Abstract
Aggressive behavior is widespread throughout the animal kingdom, and is a complex social behavior influenced by both genetics and environment. Animals typically fight over resources that include food, territory, and sexual partners. Of all the neurotransmitters, serotonin has been the most implicated in modulating aggressive behaviors in mammalian systems. In the fruit fly, Drosophila melanogaster, the involvement of serotonin itself in aggressive behaviors has been recently established, however, the underlying mechanisms have largely remained elusive. Here we describe the influence of different serotonin receptor subtypes on aggressive behaviors in Drosophila. Drosophila express homologs of three mammalian serotonin receptors: the 5-HT1A, 5-HT2, and 5-HT7 receptors. Significantly, these receptors mediate important behaviors in mammalian systems ranging from feeding, aggression, and sleep, to cognition. To examine the role of the 5-HT2Dro receptor, we utilized the selective 5-HT2 receptor agonist (R)-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI), and the 5-HT2 receptor antagonist, ketanserin. To examine the role of 5-HT1A-like receptors we used the 5-HT1A receptor agonist 8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT), and the 5-HT1A receptor antagonist WAY100635. We find that activation of 5-HT2 receptors with (R)-DOI appears to decrease overall aggression, whereas activation of 5-HT1A-like receptors with 8-OH-DPAT increases overall aggression. Furthermore, the different serotonin receptor circuitries appear to mediate different aspects of aggression: 5-HT2 receptor manipulation primarily alters lunging and boxing, whereas 5-HT1A-like receptor manipulation primarily affects wing threats and fencing. Elucidating the effects of serotonergic systems on aggression in the fly is a significant advancement not only in establishing the fly as a system to study aggression, but as a system relevant to elucidating molecular mechanisms underlying aggression in mammals, including humans.
Keywords: Aggression, neuropharmacology, 5-HT, DOI, 8-OH-DPAT
The study of aggression in flies has a long history (Jacobs, 1960, 1978); Drosophila exhibits aggressive behavior in the acquisition of food, territory, and mates, and these behaviors can differ among fly strains (Jacobs, 1960, Dow and Schilcher, 1975, Jacobs, 1978, Hoffmann, 1987). Only recently, however, have modern techniques been used to explore this behavior and establish the fly as an accepted model system to study molecular mechanisms underlying aggression. Drosophila exhibit complex multi-component aggressive behaviors that are easily discernable and scored (Chen et al., 2002, Nilsen et al., 2004). These behaviors, along with many others, are often very similar to those observed in mammals (Nichols, 2006).
It is known that male flies will fight with each other, but not with females, to establish social dominance (Yurkovic et al., 2006). Interestingly, sexually dimorphic aggressive behavioral patterns have been shown to be mediated by the fruitless gene (Vrontou et al., 2006, Chan and Kravitz, 2007). Recently, the neurotransmitter octopamine also has been found to mediate aspects of aggression in the fly (Hoyer et al., 2008, Zhou et al., 2008). Genetic studies with fly strains bred for hyper- and hypoaggressiveness, combined with microarray analysis, has delineated some of the molecular factors underlying aggression (Dierick and Greenspan, 2006). Although several differentially expressed genes have been identified, only one, for a cytochrome P450 (Cyp6a20), was determined to affect aggression directly. Somewhat surprisingly, serotonin levels in these strains did not differ.
Serotonin has been strongly implicated in mediating aggressive behaviors in other animals, ranging from the more evolutionarily related crustacean, to birds, and mammals (Kravitz, 2000, Sperry et al., 2003, Popova, 2006). In the lobster, infusion of serotonin into the hemolymph elicits a socially dominant posture (Livingstone et al., 1980), as well as encouraging subordinate engagement of dominants (Huber et al., 1997). Attempts to alter serotonin levels in crayfish using biosynthetic precursors and inhibitors also have been found to modulate aggressive and social behaviors (Panksepp and Huber, 2002). The effects of serotonin in crustaceans are likely mediated by receptors similar to mammalian 5-HT1A and 5-HT2 receptors, although they are more dissimilar to their mammalian counterparts with regard to ligand binding profiles than the corresponding Drosophila receptors (Spitzer et al., 2008). In mammals, many aspects of the serotonin system including biosynthesis, metabolism, and receptor activation, primarily of 5-HT1A, 5-HT1B, and 5-HT2 receptors, have been demonstrated to modulate aggressive behaviors (Popova, 2006).
In the fly, the effect of manipulating serotonin levels on aggression has been studied by Baier and coworkers (Baier et al., 2002), who examined the potential role of serotonin by feeding flies a metabolic precursor to serotonin, 5-HTP, and a biosynthesis inhibitor, pCPA, to raise and lower endogenous levels, respectively. Neither of these treatments, however, was found to alter aggression in their assays. These unexpected results should not be surprising, however, because two of the three families of serotonin receptors in Drosophila are functional orthologs of the mammalian 5-HT1A and 5-HT2 receptors (there is also a 5-HT7 receptor ortholog). In mammalian systems these two receptor subtypes often act to oppose one another functionally. Moreover, in previous crayfish and lobster studies, it was found that the rate of manipulation of serotonin levels (i.e. rapidly or slowly) was more important for modulation of aggressive behaviors than absolute levels (Ma et al., 1992, Panksepp and Huber, 2002, Panksepp et al., 2003), and it may be that a different treatment protocol would have uncovered a role for serotonin. Nevertheless, in the flies bred for hyperaggressivness by Dierick and Greenspan, mentioned above, follow-up studies manipulating 5-HT levels demonstrated that serotonin does modulate aspects of aggression in these flies, implicating serotonin in this behavior (Dierick and Greenspan, 2007).
