Abstract
Background
Heterogeneity manifest as more severe disease in successive generations has been attributed to genetic anticipation in patients with autosomal dominant polycystic kidney disease (ADPKD). We evaluated variation in age at end-stage renal disease (ESRD) in ADPKD families for evidence of anticipation.
Study Design
Retrospective.
Setting & Participants
413 families with ADPKD seen at our single center between 1985 and 2004 (including 95 families with documented polycystic disease type 1 [PKD1] and 213 ADPKD families with parents born before 1930).
Predictor
Generational status.
Outcome
Age at ESRD onset.
Measurements
Time to ESRD was evaluated by using survival analysis, Cox regression, and descriptive statistics. Unstable trinucleotide repeat expansion was evaluated by means of genotyping in 6 PKD1 families.
Results
We analyzed 413 ADPKD families (1,391 parent-offspring pairs) with known age at ESRD or last known age without ESRD (informative pairs). There was no difference in age at ESRD between parents and offspring by means of Cox regression after adjusting for correlations among family members and sex (hazard ratio, 1.019; 95% confidence interval, 0.919 to 1.13; P = 0.7). Similar analysis of PKD1 informative pairs and those with parents born before 1930 showed no differences in age at ESRD. Male ADPKD patients were 42% more likely to reach ESRD (P < 0.001), and male patients with documented PKD1 were 41% more likely to reach ESRD (P = 0.01) than female patients.
Limitations
Hypertension treatment unknown.
Conclusions
We found no evidence for anticipation of ESRD in patients with ADPKD; thus, the observed variation in age at ESRD may result from other genetic, sex, or environmental causes.
INDEX WORDS: End-stage renal disease, autosomal dominant polycystic kidney disease (ADPKD), genes, renal disease
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening hereditary kidney disease.1 The clinical course ofADPKD is variable, with end-stage renal disease (ESRD), ie, stage 5 chronic kidney disease, in our ADPKD center ranging from age 3 years to patients reaching their 80s without ESRD. Dalgaard,2 in his seminal treatise in 1957, found no tendency toward anticipation and observed “a certain familial uniformity and characteristic in the course of the disease.”2 However, this is not always the case because differences in age of onset of ESRD in the same family of 20 or more years were reported.3 Extreme differences in age at ESRD have occurred in which the offspring reached ESRD earlier than the parent. This observation raised the possibility that the phenomenon of genetic anticipation, ie, earlier onset and more severe disease in successive generations, occurs in ADPKD.3 Although genetic anticipation caused by unstable expansion of nucleotide repeats was reported for patients with several diseases,4–9 the issue of genetic anticipation in patients with ADPKD remains controversial.10–16
Previously, 242 ADPKD families from the University of Colorado Health Sciences Center (UCHSC; Denver, CO) ADPKD database were examined for evidence of anticipation.3 Of 242 families, 86 were informative for age at ESRD. Anticipation of ESRD (defined as a 10-year earlier onset of ESRD in an affected offspring than in the affected parent) was reported in 49% of 86 families that were informative for age at ESRD and 24% of parent-offspring pairs from these families. However, it should be noted that 178 of the 242 families were noninformative for age at ESRD at the time of the previous study. Moreover, in patients with ADPKD, when the offspring has worse renal survival than the parent, this obviously is observed earlier than when the offspring has longer renal survival than the parent. Considering this bias and with a larger ADPKD population in the present study, the possibility of genetic anticipation in ADPKD was examined further.
METHODS
ADPKD Study Subjects and Families
The polycystic disease (PKD) clinical database at the UCHSC containing 1,228 families and 4,618 individuals was searched, and families with a confirmed diagnosis of ADPKD were identified. Selection of study families was limited to those in which at least 1 member had been clinically evaluated at the General Clinical Research Center at UCHSC and those with the added criteria that the family have at least 1 affected child and parent (parent-offspring pair) for study. A total of 413 families with 2,409 affected individuals was identified based on selection criteria. A description of data available for these subjects is listed in Table 1. All subjects provided written informed consent in accordance with protocols approved by the Colorado Multi-Institutional Review Board.
Table 1.
Description of the Autosomal Dominant Polycystic Kidney Disease Study Population
| Total patients for potential analysis | 2,409 |
| Male | 1,102 (46) |
| Female | 1,307 (54) |
| No. with data on year of birth | 2,096 |
| Median year of birth (range) | 1949 (1860–2004) |
| No. with known age at ESRD or last known age without ESRD | 2,048 |
Note: Values expressed as number (percent) or median (range) unless noted otherwise.
Abbreviation: ESRD, end-stage renal disease.
