresponds to Genbank identifier NM_006139. The sequence includes all amino acids starting with the amino acid sequence IEVMYPPY and continuing all the way to the carboxy-terminus of the protein. To encode the anti-CD19 scFv component of the vector, we designed a DNA sequence which was based on a portion of a previously published CAR 40. This sequence encodes the following components in frame from the 5′ end to the 3′ end: an XhoI site, the human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor α-chain signal sequence, the FMC63 light chain variable region 39, a linker peptide 40, the FMC63 heavy chain variable region 39, and a NotI site. This sequence was synthesized by GeneArt AG (Regensburg, Germany), and a plasmid encoding this sequence was digested with XhoI and NotI (New England Biolabs). To form the MSGV-FMC63-28Z retroviral vector, the XhoI and NotI-digested fragment encoding the FMC63 scFv was ligated into a second XhoI and NotI-digested fragment that encoded the MSGV retroviral backbone 14 as well as part of the extracellular portion of human CD28, the entire transmembrane and cytoplasmic portion of human CD28, and the cytoplasmic portion of the human TCR-ζ molecule 41.
We also designed a second recombinant retroviral vector called MSGV-FMC63-CD828BBZ that consists of the following components in order from 5′ to 3′: the MSGV retroviral backbone, the FMC63 scFv, the hinge and transmembrane regions of the CD8 molecule, the cytoplasmic portions of CD28 and 4-1BB, and the cytoplasmic component of the TCR-ζ molecule. MSGV-FMC63-CD828BBZ was constructed using a multistep strategy. A fragment encoding the CD8, CD28, 4-1BB, and TCR-ζ components was generated using an overlapping PCR method 42 using the following primers:
NotI-CD8F: 5′-acgGCGGCCGCAttcgtgccggtcttcctgc-3′;
28cyto-CD8R: 5′- gcctgctcctcttactcctgttcctgtggttgcagtaaag-3′;
CD8-28cytoF: 5′- ctttactgcaaccacaggaacaggagtaagaggagcaggc-3′;
BamHI-zetaR: 5′- ttat GGATCC ttagcgagggggcagggcc-3′.
The NotI-CD8F and 28cyto-CD8R oligonucleotide primers were used to generate a PCR product that encoded the hinge and transmembrane region of CD8 by using human CD8α cDNA as a template. An overlapping fragment encoding the cytoplasmic portions of CD28, 4-1BB and TCR-ζ components in the order of CD28-4-1BB-CD3ζ was generated using forward primer CD8-28cytoF and the BamHI-zetaR reverse primer. The two PCR products were combined and the full-length construct generated using the NotI-CD8F and the BamHI-zetaR primers. DNA encoding this full-length construct and DNA encoding the FMC63 scFv were ligated into the MSGV retroviral backbone to form the MSGV-FMC63-CD828BBZ retroviral vector.
A plasmid encoding the SP6 scFv 43 was kindly provided by Z. Eshhar. This scFv was cloned into the MSGV retroviral vector along with a portion of the CD28 gene and the gene for cytoplasmic portion of the CD3ζ molecule to form the MSGV-SP6-28Z plasmid. This plasmid encoded a receptor that is referred to as SP6-28Z in this paper. The SP6-28Z receptor recognizes the hapten 2, 4, 6-trinitrobenzenesulfonic acid and served as a negative control.
Culture Media
T cells were always cultured in T cell medium which consisted of AIM V medium plus 5% AB serum (Gemini Bioproducts, Woodland, CA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1.25 μg/mL amphotericin B. R10 medium consisted of Roswell Park Memorial Institute (RPMI)1640 plus 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine. D10 medium consisted of Dubecco's Modified Eagle Medium, 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, nonessential amino acids, and 2 mM L-glutamine. SupB15 medium consisted of Iscove's Modified Dulbecco's Medium plus 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 0.055 mM 2-mercaptoethanol. Cytotoxicity medium was phenol red-free RPMI plus 5% AB serum (Gemini Bioproducts), 100 U/mL penicillin, and 100 μg/mL streptomycin. All cell culture media ingredients except the AB serum were from Invitrogen (Carlsbad, CA). Recombinant human IL-2 was obtained from Chiron (Emeryville, CA).
Transient Retrovirus Production
To transiently produce retroviruses, 293GP packaging cells 44 were transfected with either the MSGV-FMC63-28Z plasmid, the MSGV-FMC63-CD828BBZ plasmid, or the MSGV-SP6-28Z plasmid along with a plasmid encoding the RD114 envelope protein 45 using Lipofectamine 2000 (Invitrogen). The transfected cells were incubated at 37°C for 6-8 hours in D10 medium without antibiotics. The medium used for transfection was then replaced with fresh D10 medium and the cells were incubated for another 36-48 hours. During and after transfection, the 293GP cells were cultured on poly-D-lysine coated dishes (BD Biosciences, San Jose, CA). Supernatant containing retroviruses was removed from the dishes and centrifuged to remove cellular debris. The supernatant was snap frozen on dry ice and stored at -80C°. Transiently produced retroviruses were used in the experiments described in Figures 1 and 2 and in Table 1. In addition, all retroviruses encoding the SP6-28Z CAR were produced transiently.
Figure 1.
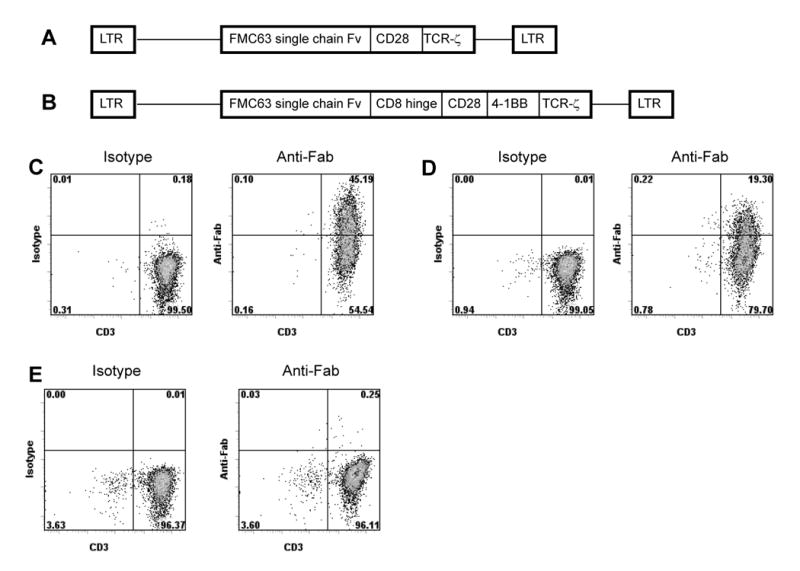
A comparison of the design and transduction efficiency of two anti-CD19 CARs. (A) A diagram of the recombinant retroviral vector MSGV-FMC63-28Z is shown (LTR, long terminal repeat; Fv, variable regions; CD28, part of the extracellular region and all of the transmembrane and intracellular regions of CD28; TCR-ζ, the entire cytoplasmic region of the TCR-ζ molecule). (B) A diagram of the recombinant retroviral vector MSGV-FMC63-CD828BBZ is shown (LTR, long terminal repeat; Fv, variable regions; CD8, CD8 hinge region; CD28, CD28 cytoplasmic region; 4-1BB, 4-1BB cytoplasmic region; TCR-ζ, the cytoplasmic region of the TCR-ζ molecule. (C) PBMC were started in culture with OKT3 and IL-2 on day 0. The cells were transduced with retroviruses encoding FMC63-28Z on days 2 and 3. On day 8, expression of FMC63-28Z was detected on 45% of T cells when the cells were stained with anti-Fab antibodies and anti-CD3. Staining with isotype-matched control antibodies and anti-CD3 is also shown. (D) A PBMC culture from the same donor as in (C) was initiated on day 0. Cells were transduced with retroviruses encoding FMC63-CD828BBZ on days 2 and 3, and stained with either anti-Fab antibodies or isotype-matched control antibodies on day 8 in the same manner as in (C). Expression of FMC63-CD828BBZ was detected on 19% of T cells. (E) A PBMC culture from the same donor as in (C) and (D) was initiated on day 0. The cells were not transduced. On day 8 the cells were stained with either anti-Fab or isotype-matched control antibodies in the same manner as in (C) and (D). This experiment was performed using cells from the same cultures that were tested in the experiments described in Table 1 and Figure 2. The results presented in (C), (D), and (E) are representative of six experiments that used cells from six different donors.
Figure 2.
