Abstract
Recombinant human activated protein C (rhAPC) was developed to reduce excessive coagulant and inflammatory activity during sepsis. Basic and clinical research has suggested these pathways contribute to the pathogenesis of this lethal syndrome and are inhibited by rhAPC. Based in large part on the results of a single multicenter randomized controlled trial, rhAPC was first approved in 2001 by the US Food and Drug Administration (FDA) as adjunctive therapy in septic patients with a high risk of death. This was followed closely by approval in Europe, Australia, and New Zealand. At the original FDA review of rhAPC, concerns were raised as to whether a confirmatory trial should be done before final regulatory approval because of concerns that rhAPCs bleeding risk might outweigh its potential benefit during clinical use. Since 2001, continuing basic and clinical research has further elucidated the complex role activated protein C may have in both adaptive and maladaptive responses during sepsis. Moreover, subsequent controlled trials in other types of septic patients and observational studies appear to support earlier concerns that the benefit-to-risk ratio of rhAPC may not support its clinical use. This experience has prompted additional trials presently underway, to define whether treatment with rhAPC as it was originally indicated in septic patients with persistent shock, is safe and effective. Until such trials are complete, physicians employing this agent must carefully consider which patients may be appropriate candidates for rhAPC administration.
Keywords: rhAPC, treatment, sepsis
Introduction
Septic shock is a major cause of morbidity and mortality in intensive care units and its incidence is increasing.1–4 While excessive release of host inflammatory mediators (eg, cytokines, prostaglandins) is closely associated with the pathogenesis of sepsis, increased host coagulant activity has been implicated as well. Microvascular thrombosis not only disrupts organ perfusion and function, but it may also stimulate further inflammatory mediator release and injurious processes such as apoptosis.5–8 Both preclinical and clinical studies have suggested that this excessive coagulant activity results in part from depletion or depression of endogenous anticoagulant systems.8 One system central to normal coagulant homeostasis is the protein C (PC) system. Reductions in endogenous protein C and its active form, activated protein C (APC), have been associated with worsened outcome in sepsis.9–13 Based in large part on this association, recombinant human APC (rhAPC) was developed for use in patients with severe sepsis.
In contrast to a long list of other immunomodulators developed for sepsis, rhAPC was the first one reported to significantly improve survival in a single phase III multicenter trial: the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study.14 During the regulatory approval process questions were raised about the consistency of its effect in this trial, its mechanism of action and its safety profile. All of these concerns were addressed by the manufacturer and as a result, the US Food and Drug Administration (FDA) approved rhAPC for clinical use. Based on a significant relationship noted in the original trial between severity of disease (ie, Acute Physiology and Chronic Health Evaluation II Score) and the beneficial effect of rhAPC on mortality rates, use was restricted to patients with severe sepsis and a high risk of death. Furthermore, the FDA requested that the manufacturer of rhAPC conduct additional randomized controlled trials (RCTs) testing the effects of the agent in septic adults with a low risk of death, in pediatric patients, and in patients receiving prophylactic heparin. These were subgroups which had not been tested or where rhAPC appeared to show no benefit, or possible harmful trends.
rhAPC has now been in use clinically for almost eight years. During this time growing data from basic research has further defined the potential effects of rhAPC. In addition, the results of the RCTs requested by the FDA as well as clinical surveys assessing the use of rhAPC are available. The purpose of the present article is to review the growing insights this experience provides regarding the potential benefits and risks associated with use of rhAPC in sepsis.
