Abstract
A series of quinolines, including chloroquine and quinine, were identified as potent pigmentation inhibitors through screening a compound library in murine melanocytes. Structure-activity relationship analysis indicated that 4-substituted amino groups with a tertiary amine side chain, such as chloroquine, were associated with robust inhibitory activity. In contrast to many previously identified pigmentation inhibitors, these newly identified inhibitors had no effect on either the level or the enzymatic activity of tyrosinase, the rate-limiting enzyme in melanin production. Rather, our results showed that these quinolines inhibited melanogenesis by disrupting the intracellular trafficking of tyrosinase-related proteins and lysosome-associated membrane protein 1 (Lamp-1). In treated melanocytes, tyrosinase and tyrosinase-related protein 1 accumulated in Lamp-1-positive perinuclear organelles instead of melanosomes, thus preventing melanogenesis. The depigmenting abilities of chloroquine and quinine salicylate were assessed in a human skin equivalent model (MelanoDerm). Both compounds were considerably more effective than arbutin, a widely used lightening agent. Our results indicate that quinolines may be useful agents for “cosmeceutical” skin lightening and treatment of hyperpigmentation disorders.
Constitutive skin pigmentation depends primarily on the amount and type of melanin synthesized by melanocytes located in the basal layer of epidermis and its distribution toward the surface of the epidermis (Hearing, 2005; Yamaguchi et al., 2007). Melanin is synthesized within a specialized organelle termed the melanosome (Jimbow et al., 1976; Shen et al., 2001; Hearing, 2005). An essential enzyme in the synthesis of melanin is tyrosinase (Tyr), a melanosomal membrane glycoprotein that catalyzes the initial and rate-limiting steps of melanogenesis (Körner and Pawelek, 1982; Tripathi et al., 1992). After initial synthesis and processing in the endoplasmic reticulum (ER), tyrosinase traffics through the Golgi network and from there to its final destination, the melanosome, where melanin synthesis and deposition takes place (Raposo et al., 2001; Toyofuku et al., 2001). Ultimately, melanins packed in melanosomes are transferred from melanocytes to the adjacent keratinocytes. The distribution pattern of melanosomes and their breakdown in keratinocytes are also processes that play important roles in determining skin pigmentation (Van Den Bossche et al., 2006; Goding, 2007; Yoshida et al., 2007).
The ability to control skin pigmentation may be used to address a variety of medical conditions, such as acquired hyperpigmentation. A number of lightening agents, such as hydroquinone, arbutin, and kojic acid, are also commonly used as cosmetic additives. However, they are only modestly active; some, such as hydroquinone, may be associated with adverse side effects (Abramowitz and Chavin, 1980; Curto et al., 1999). There is a need for more effective and safe lightening agents to be used in cosmetic and medicinal products for the treatment of hyperpigmentation disorders.
Despite remarkable progress in the knowledge of melanocyte biology and the mechanism of melanin synthesis, the regulation of mammalian pigmentation remains incompletely understood because of its complexity. For example, other types of cells in the skin also contribute to the regulation of melanogenesis, and depigmenting compounds identified by using melanocytes alone in vitro might not prove effective in reducing human skin pigmentation. In recent years, reconstituted human skin equivalents such as MelanoDerm have provided a means to evaluate cosmetic and pharmaceutical agents designed to modulate pigmentation (Yoon et al., 2003; Takeyama et al., 2004; Matsuda et al., 2008). MelanoDerm consists of normal human-derived epidermal keratinocytes and melanocytes that undergo spontaneous melanogenesis in a morphological and growth environment similar to human epidermis. These human skin equivalents may provide a more physiologically relevant system to study the three-dimensional interactions of melanocytes and keratinocytes and to elucidate the regulatory mechanism of pigmentation modulators.
Our laboratory has successfully employed chemical genetic screening to identify novel compounds and their targets involved in the regulation of mammalian pigmentation (Ni-Komatsu and Orlow, 2007; Ni-Komatsu and Orlow, 2008). In the current study, we screened a commercially available library of natural products and marketed drugs for their ability to inhibit pigmentation in cultured murine melanocytes in order to discover alternative treatments for hyperpigmentation disorders. A group of quinolines was identified as potent pigmentation inhibitors. The mechanism by which they inhibit melanogenesis was further characterized and found to involve disruption of tyrosinase family member trafficking. Two compounds, chloroquine and quinine salicylate, were selected to verify their effects on pigmentation in the MelanoDerm model. Our results indicate that both quinolines are much more potent melanogenic inhibitors than arbutin.
Materials and Methods
Materials
Chloroquine, cinchonidine, hydrocinchonine, hydroquinidine, hydroquinidine 4-chlorobenzoate, hydroquinine, hydroquinine hydrobromide hydrate, N-benzylcinchonidinium chloride, mefloquine hydrochloride, primaquine diphosphate, quinacrine hydrochloride, quinidine and quinine were purchased from Sigma-Aldrich (St. Louis, MO). Amodiaquine dihydrochloride, dibucaine hydrochloride, and quinine ethyl carbonate were purchased from MicroSource Discovery Inc. (Gaylordsville, CT).
αPep7 antiserum was raised against the C terminus of mouse Tyr based on reported antigenic peptide sequences (Jiménez et al., 1991). The following antibodies were also used: Mel-5, a monoclonal antibody reactive with mouse Tyrp1 (Signet Laboratories Inc., Dedham, MA); 1D4B, a monoclonal antibody reactive with mouse Lamp-1 (The Developmental Studies Hybridoma Bank, Iowa City, IA); and sheep anti-TGN38, a polyclonal antibody reactive with mouse TGN38, a trans-Golgi network (TGN) marker (Serotec, Oxford, UK).
Compound Library
The library used in this study was The Spectrum Collection (MicroSource Discovery Inc., Gaylordsville, CT). The 2000 compounds in this library include many marketed drugs or natural products and are supplied as a 10 mM solution in dimethyl sulfoxide.
Cell Culture
Murine melan-a cells were cultured as reported previously (Ni-Komatsu and Orlow, 2008).
Screening of the Compound Library in Melan-a Cells and Melanin Assay in Cells
Screening and melanin assay were performed as reported previously (Ni-Komatsu and Orlow, 2008).
Immunofluorescence Microscopy
Cells were cultured on coverslips (Thermo Fisher Scientific, Waltham, MA), incubated with or without 2.5 µM chloroquine or mefloquine, 5 µM quinidine, quinine, or quinine salicylate for 72 h or the indicated time at 37°C. The cells were fixed with −20°C methanol for 5 min and stained as described previously (Shen et al., 2001). The slides were analyzed with a confocal microscope (LSM 510; Zeiss, Proctor, MN).
Culture and Treatment of Human Skin Equivalent
MelanoDerm (MatTek Corp., Ashland, MA) is a viable reconstituted three-dimensional human skin equivalent containing normal melanocytes and keratinocytes that are derived from African-American (MEL-B), Asian (MEL-A), or white (MEL-C) donors. MEL-A and MEL-B tissues were used in the current study, and they were maintained in the NMM-113 medium as recommended by the manufacturer.