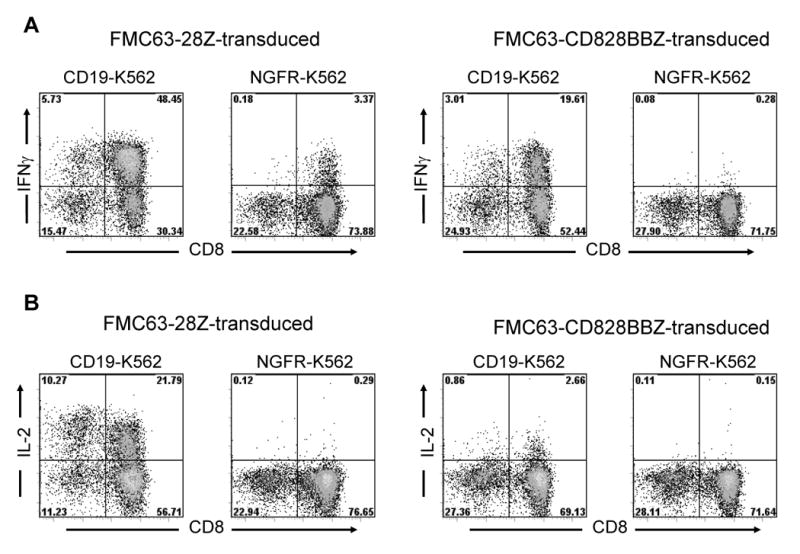
A comparison of cytokine production by T cells transduced with different anti-CD19 CARs. PBMC cultures were initiated on day 0 and transduced on days 2 and 3 after culture initiation. On day 11 after the cultures were initiated, cells that were transduced with either FMC63-28Z or FMC63-CD828BBZ were stimulated with either CD19-K562 cells or NGFR-K562 cells for 5 hours and intracellular staining for IFNγ (A) and IL-2 (B) was performed. The transduced T cells produced IFNγ and IL-2 in a CD19-specific manner. The plots are gated on CD3+ lymphocytes, and the percentage of cells in each quadrant is shown on the plots. This experiment was performed using cells from the same cultures that were tested in the experiments described in Figure 1 and Table 1. This experiment is representative of six experiments that used cells from six different donors.
Table 1.
IFNγ ELISA comparing FMC63-28Z and FMC63-CD828BBZ
| CD19+ targets | CD19 negative targets | ||||||
|---|---|---|---|---|---|---|---|
| bv173 | SupB15 | CLL | MDA231 | A549 | CCRF-CEM | Effectors alone | |
| Effector cells | |||||||
| FMC63-28Z | 17450 | 6150 | 2970 | 301 | 30 | 10 | 7 |
| FMC63-CD828BBZ | 13500 | 2700 | 640 | 15 | 7 | 20 | 7 |
| Nontransduced | 118 | 57 | 17 | 16 | 6 | 15 | 8 |
100,000 effector cells were cultured overnight with 100,000 target cells, and an interferon-γ ELISA was performed. Effector cells were T cells that were transduced with the FMC63-28Z CAR, T cells that were transduced with the FMC63-CD828BBZ CAR, or nontransduced T cells from the same donor that were cultured in the same manner. This assay was performed on day 8 after initiation of cultures. Transductions were performed on day 2 and day 3 after culture initiation. All values are pg/mL of IFNγ (mean of duplicate wells). CLL refers to primary CLL cells. This data is representative of the results obtained in two separate experiments with cells from two different donors. The cells used in this experiment were from the same cultures used in the experiments reported in Figure 1 and Figure 2.
Generation of the MSGV-FMC63-28Z PG13 Producer Cell Clone, H3
PG13 packaging cell clones were generated using the PG13 gibbon ape leukemia virus packaging cell line (ATCC, Manassass, VA), and the human ecotropic packaging cell line, Phoenix ECO (kindly provided by Gary Nolan, Stanford University, Stanford, CA). All cells were cultured in D10 medium without antibiotics. Cells were maintained at 37°C and 5% CO2.
A PG13 retroviral producer cell clone was generated as described previously 14 with the following changes. Phoenix ECO cells were transfected with 9 μg of plasmid DNA (MSGV-FMC63-28) using the Lipofectamine 2000 transfection reagent (Invitrogen). After 48h, supernatant was harvested and used to transduce retroviral packaging cell line, PG13. Non-tissue culture treated 6-well plates were coated with 20 μg/ml recombinant fibronectin fragment (RetroNectin™) as described by the manufacturer (Takara USA, Madison, WI). Retroviral vector supernatant (4ml) was added to each well followed by centrifugation (2000 × g) at 32°C for 3 hours. After centrifugation, supernatant was removed and 5 × 105 PG13 cells were added to each well, and the plates were centrifuged (1000 × g) for 10 min at 32°C. The transduction was repeated the next day, and then PG13 producer cell clones were generated by limiting dilution cloning. Due to lack of a selectable marker, high titer clones were identified by RNA dot blot as described previously 14,46. Retroviral supernatant from the 6 highest titer clones was generated. Briefly, 175 cm2 tissue culture flasks (Nunc, Cole-Parmer, Vernon Hills, IL) were seeded at 4 × 104 cells/cm2. A medium exchange (30ml) was performed on day 3. Supernatant was harvested 24h later and aliquoted. The supernatant was stored at -80°C until further use. Supernatant from each clone was evaluated by transducing T cells as described below under “Retroviral Transductions” and measuring CAR expression on the surface of transduced T cells as described below under “CAR Detection on Transduced T cells”. The ability of the transduced T cells to produce interferonγ (IFNγ) in a CD19-specific manner was measured in an enzyme-linked immunosorbent (ELISA) assay. One clone, which was designated H3, was selected for production of a master cell bank and subsequent production of retroviral supernatant under Good Manufacturing Practice (GMP) conditions.
MSGV-FMC63-28Z Retrovirus Production Using PG13 Producer Cell Clone H3 under Good Manufacturing Practice (GMP) conditions
A total of 26 1700 cm2 expanded surface roller bottles were seeded on day 0 at a cell density of 4 × 104 clone H3 cells/cm2 in 200 mL of D10 medium. On day 3, the medium was exchanged and replaced with 120 mL D10 medium. Medium containing the retroviral vector was harvested daily with bottles being refed with 120 mL of medium. Glucose levels were monitored daily using Roche's Accu-check system (Roche, Basal, Switzerland). If glucose levels dropped below 2 g/l, the volume of the medium exchange was doubled to 240ml/roller bottle for all subsequent harvests. All harvests were aliquoted and stored at -80°C until further use. All clinical products were subjected to an extensive biosafety testing program in accordance with current regulatory guidelines 47-50.
Patient Samples and Cell Lines
Non-leukemic PBMC samples were obtained from melanoma patients that were enrolled on institutional review board-approved protocols in the Surgery Branch of the National Cancer Institute (NCI). Leukemic PBMC were obtained from patients with chronic lymphocytic leukemia (CLL) that were enrolled on NCI protocol number 1997-C-0178. PBMC were cryopreserved in 90% FBS plus 10% DMSO (Sigma, St. Louis, MO). The following CD19-expressing immortalized cell lines were used: bv173 (chronic myeloid leukemia in lymphoid blast crisis, a kind gift of Dr. A. Wiestner National Heart Lung and Blood Institute, Bethesda, MD), NALM-6 (acute lymphoid leukemia from DSMZ, Braunschweig, Germany), SupB15 (acute lymphoid leukemia from ATCC, Manassass, VA), Toledo (B cell diffuse large cell lymphoma from ATCC). The following CD19-negative cell lines were used: A549 (lung carcinoma, from ATCC), CCRF-CEM (T cell leukemia from ATCC), K562 (chronic myeloid leukemia from ATCC), MDA231 (breast carcinoma from ATCC), TC71 (Ewing's sarcoma, a kind gift of Dr. M. Tsokos, National Cancer Institute, Bethesda, MD), 624 (melanoma, derived in Surgery Branch, National Cancer Institute). All cell lines were maintained in R10 medium except for SupB15. SupB15 was cultured in SupB15 medium. When CLL PBMC were used as targets in assays, the cells were cultured in R10 medium for 12-18 hours prior to the assay.