In flies, serotonin has been demonstrated to modulate sleep, circadian behaviors, and sensory processing (Nichols et al., 2002, Yuan et al., 2006, Nichols, 2007). In both flies and mammals the effects of serotonin are primarily mediated through interactions with a number of G-protein coupled receptor proteins to initiate various effector pathways (Nichols and Nichols, 2008). Significantly, the orthologous serotonin receptor families present in Drosophila are the 5-HT2, 5-HT1A-like, and 5-HT7 receptors (Witz et al., 1990, Saudou et al., 1992, Colas et al., 1995). The 5-HT1A-like receptors include the 5-HT1ADro and 5-HT1BDro (formerly the 5-HTdro2A and 5-HTdro2B) receptors, which are believed to be duplicated genes and homologues to the mammalian 5-HT1A receptor (Saudou et al., 1992). Of all the serotonin receptors, the 5-HT1A, 5-HT1B, and 5-HT2, receptors have been the most strongly implicated in mammalian and human behaviors. Here, we have used pharmacological methods to probe individually 5-HT2 and 5-HT1A-like receptor circuitries for their roles in modulating aggression in Drosophila, and show that 5-HT2- and 5-HT1A-like receptors have differential effects on aggressive behaviors in the fly
EXPERIMENTAL PROCEDURES
Chemicals
Ketanserin and 8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT) were obtained from Tocris (Ellisville, MO). (R)-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (R)-DOI and WAY10065 were gifts of Dr. David E. Nichols, Purdue University. (R)-DOI is a potent hallucinogenic drug in humans that is a highly selective agonist at the mammalian 5-HT2 receptor family, with greater than 1,000-fold lower affinity at 5-HT1A type receptors, and high affinity for the Drosophila 5-HT2Dro receptor (Colas et al., 1995). Ketanserin is a highly selective antagonist for the 5-HT2 receptor family that also has been demonstrated to have affinity for the 5-HT2Dro receptor (Colas et al., 1995). 8-OH-DPAT is a selective agonist of mammalian 5-HT1A receptors, however, the affinity of 8-OH-DPAT at fly 5-HT1A-like receptors has been shown to be lower than at mammalian 5-HT1A receptors (Saudou et al., 1992). Nevertheless, 8-OH-DPAT has been demonstrated to functionally activate 5-HT1BDro receptors (Yuan et al., 2005), a homolog to the mammalian 5-HT1A receptor. Furthermore, we previously have demonstrated the ability of the 5-HT1A selective antagonist WAY100635 to block some of the behavioral effects of the mixed serotonin receptor agonist lysergic acid diethylamide (LSD) (Nichols et al., 2002).
Drosophila rearing and feeding
Male adult Oregon-R wild type Drosophila (source: Dr. John Pollock, Duquesne University, Pittsburgh, PA) were used in all experiments. For routine maintenance, flies were grown in polypropylene bottles on standard cornmeal-molasses food at 23 °C under constant light conditions. Because circadian light/dark cycling can alter 5-HT levels in flies, we maintained and isolated flies at constant light in an attempt to eliminate this variable. For selection of flies for assays, bottles were cleared, and about 72 hours later males that did not appear to be recently eclosed by visual inspection were selected under light anesthetization with CO2. We attempted to select flies that were between 2-3 days post eclosion. Within this time frame, there is some extent of initial social contact between flies before isolation. Flies isolated from the moment of eclosion may exhibit different behaviors. For example, by day two, males are expected to be non-virgin through social contact with females, and recent work has demonstrated that virgin males exhibit different behaviors than sexually experienced males (Neckameyer and Matsuo, 2008).
For the aggression assays, selected flies were placed individually into 5 mm diameter glass capillary tubes (Trikinetics, Waltham, MA). One end of the tube was plugged with 1% agar + 20% sucrose, drug added to a final concentration of 3 mM for each treatment where appropriate, and the other end of each tube was plugged with cotton. After four days, at an age of 6-7 days post-eclosion, flies were assayed for aggressive behaviors as described below. As with any pharmacological experiment, an important factor is the concentration of drug used in relation to receptor specificity. Significantly, the drug concentration of 3 mM that we utilized in the food is in accordance with accepted drug levels used in Drosophila studies by others (Hendricks et al., 2003, Leal et al., 2004, Hong et al., 2006, Liu et al., 2008, Sitaraman et al., 2008). Furthermore, our specific choice of drug concentration is based upon our previous work with serotonergic agents in the fly (Nichols et al., 2002, Nichols, 2007). Because these levels of drug have been demonstrated to influence behaviors both here and in previous published and unpublished work, we expected to achieve sufficient levels in the brain to activate receptors.
Our feeding strategy was to maintain flies on food + drug for four days. We chose long-term feeding over acute administration because we have found that there is significantly more variability in both the severity and response of adult fly behaviors such as locomotor activity to serotonergic drugs (Nichols et al., 2002) than in the four day treatment, where the flies can slowly accumulate to steady-state levels of drug. These differential effects are consistent with what has been observed for differential rates of 5-HT level manipulation in crustacean studies as is discussed below. In our previous studies we also have found that with this rate of administration it takes about 3-4 days for the response to drug-induced behaviors to develop and stabilize (Nichols, 2007). Furthermore, because the flies are adults, and the feeding only four days, we do not anticipate any significant physiological side effects as observed with larva when 5-HT systems are chronically manipulated (Dasari et al., 2007).
Locomotor Activity
The effect of each drug condition on negative geotaxis was evaluated as a measure for effects on both general, and startle-induced locomotor activity levels. To measure general locomotor activity levels, the Drosophila Activity Monitor System from Trikinetics (Waltham, MA) was used as previously described (Nichols, 2007), using 3 mM drug, and measuring total activity on Day 4 of the assay. Essentially, individual flies are placed in 5 mM diameter × 6.5 cm long glass tubes within a 32 tube array connected to a computer. Each cross of a photobeam through the middle of the long axis of the tube is scored by computer, and these photobeam breaks together represents total activity.