Chloroquine was dissolved in water to a final concentration of 0.5 mM (equal to 0.26 mg/ml). Quinine salicylate was dissolved in 30:70 water/propylene glycol (PEG) to a final concentration of 4.0 mM (equals to 1.85 mg/ml). A 25-µl aliquot of each compound was applied topically to the Asian MelanoDerm tissue (MEL-A) on days 0, 1, 3, 6, 8, 10, and 13. The MelanoDerm tissues were fed every other day with 5 ml of fresh NMM-113. Before each application, the tissues were washed with 1 ml of PBS to remove any residual test compound. Tissues were fixed on days 13 and 16 for microscopic analysis and histological evaluation. In addition, duplicate tissues were frozen on days 13 and 16 for melanin assay. Water/PEG (30:70) and water alone were used as negative controls, and arbutin (at concentration of 3 mg/ml, approximately 11 mM) was used as a positive control.
Similar experiments were performed on African-American skin equivalents (MEL-B) except that a 25-µl aliquot of each compound was applied topically to MEL-B on days 0, 1, 3, 6, 8, and 10, and the treatment was lasted for either 10 or 13 days.
Cell Viability Assay of Human Skin Equivalent
Cell viability was measured in accordance with the manufacturer’s instructions using the MTT assay kit (MatTek Corp.). A 10-or 25-µl aliquot of chloroquine (at 0.5 mM) or quinine salicylate (at 4.0 mM) was applied topically to MEL-A on days 0, 1, 3, and 6. Water/PEG (30:70) and water alone were used as negative controls. One MEL-A tissue from each condition was harvested on days 2 and 7, and MTT assay was performed. Similar experiments were performed on MEL-B.
Melanin Assay of Human Skin Equivalent
Melanin content in treated human skin equivalent (MEL-A and MEL-B) was determined as previously reported (Rosenthal et al., 1973; Takeyama et al., 2004). The frozen tissues were homogenized in 0.45 ml of 1% SDS, 50 µM EDTA, and 10 mM Tris, pH 6.8. To each homogenate, 20 µl of protease K was added at 5 mg/ml. Digestion proceeded overnight at 45°C, and an additional 20 µl of protease K was added for an additional 4 hours of incubation. Then 50 µl of 500 mM sodium carbonate and 10 µl of 30% H2O2 were added to the homogenates. Samples were maintained at 80°C for 30 min and cooled to room temperature. The mixture was extracted with 100 µl of chloroform/methanol (2:1). After centrifugation at 10,000g for 30 min, the optical density of the top phase at 490 nm was measured. Synthetic melanin (Sigma-Aldrich) was subjected to the same procedure as a control, and a standard curve was constructed so that the melanin content of the unknowns could be determined for each cultured tissue.
Histological Analysis of Human Skin Equivalent
Treated African-American and Asian human skin equivalent (MEL-B and MEL-A) samples were fixed with 10% formalin (Sigma-Aldrich) at 4°C overnight. Samples were observed under phase-contrast microscopy. To visualize the melanin distribution throughout the tissue, Fontana-Masson silver stain was performed on the paraffin-embedded tissues as described previously (Bancroft and Stevens, 1982; Yoon et al., 2003).
Results
Identification of Quinoline Analogs as Pigmentation Inhibitors by Screening the Spectrum Collection Library in Murine Melanocytes
A commercial library of 2000 drugs and natural products was screened to identify novel compounds that inhibit melanin synthesis in cultured murine melanocytes (melan-a cells). Screening was performed at final compound concentration of 1 µM (0.1 µl of stock) in 24-well plates followed by melanin assay. DMSO (0.1 µl) was used as a negative control and the tyrosinase inhibitor phenylthiourea at 300 µM was used as a positive control on every plate (Hall and Orlow, 2005). Positive candidates were rescreened in duplicate at final concentrations of 1 and 5 µM, resulting in the initial identification of four quinolines amodiaquine dihydrochloride, primaquine diphosphate, quinacrine hydrochloride, and quinine as potential pigmentation inhibitors.
These drugs all belong to a family of quinolines that function as antimalarials (Goodman and Gilman, 1996). Additional quinolines were tested for their effect on pigmentation and seven more compounds, chloroquine, cinchonidine, dibucaine hydrochloride, mefloquine hydrochloride, quinidine, quinine ethyl carbonate, and quinine salicylate, were also found to inhibit melanin synthesis to varying extents in melan-a cells. Inhibition of melanin synthesis in a dose-dependent manner in melan-a cells was observed with all 11 tested quinolines (data not shown). Cinchonidine, dibucaine hydrochloride, quinine ethyl carbonate, and quinine salicylate did not exhibit cytotoxicity at concentrations below 20 µM; primaquine diphosphate, quinidine, and quinine were not cytotoxic at concentration below 10 µM; amodiaquine dihydrochloride, chloroquine, and mefloquine hydrochloride did not show any cytotoxicity at concentrations below 5 µM; and quinacrine hydrochloride showed cytotoxicity at concentrations above 2.5 µM (data not shown).
The IC50 values for inhibition of cellular melanin synthesis for the 11 quinolines were determined by culturing melan-a cells with various concentrations of each compound for 72 h (Table 1). Chloroquine was the most potent pigmentation inhibitor with an IC50 less than 1 µM; amodiaquine dihydrochloride, mefloquine hydrochloride, primaquine diphosphate, quinacrine hydrochloride, quindine, quinine, quinine ethyl carbonate, and quinine salicylate were less potent pigmentation inhibitors with IC50 values between 1 and 10 µM; cinchonidine and dibucaine hydrochloride showed the least potency in inhibiting pigmentation with IC50 values above 10 µM (Table 1). By comparison, in our system, the IC50 values of two previously recognized pigmentation inhibitors, phenylthiourea and hydroquinone, were 29 and 36 µM, respectively (Hall and Orlow, 2005). These data suggest that when tested on melanocytes in vitro, all 11 quinolines are more potent pigmentation inhibitors than currently available depigmenting compounds. Although quinacrine hydrochloride was a potent pigmentation inhibitor, it was eliminated from further study because of its high cytotoxicity.
TABLE 1.
Structures of quinoline analogs that inhibit pigmentation and their IC50 values on melanin synthesis in melan-a cells
| Compound | Structure | IC50 |
|---|---|---|
| µM | ||
| Amodiaquine dihydrochloride |
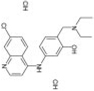
|
2.6 ± 0.15 |
| Chloroquine |
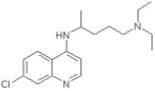
|
0.7 ± 0.18 |
| Cinchonidine | 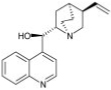 |
10.9 ± 0.22 |
| Dibucaine hydrochloride |
|
10.2 ± 0.28 |
| Mefloquine hydrochloride |
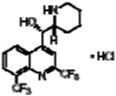
|
2.0 ± 0.15 |
| Primaquine diphosphate | 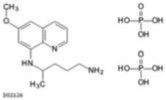 |
6.8 ± 0.37 |
| Quinacrine hydrochloride |
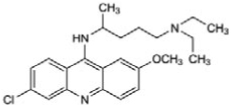
|
1.8 ± 0.17 |
| Quinidine |
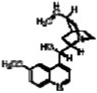
|
2.7 ± 0.14 |
| Quinine | 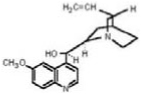 |
1.6 ± 0.15 |
| Quinine ethyl carbonate |
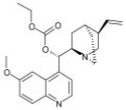
|
5.1 ± 0.12 |
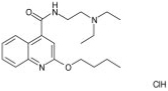
| Quinine salicylate |
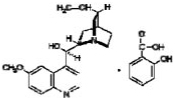
|
3.6 ± 0.28 |
Structure-Activity Relationship Analysis of Quinoline Analogs as Pigmentation Inhibitors
To gain insight into structure-activity relationships, additional quinoline analogs were tested for their ability to inhibit melanin synthesis in melan-a cells at final compound concentrations of 1 and 5 µM. The effects on pigmentation in melan-a cells are presented in Table 2.