Information for ADPKD diagnosis and ESRD status for deceased family members and those who were not contacted was obtained from participating family members when possible. In subjects participating in clinical studies, ADPKD diagnosis was established by means of renal ultrasound.1,17 A diagnosis of PKD type 1 (PKD1) or PKD2 was based on demonstrated linkage to gene loci on chromosomes 16 or 4, respectively, or by means of mutation identification.18 Families suitable for linkage analysis were the largest multigen-eration families in our database. Age at ESRD, ie, stage 5 chronic kidney disease, was defined as age at initiation of dialysis therapy or renal transplantation. Age without ESRD was defined as the last known age without ESRD. Pairs informative for ESRD were those in which either age at ESRD or last known age without ESRD were available for both child and parent. For survival analyses, an observation was considered an event if the individual reached ESRD before the last observation time. An observation was censored if an individual did not reach ESRD by the last observation time; however, such observations were informative for last known age without ESRD. Pairs informative for the difference in age at ESRD existed when 1 of 3 conditions were present: (1) both parent and offspring had reached ESRD, (2) the parent had reached ESRD and the child was now older than the parent’s age at ESRD, or (3) the child reached ESRD earlier than the last known parental age without ESRD. Pairs in which the child was without ESRD and younger than the parent’s age at ESRD were excluded in this definition. Selection criteria for study patients are shown in Fig 1 and Table 2.
Figure 1.
Selection criteria for families with parent-offspring pairs for study. The polycystic kidney disease (PKD) database at University of Colorado Health Sciences Center containing 1,228 PKD families with 4,618 individuals was queried, and 413 families (2,409 affected) with 1,807 parent-offspring pairs for potential analysis were identified. Detailed distribution of the 1,391 parent-offspring pairs informative for age at end-stage renal disease (ESRD) or last known age without ESRD identified is listed in Table 2. Abbreviation: ADPKD, autosomal dominant PKD.
Table 2.
Distribution of All Autosomal Dominant Polycystic Kidney Disease Parent-Offspring Pairs
| Total Pairs | Pairs Informative for Age at ESRD or Last Known Age Without ESRD | Parent Died Without ESRD | Missing Data for ESRD | No ESRD in Parent or Offspring | Offspring Younger Than Parent’s Age at ESRD | Informative for Difference Between Parent and Offspring Age at ESRD | |
|---|---|---|---|---|---|---|---|
| All PKD | 1,807 | 1,391 | 59 | 403 | 468 | 452 | 425 |
| All parent-offspring pairs with parent born before 1930 | 925 | 725 | 56 | 194 | 139 | 170 | 366 |
| PKD1 | 689 | 585 | 28 | 100 | 185 | 202 | 174 |
| PKD1 parent-offspring pairs with parent born before 1930 | 341 | 283 | 26 | 56 | 44 | 67 | 148 |
Abbreviations: PKD1, polycystic kidney disease type 1; ESRD, end-stage renal disease.
ADPKD Study Subjects With Parental Birth Date Before 1930
In an attempt to eliminate the effect of antihypertensive therapy, we also selected older parent-offspring pairs based on birth date of parent before 1930 (or if parent’s birth date was unknown, birth date of offspring before 1945).
PKD1 Study Subjects and Families
Ninety-five of 413 ADPKD families with 785 affected were determined to have PKD1 by means of gene linkage (N = 62 families), mutation screening (N = 24 families), or both gene linkage and mutation screening (N = 9 families). Three hundred fifty-one individuals with PKD1 from these 95 families participated in clinical studies at UCHSC, and 94 additional affected family members supplied DNA for genetic studies.
PKD1 Study Subjects With Birth Date Before 1930
Older PKD1 parent-offspring pairs were selected for separate analysis by using the same criteria described for ADPKD subjects born before 1930.
Genotype Analysis
DNA was prepared from either whole blood or saliva by using a Pure Gene DNA extraction kit (Gentra Biosystems, Minneapolis, MN). To identify potential unstable repeats in the PKD1 gene, we analyzed the sequence (Genbank Accession No. L39891) for the repeats (CAG)n, (GAA)n, (CGG)n, and (CTG)n, which previously were shown to undergo unstable expansion in human disease.4–9 Six PKD1 families were selected for analysis of the candidate repeats based on the occurrence of ESRD 10 years earlier in the offspring compared with the affected parent’s age at ESRD and availability of DNA from affected parent and child. Selection criteria are shown in Fig 1. Fragments of the PKD1 gene were amplified as previously described,19 and areas of interest subsequently were amplified by means of nested polymerase chain reaction from these long primary products. The following primers and conditions were used to amplify the specific repeat-containing regions: primers N1F and N1R and conditions as described previously20 were used to generate a 326–base pair (bp) amplicon containing the repeat c.-132CAG(3) (ie, 3 repeats of the trinucleotide sequence CAG starting at a position 132 bases upstream of the start of the coding sequence [numbering based on complementary DNA sequence; thus, the first nucleotide of the translation initiation codon is position 1]; reference sequence NM_ 000296.2). The potential repeat c.7,274+400CTG(3) (ie, 3 CTG repeats in intron 16 at a position 400 bases beyond coding DNA nucleotide 7,274) was amplified by using primers Int16F 5′-CAGAGGTAGCCACTGTCC-3′and Int16R 5′-ATCAG-GCCAGCTGAGGAA-3′; this generated a 206-bp amplicon. The candidate repeat c.10,708+724CGG(3) was amplified directly from genomic DNA using primers Int34F 5′-ATGGTCATATAGAGGTTACC-3′and Int34R 5′-AGCA-CACCTGAGCATAG-3′, which generated a 137-bp amplicon. Polymerase chain reaction conditions used for amplification were initial denaturation for 10 minutes at 94°C, followed by 35 cycles of 1 minute at 94°C and 1 minute at 56°C for intron 16 or 1 minute at 62°C for intron 34, 1 minute at 72°C, and a final incubation of 7 minutes at 72°C. The candidate repeat c.11,928CTG(3) was amplified directly from genomic DNA by using primers and conditions as previously described.19 Amplicon sizes were compared in the affected offspring/parent pairs after electrophoresis on a 2% agarose gel with visualization by means of ethidium bromide staining. In each analysis, a nonaffected control DNA sample was included for size comparison.