Function and structure of activated protein C
Protein C is a vitamin K-dependent protein that normally circulates in a free state. In the setting of increased coagulant activity, thrombin (T) cleaves PC and generates APC. Formation of APC is increased 10,000-fold when thrombin is bound to thrombomodulin (TM), present on the endothelial surfaces of both large and small vessels.15 This conversion is increased 20-fold further when protein C is bound in a Ca2+-dependent reaction by endothelial protein C receptor (EPCR) adjacent to the thrombin-thrombomodulin (TTM) complex.15,16 EPCR is a transmembrane protein with a structure similar to the major histocompatibility class1/D1 family of molecules.15,17,18 APC then complexes to protein S and inactivates factor Va and VIIIa to prevent further thrombin formation and coagulation.19
While anticoagulation with APC may be beneficial, its binding to EPCR also results in proteolytic activation of protease activated receptor-1 (PAR-1), providing alternative mechanisms for potential benefit in sepsis.20–23 The cleavage of PAR-1 exposes a tethered ligand that in turn stimulates G-protein mediated activation of mitogen activated protein kinase (MAPK) cascades.24 While thrombin can also bind to PAR-1, it produces inflammatory and apoptotic effects as opposed to APC-cleaved PAR-1, which elicits protective cell signaling responses.25,26
APC consists of a Gla domain necessary for EPCR binding, followed by two epidermal growth factor (EGF) domains, and finally a serine protease domain.27 The active site for inactivation of Va and VIIIa by APC resides in the serine protease domain on two surface loops.17,28–33 While the position of the site necessary for PAR-1 activation is unclear, mutant deletion studies show it is distinct from the anticoagulant site.34–36 In genetically engineered animals, improved survival following lipopolysaccharide (LPS) challenge required intact enzymatic activity of APC, EPCR, and PAR-1.37 Furthermore, a recombinant APC variant with normal PAR-1 signaling but reduced anticoagulant activity improved survival with LPS challenge in mice similar to wild-type protein.37
APC and anticoagulation in sepsis
During infection, stimulation of monocytes, macrophages and endothelial cells by microbial toxins and host inflammatory mediators causes release of tissue factor.38 This results in a procoagulant state which is aggravated by sepsis related depression of counter regulatory anticoagulant mechanisms including the PC system.9,10,12,13,39,40 Septic patients have a reduction in endogenous PC, APC, and protein S, which may be due to decreased production by the liver or by degradation by neutrophil elastase.41 In addition, soluble forms of EPCR and TM released from injured endothelial cells bind to APC and competitively inhibit its uptake by the intact cellular proteins.39,42 Finally sepsis induced downregulation of EPCR and TM can further decrease endogenous APC generation.39
Analysis of PC levels in patients from the PROWESS trial showed an association between PC deficiency and death. Severe PC deficiency was associated with increased odds of death at 28 days as compared to subjects without PC deficiency. Furthermore, increased PC levels were associated with better outcome.11 Administration of rhAPC during sepsis decreased pro-coagulant markers (eg, prothrombin fragment and thrombin generation) and increased anticoagulant ones (eg, plasminogen, antithrombin and protein C).43 Perhaps as a consequence, rhAPC infusion increased the rate of bleeding.14
Potential cytoprotective effects of APC in sepsis
Although the anticoagulation effects of rhAPC were believed important for its benefit in the PROWESS trial, two other antithrombotic agents (ie, anti-thrombin III and tissue factor pathway inhibitor), were not beneficial in phase III sepsis trials.44,45 This finding and others have suggested that the observed benefit of rhAPC in septic patients may have been related to mechanisms possibly mediated by PAR-1.22,46,47 These nonanticoagulant mechanisms, now under study and collectively referred to as cytoprotective ones, include anti-inflammatory and antiapoptotic activities and endothelial barrier stabilization.
APC has been shown to have several effects on monocytes, endothelial cells, and neutrophils that could be anti-inflammatory ones. Either in LPS-stimulated monocytic cell lines or in monocytes from septic patients, APC 1) inhibits nuclear factor-kappaB (NF-κB) and the downstream pro-inflammatory cytokines NF-κB upregulates, 2) inhibits macrophage inflammatory protein-1α release and 3) stimulates production of the anti-inflammatory cytokine interleukin-10 (IL-10).48,50,51 In endothelial cells, APC 1) inhibits NF-κB binding to DNA, 2) suppresses tumor necrosis factor (TNF)-mediated expression of the adhesion molecules vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), and E-selectin, and 3) reduces E-selectin-dependent leukocyte rolling.49,52,53 Finally, APC inhibits IL-8, antithrombin, and C5a-mediated neutrophil chemotaxis.54 Consistent with such in vitro findings, in vivo APC inhibition increased inflammatory cytokine levels in Escherichia coli-challenged baboons55 while rhAPC treatment in rats reduced leukocyte adherence to intestinal endothelial cells and improved microvascular perfusion.56 In normal human volunteers challenged with intratracheal LPS, rhAPC reduced pulmonary neutrophil recruitment.57 Finally, in the PROWESS trial itself, rhAPC treatment was associated with reduced IL-6 levels over time.43