TABLE 2.
Structures of cinchona alkaloids and quinolines and their effects on melanin synthesis in melan-a cells
| Compound | Structure | Inhibition | |
|---|---|---|---|
| At 1 µM | At 5 µM | ||
| % | |||
| Cinchonidine |
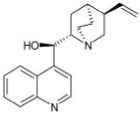
|
25.0 | 35.7 |
| Hydrocinchonine | 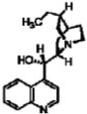 |
24.0 | 37.3 |
| Hydroquinidine | 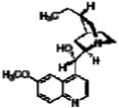 |
30.9 | 52.3 |
| Hydroquinidine 4-chlorobenzoate | 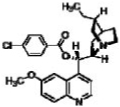 |
10.0 | 25.5 |
| Hydroquinine | 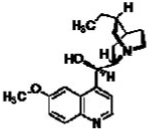 |
30.5 | 67.5 |
| Hydroquinine hydrobromide hydrate | 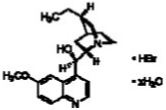 |
26.8 | 67.4 |
| N-Benzylcinchonidinium chloride | 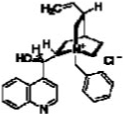 |
9.6 | 16.4 |
Our data indicate that 4-substituted amino groups with a tertiary amine side chain provide good inhibitory activity. For example, chloroquine inhibited pigmentation with an IC50 of 0.7 µM. Similar activity was observed for amodiaquine dihydrochloride and quinacrine hydrochloride; both can be considered 4-substituted aminoquinolines where the amino group bears an additional tertiary amine side chain. Furthermore, compounds having a 4-alkyl group containing a tertiary amine side chain, such as quinine and quinidine derivates, also exhibited good activity. The chirality of the α-carbon of the 4-substituent in quinine and quinidine derivates does not appear to have any impact on the activity. Finally, substitutions in the phenyl ring of the quinoline core seem to enhance the inhibitory activity. For example, unsubstituted compounds, such as cinchonine and cinchonidine, which are close analogs of quinine and quinidine, showed low (cinchonidine) or no (cinchonine) activity as pigmentation inhibitors.
Quinolines Inhibit Pigmentation via Altering the Intracellular Trafficking of Tyrosinase and Tyrosinase-related Protein 1
Because tyrosinase plays a central role in melanogenesis, it is not surprising that many depigmenting compounds act via effects on the expression, processing, maturation, degradation, and enzymatic activity of this enzyme (Parvez et al., 2006; Solano et al., 2006; Ni-Komatsu and Orlow, 2008). Therefore, we performed several assays to determine whether these quinoline pigmentation inhibitors affected tyrosinase.
None of the quinolines seemed to alter levels of tyrosinase, Tyrp1, and Tyrp2/Dct proteins as assessed by immunoblotting (data not shown). The effect of these compounds on tyrosinase activity was studied in vitro using melan-a cell lysates. None of the tested quinolines directly inhibited tyrosinase activity (data not shown). No obvious difference in tyrosinase activity of melan-a cell lysates was observed when the cells were preincubated with each compound for 72 h before harvesting (data not shown). These data suggested that quinolines decreased pigmentation through mechanisms other than inhibiting tyrosinase levels or enzymatic activity.
Many genetic disorders of hypopigmentation are due to alterations in the subcellular distribution of tyrosinase gene family proteins (Chen et al., 2002; Richmond et al., 2005; Wei, 2006). Therefore, compounds that inhibit pigmentation could act through altering the subcellular distribution of these proteins, a phenomenon reported previously by our laboratory (Hall et al., 2003; Ni-Komatsu and Orlow, 2008).
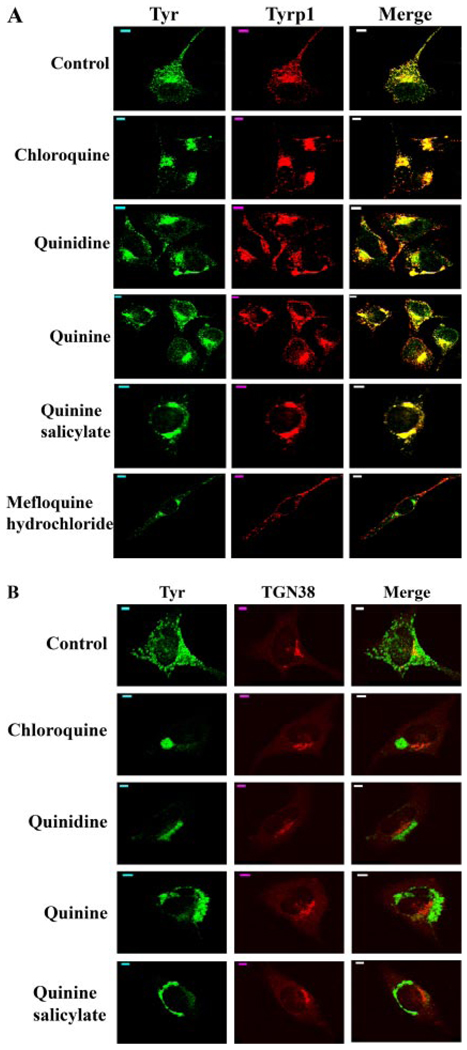
We evaluated the subcellular localizations of tyrosinase and Tyrp1 in melan-a cells treated with quinolines by immunofluorescence (Fig. 1A). As observed previously in these cells (Chen et al., 2002; Toyofuku et al., 2002), in both vehicle-treated and untreated cells, tyrosinase and Tyrp1 are found in melanosomes distributed throughout the cytoplasm, as well as in the perinuclear area where the endoplasmic reticulum (ER), Golgi, and stage I melanosomes are located. Dramatic differences in the subcellular distribution of tyrosinase and Tyrp1 were observed after treatment of melan-a cells with the newly identified quinoline pigmentation inhibitors. In melan-a cells treated with chloroquine, quinidine, quinine, and quinine salicylate, both tyrosinase and Tyrp1 were almost exclusively restricted to the perinuclear region (Fig. 1A). Similar results were observed in melan-a cells treated with each of the other quinoline analogs (data not shown), except mefloquine hydrochloride. In melan-a cells treated with mefloquine hydrochloride, tyrosinase was trapped to the perinuclear region (Fig. 1A), as observed when cells were treated with the other quinolines. However, mefloquine hydrochloride treatment did not seem to alter the subcellular localization of Tyrp1, and the colocalization of Tyr and Tyrp1 was abolished in treated cells (Fig. 1A).
Fig. 1.