Statistics
Frequency counts and percentages were used to describe numbers and proportions of offspring reaching ESRD earlier than their PKD-affected parents. Survival analysis using the Kaplan-Meier method and log-rank statistic were used to compare survival curves between parents and offspring. A Cox proportional hazards model was used to test the difference in age at onset of ESRD in parents and children, and potential correlation within members of the same family was accounted for and significance was tested by using a robust variance estimator as described by Lin and Wei.21 In addition, sex was included as a covariate. χ2 test of independence was used to compare distributions among age-of-onset categories between all patients with PKD and those with the affected parent born before 1930. Kolmogorov-Smirnov and Cramer-von Mises tests were used to test the shape of the distribution of differences in age at onset of ESRD in parents and children.
RESULTS
Analyses in All Informative PKD Pairs
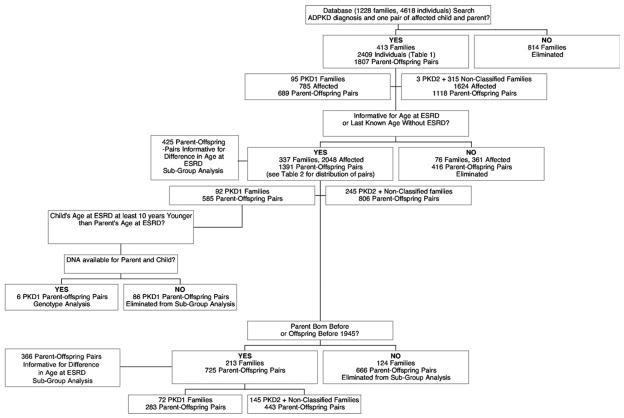
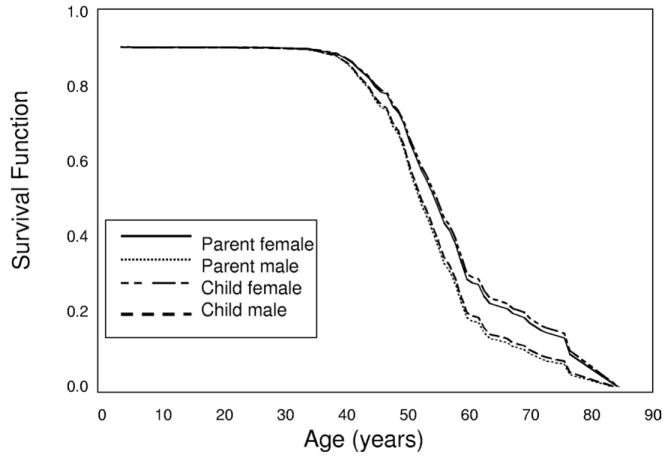
Four hundred thirteen ADPKD families (95 PKD1, 3 PKD2, and 315 nonclassified) were identified in our database, resulting in 1,807 parent-offspring pairs, as shown in Fig 1 and Table 2. Of 1,807 parent-offspring pairs, 1,391 pairs were informative for ESRD, meaning that information was available for both parent and offspring for age at ESRD or last known age without ESRD; thus, both censored and uncensored data were used. Four hundred twenty-five of 1,391 informative pairs were informative for difference in age at ESRD. Distribution of the 1,807 parent-offspring pairs is listed in Table 2. Survival analysis using all 1,391 informative pairs showed no difference in survival curves between children (median age, 57 years; 95% confidence interval [CI], 55 to 58) based on the log-rank statistic and their parents (median, 56 years; 95% CI, 55 to 57) for survival to ESRD (P = 0.9). A Cox proportional hazards method using all 1,391 informative pairs, including sex as a covariate, and accounting for correlations among families showed no difference between children and parents (hazard ratio [HR], 1.019; 95% CI, 0.919 to 1.130; P = 0.7), a significant effect of male sex (HR, 1.424; 95% CI, 1.180 to 1.719; P < 0.001), with males 42% more likely to reach ESRD (Fig 2). Using the survivor function to estimate median age of onset, survival time until ESRD was 58 years for females in the parental generation versus 57 years in female offspring and 54 years for males in the parental generation and 54 years in male offspring. To further explore those informative for difference in age of onset of ESRD in which the child was not younger than parental age at ESRD, 425 parent-offspring pairs informative for difference in age at ESRD were grouped into 5 possible categories, and the distribution of age at ESRD onset among the 425 is listed in Table 3. Overall, 107 of the parent-offspring pairs informative for the difference in the age at ESRD (25%) had an offspring who reached ESRD at least 10 years earlier than the affected parent. Two empirical distribution tests, the Kolmogorov-Smirnov and Cramer-von Mises, were performed to test the shape of the distribution of differences in age at onset of ESRD in 285 parent-offspring pairs in which both members reached ESRD during the observation time. For both tests, the null hypothesis that distribution was normal was retained (P = 0.2 and P = 0.3, respectively).