Apoptosis of lymphocytes and endothelial cells may also contribute to the pathogenesis of sepsis and evidence suggests that APC can suppress this process via EPCR and PAR-1.6,7,58,59 Treatment of endothelial cells with APC inhibits the pro-apoptotic calreticulin gene and upregulates the anti-apoptotic A1 Bcl-2 homologue and inhibitor of apoptosis protein-1 (IAP-1).60 In brain endothelial cells, APC prevents ischemia mediated apoptosis by inhibiting p53 suppressor protein and reducing caspase-3 activation.61 In a murine E. coli sepsis model, APC treatment reduced apoptotic proteins p21 and p53.62 Also APC via PAR1/sphingosine-phosphate (S1P), inhibited the endothelial cell expression and secretion of TNF-related inducing ligand (TRAIL) in a mechanism involving increased levels of early growth response factor (EEGR)-1 and of phosphorylated ERK 1/2.63
APC stabilization of endothelial cell integrity and vascular permeability via EPCR and PAR-1 may also be beneficial during sepsis.64 APC-mediated EPCR/PAR-1 binding stimulates sphingosine kinase-1 (SphK-1) to form S1P.65,66 In turn, S1P can activate the sphingonase-1-phosphate receptor (S1P1), a G-protein-coupled receptor, which results in cortical cytoskeleton rearrangement and stabilization.67–69 With endotoxin challenge, S1 administration prevented lung edema in the dog, and increased tissue permeability in the mouse.70,71
Evolving clinical experience with rhAPC
In 2001, results from the Phase III Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial were published in The New England Journal of Medicine.14 The reported findings were striking with the agent producing a statistically significant 6% absolute reduction in sepsis related mortality. Furthermore, while bleeding, a likely complication associated with rhAPC, was increased with treatment this did not reach statistical significance. This report raised great hope in health care workers treating patients with sepsis.
Further analysis of the trial by the US FDA officials and an FDA advisory panel however, raised several questions regarding the promising results of the PROWESS trial. First, while rhAPC did show an overall significant beneficial effect, this appeared to occur primarily in patients with a high risk of death as measured with the Acute Physiology and Chronic Heath Evaluation Score (APACHE II). This score was used to stratify patients for primary analysis in PROWESS, and, as determined by the FDA, was most effective in classifying patients by risk of death and by the likelihood of benefit from rhAPC.14,72 Importantly however, the decision to treat with rhAPC in the trial itself was not based on this score. Instead, APACHE II scores were determined retrospectively using the most extreme values obtained over the 24-hour period before the drug was administered. Second, while the risk of hemorrhage with treatment as compared to placebo during the overall study did not reach significance (3.5 vs 2.0, respectively; p = 0.06), serious hemorrhage during drug infusion, was significantly increased with rhAPC (0.2 vs 0.1; p = 0.02). An ongoing open label trial at the time supported concern regarding this bleeding risk.73 While other questions also arose regarding changes in patient selection criteria and treatment preparation during the trial, the most concerning issues for members of the advisory panel were whether it was clear which patient population would benefit from rhAPC, and whether the potential risk of hemorrhage would outweigh that benefit during clinical use.74 These questions about the safety and efficacy of rhAPC in the PROWESS trial prompted half the FDA advisory panel members (10 of 20) to vote that the agent undergo additional phase III testing.74 Nonetheless, the FDA approved rhAPC, but restricted its use to septic patients with a high risk of death. It also directed the manufacturer to perform phase IV studies to clarify its effects in adult septic patients with a low risk of death, pediatric patients, and in combination with prophylactic heparin.
Unfortunately, since a confirmatory trial was not originally performed in the population the agent was prescribed for, early questions raised by the PROWESS trial have persisted.75–85 At present, answers to those questions can only be sought from controlled trials that have been conducted subsequently, and in the increasing number of uncontrolled studies assessing the clinical experience published since rhAPC’s approval. While such data lack the clarity a randomized control trial might offer, the combined experience to date with rhAPC supports concerns that the benefit from rhAPC may not be as great as originally reported, and the risk of hemorrhage is very real and likely increases during clinical use.