T cell Culture
Peripheral blood mononuclear cells (PBMC) were thawed and washed once in T cell medium. PBMC were suspended at a concentration of 1×106 cell per mL in T cell medium containing 50 ng/mL of the anti-CD3 monoclonal antibody OKT3 (Ortho, Bridgewater, NJ) and 300 IU/mL of IL-2. Twenty mL of this suspension were added to 75 cm2 culture flasks (Corning, Corning, NY). The flasks were cultured upright at 37°C and 5% CO2. This method of T cell stimulation in which OKT3 was added directly to media is referred to as solubilized OKT3 and was used for the initial stimulation of PBMC in all experiments reported in this paper except for the results reported in Figure 6 and patients 1-3 in Table 3.
Figure 6.
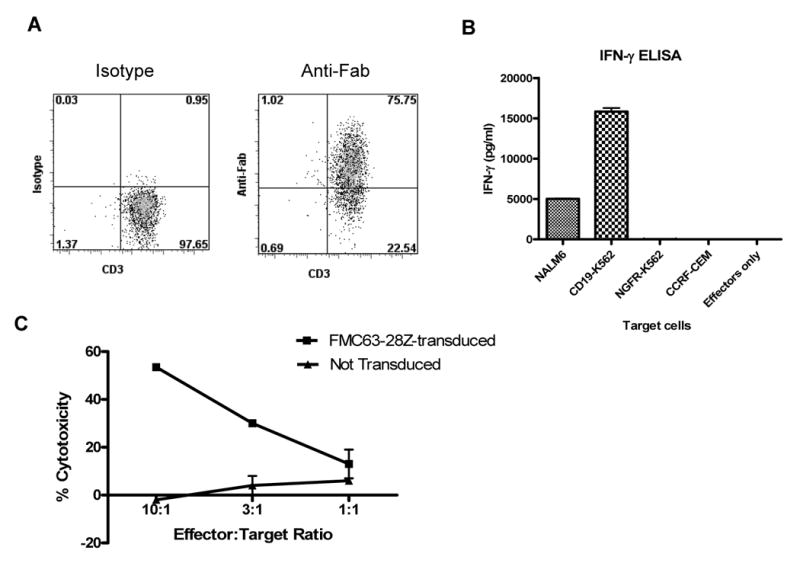
Large numbers of functional anti-CD19 T cells can be generated from the blood of CLL patients. (A) T cells from a patient with CLL that had received fludarabine plus rituximab were transduced with FMC63-28Z and induced to proliferate in a REP. On day 10 after initiation of the REP 75% of the T cells expressed the FMC63-28Z CAR as measured by staining with anti-Fab antibodies. Cells from six different CLL patients were transduced in a similar manner, and the percentage of T cells from each of these patients that expressed the FMC63-28Z CAR ten days after the initiation of a REP is reported in Table 3. (B) Ten days after initiation of a REP, cells from the same culture used for the experiment reported in (A) produced IFNγ upon stimulation with the CD19-expressing cell lines NALM6 and CD19-K562 but not the CD19-negative cell lines NGFR-K562 and CCRF-CEM. These results are representative of six experiments in which cells from six different CLL patients were used. (C) FMC63-28Z-transduced T cells from a CLL patient specifically killed autologous CLL cells while nontransduced T cells from the same patient exhibited minimal cytotoxicity 12 days after initiation of a REP. Cytotoxicity is reported as the mean +/- the SEM of duplicate determinations. These results are representative of two experiments in which cells from two different patients were used.
Table 3.
Summary of transduction and proliferation of cryopreserved leukemic PBMC samples
| Patient number | Blood lymphocyte count (cells/μL) | Chemotherapy Treatment status | Type of OKT3 | Proliferation day 0 until day 10 of REP | % Transduced |
|---|---|---|---|---|---|
| 1 (depleted) | 9800 | yes | plate | 255X | 52 |
| 2 (depleted) | 28600 | no | plate | 727X | 75 |
| 3 (depleted) | 47800 | no | plate | 14,024X | 67 |
| 4 | 7200 | yes | solubilized | 33X | 27 |
| 5 | 4400 | yes | solubilized | 1051X | 67 |
| 6 | 1900 | yes | solubilized | 1013X | 46 |
Patient samples 1-3 were depleted of CD19+ cells. The normal range for blood lymphocytes is 980-2910 cells/μL. Patients with chemotherapy treatment “yes” had all received prior treatment with fludarabine and rituximab. The type of OKT3 stimulation was either solubilized or plate-bound as indicated. Proliferation is presented as the fold increase in total cell number as measured by counting with trypan blue for dead cell exclusion. For samples that were depleted of CD19+ cells, the starting number was the number of live cells after depletion. For non-depleted samples the starting number was the number of PBMC immediately after thawing. Transduction percentage was measured by detecting the scFv by flow cytometry with anti-Fab antibodies.
In some experiments, leukemic PBMC samples from patients with CLL were used. These leukemic PBMC sometimes included a large number of CD19+ leukemia cells. In these cases (experiments shown in Figure 6 and Table 3 patients 1-3), CD19+ cells were depleted from the PBMC using CD19 microbeads and LD columns (Miltenyi, Auburn, CA) according to the manufacturer's instructions. When CD19+ cells were depleted, T cell culture was initiated by adding the CD19-depleted PBMC to plates that were coated with OKT3. OKT3 was diluted in PBS to a concentration of 10 μg/mL. One mL of this OKT3 solution was added to each well of a 24 well plate (Corning). The plate was incubated for 4 hours at RT and then washed once with PBS. Next, CD19-depleted PBMC were suspended at 1×106 cells per mL in T cell medium plus 300 IU/mL of IL-2, and 1 mL of this solution was added to each well of the OKT3-coated plate.
Retroviral Transductions
RetroNectin™ (Takara) was dissolved at a concentration of 15 μg/mL in PBS and two mL of this RetroNectin™ in PBS solution were added to each well of nontissue-culture-coated 6 well plates (BD Biosciences). The plates were incubated for 2 hours at room temperature (RT). After the incubation, the RetroNectin™ solution was aspirated and 2 mL of a blocking solution consisting of Hanks' balanced salt solution (HBSS) plus 2% bovine serum albumin (BSA) were added to each RetroNectin™-coated well. The plates were incubated for 30 minutes at RT. The blocking solution was aspirated, and the wells were rinsed with a solution of HBSS+2.5% HEPES. Retroviral supernatant was rapidly thawed and diluted 1:1 in T cell media. When transiently produced retroviral supernatant was used, two mL of the diluted supernatant were then added to each RetroNectin™-coated well. When supernatant from the H3 producer cell clone was used, 4 mL of 1:1 diluted supernatant were added to each well. After addition of the supernatants, the plates were centrifuged at 2000×g for 2 hours at 32°C. The supernatant was then aspirated from the wells, and 2×106 T cells that had been cultured with OKT3 and IL-2 for 2 days were added to each well. When the T cells were added to the retrovirus-coated plates, they were suspended at a concentration of 0.5×106 cells per mL in T cell medium plus 300 IU/mL of IL-2. After the T cells were added to each well, the plates were centrifuged for 10 minutes at 1000×g. The plates were incubated at 37°C overnight.
The transduction was repeated the next day. New 6 well nontissue culture-coated plates (BD Biosciences) were coated with RetroNectin™ and blocked in the same manner as in the first transduction. Retroviral supernatant was added to each well and the plates were centrifuged identically as in the first transduction. The T cells from the first transduction were then transferred onto the new retrovirus-coated plates and the plates were centrifuged at 1000×g for 10 minutes. After a twenty-hour incubation, the T cells were removed from the plates and suspended in fresh T cell medium with 300 IU/mL of IL-2 at a concentration of 0.5×106 cells per mL and cultured at 37° and 5% CO2.
Rapid Expansion Protocol (REP)
In order to generate a large number of transduced T cells, the cells were induced to proliferate using a rapid expansion protocol (REP) 11,51. Prior to being used in REPs, T cells had been started in culture with OKT3 and IL-2 and transduced on the second and third days after the initiation of culture as detailed above. Ten days after the start of the cultures, 0.5×106 transduced T cells were combined with 100 ×106 allogeneic, irradiated (5000 rad) PBMC, and these cells were suspended in 35 mL of T cell medium with 30 ng/mL of OKT3 and 300 IU/mL of IL-2. The cells were cultured in a 75 cm2 flask at 37°C and 5% CO2. Five days later, cells were counted and suspended at a concentration of 0.5×106 cells/mL in fresh T cell medium with 300 IU/mL of IL-2. The cells were counted and suspended at a concentration of 0.5×106 cells/mL in fresh T cell medium with 300 IU/mL of IL-2 every two days for the remainder of the time they were kept in culture.