Quinolines alter the intracellular trafficking of tyrosinase (Tyr) and tyrosinase-related protein 1 (Tyrp1). A, alterations of the subcellular localization of Tyr and Tyrp1 by quinolines. Immunofluorescence confocal microscopy images are shown with immunostaining of Tyr (green) and Tyrp1 (red). The colocalization of tyrosinase with tyrosinase-related protein 1 is indicated in yellow in the merged image. Scale bars, 5 µm. B, tyrosinase traffics beyond the TGN in melan-a cells treated with quinolines. Immunofluorescence confocal microscopy images are shown with immunostaining of Tyr (green) and TGN38 (red). The colocalization of tyrosinase with TGN38 is indicated as yellow in the merged image. Scale bars, 5 µm.
Tyrosinase (Tyr) is a type I membrane glycoprotein with 6 or 7 asparagine residues that undergo N-glycosylation (Körner and Pawelek, 1982; Ujvari et al., 2001). After initial glycosylation and processing in the ER, Tyr traffics through the Golgi network and from there to its final destination, the melanosome (Raposo et al., 2001; Toyofuku et al., 2001). No obvious change in the endoglycosidase H (Endo-H) sensitivity of tyrosinase was observed in melan-a cells treated with these quinolines (data not shown), suggesting that in these cells, tyrosinase has been processed beyond the ER and the early Golgi compartments. The perinuclear pool of tyrosinase in melan-a cells treated with chloroquine, quinidine, quinine, and quinine salicylate showed no obvious colocalization with TGN38, a trans-Golgi network (TGN) marker (Fig. 1B, data not shown for the other quinoline pigmentation inhibitors), indicating that tyrosinase trafficked beyond the TGN in melan-a cells treated with these compounds. Post-Golgi sorting of tyrosinase is thought to involve trafficking through endosomal compartments (Theos et al., 2005). However, quinoline treatment did not seem to affect the subcellular localizations of either adaptor protein complex 3 or mannose 6-phosphate receptor (data not shown), two proteins involved in the transport of melanosomal/lysosomal proteins from TGN to endosomes (Shen et al., 2001; Theos et al., 2005; Ni et al., 2006).
Induction of Tyrosinase and Lysosome-Associated Membrane Protein 1 Colocalization by Quinoline Pigmentation Inhibitors
A common lineage among melanosomes and lysosomes is well accepted (Orlow, 1995; Raposo and Marks, 2002; Dell’Angelica, 2003). Both organelles contain certain proteins in common and both are affected in several single gene disorders, such as Hermansky-Pudlak Syndrome (HPS) subtype 1 (HPS-1), that alter the subcellular trafficking of lysosome-related organelles (Boissy et al., 1998; Richmond et al., 2005). Our laboratory has reported previously that certain tricyclic compounds such as tricyclic antidepressants and the steroidal compound U18666A inhibit pigmentation by altering the subcellular distribution of both tyrosinase gene family proteins and lysosome-associated membrane protein 1 (Lamp-1) (Hall et al., 2003; Ni-Komatsu and Orlow, 2008). Therefore, the subcellular localization of Lamp-1 in melan-a cells treated with quinoline analogs was also investigated by immunofluorescence.
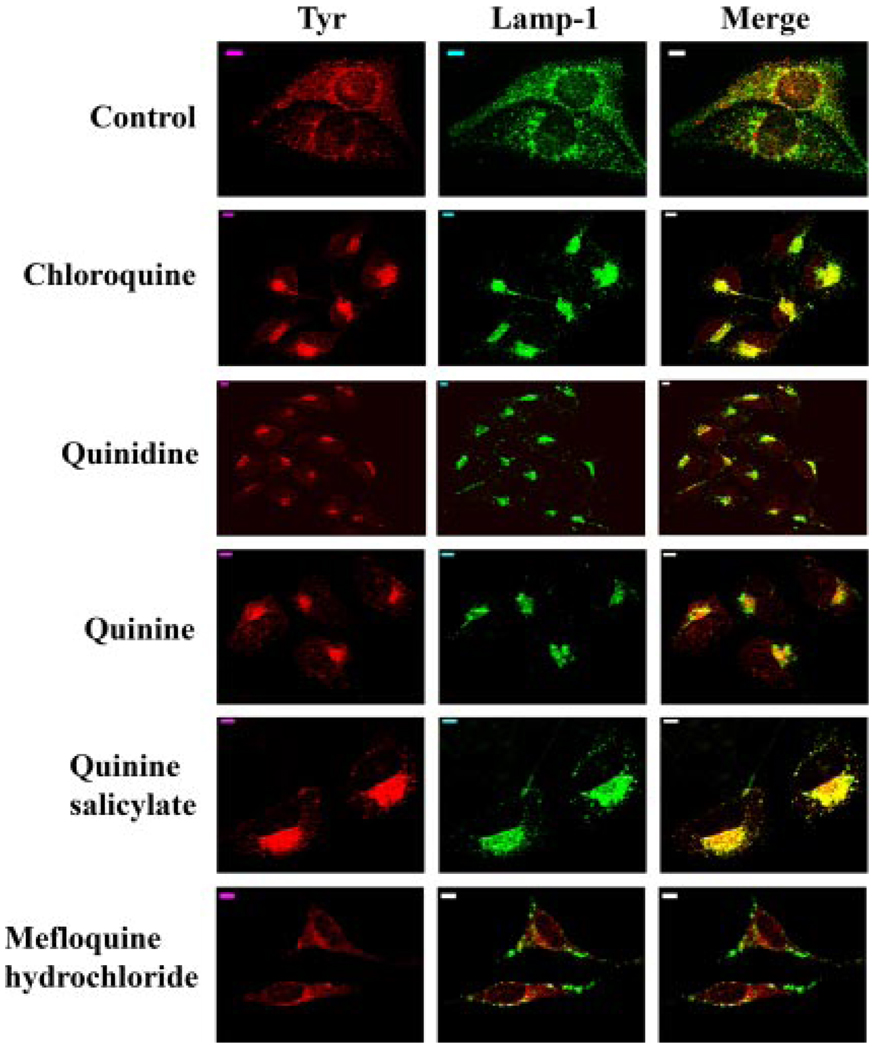
In untreated melan-a cells, Lamp-1 was localized mainly in cytoplasmic granules with some in the perinuclear area (Fig. 2). No obvious colocalization of Lamp-1 with tyrosinase was observed in untreated cells, consistent with the previous report that tyrosinase is not detected in late endosomes/lysosomes in these cells (Hall et al., 2003). By contrast, in the melan-a cells treated with chloroquine, quinidine, quinine, or quinine salicylate, Lamp-1 was localized to the perinuclear region, similar to that observed for the tyrosinase gene family proteins (Fig. 1). In addition, a significant colocalization of tyrosinase and Lamp-1 was also observed in the cells treated with these compounds for 72 h (Fig. 2). Similar to its lack of effect on the subcellular localization of Tyrp1, mefloquine hydrochloride treatment did not seem to have any obvious effects on Lamp-1 subcellular localization (Fig. 2).
Fig. 2.