Assessment of rhAPC’s benefit since the PROWESS trial
One can try to assess the effect of rhAPC on survival in sepsis (ie, its beneficial effect) since the PROWESS trial in at least two ways. First, one can compare its effects to placebo treated patients in subsequent controlled trials. Second, one can compare survival rates in patients receiving rhAPC in uncontrolled studies to patients in the original PROWESS trial after attempting to control for severity of sepsis or risk of death. The APACHE II score is employed primarily for this purpose here since, as outlined above, it provided the basis for primary analysis in PROWESS and was the best predictor of death and of the likelihood of benefit with rhAPC in the FDA’s review of this trial.14,72 Furthermore, differentiation of the effects of rhAPC based on the APACHE II score is common in both controlled and uncontrolled studies (Table 1 and 2).14,73,86–90 In addition, mean organ injury scores are presented when reported. Two points of caution are necessary. First, injury severity scores such as these have not been developed for the purpose of assessing the effectiveness of treatments across subgroups and may have major limitations in this setting. Second, even after accounting for severity of illness, differences in other variables may weaken comparisons between controlled and uncontrolled trials.
Table 1.
Summary of controlled studies
| Number of patients
|
Age (year) (mean ± SD)
|
APACHE II score (mean ± SD)
|
Organ injury score (mean ± SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Authora | Name | Dates of enrollment | Follow-up (days) | Control | rhAPC | Control | rhAPC | Control | rhAPC | Control | rhAPC |
| Bernard72 | Phase II study | 7/96–12/97 | 28 | 15 | 15 | NAb | NA | NA | NA | NA | NA |
| Bernard14 | PROWESS | 8/98–6/00 | 28 | 840 | 850 | 61 (17) | 61 (17) | 25 (8) | 25 (8) | 2.4 (1.1) | 2.4 (0.1) |
| Abraham91 | ADDRESS | 9/02–2/04 | 28 | 1307 | 1333 | 59 (17) | 59 (17) | 18 (6) | 18 (7) | NA | NA |
| Nadel93 | RESOLVE | 11/02–3/05 | 28 | 237 | 240 | 3 (1,9)c | 2 (1, 8)c | NA | NA | NA | NA |
| Liu94d | NA | 1/05–2/07 | 60 | 38 | 37 | 52 (19) | 52 (16) | 20 (7) | 20 (7) | NA | NA |
| Dhainaut95e | NA | 1/04–12/07 | 28 | 99 | 94 | 62 | 62 | 28 | 28 | NA | NA |
Notes:
All trials were provided support by the manufacturer of rhAPC (Eli Lilly and Company);
Not available;
Interquartile range;
Evaluated rhAPC in critically ill patients with acute lung injury, of whom 18% and 11% of placebo and rhAPC groups respectively, had sepsis as the primary etiology of lung injury;
Randomized patients at the completion of 96 hours of rhAPC treatment to an additional 72 h of rhAPC or placebo.
Table 2.
Summary of uncontrolled studies
| Nonsurvivors/Total number of patients
|
|||||||
|---|---|---|---|---|---|---|---|
| Author | Name | Dates of enrollment | 28 days | Hospital or ICU discharge or 90 daysa | Age (year)* | APACHE II score* | Organ injury score* |
| Tanzi102 | NAb | 5/02–11/03 | NA | 265/599 | 57 | NA | 3.0 (1.1) |
| Vincent73c | ENHANCE | 3/01–1/03 | 601/2378 | NA | 59 (17) | 19 (7) | 2.7 (1.1) |
| Kübler100 | NA | 4/03–11/05 | NA | 118/302 | 45 (18) | 25 (10) | NA |
| Spriet101 | NA | 7/03–1/06 | 17/23 | 11/23 | 59 | 25 | 3.0 (4) |
| Levi87d | XPRESS | 12/02–8/05 | 580/1927 | NA | 59 (17) | 24 (7) | 3.0 (1.2) |
| Kanji99 | NA | 3/03–2/04 | NA | 118/261 | 56 | 32 (26,36)e | 3.4 (1.0) |
| Bertolini97 | NA | 8/03–3/06 | NA | 310/668 | 58 | NA | NA |
| Wheeler104 | NA | 11/01–12/02 | NA | 115/274 | 57 (18) | 27 (9) | 2.9 (1.1) |
| Ridley88 | NA | 12/02–11/05 | NA | 164/351 | 61.8 | 23 (7) | 3.3 (1.0) |
| Vincent90 | NA | 7/03–9/04 | 184/436 | 224/436 | NA | 24 (8) | 3.1 (1.0) |
| Rowan89 | NA | 12/02 | NA | 398/1079 | 59 (16) | 22 (7) | 3.3 (0.9) |
| Taylor103 | NA | 1/01–12/04 | 32/99 | NA | 64.6 | 28 (8) | NA |
| Gentry86 | NA | 1/02–12/05 | NA | 26/73 | 57 | 24 | NA |
| Decruyenaere98 | BOOST | 12/03–10/03 | 31/97 | 41/97 | 61.4 (19) | 25 (9) | 3.4 (1) |
Notes:
All studies assessed hospital discharge except the ones by Kubler and Bertolini that assessed ICU discharge and by Decruyenaere that assessed 90 days;
Not available;
Bolded studies were provided support by the manufacturer of rhAPC (Eli Lilly and Company);
Compared prophylactic heparin treatment to placebo in critically ill patients, all of whom were receiving rhAPC;
Interquartile range.