Figure 2.
Survival until end-stage renal disease (ESRD) in all polycystic kidney disease parents and offspring. Survival functions from the Cox regression are plotted against age (years) for parents and offspring from 1,391 parent-offspring pairs informative for age at ESRD onset or survival age without ESRD. Male and female affected parents and children are plotted separately. A Cox proportional hazards method showed a significant effect of male sex (hazard ratio, 1.424; 95% confidence interval, 1.180 to 1.719; P < 0.001) on survival until ESRD. The lines for female parent and female child overlay each other and are indicated by an arrow on the right of the figure. An arrow to the left indicates the position of the lines for male parent and male child that are similarly overlaid.
Table 3.
Summary of Autosomal Dominant Polycystic Kidney Disease Parent-Offspring Pairs Informative for Difference in Age of Onset of ESRD Between Parent and Offspring
| Parent-Offspring Pairs |
|||
|---|---|---|---|
| Category | Definition | All PKD (N = 425) | PKD With Parent Born Before 1930 (N = 366) |
| Offspring ESRD ≥10 y earlier | Offspring reached ESRD ≥10 y earlier than parent (or ≥10 y earlier than parent’s death without ESRD) | 107 (25) | 86 (24) |
| Offspring ESRD 2–≥9 y earlier | Offspring reached ESRD 2–9 y earlier than parent (or 2–9 years earlier than parent’s death without ESRD) | 102 (24) | 89 (24) |
| Same age at ESRD onset | Offspring reached ESRD within 1 y of the parent’s age of ESRD onset | 39 (9) | 31 (8) |
| Offspring ESRD 2–≥9 y later | Offspring reached ESRD 2–9 y later than parent (or is 1–9 y older than age at which the parent reached ESRD) | 123 (29) | 109 (30) |
| Offspring ESRD ≥10 y later | Offspring reached ESRD ≥10 y later than parent (or is ≥10 y older than age at which parent reached ESRD) | 54 (13) | 51 (14) |
Note: Values expressed as number (percent).
Abbreviations: ESRD, end-stage renal disease; PKD, polycystic kidney disease.
Analysis of PKD Pairs With Parental Birth Dates Before 1930
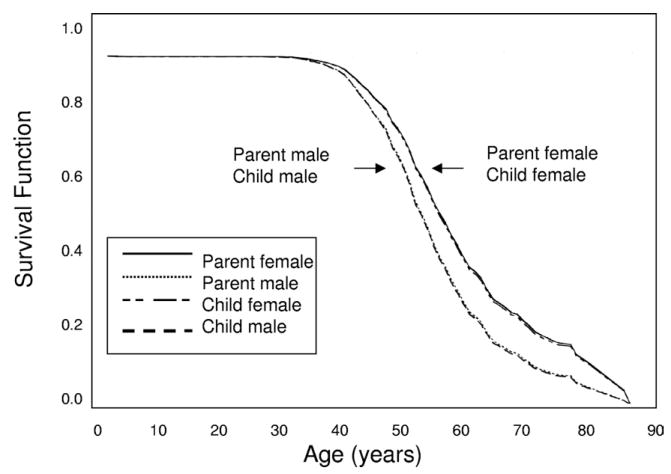
Because many pairs (76%) were not informative for difference in age of onset of ESRD and also because antihypertensive medication may have an impact on age at ESRD onset, we also examined older pairs in which the parent was born before 1930 (or if the parent’s year of birth was unknown, the offspring was born before 1945). Distribution of 925 pairs from this category is listed in Table 2. In this category, 725 pairs were informative for ESRD and 366 pairs (24%) were informative for difference in age at onset of ESRD. Distribution of the 366 informative pairs with the parent born before 1930 is listed in Table 3. χ2 test of independence showed that distribution of age at ESRD onset among informative pairs with the parent born before 1930 closely resembled the distribution seen in the total group (P = 0.9; Table 3). The log-rank statistic showed no difference in survival curves between children (median, 57 years; 95% CI, 56 to 59) and their parents (median, 57 years; 95% CI, 55 to 58) for survival to renal failure (P = 0.4). A Cox proportional hazards method showed no difference in survival to ESRD between children and parents (HR, 1.080; 95% CI, 0.950 to 1.227; P = 0.2). However, a significant effect of male sex was observed (HR, 1.431; 95% CI, 1.149 to 1.781; P = 0.001), with males 43% more likely to experience ESRD than females (Fig 3).
Figure 3.