Quinolines induce partial colocalization of tyrosinase and Lamp-1. Immunofluorescence confocal microscopy images are shown with immunostaining of Tyr (red) and Lamp-1 (green). The colocalization of tyrosinase with Lamp-1 is indicated as yellow in the merged image. Scale bars, 5 µm.
Our cell-based assay data suggested that with the exception of mefloquine hydrochloride, quinolines tested in this study inhibited melanogenesis by altering the trafficking of tyrosinase and Tyrp1 in melan-a cells, causing them to be trapped in a Lamp-1-positive perinuclear compartment. Even though no obvious effects on the intracellular trafficking of Tyrp1 and Lamp-1 were observed, mefloquine hydrochloride treatment did alter the trafficking of tyrosinase in treated cells. The correct regulation of intracellular trafficking of tyrosinase and related proteins plays an important role in melanogenesis, because tyrosinase and Tyrp1 cannot synthesize melanin if they do not traffic to melanosomes.
Chloroquine and Quinine Salicylate Inhibit Pigmentation in a Three-dimensional Asian Human Skin Equivalent Model
Human skin equivalent models have been used increasingly to evaluate pigmentation modulators identified through in vitro assays for their abilities to affect pigmentation in a system more closely representative of human skin (Yoon et al., 2003; Takeyama et al., 2004; Matsuda et al., 2008). We chose two quinolines, chloroquine and quinine salicylate, for testing on the commercially available human skin equivalent, MelanoDerm. Their effects on melanogenesis were compared with both vehicle-treated controls and MelanoDerm treated with arbutin, an agent widely used in “cosmeceutical” products for the treatment of hyperpigmentary disorders. Both chloroquine and quinine salicylate were potent pigmentation inhibitors identified in our cell-based screening, with chloroquine standing as the most effective among all the tested quinoline compounds. Second, both chloroquine and quinine salicylate have a long history of human oral usage in the treatment of malaria (Goodman and Gilman, 1996). Finally, neither compound exhibited substantial cytotoxicity to melanocytes.
MelanoDerm is a living, reconstituted, highly differentiated, three-dimensional human epidermal-like tissue containing melanocytes and keratinocytes that are derived from African-American, Asian, or white donors (termed MEL-B, MEL-A, and MEL-C, respectively). Both MEL-B and MEL-A tissues were used in the current study. In this model, substantial accumulation of newly synthesized melanin occurs over a 2-to 3-week period.
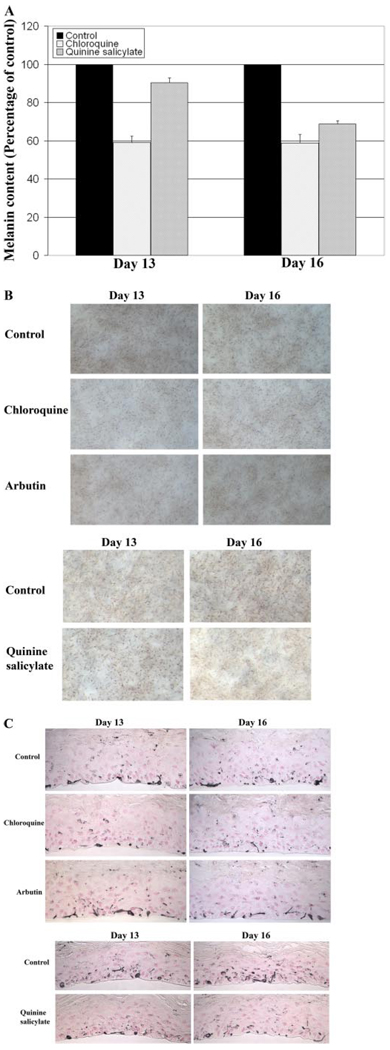
There was a considerable decrease in relative melanin accumulation in Asian human skin equivalents (MEL-A) after topical treatment with either 0.5 mM chloroquine or 4.0 mM quinine salicylate (Fig. 3A). A 40 to 50% decreased melanin content was observed in MEL-A treated with 0.5 mM chloroquine for both 13 and 16 days. Quinine salicylate was less effective at day 13, but by day 16, a 30 to 40% lesser melanin content was observed in MEL-A treated with 4.0 mM quinine salicylate. By comparison, the widely used melanogenic inhibitor arbutin at a concentration of 3 mg/ml (approximately 11 mM) did not seem to have an obvious effect on the melanin content of MEL-A after either 13 or 16 days of treatment (data not shown). The decreased melanin content in MEL-A treated with either chloroquine or quinine salicylate was probably not due to cytotoxicity. The cell viability of either 0.5 mM chloroquine-or 4.0 mM quinine salicylate-treated MEL-A was examined by cytotoxicity testing performed according to the manufacturer’s instructions, and no detectable cytotoxicity was observed with either compound treatment (data not shown). Neither compound at the indicated concentration seemed to alter cell proliferation or morphology (data not shown).
Fig. 3.
Chloroquine and quinine salicylate decrease melanin accumulation of Asian human skin equivalent (MEL-A). MEL-A were treated with 0.5 mM chloroquine, 4.0 mM quinine salicylate, 3 mg/ml arbutin, or controls (either water or 30:70 water/PEG) for 13 or 16 days. Representative data from multiple experiments are presented. A, changes in melanin content of MEL-A. Data are presented as the percentage of control, and each column represents the mean ± S.D. of multiple experiments. B, phase-contrast microscopic images of MEL-A. C, immunohistochemical appearances of MEL-A after Fontana-Masson staining to emphasize melanin content.
The effects of chloroquine and quinine salicylate on melanin synthesis in MEL-A were evaluated by light microscopy. A visible difference in pigmentation was observed 13 days after chloroquine treatment, compared with either the vehicle-treated control or MEL-A treated with quinine salicylate or arbutin (Fig. 3B). In MEL-A treated with chloroquine or quinine salicylate, the degree of pigmentation was strikingly decreased by day 16 compared with controls (Fig. 3B). This is consistent with the melanin assay data, suggesting that chloroquine is a more effective whitening agent than quinine salicylate. However, compared with arbutin, either quinoline is a more potent melanogenic inhibitor.
The transfer of melanin from melanocytes to keratinocytes and their distribution in keratinocytes are processes that play important roles in the regulation of skin color (Van Den Bossche et al., 2006; Goding, 2007; Yoshida et al., 2007). The distribution of melanin in the treated MEL-A was assessed by image analysis of histological sections stained with the Fontana-Masson method that highlights melanin (Bancroft and Stevens, 1982; Yoon et al., 2003) (Fig. 3C). At both days 13 and 16, chloroquine treatment resulted in a significant decrease in the relative melanin content of MEL-A. Although no noticeable difference in melanin content was observed in MEL-A treated with quinine salicylate for 13 days, an obvious decrease was observed with 16 days of quinine salicylate treatment. Quantitative analysis was performed on 8 to 10 Fontana-Masson stained histological sections of either vehicle- or compound-treated MelanoDerm. The results indicated a 47.1% (P < 0.001, day 13) and 56.2% (P < 0.0005, day 16) decrease in melanin content in MelanoDerm treated with chloroquine and a 19.8% (day 13) and 42.2% (P < 0.001, day 16) decrease with quinine salicylate treatment, compared with the vehicle-treated tissues. Arbutin did not exhibit a stronger effect on the melanin content of treated MEL-A. Quantitative analysis indicated only a 17.2% (day 13) and 26.8% (P ~ 0.05, day 16) decrease in melanin content of treated MEL-A. None of the treatments seemed to promote a significant redistribution of melanin pigment in MEL-A (Fig. 3C).