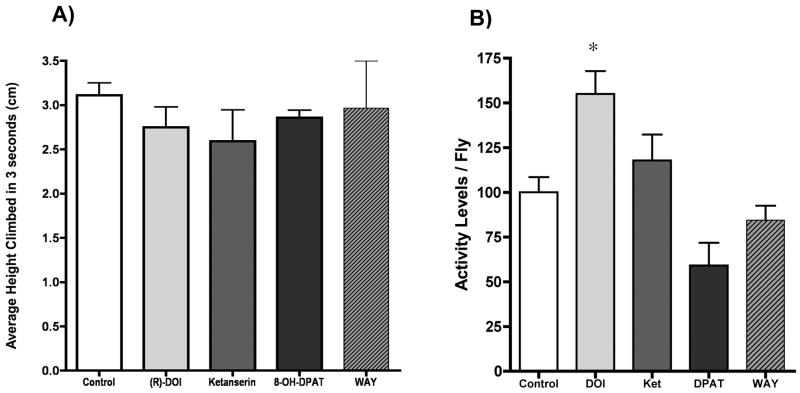
For the negative geotaxis assay, 25 flies were isolated under CO2 and placed into 1.5 ml microfuge tubes containing 200 μl of 1% agarose + 10% sucrose + 3 mM drug (where appropriate) at the bottom, and air holes for ventilation at the top, on their side at 23 °C under constant light conditions for four days without disturbing them. On the fourth day, groups of flies were transfered without anesthetization into an apparatus similar to that described by Gargano (Gargano et al., 2005). Essentially, this device consists of five narrow polystyrene fly vials with ruler markings taped to the sides, with each tube being capped with a lid from a 50 mL polypropylene centrifuge tube. Each vial was used only for one set of experiments before being discarded and replaced. For the assay, flies were placed in the vials and allowed to acclimate for 15-20 minutes without disturbing them. Then, the apparatus was sharply tapped down onto a flat surface three times, and remained on the surface after the third tap to initiate the negative geotaxis responses. After precisely three seconds, a consumer digital camera placed 1 m from the apparatus was used to take an image. After 30 seconds of rest, another trial was repeated, for a total of 5-6 trials for each experiment. The captured images were uploaded onto a computer, and the height of each fly in each tube/treatment condition was scored against the ruler taped to the back of the vial by an observer blind to the treatments. The average height and SEM for each tube/treatment was then calculated. Each drug treatment was independently tested in at least three separate experiments. Using these methods, the average height climbed for OR-wild type flies was virtually identical to that reported by Gargano (Gargano et al., 2005). Furthermore, no effect was observed for any of the drug treatments on negative geotaxis behavior (Figure 1). Statistical analysis was performed with GraphPad Prism4 (San Diego, CA).
Figure 1.
Negative geotaxis and general locomotor activity assays were performed to assess the effects of 3mM drug on locomotor activity levels. A) No statistically significant effect for any of the treatments was observed on negative geotaxis. (n = 25 flies/tube × 3 replicate tubes/experiment). B) General locomotor activity was, however, increased by (R)-DOI treatment. Activity Levels / Fly are a measure of total beam breaks / fly / 24 hours on Day 4 normalized to control values of 100. (n=16 flies/treatment; * p<0.01 vs control; one way ANOVA with Dunnett’s post hoc test for multiple comparison).
Aggression Assay
In these tests, eight different behavioral phenotypes related to aggression were scored based on the scale described by Chen and colleagues (Chen et al., 2002). Flies were scored individually, and interactive aggressive behaviors between flies counted separately. These included: slow approach, where one fly orients towards the other and slowly approaches; fast approach, where one fly orients towards the other and quickly approaches the other; lunging, where one fly orients towards the other and dramatically and quickly moves toward the other and ‘pounces’ or ‘head-butts’ the opponent, often rearing up on hind legs to strike; wing threats, where one fly orients his body in parallel to the other and lifts one wing and vibrates it; fencing, where one fly orients his body in parallel to the other and extends a leg to the other, which is then met by an extended leg from the opponent where they then ‘fence’; holding, where both flies are head-on to each other and extend their front legs and hold on to each other; boxing, where flies are closely facing each other and ‘boxing’ with their front legs, often rising on their hind legs; and retreat, when one fly retreats from the aggressive advance of another fly. Aggression can exist as a continuum from a low aggressive behavior such as slow approach, and progress to higher levels of aggression such as fencing, which can progress to boxing. Alternatively, these incidents can present in isolation. When two flies are seemingly ignoring each other, but in close proximity, one may suddenly charge the other without warning. There are a few more distinct aggressive behaviors described in the literature (Chen et al., 2002), but we have chosen to score only these major behaviors.
For the social housing experiment, the small groups consisted of six male flies placed in a 1.5 ml microcentrifuge tubes containing 200 ml of 1% agarose + 20% sucrose at the bottom, with air holes present in the cap, and did not receive any drug treatment, except for the 8-OH-DPAT group, which had 3 mM drug present. The large groups consisted of about 50 flies in a standard large diameter polystyrene vial, 10 of which were randomly selected for testing. Each of the treatment environments were placed under constant light conditions at 23 °C for 4 days without disturbances. On the fourth day individual flies were paired with another fly of the same treatment condition in the testing chamber.
The testing chamber was a modification of that described by Chen and coworkers (Chen et al., 2002), and consisted of a round optically clear 2.5 cm ID clear Plexiglas tube chamber with a food cup placed at the center. The food cup was an inverted 0.5 cm lid of a cryovial containing standard cornmeal molasses food smoothed to a flat surface with a drop of yeast paste at the center. A round 5 cm diameter lid was constructed from the top portion of a standard plastic Petri dish, which contained at its edge a small 3 mm hole for placing the flies into the chamber. Flies were placed in the aggression apparatus by gently tapping the glass tubes containing the flies over the insertion hole in the lid of the aggression apparatus. Testing was performed at 23 °C under standard room lighting conditions that did not vary between trials or experiments.
For the recording and scoring, a digital camcorder was placed at the edge of the chamber and focused on the food cup. Once both flies were on the food source, videotaping commenced for 20 minutes. After recording, the video was uploaded into iMovie HD on MacOS X, and scored for aggressive interactions by an observer blinded to the treatment of the flies. A total of eight behaviors were scored, and included slow approach, fast approach, wing threat, lunging, fencing, boxing/tussling, holding, and retreating, as described above. At least 2 seconds of inactivity between two behaviors marked the end of one behavior and beginning of the next.