Survival until end-stage renal disease (ESRD) in all polycystic kidney disease (PKD) parents and offspring with parental birth dates before 1930. Survival functions from the Cox regression are plotted against age (years) for parents and offspring from 725 parent-offspring pairs with parental birth dates before 1930 (or if the parent’s year of birth was unknown, the offspring was born before 1945) that were informative for age at ESRD onset or survival age without ESRD. Male and female affected parents and children are plotted separately. Cox proportional hazards method showed a significant effect of male sex (hazard ratio, 1.431; 95% confidence interval, 1.149 to 1.781; P < 0.01) on survival until ESRD.
Analyses in All Informative PKD1 Pairs
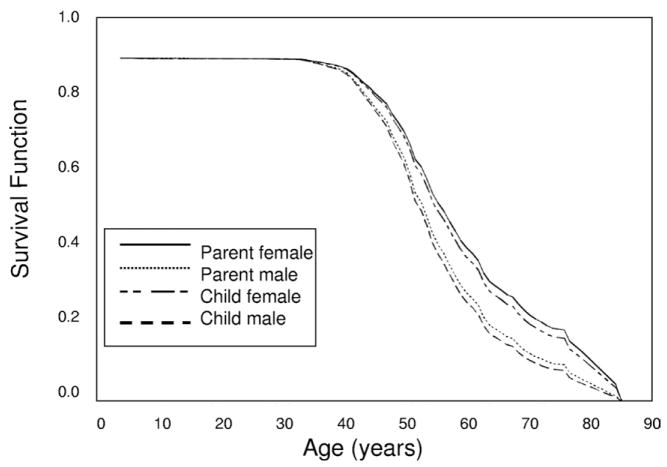
Ninety-three PKD1 families with 689 affected parent-offspring pairs were studied. Information for age at ESRD or last known age without ESRD was available for 585 PKD1 parent-offspring pairs from 92 families. Distribution of these pairs is listed in Table 2. One hundred seventy-four PKD1 pairs (25%) were informative for difference in age of onset of ESRD. Distribution of informative pairs is listed in Table 4. Log-rank statistic showed no difference in survival curves between children (median, 57 years; 95% CI, 55 to 59) and their parents (median, 55 years; 95% CI, 55 to 56) for survival to ESRD (P = 0.5). A Cox proportional hazards method showed no difference between children and parents (HR, 0.947; 95% CI, 0.803 to 1.117; P = 0.5), a significant effect of male sex (HR, 1.412; 95% CI, 1.084 to 1.839; P = 0.01), with males 41% more likely to reach ESRD than females (Fig 4).
Table 4.
Summary of PKD1 Parent-Offspring Pairs Informative for Difference in Age of ESRD Onset Between Parent and Offspring
| Parent-Offspring Pairs |
|||
|---|---|---|---|
| Category | Definition | PKD1 (N = 174) | PKD1 With Parent Born Before 1930 (N = 148) |
| Offspring ESRD ≥10 y earlier | Offspring reached ESRD ≥10 y earlier than parent (or ≥10 y earlier than parent’s death without ESRD) | 38 (22) | 30 (20) |
| Offspring ESRD 2–≥9 y earlier | Offspring reached ESRD 2–9 y earlier than parent (or 2–9 years earlier than parent’s death without ESRD) | 40 (23) | 32 (22) |
| Same age at ESRD onset | Offspring reached ESRD within 1 y of the parent’s age of ESRD onset | 13 (7) | 10 (7) |
| Offspring ESRD 2–≥9 y later | Offspring reached ESRD 2–9 y later than parent (or is 1–9 y older than age at which the parent reached ESRD) | 56 (32) | 52 (35) |
| Offspring ESRD 10+ years later | Offspring reached ESRD ≥10 y later than parent (or is ≥10 y older than age at which parent reached ESRD) | 27 (16) | 24 (16) |
Note: Values expressed as number (percent).
Abbreviations: ESRD, end-stage renal disease; PKD1, polycystic kidney disease type 1.
Figure 4.
Survival until end-stage renal disease (ESRD) in all informative polycystic kidney disease type 1 (PKD1) parents and offspring. Survival functions from the Cox regression are plotted against age (years) for parents and offspring from 585 PKD1 parent-offspring pairs informative for age at ESRD onset or survival age without ESRD. Male and female affected parents and children are plotted separately. A Cox proportional hazards method showed a significant effect of male sex (hazard ratio, 1.412; 95% confidence interval, 1.084 to 1.839; P = 0.01) on survival until ESRD.
Analyses in All Informative PKD1 Pairs With Parental Birth Dates Before 1930
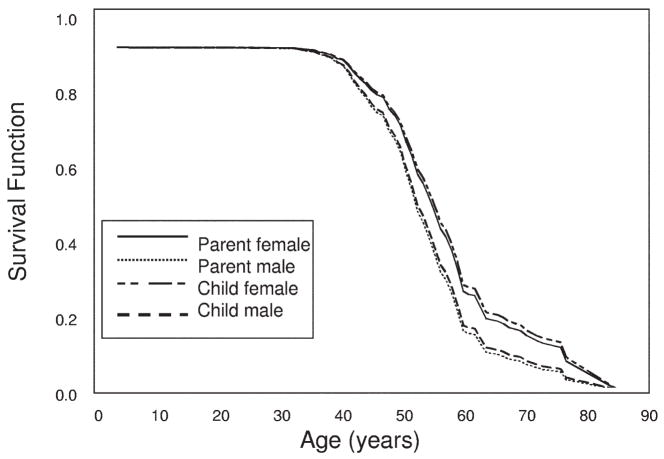
We also examined older pairs in which the parent was born before 1930 (or if the parent’s year of birth was unknown, the offspring was born before 1945). Distribution of 341 pairs in this category is listed in Table 2. Two hundred eighty-three pairs were informative for ESRD. Using all 283 informative pairs, there was no significant difference in survival time until ESRD between parents and children by means of either the Kaplan-Meier method (P = 0.5) or Cox regression (P = 0.7; Fig 5). However, a Cox proportional hazards method showed a significant effect of male sex (HR, 1.380; 95% CI, 1.027 to 1.855; P = 0.03) on survival until ESRD, with males 38% more likely to reach ESRD than females.