Preparation of CD19-K562 Cells Expressing CD19 and NGFR-K562 Cells Expressing Low-affinity Nerve Growth Factor Receptor
The cDNA for full-length CD19 was purchased from Invitrogen and cloned into the MSGV retroviral backbone. Retrovirus-containing supernatant was prepared as described above under transient retrovirus production. K562 cells were transduced with this supernatant and then CD19-expressing cells were sorted by flow cytometry to obtain a population of K562 cells that uniformly expressed high levels of CD19.
Similarly, the gene for the low-affinity nerve growth factor receptor (NGFR) was cloned into MSGV, and retrovirus-containing supernatant was produced. K562 cells were transduced with this supernatant and NGFR-expressing cells were sorted by flow cytometry to obtain a uniform population of K562 cells that expressed high levels of NGFR. CD19-K562 and NGFR-K562 were maintained in R10 medium. The NGFR-K562 cells were used as negative control cells. The NGFR-K562 cells were identical to the CD19-K562 cells except that NGFR-K562 cells expressed NGFR and CD19-K562 cells expressed CD19. Using these two cell lines, we could rigorously demonstrate specific recognition of CD19 by CAR-transduced T cells.
CAR Detection on Transduced T Cells
Cells were washed and suspended in FACs buffer (Phosphate-buffered saline (PBS) plus 0.1% sodium azide and 0.4% BSA). Fc receptors were blocked with normal goat IgG (Invitrogen). For each T cell culture to be analyzed, two tubes of cells were prepared. Biotin-labeled polyclonal goat anti-mouse-F(ab)2 antibodies (anti-Fab, Jackson Immunoresearch, West Grove, PA) were added to one tube to detect the FMC63 scFv, and biotin-labeled normal polyclonal goat IgG antibodies (Jackson Immunoresearch) were added to the other tube to serve as an isotype control. The cells were incubated at 4°C for 25 minutes and washed once. The cells were suspended in FACs buffer and blocked with normal mouse IgG (Invitrogen). The cells were then stained with phycoerythrin (PE)-labeled streptavidin (BD Pharmingen, San Diego, CA) and allophycocyanin (APC)-labeled CD3 (eBiocience, San Diego, CA). Flow cytometry acquisition was performed with a BD FacsCanto II (BD Biosciences), and analysis was performed with FlowJo (Treestar, Inc. Ashland, OR).
Interferon-γ Enzyme-linked Immunosorbent Assay (ELISA)
Target cells were washed and suspended at 1 ×106 cells per mL in T cell media without IL-2. One-hundred-thousand target cells of each target cell type were added to each of two wells of a 96 well round bottom plate (Corning). Effector T cell cultures were washed and suspended at 1 ×106 cells per mL in T cell media without IL-2. One-hundred-thousand effector T cells were combined with target cells in the indicated wells of the 96 well plate. In addition, wells containing T cells alone were prepared. The plates were incubated at 37°C for 18-20 hours. Following the incubation, an IFNγ ELISA assay was performed using standard methods (Pierce, Rockford, IL).
Intracellular Cytokine Staining Assay (ICCS)
CD19-K562 and NGFR-K562 cells were used as targets in the ICCS assay. For each T cell culture that was tested, two tubes were prepared. One tube contained 0.5 ×106 CD19-K562 cells and the other tube contained 0.5 ×106 NGFR-K562 cells. Both tubes contained 0.5 ×106 T cells, 1 ml of T cell medium without IL-2, and 1 μL of Golgi Plug (BD Pharmingen). All tubes were incubated at 37°C for 5 hours and then transferred to 4C° for overnight storage. The next morning, the cells were washed and surface stained with the following antibodies: CD3 (eBioscience, clone UCHT1), CD4 (eBioscience, clone OKT4), and CD8 (eBioscience, clone RPA-T8). The cells were permeabilized and intracellular staining was conducted for IFNγ (BD Pharmingen, clone B27) and IL-2 (BD Pharmingen, clone MQ1-17H12) according to the instructions of the Cytofix/Cytoperm kit (BD Pharmingen). Flow cytometry acquisition was performed with a BD FacsCanto II (BD Biosciences), and analysis was performed with FlowJo (Treestar).
Cytotoxicity Assay
We used a slightly modified version of a flow cytometry cytotoxicity assay 52. In this assay, the cytotoxicity of target cells is measured by comparing survival of target cells relative to the survival of negative control cells. The negative control cells and the target cells are combined in the same tube with effector T cells. In our experiments, the target cells were unmanipulated PBMC from patients with CLL that were made up of greater than 55% B cells, and the negative control cells were CCRF-CEM cells.
CCRF-CEM cells were suspended in R10 medium at a concentration of 1.5×106 cells/mL, and the fluorescent dye 5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine (CMTMR) (Invitrogen) was added at a concentration of 5 μM. The cells were mixed and then incubated at 37°C for 30 minutes. The cells were then washed and suspended in cytotoxicity medium. Next, the CCRF-CEM cells were incubated at 37°C for 60 minutes. The cells were then washed twice and suspended in cytotoxicity medium.
CLL PBMC were suspended in PBS+0.1% BSA at 1×106 cells/mL. The fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) was added to this cell suspension at a concentration of 1 μM. The cells were incubated 10 minutes at 37°C. After the incubation, the labeling reaction was stopped by adding a volume of FBS that was equal to the volume of cell suspension and the cells were incubated for 2 minutes at RT. The cells were washed and suspended in cytotoxcity medium.
Effector T cells were washed and suspended at 5×106 cells/mL in cytotoxicity medium. In all experiments, the cytotoxicity of effector T cells that were transduced with the anti-CD19 CAR FMC63-28Z was compared to the cytotoxicity of negative control effector T cells from the same patient that were transduced with the SP6-28Z control CAR or were not transduced. For FMC63-28Z-transduced effector T cells and negative control effector T cells, cultures were set up in sterile 5 mL test tubes (BD Biosciences) in duplicate at the following T cell:target cell ratios: 10:1, 3:1, and 1:1. The target cells were always 50,000 PBMC from a CLL patient. Each culture also contained 50,000 CCRL-CEM negative control cells. In addition, tubes were set up that contained only CLL target cells plus CCRL-CEM negative control cells. The cultures were incubated for 4 hours at 37°C. Immediately after the incubation, 7AAD (7-amino-actinomycin D) (BD Pharmingen) was added as recommended by the manufacturer, and flow cytometry acquisition was performed with a BD FacsCanto II (BD Biosciences). Analysis was performed with FlowJo (Treestar, Inc. Ashland, OR). Analysis was gated on 7AAD-negative (live) cells, and the percentages of live CLL target cells and live CCRF-CEM negative control cells were determined for each T cell+target cell culture. For each T cell+target cell culture, the percent survival of CLL PBMC was determined by dividing the percent live CLL PBMC by the percent live CCRF-CEM negative control cells. The corrected percent survival of CLL PBMC was calculated by dividing the percent survival of CLL PBMC in each T cell+target cell culture by the ratio of the percent CLL target cells:percent CCRF-CEM negative control cells in tubes containing only CLL target cells and CCRF-CEM negative control cells without any effector T cells. This correction was necessary to account for variation in the starting cell numbers and for spontaneous target cell death. Cytotoxicity was calculated as the percent cytotoxicity of CLL PBMC=100-corrected percent survival of CLL PBMC. For all effector:target ratios, the cytotoxicity was determined in duplicate and the results were averaged.
Results
We compared two CARs, FMC63-28Z and FMC63-CD828BBZ that contained a mouse-anti-human-CD19 scFv derived from the FMC63 hybridoma 39. The MSGV-FMC63-28Z retroviral vector encoded the FMC63 scFv, a portion of the CD28 costimulatory molecule, the cytoplasmic component of the TCR-ζ molecule, and the MSGV retroviral backbone (Figure 1A). The MSGV-FMC63-CD828BBZ retroviral vector consisted of the FMC63 scFv, the hinge and transmembrane regions of the CD8 molecule, the cytoplasmic domain of the CD28 costimulatory molecule, the cytoplasmic domain of the 4-1BB molecule, the cytoplasmic portion of the TCR-ζ molecule, and the MSGV retroviral backbone (Figure 1B). The combinations of signaling elements in these two CARs were the two most effective combinations of signaling elements that we tested in our ongoing work with CARs incorporating scFvs other than FMC63 (data not shown).