Gene Expression
The heads from 12 male adult OR flies housed under isolated conditions, and the heads from 12 flies randomly selected from large group conditions, as described above, were rapidly dissected with a clean razor blade from flies anesthetized on ice and immediately placed on dry ice. Total RNA was isolated from the combined heads from each treatment group using TRI Reagent (MRC, Cincinnati, OH) following the manufacturers protocol. First strand cDNA synthesis was performed using the Improm-II kit from Promega using 1 μg total RNA per reaction following manufactures directions using random hexamer primers. Quantitative real-time PCR assays were designed using the Universal ProbeLibrary system (Exiqon, Vedbaek, Denmark; Roche, Indianapolis, IN; https://www.roche-applied-science.com). Amplicon primers and universal probes utilized for each of the four receptor genes and the reference standard, ribosomal protein L32 (RpL32) were as follows: 5-HT1ADro (U#56) F:5’-GTGGCCAATACC-3’, R: 5’-ATCTGGTTGCCAGAAGTGCT-3’; 5-HT1BDro (U#62) F: 5’-CAGCGATGCGGATGATTA-3’, R: 5’-CGAGGCTATCAGATGGTGCT-3’; 5-HT2Dro (U#105) F: 5’-CGATCTCCTGGTCAGTTTGTT-3’, R: 5’AAGCCAAGTGGCCAATACC-3’; 5-HT7Dro (U#44) F: 5’-AATGATTCTGAGGCTCGAAGA-3’, R: 5’ TATGAGCAACCCAGTGCTGA-3’; RpL32 (U#105) F: 5’-CGGATCGATATGCTAAGCTGT-3’, R: 5’--GCGCTTGTTCGATCCGTA-3’. Q-PCR was performed with the BioRad iCycler IQ5 (BioRad, Hercules, CA) using the HotStart-IT Probe qPCR Master Mix (USB, Cleveland, OH) following the manufacturers instructions (25 μl reaction volume; cycle parameters: initial 95 °C for 2 min, followed by 44 cycles of 95 °C 15 sec, 60 °C 45 sec) in 96 well plate format. Reactions were performed in quadruplicate for each gene. Expression of RpL32 was used as the reference control to normalize expression between treatment groups. Expression levels were calculated using the ΔΔCT method (ABI: User Bulletin #2, ABI Prism 7700 Sequence Detection System, 10/2001).
Statistical analysis
All analysis was performed with GraphPad Prism 4 (San Diego, CA), and used ANOVA with appropriate pos-hoc analysis for multiple comparisons.
RESULTS
Effects of drugs on locomotor activity
The effects of the drugs on locomotor activity were tested. There are many different methods to measure locomotor activity, including phototaxis, counting the number of lines walked over within a defined time, photobeam breaks in glass tubes (DAMS), and negative geotaxis, to name a few. Whereas all are informative with regard to activity levels, each assay has unique individual variables. For example, negative geotaxis includes startle response, phototaxis includes light response, line crossing can include exploratory behavior, and DAMS can involve circadian effects. We chose two of these behaviors to investigate locomotor response. In the negative geotaxis test, no drug had any statistically significant effect (Figure 1A). In the DAMS photobeam break measurement, (R)-DOI produced a statistically significant increase, and although there was a trend towards decreased activity with 8-OH-DPAT and WAY100635 it was not statistically significant (Figure 1B). It is possible that other locomotor assays may or may not show an effect of our panel of drugs on activity. Because of the effects observed in the DAMS experiment, there may be a confounding effect at some level on locomotor activity. This effect, however, is unclear because the locomotor changes induced by (R)-DOI (increased) and the trend produced by 8-OH-DPAT (decreased) are opposite to our results with respect to aggression, where (R)-DOI decreases, and 8-OH-DPAT increases aggression, as described below.
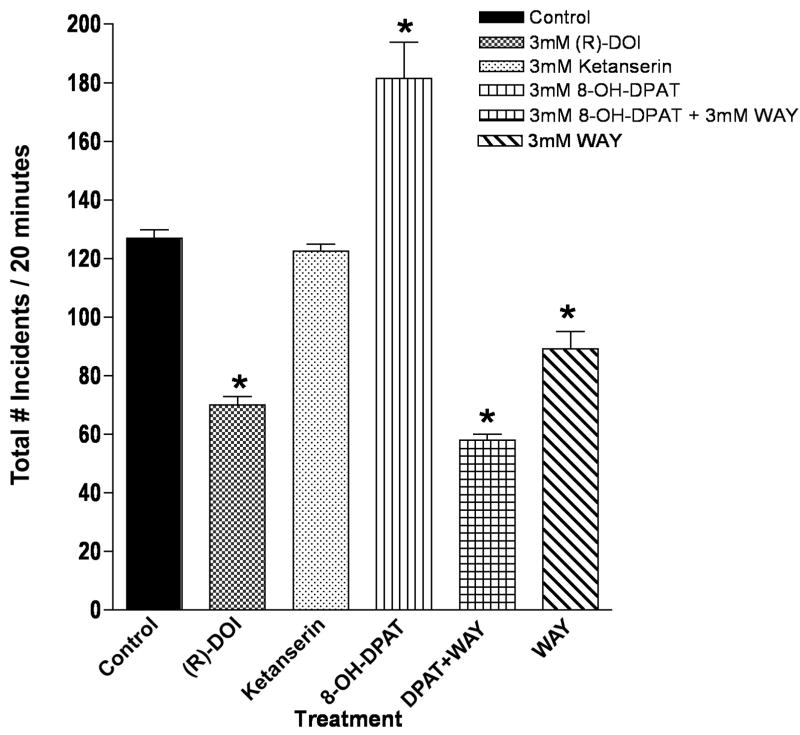
5-HT2 and 5-HT1A-like receptors modulate overall aggressive interactions
The effect of each drug on total aggression was examined as described in Experimental Procedures. Alterations in aggressive incidents included both changes in absolute numbers of incidents (e.g. wing threats) and the duration of a particular incident (e.g. holding). Because we used a criteria of at least 2 seconds of inactivity to define the next bout, however, results largely represent frequency rather than duration of a particular behavior. Control flies showed about 125 total aggressive incidents per 20 minute assay. The 5-HT2 receptor agonist (R)-DOI markedly reduced total aggression 50%, but the 5-HT2 receptor antagonist ketanserin had no effect (Figure 2). Whereas the 5-HT1A/7 receptor agonist 8-OH-DPAT increased total aggression by 50% (Figure 2), the 5-HT1A receptor antagonist WAY100635 reduced aggression (Figure 2). These data suggest that there is normally a tonic level of serotonin acting at the 5-HT1A-like receptors in Drosophila, and that either increasing or decreasing 5-HT1A-like receptor activity levels perturbs normal homeostasis with respect to aggressive behaviors. Interestingly, the combination of 8-OH-DPAT and WAY10635 even further reduced aggressive incidents (Figure 2). 8-OH-DPAT is also expected to be an agonist at the 5-HT7Dro receptor. Therefore, it may be that activation of this receptor suppresses aggressive behaviors, and that this is only revealed upon blockade of the effects of 8-OH-DPAT by WAY100635. Unfortunately there are no selective agonists of the 5-HT7 receptor to test this hypothesis.
Figure 2.
Total aggression is differentially affected by serotonin receptor agents. Isolated male flies under various treatment conditions, as indicated, were tested and scored for total aggressive incidents over a 20 minute period. The 5-HT2 receptor agonist (R)-DOI reduced aggression, whereas the 5-HT1A receptor agonist 8-OH-DPAT increased aggressive behaviors (n=10 flies/treatment; * p<0.05 compared to control, one-way ANOVA with Dunnett’s post hoc test for multiple comparison).