Figure 5.
Survival until end-stage renal disease (ESRD) in all polycystic kidney disease type 1 (PKD1) parents and offspring with parental birth dates before 1930. Survival functions from Cox regression are plotted against age (years) for parents and offspring from 725 PKD1 parent-offspring pairs with parental birth dates before 1930 (or if the parent’s year of birth was unknown, the offspring was born before 1945) that were informative for age at ESRD onset or survival age without ESRD. Male and female affected parents and children are plotted separately. There were no significant differences in survival time until ESRD between parents and children. A Cox proportional hazards method showed a significant effect of male sex (hazard ratio, 1.380; 95% confidence interval, 1.027 to 1.855; P = 0.03) on survival until ESRD.
Ninety-two PKD1 families with older parents had at least 1 parent-offspring pair informative for ESRD, and 148 PKD1 pairs (43%) with older parents were informative for the difference in age at ESRD. χ2 test of independence showed that distribution of age at ESRD onset in the subgroup of 148 informative parent-offspring pairs with the parent born before 1930 closely resembled distribution in the total PKD1 group, as listed in Table 4 (P = 0.9). Only 3 PKD2 families were informative for age at ESRD; therefore, no analysis of PKD2 families was performed.
In 13 families with offspring who reached ESRD at least 10 years earlier than the affected parent, age at ESRD was available for 3 generations (grandparent, parent, and offspring). In these multigeneration families, there was no consistent trend toward earlier onset of ESRD in successive generations indicative of genetic anticipation.
Genotype Analysis
The PKD1 gene was analyzed for all triplet repeats previously associated with unstable expansion. Four candidate repeats were chosen for genotype analysis in 6 affected parent-offspring pairs, including c.-132CAG(3), c.7,274+400CAG(3), c.10,708+724CGG(3), and c.11,928CTG(3), based on location within 750 bp of an exon or within an exon. Each of these repeats comprised 3 replicates of the trinucleotide. We previously identified a germ-line mutation in 3 of the families chosen for genotype analysis. There was no evidence for expansion of the analyzed repeats in the 6 parent-offspring pairs, as listed in Table 5; in each instance, the size of the respective amplicon was the same in both the control, affected parent, and offspring with apparent evidence for anticipation.
Table 5.
Analysis of Trinucleotide Repeat Expansion in 6 PKD1 Families With Early Onset of ESRD in Offspring
| Parent |
Offspring |
Repeat Expansion |
||||||
|---|---|---|---|---|---|---|---|---|
| Family | Sex | ESRD Age (y) | Sex | ESRD Age (y) | c.-132CAG(3) | c.7,274+400CTG(3) | c.10,708+724CGG(3) | c.11,928CTG(3) |
| 1 | M | 61 | F | 44 | No | No | No | No |
| 2* | M | 69 | M | 37 | No | No | No | No |
| 3 | F | 43 | M | 25 | No | No | No | No |
| 4* | F | 51 | F | 17 | No | No | No | No |
| 5* | M | 48 | F | 38 | No | No | No | No |
| 6 | F | 52 | M | 41 | No | No | No | No |
Abbreviations: ESRD, end-stage renal disease; PKD1, polycystic kidney disease type 1.
PKD1 mutation identified in family.
DISCUSSION
ADPKD is a clinically heterogeneous disease. It is not uncommon to find significant variation in disease severity in members of the same family who carry the same germline mutation. This intrafamilial variation may be attributed to both genetic and/or environmental causes. Several earlier studies proposed genetic anticipation as an explanation for the extreme variability in disease severity in certain pedigrees.3,11–13 However, the occurrence of anticipation in patients with ADPKD has been controversial.12,14–16 Several studies identified specific stable mutations in families with evidence of anticipation,14–16 and it was proposed that this ruled out the possibility of a dynamic mutation in PKD1. To date, no evidence for repeat instability in the PKD1 gene was reported, although no study specifically screened for repeat expansion in parent-offspring pairs with potential evidence of anticipation.