MSGV-FMC63-28Z and MSGV-FMC63-CD828Z were used to transduce peripheral blood mononuclear cells (PBMC) that were stimulated with the anti-CD3 monoclonal antibody OKT3 and IL-2. To measure gene transfer efficiency, the cells were stained with polyclonal goat-anti-mouse-Fab antibodies that bind to the FMC63 scFv. The percentage of T cells that expressed detectable levels of the scFv was higher for cells that were transduced with FMC63-28Z compared to cells that were transduced with FMC63-CD828BBZ (Figure 1C and 1D). As a control, nontransduced cells were also stained with the polyclonal goat-anti-mouse-Fab antibodies (Figure 1E). The anti-mouse-Fab antibodies did not bind to nontransduced cells.
T cells that were transduced with retroviruses encoding either FMC63-28Z or FMC63-CD828BBZ produced IFNγ specifically after stimulation with CD19-expressing target cells including primary chronic lymphocytic leukemia (CLL) cells (Table 1). FMC63-28Z-transduced T cells produced greater amounts of IFNγ than FMC63-CD828BBZ-transduced T cells in response to CD19-expressing target cells.
The percentages of FMC63-28Z and FMC63-CD828BBZ-transduced T cells that produced IFNγ and IL-2 after a five hour culture with K562 cells expressing CD19 (CD19-K562) or the low affinity nerve growth factor receptor (NGFR-K562) was determined by intracellular cytokine staining. The percentage of T cells transduced with either of the two CARs that produced IFNγ when cultured with CD19-K562 cells (Figure 2A) was similar to the percentage of T cells that expressed the FMC63 scFv as measured by staining with anti-Fab antibodies (Figure 1C and 1D). In contrast, a higher percentage of FMC63-28Z-transduced T cells than FMC63-CD828BBZ-transduced T cells produced IL-2 when the T cells were cultured with CD19-K562 cells (Figure 2B). Due to the superior recognition of primary CLL cells and the superior production of IL-2 by T cells that were transduced with FMC63-28Z, we used this receptor in the remainder of our experiments.
We prepared a stable producer cell clone that produced retroviruses encoding the FMC63-28Z CAR. This producer cell clone was designated H3. When we carried out transductions using the retrovirus-containing supernatant from producer cell clone H3, 45-67% of T cells expressed FMC63-28Z as measured by staining for the FMC63 scFv four to five days after transduction (data not shown).
In order to obtain enough T cells for clinical adoptive T cell transfer, transduced T cells must be induced to rapidly proliferate after transduction. Thus, transduced T cells were induced to rapidly proliferate using a rapid expansion protocol (REP) 11,51 in which the transduced T cells are cultured with OKT3, allogeneic feeder cells, and IL-2 (Figure 3A). Figure 3B shows that expression of FMC63-28Z was maintained on transduced T cells after they had undergone a 706-fold increase in cell number during a REP. We utilized a CAR that is specific for the hapten 2, 4, 6-trinitrobenzenesulfonic acid as a negative control. This CAR was designated SP6-28Z and it was encoded by the MSGV-SP6-28Z retroviral vector. Like FMC63-28Z this CAR includes CD28 and TCR-ζ signaling components. Expression of the SP6-28Z CAR was maintained on transduced T cells after a REP (Figure 3C). FMC63-28Z-transduced T cells but not SP6-28Z-transduced T cells produced IFNγ specifically after stimulation with CD19-expressing target cells including primary CLL cells nine days after initiation of a REP (Table 2). FMC63-28Z-transduced CD4+ and CD8+ T cells produced IFNγ and IL-2 in a CD19-specific manner fourteen days after initiation of a REP (Figure 4) and specifically killed allogeneic primary CLL cells before initiation of a REP (Figure 5A and 5B) and twenty-one days after initiation of a REP (Figure 5C).
Figure 3.
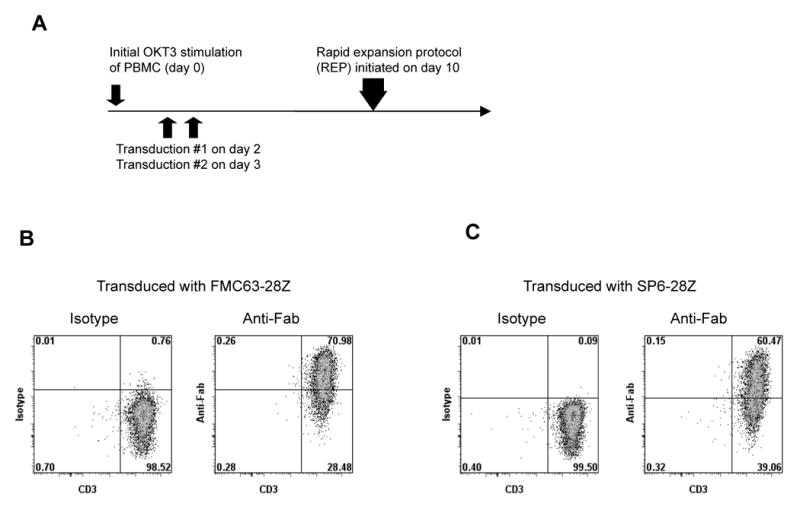
Expression of CARs is maintained in transduced T cells after extensive proliferation. (A) The design of the experiments reported in Figures 3 through 6 and Tables 2 and 3 is shown. PBMC were started in culture with OKT3 and IL-2 on day 0. On day 2, cultures were suspended in fresh medium with IL-2 and the first transduction was performed. On day 3 the transduction was repeated. On day 10, a rapid expansion protocol (REP) was initiated to generate large numbers of transduced T cells. (B) Ten days after initiation of a REP, FMC63-28Z expression was detected on 70% of T cells that had been transduced with FMC63-28Z when the T cells were stained with anti-Fab antibodies. Staining with isotype-matched control antibodies is also shown. This experiment is representative of three separate experiments using cells from three different donors. (C) Ten days after initiation of a REP, SP6-28Z expression was detected on 60% of T cells when T cells that had been transduced with SP6-28Z were stained with anti-Fab antibodies. Staining with isotype-matched control antibodies is also shown. The cells used in the experiments reported in (B) and (C) were from the same donor. The experiments reported in (B) and (C) were performed with cells from the same cultures tested in the experiments described in Figure 4, Figure 5, and Table 2.
Table 2.
IFNγ ELISA day 9 after initiation of REP
| CD19+ targets | CD19 negative targets | |||||||
|---|---|---|---|---|---|---|---|---|
| bv173 | SupB15 | CLL | 624 | TC71 | A549 | CCRF-CEM | Effectors alone | |
| Effector cells | ||||||||
| FMC63-28Z-transduced | 16070 | 7758 | 2321 | 108 | 65 | 37 | 20 | 20 |
| SP6-28Z-transduced | 78 | 16 | 27 | 342 | 60 | 198 | 15 | 10 |
| No effectors (target cells alone) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
Effector cells were cultured overnight with target cells, and an interferon-γ ELISA was performed. The effector cells were T cells that were transduced with retroviruses encoding the indicated receptors. The effector cells used in this assay were from the same cultures that were analyzed for scFv expression in the experiments reported in Figures 3B and 3C. SP6-28Z-transduced T cells recognize the hapten 2, 4, 6-TNP. All values are pg/ml of IFNγ (mean of duplicate wells). CLL refers to primary CLL cells. This experiment is representative of two experiments using cells from two different donors.
Figure 4.
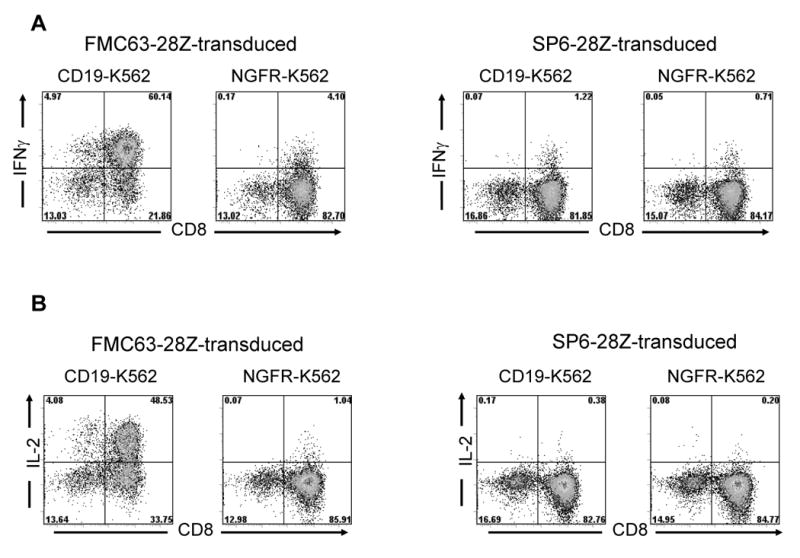
Transduced T cells can produce IFNγ and IL-2 after rapid expansion. On day 14 after initiation of REPs, PBMC from the same donor that had been cultured identically and transduced with either FMC63-28Z or SP6-28Z were stimulated with either CD19-K562 cells or NGFR-K562 cells for 5 hours, and intracellular staining for IFNγ (A) and IL-2 (B) was performed. The transduced T cells produced IFNγ and IL-2 in a CD19-specific manner. The plots are gated on CD3+ lymphocytes, and the percentage of cells in each quadrant is shown on the plots. This experiment is representative of three experiments that used cells from three different donors.
