Abstract
Recent studies indicate that the biophysical properties of the cellular microenvironment strongly influence a variety of fundamental cell behaviors. The extracellular matrix’s (ECM) response to mechanical force, described mathematically as the elastic modulus, is believed to play a particularly critical role in regulatory and pathological cell behaviors. The basement membrane (BM) is a specialization of the ECM that serves as the immediate interface for many cell types (e.g. all epithelial cells) and through which cells are connected to the underlying stroma. Matrigel is a commercially available BM-like complex and serves as an easily accessible experimental simulant of native BMs. However, the local elastic modulus of Matrigel has not been defined under physiological conditions. Here we present the procedures and results of indentation tests performed on Matrigel with atomic force microscopy (AFM) in an aqueous, temperature controlled environment. The average modulus value was found to be approximately 450 Pa.. However, this result is considerably higher than macroscopic shear storage moduli reported in the scientific literature. The reason for this discrepancy is believed to result from differences in test methods and the tendency of Matrigel to soften at temperatures below 37° C.
Keywords: Matrigel, extracellular matrix, modulus, atomic force microscopy (AFM)
Introduction
Cells actively sense and respond to both chemical and physical stimuli in their surrounding microenvironments. Cellular responses to chemical stimuli have been studied for many years, but a number of recent reports suggest that biophysical cues such as substrate topography (Flemming et al., 1999; Karuri et al., 2004; Teixeira et al., 2006) and compliance (Discher et al., 2005; Flanagan et al., 2002; Pelham and Wang, 1997) play an important role in both normal and pathological behaviors. Stem cell lineage specification (Engler et al., 2006), malignant behavior in mammary gland epithelia (Paszek et al., 2005), and fundamental cell behaviors such as migration (Pelham and Wang, 1997; Wong et al., 2003), proliferation (McDaniel et al., 2007; Thompson et al., 2005), and differentiation (Boontheekul et al., 2007; Peyton et al., 2006) have been shown to be affected by substrate compliance. These studies collectively imply that extracellular matrix (ECM) compliance acts as a homeostatic regulator of normal tissue function. This finding directly applies to the biocompatibility of implantable prosthetic devices, the efficacy of tissue engineering processes, and the relevance of cell culture studies. Therefore, it has become apparent that mechanical characterization of the cellular microenvironment, both in its natural state and in the laboratory, is increasingly necessary to develop a more complete description of biological systems.
Within the context of environmental cueing of cell behavior, a specialized layer of ECM known as the basement membrane (BM) is of particular interest. The BM is a protein complex consisting mainly of collagens, laminins, nidogen/entactin, and perlecan (LeBleu et al., 2007). It is in direct contact with the basal layer of epithelial and vascular endothelial cells and is a ubiquitous structure found in a wide range of tissues across all vertebrate species. However, experimentation with BMs is complicated by the relative difficulty and expense associated with obtaining and isolating intact, native samples in a laboratory setting. Additionally, the use of primarily isolated basement membranes from native tissues introduces the intrinsic individual variability associated with genetic differences, age and health of the individual organism.
Matrigel (Kleinman and Martin, 2005), a reconstituted BM-like complex harvested from Engelbreth-Holm-Swarm mouse tumor cultures, is a commercially available simulant of native BMs. It has found widespread use as a model system for the study of tumor cell invasion (Zaman et al., 2006), hepatocyte aggregation (Semler et al., 2000), stem cell differentiation (Ma et al., 2008), and the formation of tube-like structures by vascular endothelial cells (Kobayashi et al., 2004). Matrigel is relatively versatile and can be molded into thick gels, used to coat cell culture substrates with ECM proteins, or geometrically patterned using soft lithography techniques (Sodunke et al., 2007). The nanoscale topographic features of Matrigel have been shown to be similar to those of several native BMs (Abrams et al., 2000), but the appropriateness of its use as a mechanical surrogate has not been established. In order to quantify the stiffness of Matrigel, an atomic force microscope (AFM) was used to perform indentation tests under physiological conditions (37° C, in liquid). AFM has been widely used as a mechanical testing device for soft, hydrated materials because it allows small forces to be applied over short distances in an aqueous environment (Domke and Radmacher, 1998). Therefore, the elastic modulus reported herein is highly relevant because it more closely mimics the conditions experienced by living cells in vivo.