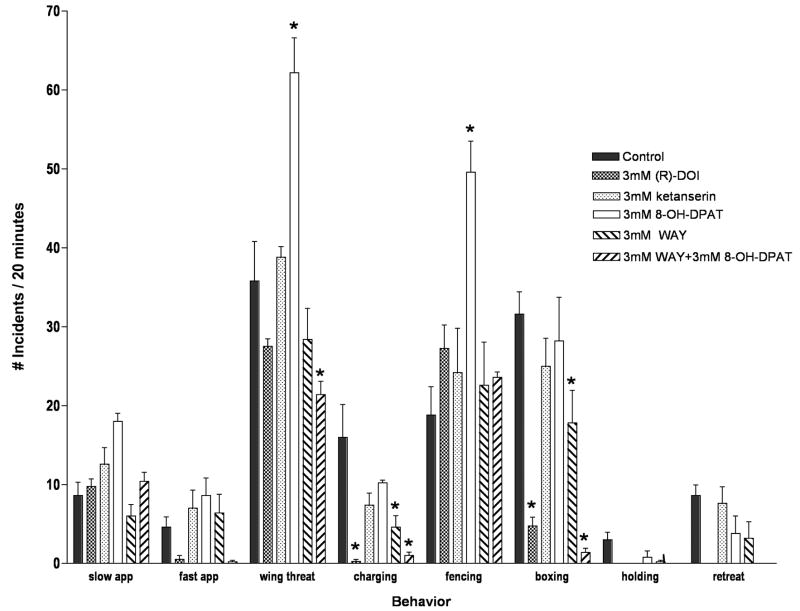
Different serotonin receptors modulate different aspects of aggression
The total aggressive behavior that we measured was comprised of the sum of eight distinct behaviors. When total aggressive incidents were categorized into these eight behaviors, surprisingly, differential effects of 5-HT2 and 5-HT1A-like receptor manipulation were unmasked. Specifically, we found that 5-HT2- and 5-HT1A-like serotonin receptors modulate different behaviors. Activation of 5-HT2 receptors with DOI primarily affected lunging and boxing, and significantly reduced these aggressive behaviors without altering levels of other others such as wing threats and fencing. 5-HT1A receptor activation by 8-OH-DPAT, however, primarily increased levels of the different behaviors of wing threats and fencing (Figure 3). The 5-HT1A receptor antagonist WAY100635 alone decreased wing threats, lunging, and boxing, but in combination with 8-OH-DPAT blocked the effects of 8-OH-DPAT on lunging and fencing, (Figure 3). Interestingly, the combination of WAY100635 and 8-OH-DPAT further decreased and nearly eliminated lunging and boxing behaviors, suggesting that these behaviors may be modulated by the 5-HT7Dro receptor, as described above.
Figure 3.
Different serotonin receptor agents had different effects on individual aggressive behaviors. Total aggressive behaviors were categorized into eight distinct categories. 5-HT2 receptor activation repressed aggression where indicated, whereas 5-HT1A-like activation elevated aggression where indicated. (n=10 flies/treatment; * p<0.05 compared to controls, two-way ANOVA with Bonferroni post hoc test for multiple comparisons).
In previous studies by others examining aggression, lunging has been observed to be the most common higher intensity behavior, with boxing a more rare event. Here, we have observed boxing to be the most commonly observed higher intensity behavior. This may be due to differences in how behaviors were scored between laboratories. Another, perhaps more likely reason could be the result of strain differences. Each of the other reports examined Canton-S (CS) flies, whereas we examined Oregon-R (OR) flies. Significant differences between these wild type strains, and even between the same strain depending on the source, have been observed for various behaviors including olfactory learning and memory, and gravitaxis (Tully and Quinn, 1985, Armstrong et al., 2006).
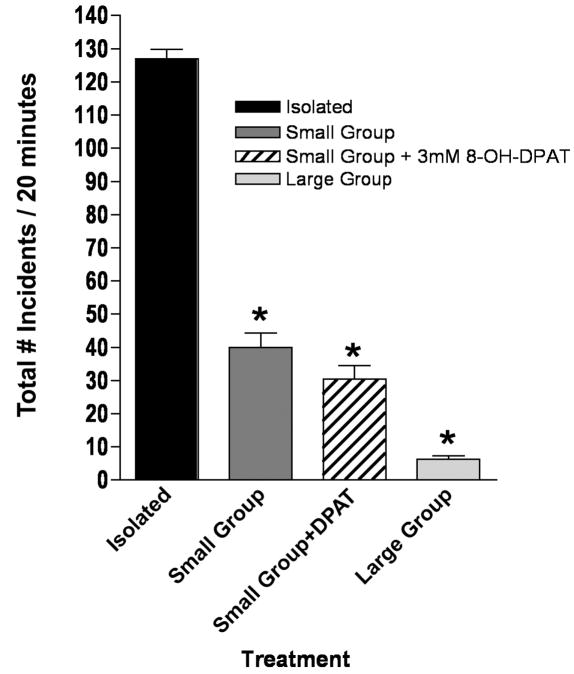
Social housing affects aggression – involvement of the 5-HT1A-like receptors
We examined the effects of social housing on aggression in the fly, and whether or not serotonergic processes may be involved in potential differences, because socially isolated mammals can demonstrate increased aggression (Matsumoto et al., 2005). In general, as the number of flies kept together before testing increased, the total number of aggressive incidents decreased, with flies kept in a large group environment demonstrating hardly any aggressive interactions (Figure 4). Based upon our results indicating that increased 5-HT1A-like receptor activation leads to increased aggression in isolated flies, and that 8-OH-DPAT had no effect on overall aggression in the small group-housed flies (Figure 4), we hypothesized that this decrease in aggression in the large social group may be due adaptive mechanisms involving reduction of 5-HT1A-like receptor function in the group-housed flies.
Figure 4.
Social environment affects aggression. Male flies under different social environments were tested and scored for total aggressive incidents over a 20 minute period. Flies maintained in groups of six had dramatically reduced aggression, and flies in large group environments (50) had virtually no aggression. Small groups of flies treated with 8-OH-DPAT showed no increases in aggression. (n=10 flies/treatment; * p<0.05 compared to control, one-way ANOVA with Dunnett’s post test for multiple comparison).