Figure 5.
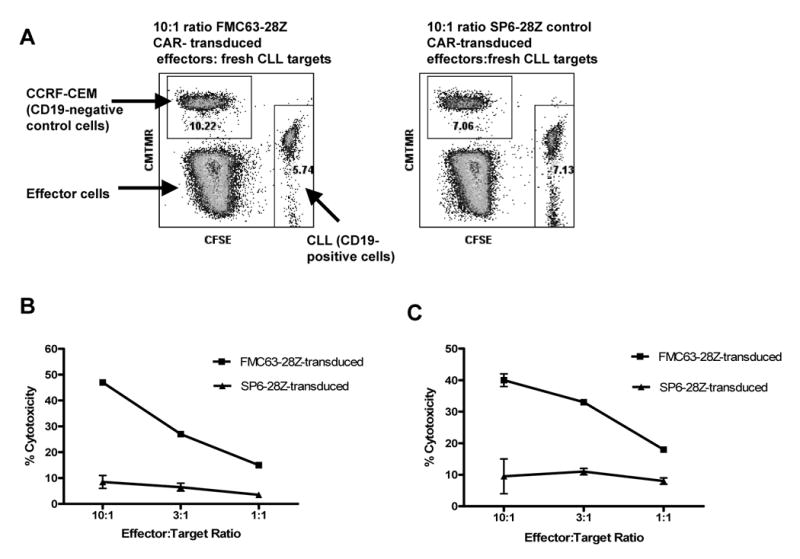
FMC63-28Z-transduced T cells can kill primary CLL cells before and after a REP. (A) An example of the flow cytometry plots obtained when we performed a flow cytometry cytotoxicity assay is shown. The cytotoxicity of target cells is measured by comparing survival of target cells relative to the survival of negative control cells. The negative control cells and the target cells are combined in the same tube with effector T cells. Note that the percentage of CD19-expressing CLL cells relative to the control CCRF-CEM cells was less after incubation with FMC63-28Z-transduced T cells than after incubation with SP6-28Z-control transduced T cells. These data were part of the data used to construct the cytotoxicity plot shown in (B). (B) FMC63-28Z-transduced T cells could specifically kill allogeneic primary CLL cells 11 days after initial OKT3 stimulation. T cells from the same donor that were transduced with SP6-28Z caused only a low level of cytotoxicity against the same primary allogeneic CLL cells. Cytotoxicity is reported as the mean +/- the SEM of duplicate determinations. This experiment is representative of three similar experiments that used cells from three different donors. (C) Twenty-one days after initiation of a REP, FMC63-28Z-transduced T cells could kill primary allogeneic CLL cells. The FMC63-28Z-transduced T cells were from the same donor as in (B). The allogeneic CLL cells used in this experiment were from a different patient than the CLL cells that were used in the experiment presented in (B). T cells from the same donor that were transduced with SP6-28Z caused only a low level of cytotoxicity against the same primary allogeneic CLL cells. Cytotoxicity is reported as the mean +/- the SEM of duplicate determinations. This experiment is representative of three similar experiments that used cells from three different donors.
Because our goal is to conduct a clinical trial in which anti-CD19-CAR-transduced T cells will be used to treat patients with lymphoma and CLL, we carried out a series of experiments in which PBMC from CLL patients were transduced and rapidly expanded using the approach summarized in Figure 3A. After transduction and rapid expansion, these cells were almost all T cells and usually expressed a high level of the FMC63-28Z CAR (Figure 6A). In addition, the T cells could produce IFNγ in a CD19-specific manner (Figure 6B), and could kill autologous CLL cells (Figure 6C). We transduced and rapidly expanded T cells from six different CLL patients. The patients varied in their leukemia burden as measured by the peripheral blood lymphocyte count. Four of the patients had received prior treatment with fludarabine and rituximab. PBMC from three patients with peripheral blood lymphocyte counts of at least 9800 cells/μL were depleted of CD19+ cells. This depletion eliminated a mean of 96% of the B cells from the PBMC samples (n=3, data not shown). Because CLL cells express CD19, this depletion eliminated most of the leukemia cells from these samples. Immediately after depletion, the CD19-depleted PBMC from these three patients were stimulated with plate-bound OKT3. PBMC samples from three other CLL patients with peripheral blood lymphocyte counts of 7200 cells/μL or less received their initial OKT3 stimulation with OKT3 which was added directly to their culture media (solubilized OKT3). Cells from all of the patients were transduced, and ten days after initial OKT3 stimulation they were induced to proliferate using a REP. The results of these experiments are summarized in Table 3. Notably, the worst result in both the percentage of T cells that were transduced and proliferation occurred in a sample from a patient with a peripheral blood lymphocyte count of 7200 cells/μL that was not depleted of CD19+ cells. Better transduction and proliferation results were obtained with CLL PBMC that had either a low percentage of leukemia cells or a large number of leukemia cells that were depleted of CD19+ cells prior to initiating cultures with plate-bound OKT3.
Producer cell clone H3 was the source of the retrovirus-containing supernatant used in all of our transduction experiments except the ones reported in Figures 1 and 2 and in Table 1. In order to conduct a clinical trial using FMC63-28Z-transduced T cells, the H3 cells were used to generate a master cell bank and subsequently to produce retrovirus-containing supernatant under good manufacturing practice (GMP) conditions. This supernatant was produced in six batches. A summary of both the transduction efficiency and the ability of T cells that were transduced using this supernatant to produce IFNγ in a CD19-specific manner is shown in Table 4.
Table 4.
IFNγ ELISA of T cells transduced with a clinical-grade retroviral vector
| CD19+ targets | CD19 negative targets | ||||
|---|---|---|---|---|---|
| H3 batch (% FMC63-28Z+) |
NALM6 | Toledo | CCRF-CEM | K562 | Effectors alone |
| 1 (53.4) |
6150 | 9350 | 51 | 222 | 37 |
| 2 (65.3) |
11000 | 6050 | 91 | 294 | 76 |
| 3 (62.5) |
12700 | 18300 | 84 | 420 | 63 |
| 4 (52.0) |
18450 | 16600 | 81 | 510 | 100 |
| 5 (68.3) |
17700 | 17950 | 59 | 246 | 59 |
| 6 (58.8) |
13900 | 15500 | 116 | 590 | 98 |
| Nontransduced T cells (0.1) |
111 | 114 | 21 | 41 | 9 |
The effector T cells in this experiment were transduced with supernatant that contained retroviruses encoding FMC63-28Z. The supernatant was produced under GMP conditions with the H3 producer cell clone. This supernatant was produced in 6 separate batches. The percentage of T cells that expressed FMC63-28Z after transduction with supernatant from each batch (% FMC63-28Z+) is shown in the first column. Effector cells were cultured overnight with target cells, and an interferon-γ ELISA was performed. Values are pg/ml of IFNγ (mean of duplicate wells). The results are representative of two experiments using cells from two different donors. The experiments were performed 6 days after the cells were transduced.
Discussion
CD19 is a promising target for antigen-specific immunotherapy because it is expressed on most malignant B cells 32,33, while expression on normal tissues is limited to B cells and perhaps follicular dendritic cells 33,34. The attractiveness of CD19 as a target has led several groups to construct anti-CD19 chimeric receptors 30,40,53-55. The only clinical trial of an anti-CD19 CAR that has been completed used electroporation to transfect T cells prior to adoptive transfer 56. In this trial, T cell persistence was limited and an anti-lymphoma effect was not detected 56.