Means ± standard deviation.
Placebo-controlled trials
Two subsequent placebo-controlled trials have assessed the effect of a similar regimen of rhAPC to the one used in PROWESS in patients with sepsis. The Drotrecogin Alfa (activated) for Adults with Severe Sepsis and Low Risk of Death (ADDRESS) was a placebo-controlled double blind multicenter RCT of rhAPC in septic patients with a low risk of death (APACHE < 25 in the United States or single organ failure in Europe)91 (Table 1). In ADDRESS, investigators could at their discretion include patients with an APACHE II score ≥ 25 or multiorgan failure if they felt the patient was still at low risk of death. In contrast to PROWESS, in which APACHE II scores were determined retrospectively, in ADDRESS, APACHE II scores (or other measures of risk) were calculated prospectively. Patients had to start therapy within 48 hours of first organ dysfunction and exclusion criteria were similar to PROWESS. While the overall estimated low mortality rate of the targeted population required enrollment of 11,444 subjects to show efficacy if one existed, the trial was halted after enrolling only 2,640 due to futility. Overall 28-day mortality was not significantly different with rhAPC compared to placebo (18.5% vs 17.0%, respectively; p = 0.34) (Table 1). As designed, 88% of all enrolled patients had an APACHE II score < 25. After stratification by APACHE II, 28 day mortality rates with rhAPC were not statistically different when compared with placebo whether scores were <25 (16.9% vs 16.0%, respectively; p = 0.55) or ≥25 (29.5% vs 24.7%, respectively; p = 0.34) (Figure 1). Notably, the absence of increased survival in patients with APACHE II ≥ 25 (324 total patients) in ADDRESS was very different from the improvement noted in this subgroup in PROWESS (Odds ratio of survival [95% CI]: 0.9 [0.8, 2.1] vs 1.7 [1.3, 2.3], respectively). Although this difference is highly concerning, it must be recognized that control mortality rate in ADDRESS patients was also lower based on investigators original estimate of enrollees’ risk of death (Figure 1).75,81,82
Figure 1.
The effect of recombinant human activated protein C (rhAPC) on the odds ratio of survival in controlled trials comparing this agent to placebo. Patients are stratified into subgroups with acute physiology and chronic health evaluation II scores (APACHE II) ≥ or <25 where data were available since this score was employed by the US Food and Drug Administration (FDA) in its approval of rhAPC. Notably, over the more than 5000 patients studied, the only subgroup showing benefit was the 421 patients with APACHE II ≥ 25 receiving rhAPC in the original PROWESS trial.
Notes: aPhase II study results from the subgroup of patients testing a 96-hr infusion of rhAPC (24 mg/kg/hr), the regimen employed in later control trials and approved by the FDA.
Severe sepsis is a leading cause of death in infants and children, with a hospital mortality rate of 13%.92 Beyond supportive care and antibiotics, there are no approved adjunctive therapies. Despite the difficulties associated with pediatric investigations, and based on the reported benefits of rhAPC in PROWESS, a trial with this agent was conducted in children with sepsis. The Researching Severe Sepsis and Organ Dysfunction in Children: a Global Perspective (RESOLVE) was an open-label placebo-controlled trial in pediatric patients93 (Table 1). Children with sepsis were enrolled within 48 hours of first organ dysfunction or within 24 hours of dual cardiac and respiratory dysfunction. Severity of sepsis for a pediatric population was great (ie, all patients required mechanical ventilation and vasopressors). While the primary endpoint was a composite time to complete organ failure resolution (CTCOFR) score, 28 day mortality was a secondary one. Compared to placebo, rhAPC did not alter the CTCOFR score (6% vs 6%; p = 0.72) nor did it improve mortality (17.5% vs 17.2%, respectively; p = 0.93) (Figure 1).