Social housing influences serotonin receptor gene expression levels
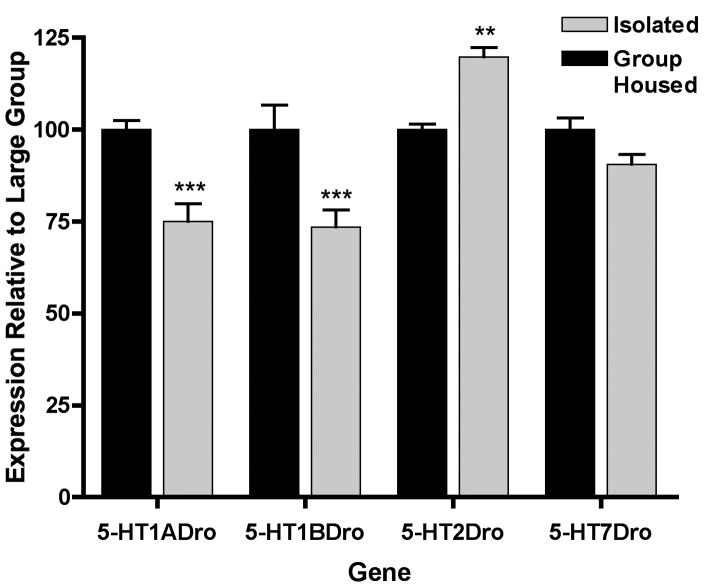
To test our hypothesis that social housing elicits adaptive mechanisms to modulate serotonin receptor activity, for example through receptor downregulation or desensitization, or a combination of both, we examined receptor expression by quantitative real-time PCR. Surprisingly, we found that isolation significantly reduced expression of the 5-HT1A-like receptor genes about 25% compared to the large group environment, and isolation significantly increased expression of the 5-HT2Dro receptor gene about 20% compared to the large group housed flies (Figure 5). There was no observable effect of isolation compared to the large group environment for 5-HT7Dro receptor mRNA expression (Figure 5). These results with respect to expression of 5-HT1A-like and 5-HT2 receptors are opposite of what would be predicted if alterations in receptor levels were playing a significant role in attenuating aggression in social housing.
Figure 5.
Serotonin receptor mRNA expression is altered by social condition. Levels of mRNA expression were examined for all four serotonin receptor genes by quantitative real-time PCR in the heads of large group-housed flies, and the heads of flies isolated for 4 days. Isolation resulted in a significant reduction in the expression of 5-HT1ADro and 5-HT1BDro receptor mRNA, and a significant increase in 5-HT2Dro receptor mRNA (*** p<0.001, ** p<0.01 vs group housed; 2-way ANOVA with Bonferroni post-hoc test for multiple comparison).
DISCUSSION
We have found that different aspects of aggressive behaviors in the fly are mediated by different serotonin receptors. We observed that the 5-HT2 receptor agonist (R)-DOI dramatically reduced total aggressive interactions in flies maintained in isolation, indicating that activation of 5-HT2Dro receptors represses aggression. We believe that the (R)-DOI induced reduction in aggression is not likely due to a general decrease in locomotor activity because assays testing the effects of (R)-DOI on negative geotaxis showed no statistical difference in activity levels, and that (R)-DOI actually increased activity in the DAMS test. Significantly, when total aggressive incidents were partitioned into eight subcategories, decreases in aggression were observed only within a subset of these behaviors: lunging and boxing. Other behaviors, such as fencing and wing threat, were not significantly altered. The 5-HT2 receptor antagonist ketanserin, however, had no effect, indicating that there is not normally constitutive activity from this receptor associated with aggressive behaviors. The absence of a behavioral response to ketanserin alone might raise the question of whether the dose was sufficiently high. Although we have not measured brain concentrations of ketanserin in these flies, in mammalian studies ketanserin has no overt observable effect in control animals. Further the cLogP of the antagonist WAY100635, which produces a behavioral effect, is 2.66, and that of ketanserin is 3.00, indicating that ketanserin is slightly more lipid soluble than WAY, and thus more likely to accumulate in lipid tissues such as neuronal cells.
By contrast, 8-OH-DPAT dramatically increased total aggressive incidents in isolated flies, indicating that 5-HT1A-like receptors positively mediate aggressive behaviors. Again, we believe that the effects of 8-OH-DPAT are not overtly influenced by alterations in locomotor activity. 8-OH-DPAT did not have any significant effects in either the negative geotaxis or DAMS assays. Although there was a trend towards decreased activity in the DAMS test, we observed an increase in aggressive behaviors in response to 8-OH-DPAT.
Because the 5-HT1A receptor antagonist WAY100635 not only blocked this increase, but significantly reduced overall aggressive behaviors to below control levels, there is likely a constitutive level of activation of these receptors normally modulating aggression. An unexpected finding was that feeding isolated flies both 8-OH-DPAT and WAY100635 reduced aggressive incidents even further than WAY100635 alone. This effect may be due to the participation of the 5-HT7Dro receptor, as described below. Alternatively, these results could potentially be due to differential effects of the drugs at pre- and postsynaptic receptors. The 5-HT1BDro receptor has been shown to have presynaptic expression (Yuan et al., 2005), and the same receptor can have different physical properties with respect to affinities and effector coupling through functionally selective mechanisms (Urban et al., 2007). If the antagonist WAY100635 has higher affinity or efficacy at the postsynaptic 5-HT1A-like receptors, and the agonist 8-OH-DPAT has higher affinity or efficacy at the presynaptic receptors, a possible scenario could be that antagonist is binding to the postsynaptic receptors and blocking activation by both 8-OH-DPAT and 5-HT, whereas 8-OH-DPAT activation of presynaptic autoreceptors is producing a reduction of 5-HT release. Together, these effects would lead to an even lower level of 5-HT1A-like receptor activation, and reduced aggression below the levels of antagonist alone.