Several factors could affect the efficacy of adoptive T cell therapy with anti-CD19-CAR-transduced T cells. The attributes of the CAR such as which signaling elements are included, the methods used to culture and transduce the T cells, the number of T cells transferred, the phenotype of the infused T cells, and administration of cytokines such as high-dose IL-2 after T cell transfer may all be important. Immunosuppression prior to adoptive T cell therapy enhances anti-tumor activity in mice 57 and humans 11. The variable regions of CARs are derived from murine antibodies, and thus the development of human anti-mouse antibodies directed against the CAR-transduced T cells may limit their effectiveness 23,58. In order to evaluate some of the many factors that could affect adoptive T cell therapy with T cells expressing anti-CD19 chimeric receptors, we are planning a clinical trial in which patients with B cell lymphoma or CLL will be treated with anti-CD19-CAR-transduced T cells and high-dose IL-2. This trial will also assess the effect of immunosuppression prior to T cell transfer.
In order to determine the optimal CAR for clinical use, we compared two CARs that contained combinations of signaling elements that we have found to be effective in CARs incorporating scFvs other than FMC63. Both CARs included the same anti-CD19 scFv component and the signaling moiety of the CD28 costimulatory molecule (Figure 1A). We decided to include a CD28 moiety because of several previous reports that CARs containing a CD28 signaling moiety had improved in vivo persistence and anti-tumor efficacy 22,25,26. We selected the specific CD28 and TCR-ζ sequences used in the FMC63-28Z CAR because these sequences were associated with high levels of CAR expression and antigen-specific cytokine production when combined with several different scFv moieties in unpublished work conducted by our group. One of the CARs that we compared, FMC63-CD828BBZ, contained the cytoplasmic region of the 4-1BB molecule. The other CAR that we evaluated, FMC63-28Z, did not contain a 4-1BB moiety. The 4-1BB molecule has been shown previously to enhance proliferation and in vivo persistence of antigen-specific T cells 28,29, and it has been shown by other investigators that inclusion of a 4-1BB moiety can enhance the in vitro function of CARs 30,31. The specific CD828BBZ sequence contained in the FMC63-CD828BBZ CAR was used in the experiments reported in this paper because in unpublished work by our group using CARs with scFvs other than FMC63 this sequence improved in vitro survival and increased antigen-specific cytokine production compared to CARs without a 4-1BB moiety. The two CARs that we evaluated also differed in their extracellular hinge regions (Figure 1). When we compared the expression of the scFv component of the anti-CD19 CARs on the surface of transduced T cells, we found that there was consistently higher expression on FMC63-28Z-transduced T cells (Figure 1), and FMC63-28Z-transduced cells produced higher levels of IL-2 (Figure 2B) even after making allowance for the lower expression of the anti-CD19 CAR on FMC63-CD828BBZ-transduced T cells compared to FMC63-28Z-transduced T cells (Figure 2B). The decreased IL-2 production is possibly due differences in the structure of the expressed CAR proteins due to either the CD8 hinge region or the presence of the 4-1BB component. The decreased IL-2 production by T cells that were transduced with CARs containing a 4-1BB moiety was unexpected because IL-2 production by T cells has been shown to be increased either substantially 59, or modestly 60, by ligation of the natural 4-1BB molecule that is expressed on T cells to the 4-1BB ligand molecule. Our purpose in comparing FMC63-28Z and FMC63-CD828BBZ was to select the best of two promising CARs for use in a clinical trial, not to formally evaluate how the addition of 4-1BB signaling affects CAR-transduced T cells. Because the two CARs that we compared contained different extracellular hinge regions, our experiments do not constitute an assessment of the function of 4-1BB in CARs. 4-1BB is a molecule that might enhance CAR function in vivo and further in vivo studies are warranted to assess the ability of 4-1BB to enhance the anti-tumor efficacy of CAR-expressing T cells.
We selected the FMC63-28Z CAR for further testing and prepared a producer cell clone that made retroviruses encoding this receptor. T cells were transduced efficiently with these FMC63-28Z-encoding retroviruses. The transduced T cells could produce IFNγ and IL-2 in a CD19-specific manner, and they could kill primary CLL cells. Importantly, these T cell functions were all exhibited when T cells were tested after the transduced T cells had been induced to proliferate to quantities sufficient for clinical adoptive T cell transfer (Figures 3-5 and Table 2).
When tumor tumor infiltrating T cells from melanoma patients undergo extended periods of in vitro culture after OKT3 stimulation in the presence of high concentrations of IL-2, the T cells generally express low levels of CD28, CD27, CD62L, CCR7, and CD127 compared to the levels of these proteins found on the surface of normal circulating T cells ex vivo 61. The phenotype of these T cells is generally that of an effector memory T cell 62. These effector memory T cells can mediate objective regressions of melanoma in approximately 50% of patients when they are infused as part of a treatment protocol that includes lymphocyte-depleting chemotherapy before cell infusion and high-dose IL-2 after T cell infusion 12. Most of the FMC63-28Z-transduced T cells also have an effector memory phenotype by the end of the culture period of 20 to 24 days that is required for preparation of sufficient cells for clinical adoptive T cell transfer (data not shown). One important point to remember is that the phenotypes of cultured T cells might change after infusion. Our group has published data showing that the percentage of infused melanoma-antigen-specific T cells expressing CD127 and CD28 increased rapidly within one week after cell infusion 61.
The CD28 sequence contained in FMC63-28Z includes the sequence (MYPPY) that is predicted to form the binding site for the B7 family molecules 63. Of course, just because the sequence is present does not mean that it is folded in a way to give the three-dimensional structure necessary for binding to the B7 molecules. We accumulated data that assured us that FMC63-28Z-transduced T cells do not recognize B7 molecules. B7 molecules are known to be expressed on activated T cells 64. We confirmed in our own repeated experiments that FMC63-28Z-transduced T cells uniformly and strongly express CD80 after being subjected to a rapid expansion protocol (REP) (data not shown). In addition, these activated T cells also express CD86 (data not shown). These T cells that express CD80 and CD86 do not produce significant amounts of interferon-gamma (IFNγ) as measured by ELISA assay when they are cultured alone (without a CD19-expressing target cell) (See Tables 1, 2, and 4, and Figure 6B). This lack of IFNγ production indicates that the FMC63-28Z-transduced T cells cannot recognize CD80 and CD86 molecules that are expressed on both the transduced and untransduced T cells in the culture.
Adoptive T cell therapy of patients with B cell malignancies poses some unique challenges. CLL cells produce several potentially immunosuppressive cytokines such as transforming growth factor-β interleukin-4, and interleukin-10 65. In addition, patients that will be treated on our proposed clinical trial will all have a history of treatment with immunosuppressive chemotherapy drugs such as fludarabine 66 prior to enrollment on the trial. These factors made evaluation of the proliferative capability of T cells from CLL patients an important focus of our pre-clinical experiments. Some patients with B cell malignancies have a large number of circulating malignant cells that could interfere with T cell culture and transduction procedures, so testing of processes to deplete PBMC of these malignant cells prior to T cell culture and transduction was necessary. Therefore, we conducted a series of experiments using cells from patients with CLL. We found that T cells from patients with CLL that had previously been treated with fludarabine and the anti-CD20 monoclonal antibody rituximab could be induced to proliferate using solubilized OKT3 and transduced efficiently when the T cells were obtained from patients with relatively low levels of leukemic cells in their blood (peripheral blood lymphocyte count 4400 thousand cells/μL or less). In contrast, when higher levels of leukemia cells were present in the blood, proliferation in response to solubilized OKT3 and transduction were impaired (Table 3 and data not shown). We depleted CD19+ cells from PBMC samples that contained large quantities of leukemia cells, and then we applied the CD19-depleted PBMC to plates that had been coated with OKT3. Using this approach we could transduce the T cells of CLL patients and induce them to proliferate (Table 3). Notably, there was extreme variation in the proliferation of T cells from different CLL patients (Table 3).
We prefer to use depletion of CD19+ cells to separate peripheral blood T cells from leukemia cells. This approach leaves the T cells untouched and thereby avoids any potential negative consequences on T cell proliferation and viability due to binding of the cells to anti-CD3-antibody-coated beads. For example, if any of the anti-CD3-coated beads remain attached to the cells after selection, activation of the T cells by the anti-CD3 antibody OKT3 might be inhibited.
The cell culture and transduction process that is described in this manuscript can be easily adapted to good manufacturing practice (GMP) conditions. The only major modifications required are to use clinical-grade anti-CD19 beads and the CliniMacs® system for the depletion of CD19-expressing cells (both anti-CD19 beads and the CliniMacs® system are from Miltenyi) and to use larger flasks and cell culture bags for the REPs. We have tested this approach in one large-scale experiment so far. The results were consistent with those obtained in the small scale experiments reported in this paper (data not shown). The depletion, culture and transduction procedures detailed in this paper will be used in a clinical trial that has been approved by the internal review board of our institution and by the U.S. Food and Drug Administration. In this clinical trial, patients with CD19-expressing B cell malignancies will be treated with FMC63-28Z-transduced T cells.