In a previous study, 86 informative ADPKD pedigrees from patients studied at UCHSC were examined for evidence of potential anticipation of ESRD. Anticipation of ESRD was indicated in 24% of informative parent-offspring pairs from 42 families. At the time of analysis, 148 of these families were uninformative for ESRD. In an updated analysis that included additional data collected in the intervening period, including age at ESRD and last known age without ESRD, we reevaluated the original families and studied additional families by using survival analysis and Cox regression methods. These analyses were possible based on the availability of the UCHSC clinical ADPKD database. This resource is the largest comprehensive longitudinal clinical database in this country for human ADPKD, with information spanning more than 38 years. In the present study, 413 families with ADPKD were analyzed. Of these families, 337 yielded at least 1 informative parent-offspring pair, enabling study of 1,391 pairs informative for age at ESRD or last known age without ESRD and 425 total pairs informative for difference in age at onset of ESRD. There was no significant difference in survival curves between parent and offspring when both censored and uncensored data were used.
In the present analysis, we classified 95 families as PKD1 and 4 as PKD2 based on linkage analysis or mutation identification. Ninety-two PKD1 families yielded 689 parent-offspring pairs, with 585 informative for age at ESRD or last known age without ESRD, and 174 parent-offspring pairs were informative for difference in age at onset of ESRD. Median age at ESRD in PKD1 families was similar to that for the total data set, and there was no significant difference in survival curves between parents and children. Examination of pairs informative for difference in age at ESRD showed extreme variability in age at ESRD in members of the same family. For example, although 10-year earlier onset of ESRD occurred in 1 offspring, others developed ESRD at least 6 to 10 years later than a parent. Moreover, in 13 of our larger families with age at ESRD available for 3 successive generations, there was no consistent trend toward earlier age at onset of ESRD in consecutive generations. Thus, within the same family, both increased risk and increased protective influences are possible.
Because antihypertensive therapy may slow the progression of renal disease22 and children born after 1930 were more likely to have received antihypertensive therapy, we analyzed the total group of 366 ADPKD parent-offspring pairs and 148 PKD1 pairs from this category separately. Again, there were no differences in age at ESRD between parents and children.
In the previous study of ADPKD patients from UCHSC, potential anticipation was noted more frequently in offspring of an affected mother.3 This may be accounted for in part by slightly later onset of ESRD in females compared with males,23 and hence a bias toward detection of earlier ESRD onset in male offspring compared with affected mothers. Conversely, others reported earlier onset of ESRD in ADPKD offspring with paternal inheritance irrespective of sex of the offspring.12 In this study, Cox regression confirmed that males were at greater risk of ESRD than females.
Structurally, the PKD1 gene does not contain long nucleotide repeat regions that are susceptible to unstable expansion. It therefore is not surprising that we found no evidence for expansion of the studied repeats in our PKD1 families with early age at ESRD. There are long polypyrimidine tracts located in intron 21 of PKD1 that are susceptible to triple helix formation and hence occurrence of mutation.24,25 However, no mutational “hot spots” within the PKD1 gene were reported to date. Although the occurrence of a second mutation in certain patients with early onset of ESRD cannot be ruled out, similar to previous reports, we identified a single germ-line mutation that segregated with disease status in 3 of the studied PKD1 families.14–16 It should be emphasized that analysis of trinucleotide repeats in 6 PKD1 families excluded only 1 genetic cause of anticipation in these families. Because DNA samples were unavailable for the key parent-offspring pairs in our other families, we cannot eliminate the possibility of unstable trinucleotide repeat expansion in these other families. Likewise, other genetic causes may explain the apparent anticipation of ESRD in the studied families.18
This updated study using survival analysis and Cox regression suggests there is no evidence of anticipation of ESRD in patients with ADPKD. Examination of parent-offspring pairs informative for the difference in age at ESRD agrees with the outcome of an earlier study of UCHSC ADPKD families. However, bias toward detection of earlier ESRD and overall variability in age at ESRD onset in successive generations of the same family indicates other genetic influences as the cause of the extreme phenotypic variability in ADPKD. We previously showed that genetic variation of all sources (mutation, modifier genes, and wild-type allele) accounted for a significant proportion of phenotypic variation in expression of ADPKD measured by means of heritability analyses.18,26
Acknowledgments
We are especially grateful to patients and relatives from ADPKD families who kindly donated their time and biological samples for our clinical and genetic studies of ADPKD.
Support: This research was supported by grants MO1RR00051 and MO1 RR00069 from the General Research Centers Program, National Center for Research Resources, National Institutes of Health; grant DK34039 from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases); and the Zell Family Foundation.
Footnotes
Financial Disclosure: None.