Similar to the results with (R)-DOI, increases in aggression elicited by 8-OH-DPAT were observed only in a subset of aggressive behaviors. Wing threats and fencing were the only subcategories statistically different from controls, with both nearly doubled. The 5-HT1A receptor antagonist WAY100635 blocked the 8-OH-DPAT induced increases in wing threats and fencing, and significantly reduced lunging and boxing, the two behaviors reduced by (R)-DOI. In general, the effects of WAY10035 + 8-OH-DPAT were not statistically different from WAY100635 alone, except for boxing, which was virtually eliminated by the double treatment condition. These results may be due to potential functional selectivity at pre- and postsynaptic 5-HT1A-like receptors, as discussed above. Another plausible explanation could be involvement of the 5-HT7Dro receptor in boxing behaviors. In mammalian systems, 8-OH-DPAT also has significant affinity and activity at 5-HT7 receptors, and there is nearly equal affinity for this drug at fly 5-HT1A-like and 5-HT7Dro receptors (Saudou et al., 1992). Therefore, it may be that activation of the 5-HT7Dro receptor suppresses certain aggressive behaviors like boxing, and that this activity is only revealed upon blockade of the effects of 8-OH-DPAT on 5-HT1A-like receptors by WAY100635. This hypothesis, however, awaits selective 5-HT7 agonists to be tested.
In the adult fly brain, different 5-HT receptors are expressed in distinct circuitries (Yuan et al., 2005, Yuan et al., 2006, Nichols, 2007). Whereas aggressive encounters in the fly are proposed to escalate from less aggressive behaviors to more aggressive ones, our results suggest that different receptor circuitries contribute to modulation of distinct aspects of aggression that together constitute the global behavior. Interestingly, the effects of serotonergic agents in the fly appear to be opposite to those observed in mammalian systems, where 5-HT1A receptor activation generally decreases overt aggression, and 5-HT2 receptor activation increases overt aggression (Hassanain et al., 2003, de Boer and Koolhaas, 2005). That is not always the case, however, as opposite effects on aggressive behaviors in mammals can be elicited by stimulation of the same serotonin receptor in different regions of the brain (De Almeida and Lucion, 1997).
Furthermore, our results also may be dependent upon the rate of drug administration, which was slow and long-term. In crayfish studies where serotonin itself was infused into the animal, it was not the absolute amount of serotonin infused that was found to influence certain aggressive behaviors, but the rate of administration (i.e. rapidly or slowly), that was determined to be more important. (Panksepp and Huber, 2002, Panksepp et al., 2003). This finding is similar to what has been proposed for serotonin function in the lobster, where tonic and phasic exposure to serotonin each can produce different behavioral responses (Ma et al., 1992). These studies demonstrating that aggression-related behaviors do not necessarily correlate with absolute 5-HT content, but rather rate of administration, indicate that some forms of compensatory mechanisms are in place to regulate the behavioral effects of elevated 5-HT in crustaceans. These mechanisms likely include modulation of neurotransmitter release and receptor downregulation (Cooper et al., 2001). Therefore, the slow long-term administration of drugs in our studies may be affecting receptor function to produce aggressive behaviors different from those if drug is administered acutely.
We also observed that the social environment during the four days leading up to the testing had a significant influence on aggression. Flies that were isolated had the highest overall levels of aggressive behaviors, whereas those maintained in groups of six exhibited only about one-third the levels of isolated flies, and flies taken from a more crowded environment showed virtually no aggression. These results are in agreement with another recent report demonstrating that socially housed flies exhibit reduced aggression (Wang et al., 2008). Significantly, we observed that 8-OH-DPAT produced no differences in aggressive behaviors from controls in the small group-housed flies. These results, together with those demonstrating increased aggression in isolated flies treated with 8-OH-DPAT, suggested that interactions between multiple flies over a period of days may modulate neural compensatory mechanisms, similar to what likely occurs in crustaceans after serotonin level manipulation, which ultimately act to inhibit aggression in a group environment through mechanisms including downregulation or desensitization of 5-HT1A-like receptor activity. If that were occurring, one may anticipate that other 5-HT1A-like receptor mediated behaviors may be affected by housing status. Limited knowledge, however, exists on these behaviors and it remains to be investigated in future studies. Surprisingly, when we examined gene expression levels for each of the receptors in the fly head, we found the opposite of what we predicted if receptor 5-HT1A-like receptor downregulation were underlying the effects of social housing. Both 5-HT1ADro and 5-HT1BDro receptor mRNA levels were significantly decreased in isolated flies compared to flies from the large social environment. Furthermore, 5-HT2Dro mRNA was increased. These results indicate that modulatory mechanisms are indeed occurring with regards to serotonin receptor function under different social housing conditions, but their precise nature, and how they mediate alterations in aggression is likely more complex than simple measures of receptor expression can determine.
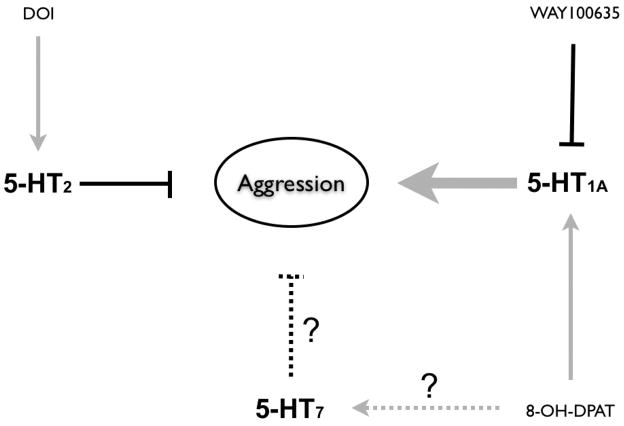
Based upon our results with total aggressive incidents, we propose a preliminary model (Figure 6) where basal activity of 5-HT2Dro receptors does not normally influence or modulate overall aggression. When they are stimulated, however, they negatively modulate certain aggressive behaviors, particularly boxing and lunging. There may be, however, a certain constitutive level of activation of 5-HT1A-like receptors (5-HT1ADro and 5-HT1BDro) by serotonin such that increased activation of these circuits increases certain aggressive behaviors, whereas inactivation of these circuits decreases behaviors. Furthermore, 8-OH-DPAT activation of 5-HT7Dro receptors may exert a weak negative modulatory influence on aggression, and these potential inhibitory influences may be overshadowed by the positive influence of 8-OH-DPAT at the 5-HT1A-like receptors. It is not until the effects of 5-HT1A-like receptor activation are masked by WAY100635 that the putative inhibitory effects of 5-HT7Dro may become apparent, particularly with regard to boxing behavior.
Figure 6.