Acknowledgments
The authors thank Arnold Mixon and Shawn Farid for assistance with flow cytometry.
This work was supported by intramural funding of the Center for Cancer Research, National Cancer Institute, NIH.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.American Cancer Society. Cancer Facts and Figures. 2007 [Google Scholar]
- 2.Gill S, Ritchie D. Therapeutic options in mantle cell lymphoma. Leukemia & Lymphoma. 2008;49:398–409. doi: 10.1080/10428190701851364. [DOI] [PubMed] [Google Scholar]
- 3.Wierda WG, O'Brien SM. Initial therapy for patients with chronic lymphocytic leukemia. Seminars in Oncology. 2006;33:202–209. doi: 10.1053/j.seminoncol.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. Journal of Clinical Oncology. 2003;21:4407–4412. doi: 10.1200/JCO.2003.05.501. [DOI] [PubMed] [Google Scholar]
- 5.Dreger P, Corradini P, Kimby E, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21:12–17. doi: 10.1038/sj.leu.2404441. [DOI] [PubMed] [Google Scholar]
- 6.Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 7.Van Besien K. The evolving role of autologous and allogeneic stem cell transplantation in follicular lymphoma. Blood Reviews. 2006;20:235–244. doi: 10.1016/j.blre.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Mandigers CM, Verdonck LF, Meijerink JP, Dekker AW, Schattenberg AV, Raemaekers JM. Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplantation. 2003;32:1159–1163. doi: 10.1038/sj.bmt.1704290. [DOI] [PubMed] [Google Scholar]
- 9.Marks DI, Lush R, Cavenagh J, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100:3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 10.Russell NH, Byrne JL, Faulkner RD, Gilyead M, Das-Gupta EP, Haynes AP. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplantation. 2005;36:437–441. doi: 10.1038/sj.bmt.1705074. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. Journal of Clinical Oncology. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA, Schumacher TN. Immunotherapy through TCR gene transfer. Nature Immunology. 2001;2:957–961. doi: 10.1038/ni1001-957. see comment. [DOI] [PubMed] [Google Scholar]
- 14.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Human Gene Therapy. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker ML, Near R, Mudgett-Hunter M, et al. Expression of a hybrid immunoglobulin-T cell receptor protein in transgenic mice. Cell. 1989;58:911–921. doi: 10.1016/0092-8674(89)90943-4. [DOI] [PubMed] [Google Scholar]
- 18.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nature Reviews Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 19.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwu P, Shafer GE, Treisman J, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. Journal of Experimental Medicine. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwu P, Yang JC, Cowherd R, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Research. 1995;55:3369–3373. [PubMed] [Google Scholar]
- 22.Haynes NM, Trapani JA, Teng MW, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors.[erratum appears in Blood. 2003 May 15;101(10):3808] Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- 23.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical Cancer Research. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes NM, Snook MB, Trapani JA, et al. Redirecting mouse CTL against colon carcinoma: superior signaling efficacy of single-chain variable domain chimeras containing TCR-zeta vs Fc epsilon RI-gamma. Journal of Immunology. 2001;166:182–187. doi: 10.4049/jimmunol.166.1.182. [DOI] [PubMed] [Google Scholar]
- 25.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Research. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 26.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clinical Cancer Research. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 27.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. Journal of Immunology. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 28.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. Journal of Immunology. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 29.Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. Journal of Experimental Medicine. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Human Gene Therapy. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 32.Nadler LM, Anderson KC, Marti G, et al. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. Journal of Immunology. 1983;131:244–250. [PubMed] [Google Scholar]
- 33.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leukemia & Lymphoma. 1995;18:385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 34.Uckun FM, Jaszcz W, Ambrus JL, et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988;71:13–29. [PubMed] [Google Scholar]
- 35.Pontvert-Delucq S, Breton-Gorius J, Schmitt C, et al. Characterization and functional analysis of adult human bone marrow cell subsets in relation to B-lymphoid development. Blood. 1993;82:417–429. [PubMed] [Google Scholar]
- 36.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. Journal of Clinical Oncology. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 37.Rafailidis PI, Kakisi OK, Vardakas K, Falagas ME. Infectious complications of monoclonal antibodies used in cancer therapy: a systematic review of the evidence from randomized controlled trials. Cancer. 2007;109:2182–2189. doi: 10.1002/cncr.22666. [DOI] [PubMed] [Google Scholar]
- 38.Quinti I, Soresina A, Spadaro G, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. Journal of Clinical Immunology. 2007;27:308–316. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson IC, Lenton KA, Little DJ, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Molecular Immunology. 1997;34:1157–1165. doi: 10.1016/s0161-5890(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 40.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 41.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nature Biotechnology. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 42.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 43.Ochi A, Hawley RG, Hawley T, et al. Functional immunoglobulin M production after transfection of cloned immunoglobulin heavy and light chain genes into lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:6351–6355. doi: 10.1073/pnas.80.20.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter CD, Collins MK, Tailor CS, et al. Comparison of efficiency of infection of human gene therapy target cells via four different retroviral receptors. Human Gene Therapy. 1996;7:913–919. doi: 10.1089/hum.1996.7.8-913. [DOI] [PubMed] [Google Scholar]
- 46.Onodera M, Yachie A, Nelson DM, Welchlin H, Morgan RA, Blaese RM. A simple and reliable method for screening retroviral producer clones without selectable markers. Human Gene Therapy. 1997;8:1189–1194. doi: 10.1089/hum.1997.8.10-1189. [DOI] [PubMed] [Google Scholar]
- 47.Guidance for Industry: INDs-Approaches to Complying with cGMP during Phase I (draft), U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 2006.
- 48.Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 1998.
- 49.Reference Points to Consider in the Production and Testing of New Drugs and Biologicals Produced by Recombinant DNA Technology, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 1985
- 50.Points to Consider in the Use of Cell Lines to Produce Biologicals, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 1993.
- 51.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. Journal of Immunological Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 52.Hermans IF, Silk JD, Yang J, et al. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. Journal of Immunological Methods. 2004;285:25–40. doi: 10.1016/j.jim.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature Medicine. 2003;9:279–286. doi: 10.1038/nm827. see comment. [DOI] [PubMed] [Google Scholar]
- 54.Cheadle EJ, Gilham DE, Thistlethwaite FC, Radford JA, Hawkins RE. Killing of non-Hodgkin lymphoma cells by autologous CD19 engineered T cells. British Journal of Haematology. 2005;129:322–332. doi: 10.1111/j.1365-2141.2005.05456.x. [DOI] [PubMed] [Google Scholar]
- 55.Rossig C, Bar A, Pscherer S, et al. Target antigen expression on a professional antigen-presenting cell induces superior proliferative antitumor T-cell responses via chimeric T-cell receptors. Journal of Immunotherapy. 2006;29:21–31. doi: 10.1097/01.cji.0000175492.28723.d6. [DOI] [PubMed] [Google Scholar]
- 56.Jensen MC, Popplewell L, DiGiusto D, Raubitschek A. Abstract American Society of Hematology Meeting. 2007. A First-In-Human Clinical Trial of Adoptive Therapy Using CD19-Specific Chimeric Antigen Receptor Re-Directed T-Cells for Recurrent/Refractory Follicular Lymphoma. Abstract #288. [Google Scholar]
- 57.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. Journal of Experimental Medicine. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamers CHJ, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. Journal of Clinical Oncology. 2006;24:e20–22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 59.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. Journal of Immunology. 2002;168:4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 60.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. see comment. [DOI] [PubMed] [Google Scholar]
- 63.Kariv I, Truneh A, Sweet RW. Analysis of the site of interaction of CD28 with its counter-receptors CD80 and CD86 and correlation with function. Journal of Immunology. 1996;157:29–38. [PubMed] [Google Scholar]
- 64.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. Journal of Experimental Medicine. 1993;177:845–850. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kater AP, van Oers MHJ, Kipps TJ. Cellular immune therapy for chronic lymphocytic leukemia. Blood. 2007;110:2811–2818. doi: 10.1182/blood-2007-01-068932. [DOI] [PubMed] [Google Scholar]
- 66.Wijermans PW, Gerrits WB, Haak HL. Severe immunodeficiency in patients treated with fludarabine monophosphate. European Journal of Haematology. 1993;50:292–296. doi: 10.1111/j.1600-0609.1993.tb00165.x. [DOI] [PubMed] [Google Scholar]