Chloroquine Inhibits Pigmentation in a Three-Dimensional African-American Human Skin Equivalent Model
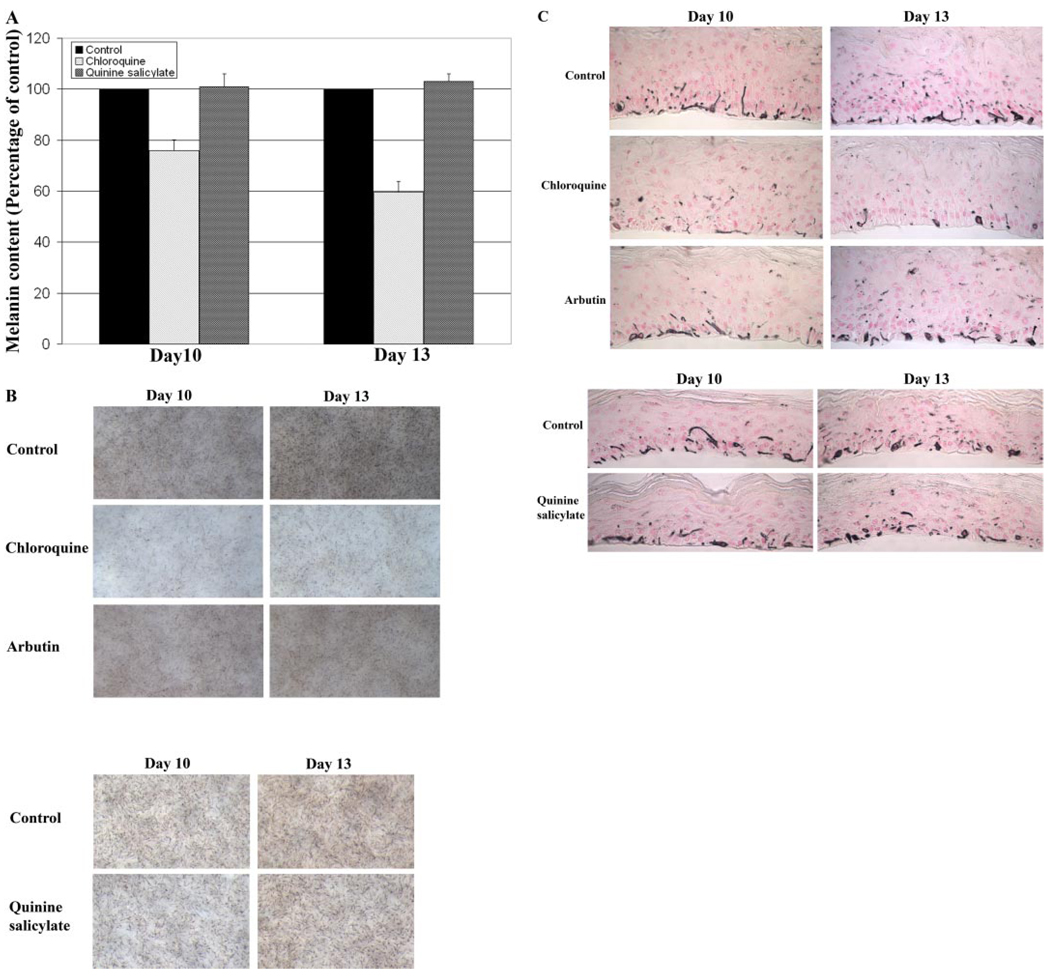
Similar experiments were performed on African-American skin equivalents (MEL-B), except that the treatment period was either 10 or 13 days because of the much higher level of melanin deposition in this system. A considerable decrease in relative melanin content of MEL-B was only observed upon topical treatment with 0.5 mM chloroquine for 13 days (Fig. 4A). A decrease in melanin content of only 20% was observed in MEL-B treated with 0.5 mM chloroquine for 10 days. However, a 40 to 50% decrease in melanin content was observed in the MEL-B treated with 0.5 mM chloroquine for 13 days. Quinine salicylate, a less effective pigmentation inhibitor on MEL-A, did not have a substantial effect on melanogenesis of MEL-B (Fig. 4A). Arbutin treatment did not significantly decrease the melanin content of MEL-B (data not shown). Both light microscopic images and Fontana-Masson stained histological results indicated that, consistent with the melanin assay data, chloroquine was the most potent melanogenic inhibitor found to be active on MEL-B (Fig. 4, B and C). Similar quantitative analysis was performed on 10 Fontana-Masson stained histological sections of either vehicle- or compound-treated MEL-B. The results indicated a 23.8% (day 10) and 45.5% (P < 0.0005, day 13) decrease in melanin content in Mel-B treated with chloroquine, compared with the vehicle-treated tissues. The quantitative analysis did not indicate any obvious difference in melanin content of MEL-B treated with quinine salicylate, compared with the vehicle-treated MEL-B, which is consistent with the melanin assay results. Arbutin had little effect on the melanin content of treated MEL-B. Quantitative analysis indicated only a 0.4% (day 10) and 10.3% (day 13) decrease in melanin content of MEL-B treated with arbutin, which is not statistically significant.
Fig. 4.
Chloroquine decrease melanin accumulation of African-American human skin equivalent (MEL-B). MEL-B were treated with 0.5 mM chloroquine, 4.0 mM quinine salicylate, 3 mg/ml arbutin, or controls (either water or 30:70 water/PEG) for 10 or 13 days. Representative data from multiple experiments are presented. A, changes in melanin content of MEL-B. Data are presented as the percentage of control, and each column represents the mean ± S.D. of multiple experiments. B, phase-contrast microscopic images of MEL-B. C, immunohistochemical appearances of MEL-B after Fontana-Masson staining to emphasize melanin content.
Discussion
Our laboratory has employed a chemical genetic screening procedure that uses immortalized mouse melanocytes (melan-a cells) in monolayer culture to identify novel compounds and their targets involved in the regulation of mammalian pigmentation (Ni-Komatsu and Orlow, 2007, 2008). In the current study, we further extend our cell-based screening to examine the response of the three-dimensional reconstituted human skin equivalent model, MelanoDerm, to a group of quinoline analogs, in particular 4-substituted amino quinolines, which were identified as novel and potent pigmentation inhibitors by screening the Spectrum Collection, a library of natural products and marketed drugs. This group of quinoline compounds inhibited melanin synthesis by altering the intracellular trafficking of tyrosinase gene family proteins in melanocytes. Most importantly, we have identified chloroquine as a potent depigmenting agent on both African-American and Asian human skin equivalent, and quinine salicylate as a whitening agent on Asian human skin equivalent.
Quinolines have been previously used in the treatment of malaria. As weak bases, quinolines are concentrated in acidic cellular compartments such as lysosomes, where they interfere with lysosomal functions. It is generally believed that these compounds raise the pH of acidic vesicles within sensitive malarial parasites (Goodman and Gilman, 1996). However, beyond the alteration of lysosomal pH, the exact mechanism by which quinolines suppress malaria is not well understood. We found that treatment with quinolines affects the lysosomal compartment in melan-a cells, altering the subcellular localization of Lamp-1.