Model for serotonergic modulation of aggression based upon total aggressive behaviors. When stimulated with DOI, 5-HT2Dro receptors may negatively modulate certain aggressive behaviors, particularly boxing and lunging. The 5-HT1A-like receptors (5-HT1ADro and 5-HT1BDro) may normally exert a tonic influence on aggression such that activation of these circuits with 8-OHDPAT increases certain aggressive behaviors, whereas inactivation of these circuits with WAY100636 decreases behaviors. Although 8-OH-DPAT activation of the 5-HT7Dro receptor may exert a weak negative modulatory influence on aggression, these potential inhibitory influences may be overshadowed by the positive influence of 8-OH-DPAT on 5-HT1A-like receptors. It is not until the effects of 5-HT1A-like receptor activation with 8-OH-DPAT are masked by WAY100635 that the putative inhibitory effects of 5-HT7Dro may become apparent, especially with regard to boxing behavior.
Although further research is necessary to determine exactly how each circuit contributes to aggression, especially with respect to the sub-categories, this work provides the first clues as to how different receptors for the neurotransmitter serotonin are involved in aggressive behaviors in Drosophila.
Acknowledgments
The authors would like to thank Lisa Bothman for technical assistance. Supported in part by MH078454 and MH078454-S1.
Abbreviations
- (5-HT)
5-hydroxytryptamine
- (R)-DOI
(R)-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane
- (8-OH-DPAT)
8-hydroxy-2-dipropylaminotetralin hydrobromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong JD, Texada MJ, Munjaal R, Baker DA, Beckingham KM. Gravitaxis in Drosophila melanogaster: a forward genetic screen. Genes, brain, and behavior. 2006;5:222–239. doi: 10.1111/j.1601-183X.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- Chan YB, Kravitz EA. Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:19577–19582. doi: 10.1073/pnas.0709803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas JF, Launay JM, Kellermann O, Rosay P, Maroteaux L. Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci U S A. 1995;92:5441–5445. doi: 10.1073/pnas.92.12.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Chase RJ, Tabor J. Altered responsiveness to 5-HT at the crayfish neuromuscular junction due to chronic p-CPA and m-CPP treatment. Brain Res. 2001;916:143–151. doi: 10.1016/s0006-8993(01)02885-2. [DOI] [PubMed] [Google Scholar]
- Dasari S, Viele K, Turner AC, Cooper RL. Influence of PCPA and MDMA (ecstasy) on physiology, development and behavior in Drosophila melanogaster. Eur J Neurosci. 2007;26:424–438. doi: 10.1111/j.1460-9568.2007.05655.x. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology (Berl) 1997;134:392–400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: A pharmacological challenge of the serotonin deficiency hypothesis. European Journal of Pharmacology. 2005;526:125. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006;38:1023. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nature genetics. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- Dow MA, Schilcher F. Aggression and mating success in Drosophila melanogaster. Nature. 1975;254:511–512. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Experimental Gerontology. 2005;40:386. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Bhatt S, Siegel A. Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1A and 5-HT2 receptors. Brain Res. 2003;981:201–209. doi: 10.1016/s0006-8993(03)03036-1. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Kirk D, Panckeri K, Miller MS, Pack AI. Modafinil maintains waking in the fruit fly drosophila melanogaster. Sleep. 2003;26:139–146. doi: 10.1093/sleep/26.2.139. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and Drosophila simulans. Anim Behav. 1987;35:807–818. [Google Scholar]
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci. 2006;26:7245–7256. doi: 10.1523/JNEUROSCI.5426-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci U S A. 1997;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ME. Influence of light on mating of Drosophila melanogaster. Ecology. 1960;41:182–188. [Google Scholar]
- Jacobs ME. Influence ofβ-alanine on mating and territorialism inDrosophila melanogaster. Behavior Genetics. 1978;8:487. doi: 10.1007/BF01067478. [DOI] [PubMed] [Google Scholar]
- Kravitz EA. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. Journal of comparative physiology. 2000;186:221–238. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Leal SM, Kumar N, Neckameyer WS. GABAergic modulation of motor-driven behaviors in juvenile Drosophila and evidence for a nonbehavioral role for GABA transport. J Neurobiol. 2004;61:189–208. doi: 10.1002/neu.20061. [DOI] [PubMed] [Google Scholar]
- Liu T, Dartevelle L, Yuan C, Wei H, Wang Y, Ferveur JF, Guo A. Increased dopamine level enhances male-male courtship in Drosophila. J Neurosci. 2008;28:5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Harris-Warrick RM, Kravitz EA. Serotonin and Octopamine Produce Opposite Postures in Lobsters. Science. 1980;208:76–79. doi: 10.1126/science.208.4439.76. [DOI] [PubMed] [Google Scholar]
- Ma PM, Beltz BS, Kravitz EA. Serotonin-containing neurons in lobsters: their role as gain-setters in postural control mechanisms. Journal of neurophysiology. 1992;68:36–54. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress (Amsterdam, Netherlands) 2005;8:85–93. doi: 10.1080/10253890500159022. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Matsuo H. Distinct neural circuits reflect sex, sexual maturity, and reproductive status in response to stress in Drosophila melanogaster. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112:677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Nichols CD. 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Developmental Neurobiology. 2007;67:752–763. doi: 10.1002/dneu.20370. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Ronesi J, Pratt W, Sanders-Bush E. Hallucinogens and Drosophila: linking serotonin receptor activation to behavior. Neuroscience. 2002;115:979–984. doi: 10.1016/s0306-4522(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Huber R. Chronic alterations in serotonin function: dynamic neurochemical properties in agonistic behavior of the crayfish, Orconectes rusticus. J Neurobiol. 2002;50:276–290. doi: 10.1002/neu.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Yue Z, Drerup C, Huber R. Amine neurochemistry and aggression in crayfish. Microscopy research and technique. 2003;60:360–368. doi: 10.1002/jemt.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK. From genes to aggressive behavior: the role of serotonergic system. BioEssays. 2006;28:495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. Embo J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry TS, Thompson CK, Wingfield JC. Effects of acute treatment with 8-OH-DPAT and fluoxetine on aggressive behaviour in male song sparrows (Melospiza melodia morphna) Journal of neuroendocrinology. 2003;15:150–160. doi: 10.1046/j.1365-2826.2003.00968.x. [DOI] [PubMed] [Google Scholar]
- Spitzer N, Edwards DH, Baro DJ. Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii. J Exp Biol. 2008;211:92–105. doi: 10.1242/jeb.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. Journal of comparative physiology. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, Hen R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci U S A. 1990;87:8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:17519–17524. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]