Since PROWESS, two controlled trials not requested by the FDA have also compared the effects of rhAPC to placebo: one in patients with acute lung injury (ALI), some of whom had sepsis,94 and another in septic patients who had completed a 96-hour course of rhAPC, but in whom shock persisted95 (Table 1). In patients with ALI, compared to placebo, rhAPC did not decrease the number of ventilator-free days, the primary outcome (Median [IQR]: 19 [14, 22] vs 19 [0, 24], respectively; p = 0.78). Sixty-day mortality, a secondary outcome, was the same between the two groups (13.5%) (Figure 1). Unfortunately, the effect of rhAPC in the subgroup of patients with sepsis as the cause of ALI was not reported. In the second study, a total of 193 patients who had already completed a 96-hour infusion of rhAPC, but who were still in shock, were randomized to placebo or an additional 72-hour rhAPC infusion (Table 1). Extended rhAPC infusion did not resolve vasopressor-dependent shock, the primary endpoint, more quickly than controls (34% vs 40%, respectively; p = 0.42). Compared to placebo, 28-day mortality was higher with rhAPC but not significantly different (32.3% vs 39.8%; p = 0.28) (Figure 1). Importantly, while fifty-three patients (27.5%) had protocol violations, most commonly due to discontinuation of study drug, the study group assignments of these patients were not identified. Furthermore, although it was reported that prothrombin and thrombin times did not differ between groups, partial thromboplastin times (PTT), which can be increased with rhAPC, were not reported. If PTT data were available for review by clinicians, it is possible that more patients in the treatment group had therapy stopped early based on increased PTT values and concern over bleeding.
Uncontrolled phase IIIB or IV studies
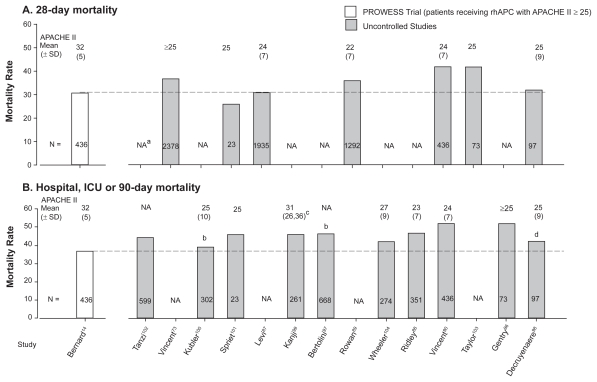
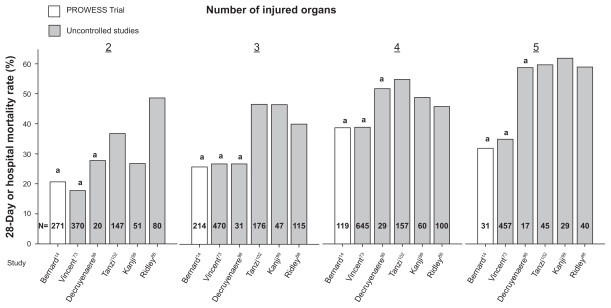
The extended evaluation of rhAPC in the treatment of severe sepsis (ENHANCE) trial was a single arm, multinational study designed to further examine the safety and efficacy of rhAPC in adult patients with sepsis73 (Table 2). Although an open label study, inclusion and exclusion criteria and time to treatment (within 48 hours of organ dysfunction) were the same as in PROWESS. It was considered a phase IIIB trial because it was initiated before final regulatory approval of rhAPC. A total of 2,378 patients were treated with rhAPC and the overall 28-day mortality rate (25.3%) was not different than in PROWESS (24.7%). However, in ENHANCE patients with APACHE II ≥ 25, mortality was higher than in similar patients in PROWESS (36.7% vs 30.9%) (Figure 2). Despite this difference, the mortality rate in this subgroup from ENHANCE was still lower than in control patients from PROWESS (43.7%). Also, when stratified by number of organs injured, the mortality rates in PROWESS and ENHANCE were similar (Figure 3). Based on these latter points, some would argue that the efficacy of rhAPC in PROWESS was reproduced in ENHANCE.