Melanosomes share in common certain biological properties with lysosomes. Previous studies suggest that many melanosomal proteins use intracellular trafficking signals and pathways similar to those of lysosomes and other acidic organelles (Raposo and Marks, 2002; Dell’Angelica, 2003). Quinolines might also alter the pH of melanosomes. Intracellular pH is an important factor in the regulation of tyrosinase function, dramatically affecting tyrosinase enzymatic activity, and it is critical to the sorting of proteins to melanosomes. In melan-a cells, quinoline analogs seem to disrupt the intracellular trafficking of tyrosinase gene family proteins. The majority of these proteins are trapped in Lamp-1 positive perinuclear compartments in treated cells, contributing to the reduction in pigmentation.
It has been reported that the melanosomes in retinal pigment epithelium (RPE) cells are directly affected by chloroquine (Schraermeyer et al., 1999). After chloroquine treatment of RPE cultured from cattle eyes, the membranes of melanin granules inside RPE fused and formed large clusters and vacuoles (Schraermeyer et al., 1999), a phenomenon similar to what we observed in chloroquine-treated melan-a cells, where Tyr and Tyrp1 were trapped in clusters near the perinuclear region. Electron microscopic analysis of melan-a cells treated with quinoline compounds indicated that, compared with the vehicle-treated control cells in which melanosomes were mostly present in the mature forms (data not shown), the melanosomes in chloroquine- and mefloquine-treated melan-a cells are predominantly of stage I and II (data not shown). However, melanofilament matrix formation seems normal, which is further supported by the fact that quinoline treatment exhibited no effect on either the protein level or subcellular localization of Pmel17, the major structural protein of the melanosome matrix (data not shown).
Human skin equivalent models such as MelanoDerm provide an alternative to animal testing for evaluating the effects of putative bioactive compounds on mammalian pigmentation. MelanoDerm is considered a more physiologically relevant model than melanocytes alone or melanocyte-keratinocyte cocultures. The three-dimensional model has the advantage of exhibiting an accurate histologic structure and allows the assessment not only of melanin synthesis in melanocytes, but also of pigment translocation and melanin distribution in keratinocytes. Human skin equivalents have been used to evaluate novel pigmentation modulators and study the regulation of mammalian pigmentation (Yoon et al., 2003; Takeyama et al., 2004; Matsuda et al., 2008). We evaluated the effects of two 4-amino-substituted quinolines on melanogenesis in MelanoDerm by measurement of melanin content, light microscopic observation, and image analysis of Fontana-Masson stained MelanoDerm. Our results indicates that compared with the widely used pigmentation inhibitor, arbutin, both quinoline compounds are more effective whitening agents on Asian human skin equivalent (MEL-A), and of the three tested compounds, chloroquine is the most potent depigmenting compound and the only melanogenic inhibitor active in human skin equivalent derived from African-American skin (MEL-B). Furthermore, the inhibitory effects were observed without detectable cytotoxicity.
Even though chloroquine, the most potent pigmentation inhibitor tested in our cell-based assay, was also the most effective one on MelanoDerm, the concentration of compound applied to MelanoDerm tissues is much higher than that applied to melanocytes in culture by virtue of the difference in sensitivity between these two assay systems. In the melanocyte monolayer culture system, compounds are added directly to the culture media and need only penetrate the cell membrane, whereas in the human skin equivalent model, compounds are topically applied to the surface of the tissues, so the final concentrations of compounds that permeate through the tissue to reach the melanocytes is expected to be much lower. In addition, there is also a time difference in the length of treatment between melanocytes and MelanoDerm, which is due to the different pigmentation stages of cultured melanocytes and MelanoDerm. Cultured melan-a cells are already pigmented before treatment with the compounds. By contrast, in the case of either MEL-A or MEL-B, it takes at least 7 to 10 days for the tissues to accumulate melanin evident as a progressive darkening during the culture, and the tissue darkening is much more apparent at 10 to 14 days.
There are limitations to the MelanoDerm model. First, the structure of the skin equivalent lasts only 3 weeks, which prevents any longer term studies. Second, certain other resident skin cells such as Langerhans cells and dermal fibroblasts are not included in MelanoDerm. It has been suggested that those cell may also contribute to the regulation of pigmentation. Testing in MelanoDerm does not obviate the need for further human clinical testing. It is noteworthy that many quinolines, including chloroquine and quinine, have a long history of human usage that would be expected to facilitate their clinical development.
Blue-black pigmentation is a rare complication of oral longterm chloroquine therapy (Dupré et al., 1985; Mahler et al., 1986; Goodman and Gilman, 1996). Its nature is uncertain, although a rapid chloroquine-induced clumping of pigment and more delayed and subtle pigment changes produced by long-term quinine or mefloquine have been reported (Dupré et al., 1985; Mahler et al., 1986; Goodman and Gilman, 1996). This most likely is due to the high affinity of these compounds for melanin. Hypopigmentation of hair and freckles has also been reported (Dupré et al., 1985; Mahler et al., 1986; Goodman and Gilman, 1996). However, our data indicated that quinolines potently inhibited melanin synthesis in melanocytes. Therefore, in treated skin, there would be less or no melanin for this group of compounds to bind to. In addition, there is a difference between oral administration of quinolines and their topical application. Although the concentration of this group of compounds required to treat malaria is much higher than the concentration needed to inhibit melanogenesis, the skin concentration achieved upon oral administration are unknown.
In conclusion, we have identified a series of quinoline compounds as potent pigmentation inhibitors in culture murine melanocytes through a chemical genetic approach. Two of the quinolines, chloroquine and quinine salicylate, inhibited melanogenesis in a three-dimensional human skin equivalent model to a greater degree than arbutin. Our results indicate that chloroquine and quinine salicylate are potential effective whitening agents for cosmetics and the medical treatment of disorders of pigmentation.
Acknowledgments
The work was supported by National Institutes of Health grant AR41880 and by Luminessence, Inc. (to S.J.O.). S.J.O. is a founder of Luminessence, Inc. and S.J.O. and L.N.K. are minority shareholders in Luminessence, Inc. C.X.T., G.C., and N.B. state no conflict of interest.