References
- 1.Ecder T, Fick-Brosnahan GM, Schrier RW. Polycystic kidney disease. In: Schrier RW, editor. Diseases of the Kidney . 8. Philadelphia, PA, Lippincott: Williams & Wilkins; 2006. pp. 502–539. [Google Scholar]
- 2.Dalgaard OZ. Bilateral polycystic disease of the kidneys: A follow-up of two hundred and eighty-four patients and their families. Acta Med Scand. 1957;158(suppl 328):S1–S225. [PubMed] [Google Scholar]
- 3.Fick GM, Johnson AM, Gabow PA. Is there evidence for anticipation in autosomal dominant polycystic kidney disease? Kidney Int. 1994;45:1153–1162. doi: 10.1038/ki.1994.153. [DOI] [PubMed] [Google Scholar]
- 4.Harper PS, Harley HG, Reardon W, Shaw DG. Anticipation in myotonic dystrophy: New light on an old problem. Am J Hum Genet. 1992;51:10–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Howeler CJ, Busch HFM, Geraedts JPM, Niermeijer MF, Staal A. Anticipation in myotonic dystrophy: Fact or fiction. Brain. 1989;112:779–797. doi: 10.1093/brain/112.3.779. [DOI] [PubMed] [Google Scholar]
- 6.Ridley RM, Frith CD, Crow TJ, Conneally PM. Anticipation in Huntington’s disease is inherited through the male line but may originate in the female. J Med Genet. 1988;25:589–595. doi: 10.1136/jmg.25.9.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 8.Orr HT, Chung M, Banfi S, et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4:221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- 9.Kremer EJ, Pritchard M, Lynch M, et al. Mapping of DNA instability at the fragile-X to a trinucleotide repeat sequence p(CCG)n. Science. 1991;252:1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- 10.Geberth S, Ritz E, Zeier M, Stier E. Anticipation of age at renal death in autosomal dominant polycystic kidney disease (ADPKD)? Nephrol Dial Transplant. 1995;10:1603–1606. [PubMed] [Google Scholar]
- 11.Gonzalo A, Gallego A, San Millan JL, Ortuno J. Anticipation of end-stage renal disease in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1996;11(suppl 6):S21–S23. doi: 10.1093/ndt/11.supp6.21. [DOI] [PubMed] [Google Scholar]
- 12.Sotirakopoulos N, Tsitsios T, Stambolidou M, Cris-todoulidou C, Spaia S, Mavromatidis K. Anticipation of end-stage renal disease in patients with autosomal dominant polycystic kidney disease in successive generations. Ren Fail. 2001;23:715–720. doi: 10.1081/jdi-100107368. [DOI] [PubMed] [Google Scholar]
- 13.Bersani E, De Fonzo V, Aluffi-Pentini F, Parisi V. On new hypotheses about autosomal dominant polycystic kidney disease type 1. Med Hypotheses. 2001;57:754–758. doi: 10.1054/mehy.2001.1482. [DOI] [PubMed] [Google Scholar]
- 14.Peral B, Ong ACM, San Millan JL, Gamble V, Rees L, Harris PC. A stable, nonsense mutation associated with a case of infantile onset polycystic kidney disease 1 (PKD1) Hum Mol Genet. 1996;5:539–542. doi: 10.1093/hmg/5.4.539. [DOI] [PubMed] [Google Scholar]
- 15.Torra R, Badenas C, Darnell A, Bru C, Escorsell A, Estivill X. Autosomal dominant polycystic kidney disease with anticipation of Caroli’s disease associated with PKD1 mutation. Kidney Int. 1997;52:33–38. doi: 10.1038/ki.1997.300. [DOI] [PubMed] [Google Scholar]
- 16.Perrichot RA, Mercier B, de Parscau L, Simon PM, Cledes J, Ferec C. Inheritance of a stable mutation in a family with early-onset disease. Nephron. 2001;87:340–345. doi: 10.1159/000045940. [DOI] [PubMed] [Google Scholar]
- 17.Sedman A, Bell P, Manco-Johnson M, et al. Autosomal dominant polycystic kidney disease in childhood: A longitudinal study. Kidney Int. 1987;31:1000–1005. doi: 10.1038/ki.1987.98. [DOI] [PubMed] [Google Scholar]
- 18.Fain PR, McFann KK, Taylor MRG, et al. Modifier genes play a significant role in the phenotypic expression of ADPKD. Kidney Int. 2005;67:1256–1257. doi: 10.1111/j.1523-1755.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- 19.Rossetti S, Chauveau D, Walker D, et al. A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int. 2002;61:1588–1599. doi: 10.1046/j.1523-1755.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Mei C, Zhang D, et al. Mutation analysis of autosomal dominant polycystic kidney disease genes in Chinese. Nephron Exp Nephrol. 2005;20:2368–2375. doi: 10.1159/000084572. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 22.Schrier RW, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal dominant polycystic kidney disease: Results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13:1733–1739. doi: 10.1097/01.asn.0000018407.60002.b9. [DOI] [PubMed] [Google Scholar]
- 23.Gabow PA, Johnson AM, Kaheny WD, et al. Factors affecting the progression of renal disease in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1992;41:1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Seidman MM, Glazwe PM. Mutagenesis in mammalian cells induced by triple helix formation and transcription coupled repair. Science. 1996;271:802–805. doi: 10.1126/science.271.5250.802. [DOI] [PubMed] [Google Scholar]
- 25.Van Raay TJ, Burn TC, Connors TD, et al. A 2.5kb polypyrimidine tract in the PKD1 gene contains at least 23 H-DN-forming sequences. Microb Com Genomics. 1996;1:317–327. doi: 10.1089/mcg.1996.1.317. [DOI] [PubMed] [Google Scholar]
- 26.Paterson AD, Magistroni R, He N, et al. Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005;16:755–762. doi: 10.1681/ASN.2004090758. [DOI] [PubMed] [Google Scholar]