Materials and methods
2.1. Matrigel sample preparation
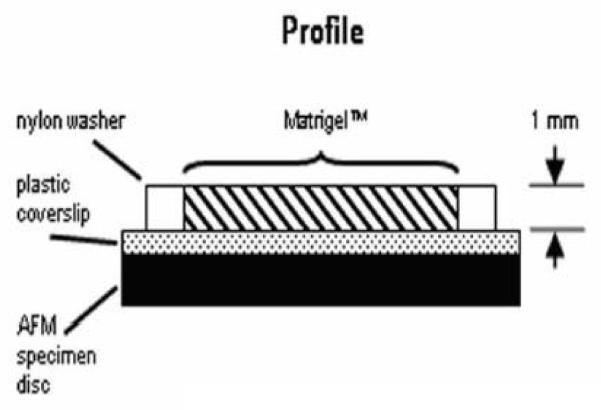
Three different lots of Matrigel (catalog number 354234, BD Biosciences, San Jose, CA USA) were tested and prepared according to the manufacturer. Samples for AFM indentation tests were created by cold-pipetting Matrigel into wells mounted on AFM specimen discs (Fig. 1). Specimens were made approximately 1 mm thick in order to avoid substrate effects during indentation (Domke and Radmacher, 1998). The Matrigel was polymerized for 30 minutes in a humidified incubator at 37° C, and then placed in 1x phosphate buffered saline (PBS — pH 7.4) at 37° C for 24 hours to allow complete gelation. Sample temperatures were not allowed to drop below 37° C before or during experimentation. In order to accomplish this, an AFM environmental chamber was built to maintain a constant temperature of 37° C. The chamber consists of a commercially available thermoelectric heater/cooler (Igloo Products Corp., Katy, TX) modified with a digital temperature controller. All AFM parts, including probe and fluid cell, were preheated in the environmental chamber for at least 30 minutes prior to use, and all tests were conducted in PBS preheated to approximately 45° C to ensure that the system did not drop below 37° C over the course of experimentation. Throughout the experiment the AFM remained inside the environmental chamber and the temperature was maintained at 37° C.
2.2. AFM indentation testing
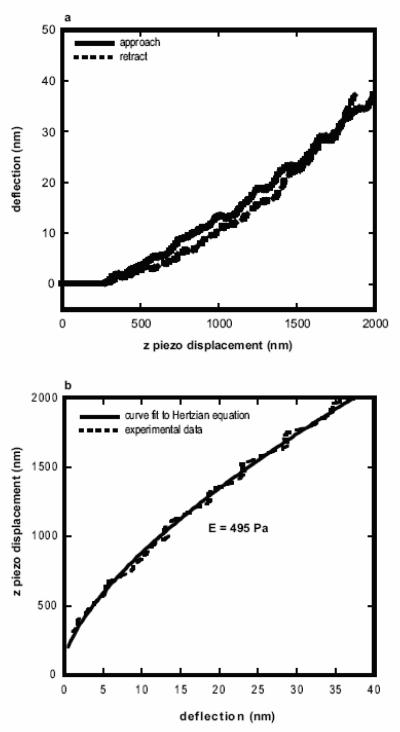
A Multimode AFM with Nanoscope IIIa controller (Veeco Instruments Inc., Santa Barbara, CA, USA ) was used for all indentation tests, as described elsewhere (Dimitriadis et al., 2002). Briefly, the elastic (Young’s) modulus was extracted from a plot of cantilever deflection versus sample (piezo) displacement by fitting the data to a spherical Hertzian contact equation (Sneddon, 1965),
| (1) |
where z and d are piezo and deflection coordinates, respectively, z0 and d0 are piezo and deflection contact coordinates, respectively, k is the cantilever spring constant, υ is Poisson’s ratio (assumed to be 0.5 for a soft, incompressible material (Boudou et al., 2006)), E is the elastic modulus, and R is the particle probe radius (500 nm). E and z0 were used as fitting parameters over the first 400 nm — 500 nm of deflection-displacement data.
Spherically tipped cantilevers (1 μm diameter) with nominal spring constants of 0.06 N/m were used in all experiments. Cantilever spring constants were more precisely calibrated by the manufacturer with the Sader method (Sader et al., 2005) prior to use. Indentations were performed at a rate of 2 - 6 μm/sec to probe elastic rather than viscoelastic behavior (Mahaffy et al., 2000), and only the piezo extension data were analyzed to avoid artifacts created by tip adhesion and uncertainty in the contact coordinates. The average maximum force applied to each sample was 3 nN. The moduli of at least four samples of each lot were calculated as an average over 3-10 sites per sample.
Results
AFM was used to obtain deflection-displacement curves on Matrigel at physiological temperatures in an aqueous environment (Fig. 2). Micron sized spherical tips were used because they provide a contact area which is larger than that of conical or pyramidal tips. A larger contact area is desirable because interactions between probe geometry and the nanoscale topography of the sample surface are reduced, and the contact area of a cellular focal adhesions is more closely approximated (Geiger et al., 2001). These factors provide a more biologically relevant measurement.