ABBREVIATIONS
- ER
endoplasmic reticulum
- TGN
trans-Golgi network
- PEG
propylene glycol
- Lamp-1
lysosome-associated membrane protein 1
- RPE
retinal pigment epithelium
- MEL-A
Asian human skin equivalent
- MEL-B
African-American human skin equivalent
- Tyr
tyrosinase
- Tyrp1
tyrosinase-related protein 1
References
- Abramowitz J, Chavin W. Acute effects of two melanocytolytic agents, hydroquinone and beta-mercaptoethanolamine, upon tyrosinase activity and cyclic nucleotide levels in murine melanomas. Chem Biol Interact. 1980;32:195–208. doi: 10.1016/0009-2797(80)90078-2. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A. Theory and practices of histological techniques. New York: Churchill Livingstone; 1982. [Google Scholar]
- Boissy RE, Zhao Y, Gahl WA. Altered protein localization in melanocytes from Hermansky-Pudlak syndrome: Support for the role of the HPS gene product in intracellular trafficking. Lab Invest. 1998;78:1037–1048. [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell. 2002;13:1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto EV, Kwong C, Hermersdörfer H, Glatt H, Santis C, Virador V, Hearing VJ, Jr, Dooley TP. Inhibitors of mammalian melanocyte tyrosinase: in vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem Pharmacol. 1999;57:663–672. doi: 10.1016/s0006-2952(98)00340-2. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica EC. Melanosome biogenesis: shedding light on the origin of an obscure organelle. Trends Cell Biol. 2003;13:503–506. doi: 10.1016/j.tcb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Dupré A, Ortonne JP, Viraben R, Arfeux F. Chloroquine-induced hypopigmentation of hair and freckles. Association with congenital renal failure. Arch Dermatol. 1985;121:1164–1166. doi: 10.1001/archderm.1985.01660090078018. [DOI] [PubMed] [Google Scholar]
- Goding CR. Melanocytes: the new black. Int J Biochem Cell Biol. 2007;39:275–279. doi: 10.1016/j.biocel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Goodman LS, Gilman A. The Pharmacological Basis of Therapeutics. 9th ed. McGraw-Hill Company; 1996. Drugs used in the chemotherapy of protozoal infections; pp. 978–998. [Google Scholar]
- Hall AM, Krishnamoorthy L, Orlow SJ. Accumulation of tyrosinase in the endolysosomal compartment is induced by U18666A. Pigment Cell Res. 2003;16:149–158. doi: 10.1034/j.1600-0749.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- Hall AM, Orlow SJ. Degradation of tyrosinase induced by phenylthiourea occurs following Golgi maturation. Pigment Cell Res. 2005;18:122–129. doi: 10.1111/j.1600-0749.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Quevedo WC, Jr, Fitzpatrick TB, Szabo G. Some aspects of melanin biology: 1950–1975. J Invest Dermatol. 1976;67:72–89. doi: 10.1111/1523-1747.ep12512500. [DOI] [PubMed] [Google Scholar]
- Jiménez M, Tsukamoto K, Hearing VJ. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991;266:1147–1156. [PubMed] [Google Scholar]
- Körner A, Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982;217:1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- Mahler R, Sissons W, Watters K. Pigmentation induced by quinidine therapy. Arch Dermatol. 1986;122:1062–1064. [PubMed] [Google Scholar]
- Matsuda S, Shibayama H, Hisama M, Ohtsuki M, Iwaki M. Inhibitory effects of a novel ascorbic derivative, disodium isostearyl 2-O-l-ascorbyl phosphate on melanogenesis. Chem Pharm Bull. 2008;56:292–297. doi: 10.1248/cpb.56.292. [DOI] [PubMed] [Google Scholar]
- Ni X, Canuel M, Morales CR. The sorting and trafficking of lysosomal proteins. Histol Histopathol. 2006;21:899–913. doi: 10.14670/HH-21.899. [DOI] [PubMed] [Google Scholar]
- Ni-Komatsu L, Orlow SJ. Identification of novel pigmentation modulators by chemical genetic screening. J Invest Dermatol. 2007;127:1585–1592. doi: 10.1038/sj.jid.5700852. [DOI] [PubMed] [Google Scholar]
- Ni-Komatsu L, Orlow SJ. Chemical genetic screening identifies tricylic compounds that decrease cellular melanin content. J Invest Dermatol. 2008;128:1236–1247. doi: 10.1038/sj.jid.5701163. [DOI] [PubMed] [Google Scholar]
- Orlow SJ. Melanosomes are specialized members of the lysosomal lineage of organelles. J Invest Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, Bae H. Survey and mechanism of skin depigmenting and lightening agents. Phytother Res. 2006;20:921–934. doi: 10.1002/ptr.1954. [DOI] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol. 2001;152:809–824. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Marks MS. The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic. 2002;3:237–248. doi: 10.1034/j.1600-0854.2002.030401.x. [DOI] [PubMed] [Google Scholar]
- Richmond B, Huizing M, Knapp J, Koshoffer A, Zhao Y, Gahl WA, Boissy RE. Melanocytes derived from patients with Hermansky-Pudlak syndrome types 1, 2, and 3 have distinct defects in cargo trafficking. J Invest Dermatol. 2005;124:420–427. doi: 10.1111/j.0022-202X.2004.23585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal MH, Kreider JW, Shiman R. Quantitative assay of melanin in melanoma cells in culture and in tumors. Anal Biochem. 1973;56:91–99. doi: 10.1016/0003-2697(73)90173-5. [DOI] [PubMed] [Google Scholar]
- Schraermeyer U, Peters S, Thumann G, Kociok N, Heimann K. Melanin granules of retinal pigment epithelium are connected with the lysosomal degradation pathway. Exp Eye Res. 1999;68:237–245. doi: 10.1006/exer.1998.0596. [DOI] [PubMed] [Google Scholar]
- Shen B, Rosenberg B, Orlow SJ. Intracellular distribution and late Endosomal effects of the ocular albinism type 1 gene product: consequences of disease-causing mutations and implications for melanosome biogenesis. Traffic. 2001;2:202–211. doi: 10.1034/j.1600-0854.2001.020306.x. [DOI] [PubMed] [Google Scholar]
- Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmentation agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- Takeyama R, Takekoshi S, Nagata H, Osamura RY, Kawana S. Quercetin-induced melanogenesis in a reconstituted three-dimensional human epidermal model. J Mol Histol. 2004;35:157–165. doi: 10.1023/b:hijo.0000023388.51625.6c. [DOI] [PubMed] [Google Scholar]
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Valencia JC, Kushimoto T, Ferrans VJ, Hearing VJ. Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. FASEB J. 2001;15:2149–2161. doi: 10.1096/fj.01-0216com. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Valencia JC, Kushimoto T, Costin GE, Virador VM, Vieira WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Res. 2002;15:217–224. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Tripathi RK, Hearing VJ, Urabe K, Aroca P, Spritz RA. Mutational mapping of the catalytic activities of human tyrosinase. J Biol Chem. 1992;267:23707–23712. [PubMed] [Google Scholar]
- Ujvari A, Aron R, Eisenhaure T, Cheng E, Parag HA, Smicun Y, Halaban R, Hebert DN. Translation rate of human tyrosinase determines its N-linked glycosylation level. J Biol Chem. 2001;276:5924–5931. doi: 10.1074/jbc.M009203200. [DOI] [PubMed] [Google Scholar]
- Van Den Bossche K, Naeyaert JM, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7:769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- Yoon TJ, Lei TC, Yamaguchi Y, Batzer J, Wolber R, Hearing VJ. Reconstituted 3-dimensional human skin of various ethnic origins as an in vitro model for studies of pigmentation. Anal Biochem. 2003;318:260–269. doi: 10.1016/s0003-2697(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Hachiya A, Sriwiriyanont P, Ohuchi A, Kitahara T, Takema Y, Visscher MO, Boissy RE. Functional analysis of keratinocytes in skin color using a human skin substitute model composed of cells derived from different skin pigmentation types. FASEB J. 2007;21:2829–2839. doi: 10.1096/fj.06-6845com. [DOI] [PubMed] [Google Scholar]