Deflection-displacement profiles obtained on Matrigel are typical of soft materials, with piezo displacements an order of magnitude larger than resultant cantilever deflections. Results from all samples are summarized in Table 1. The mean elastic modulus for each lot of Matrigel was similar. Lot 1 exhibited an average elastic modulus of 420 Pa ± 280 Pa, while the average elastic modulus of lot 2 was 400 Pa ± 175 Pa. Lot 3 exhibited some variations in modulus within each sample. The majority of the data obtained were consistent with those obtained with each of the other tested lots, with an average modulus of 480 Pa ± 240 Pa. On these samples, however, areas of higher elastic modulus were observed, with modulus values ranging from 1 – 3 kPa. The average modulus value obtained on lot 3 increased to 840 Pa ± 870 Pa when including all data points.
Table 1.
| Sample | Elastic modulus, E [Pa] |
|---|---|
| Lot 1 | 420 ± 290 |
| Lot 2 | 400 ± 175 |
| Lot 3 | 480 ± 240 |
| Lot 3* | 840 ± 870 |
|
Average (excluding *) |
440 ± 250 |
The overall average, including all three lots, was 443 Pa ± 285 Pa for the elastic modulus of Matrigel (Table 1). Variations in sample moduli are believed to be a result of slight temperature variations among samples and microscale heterogeneities within the material. However, these factors had a marginal impact on calculated moduli, and our method was observed to provide a high degree of reproducibility.
Discussion
Force curves obtained on Matrigel typically exhibited evidence of a small amount of viscoelasticity, with a separation between the approach and retract curves. The viscoelasticity was minimized by choice of a slow indentation rate and was not changed by further decreasing the rate. The approach curve, however, showed a good fit with the Hertz equation and the viscoelasticity was therefore assumed to have a minimal effect on the resulting modulus values. In addition, adhesion of the tip to the sample was occasionally observed. The adhesion may effect modulus measurements, but no difference in modulus was observed between those force curves with and without adhesion, indicating that it is valid to analyze these curves with the Hertz equation.
Although different lots of Matrigel can have varying compositions of biopolymers and protein growth factors, it was unclear if each lot would exhibit identical mechanical properties. Measurements from three separate lots have yielded very similar elastic moduli, with average values of 420 Pa, 400 Pa and 480 Pa, indicating that the mechanical properties are not directly influenced by small changes in Matrigel composition. However, one lot did contain regions of higher elastic moduli. The origin of these areas is unclear, but has been previously attributed to granular regions within the film (Reed et al., 2009).
Previous studies report shear storage modulus values of approximately 55 Pa (Zaman et al., 2006) and 34 Pa (Semler et al., 2000) for Matrigel. Approximating the material as ideally elastic (vanishing shear loss modulus), these elastic moduli are lower than those obtained in our experiments. The above-cited studies employed bulk rheometry, a dynamic mechanical testing technique by which viscoelastic material properties are determined by exposing the specimen to oscillatory shear strains. Discrepancies between rheological and AFM measurements may be due to several factors including length scale differences in test methods, different stress modes (shear versus compression), and temperature conditions. AFM indentation probes highly localized surface properties of a material, and local material properties may differ from those observed with macroscopic test methods. Shear versus compressive forces elicit different modes of mechanical response and may produce anisotropic effects. One report of the elastic modulus of Matrigel with AFM, taken at room temperature, gives a value of 120 Pa, still lower than reported here for samples maintained at 37° C (Alcaraz et al., 2008). Therefore, the most likely explanation for the observed discrepancy is specimen temperature. As observed in our experiments, allowing the specimen temperature to drop below 37° C before or during experimentation resulted in a decrease in sample stiffness. Loss of sample gelation leads to strong probe-surface attraction and adhesion, which made the contact point indiscernible in the deflection-displacement data. Such indiscriminate surface/probe interactions are indicative of a semi-fluid sample surface and rendered indentation tests inconclusive. Therefore we expect mechanical testing of Matrigel without strict temperature control to result in an underestimate of the elastic modulus. Recently, the elastic modulus of Matrigel has been reported after a gelation time of 1 hour to be similar to those reported here, with a median of 650 Pa (Reed et al., 2009), using mechanical interferometry. While it was unclear if the sample temperature was maintained at 37° C, it was stated that the sample chamber was sealed to prevent evaporation.
AFM indentation tests provide experimental conditions that more closely mimic both the length scale and environmental conditions experienced by cells in vivo. The use of micron scale probe geometries, piconewton indentation forces, and aqueous environments at physiological temperatures suggest that AFM is an appropriate instrument for the mechanical characterization of biological materials.
The elastic modulus of Matrigel reported in this study is approximately an order of magnitude lower than determined for cross-linked polyacrylamide gels both prepared in our group and reported (Engler et al., 2004) and poly electrolyte multilayer films (Schneider et al., 2006). These hydrated gels have been used extensively as cell culture substrates because they have tunable mechanical properties, can be cast as thin films, and show a high degree of biological compatibility. This modulus difference may have direct relevance when choosing an appropriate support for the study of cell behaviors. The values obtained are also lower than measurements obtained for native corneal basement membranes (Last et al., 2009).
Conclusions
AFM provides an accurate and reproducible method for characterization of the elastic properties of soft, hydrated, and temperature sensitive materials. However, detailed attention to experimental conditions is necessary for reliable and consistent results. In particular, the delicate temperature sensitivity of Matrigel demands that test samples are kept above 37° C in order to prevent a decrease in sample stiffness. Discrepancies between results reported here and in the literature are believed to be a consequence of different test methods and the tendency of Matrigel to soften at room temperature.
Acknowledgements
The authors thank Prof. Nicholas Abbott for the use of the AFM. This work was funded by the National Eye Institute (5R01EY016134-02 and 1R01CA133567-01) and the National Science Foundation MRSEC (DMR—632527).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C, Bissell MJ. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. The EMBO Journal. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boontheekul T, Hill EE, Kong HJ, Mooney DJ. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007;13:1431–42. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- Boudou T, Ohayon J, Picart C, Tracqui P. An extended relationship for the characterization of Young’s modulus and Poisson’s ratio of tunable polyacrylamide gels. Biorheology. 2006;43:721–8. [PubMed] [Google Scholar]
- Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002;82:2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey PA, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Domke J, Radmacher M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 1998;14:3320–3325. [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–88. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Karuri NW, Liliensiek S, Teixeira AI, Abrams G, Campbell S, Nealey PF, Murphy CJ. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J. Cell Sci. 2004;117:3153–64. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ito E, Honma R, Nojima Y, Shibuya M, Watanabe S, Maru Y. Dynamic regulation of gene expression by the Flt-1 kinase and Matrigel in endothelial tubulogenesis. Genomics. 2004;84:185–92. doi: 10.1016/j.ygeno.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. Determining the mechanical properties of human corneal basement membranes with Atomic Force Microscopy. J. Struct. Biol. 2009 doi: 10.1016/j.jsb.2009.03.012. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp. Biol. Med. 2007;232:1121–9. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-Extracellular Matrix Interactions Regulate Neural Differentiation of Human Embryonic Stem Cells. BMC Dev. Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffy RE, Shih CK, MacKintosh FC, Kas J. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys. Rev. Lett. 2000;85:880–3. doi: 10.1103/PhysRevLett.85.880. [DOI] [PubMed] [Google Scholar]
- McDaniel DP, Shaw GA, Elliott JT, Bhadriraju K, Meuse C, Chung KH, Plant AL. The stiffness of collagen fibrils influences vascular smooth muscle cell phenotype. Biophys. J. 2007;92:1759–69. doi: 10.1529/biophysj.106.089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–93. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Reed J, Walczak WJ, Petzold ON, Gimzewski JK. In situ Mechanical Interferometry of Matrigel Films. Langmuir. 2009;25:36–39. doi: 10.1021/la8033098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sader JE, Pacifico J, Green CP, Mulvaney P. General scaling law for stiffness measurement of small bodies with applications to the atomic force microscope. J. Appl. Phys. 2005;97:124903–124909. [Google Scholar]
- Schneider A, Francius G, Obeid R, Schwinte P, Hemmerle J, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C. Polyelectrolyte multilayers with a tunable Young’s modulus: Influence of film stiffness on cell adhesion. Langmuir. 2006;22:1193–1200. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- Semler EJ, Ranucci CS, Moghe PV. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol. Bioeng. 2000;69:359–369. doi: 10.1002/1097-0290(20000820)69:4<359::aid-bit2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sneddon IN. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965;3:47–57. [Google Scholar]
- Sodunke TR, Turner KK, Caldwell SA, McBride KW, Reginato MJ, Noh HM. Micropatterns of Matrigel for three-dimensional epithelial cultures. Biomaterials. 2007;28:4006–16. doi: 10.1016/j.biomaterials.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Teixeira AI, McKie GA, Foley JD, Bertics PJ, Nealey PF, Murphy CJ. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials. 2006;27:3945–54. doi: 10.1016/j.biomaterials.2006.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MT, Berg MC, Tobias IS, Rubner MF, Van Vliet KJ. Tuning compliance of nanoscale polyelectrolyte multilayers to modulate cell adhesion. Biomaterials. 2005;26:6836–45. doi: 10.1016/j.biomaterials.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19:1908–1913. [Google Scholar]
- Zaman MH, Trapani LM, Sieminski A, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]