Figure 2.
Comparison of mortality rates and the average APACHE II score in the subgroup of patients receiving rhAPC with APACHE II scores ≥ 25 from the PROWESS trial to mortality rates and average APACHE II scores in patients receiving rhAPC in subsequent uncontrolled trials. Mortality rates at 28 days are shown in the upper panel A) and at hospital or intensive care unit discharge or 90 days in the lower panel B). results from the subgroup of patients shown from the PROWESS trial provided the basis for approval of rhAPC. in almost all uncontrolled studies, mortality rates have been higher and average APACHE II scores lower than in the subgroup in PROWESS with APACHE II ≥ 25.
Notes: aNA, Not available; bICU mortality; cInterquartile range; d90-day mortality.
Figure 3.
Comparison of mortality rates (28 day or hospital) in subgroups of patients receiving rhAPC stratified by number of injured organs in the original PROWESS trial and subsequent uncontrolled studies. in almost all cases, mortality rates have been higher in subgroups of patients with similar numbers of injured organ in later uncontrolled studies compared to PROWESS.
Notes: a28-day mortality.
A potential benefit with earlier treatment (<24 hours) with rhAPC in ENHANCE was not borne out after multivariate logistic regression analysis adjusted for possible covariates (p = 0.08). In spite of this fact, the European Medicines Agency (EMEA) amended its indication for rhAPC to start treatment within 24 hours,96 although this was questioned.84
The Xigris and Prophylactic Heparin Evaluation in Severe Sepsis (XPRESS) trial was a double-blind randomized international trial in adult patients receiving rhAPC for severe sepsis with a high risk of death (APACHE II ≥ 25, or ≥2 organ failures) (Table 2).87 It was a phase IV trial requested by the FDA to compare the effects of prophylactic heparin therapy (either low molecular weight heparin [LMWH] 40 mg q24h or unfractionated heparin [UFH] 5000 units q12h) to placebo in patients receiving rhAPC. As such, all patients received rhAPC. In this population of septic patients, compared to placebo, 28-day mortality rate was lower with heparin treatment overall, although not significantly different (31.9% vs 28.3%; p = 0.08). The incidence of thromboembolic complications was equivalent between the two groups, however ischemic strokes were less common in the heparin group (0.3% vs 1.3%; p = 0.02). Furthermore, in patients on heparin prior to randomization, mortality rate was significantly higher in those randomized to placebo compared to those who continued heparin (35.5% vs 26.9%; p = 0.005), in spite of similar baseline characteristics for age, disease severity, and comorbidities.
Although the XPRESS study was designed to enroll patients with APACHE II scores ≥ 25, more than half the patients (53.7%) had scores less than 25. Despite mean APACHE II scores that were very similar in patients receiving rhAPC in PROWESS (n = 851) and XPRESS (n = 1927) (24 ± 8 vs 24 ± 7, respectively), 28-day mortality rate was higher in patients in XPRESS (n = 1927) (30.1% vs 24.7%, respectively). In addition, compared to patients receiving rhAPC in PROWESS with APACHE II ≥ 25 (mean 31 ± 5, n = 414), overall mortality in XPRESS (ie, both placebo and heparin patients), was only marginally different (31.9% vs 30.1%, respectively) despite having a substantially lower mean APACHE II score (Figure 2). Of note, the mean organ injury score was higher in XPRESS than in PROWESS (3.0 ± 1.2 vs 2.7 ± 1.1, respectively) and mortality rate in XPRESS was lower than in PROWESS control patients with APACHE II scores ≥ 25.
Other uncontrolled studies
In addition to ENHANCE and XPRESS, there have been a series of reports regarding clinical experience with rhAPC following completion of PROWESS and approval of the agent86,88–90,97–104 (Table 2). Some have been conducted with support from the manufacturer of rhAPC (n = 5) and others have been independent studies (n = 7). While all have reported mortality rates in septic patients receiving rhAPC, the measures have varied (eg, 28 day, 90 day, hospital or intensive care unit [ICU] mortality).
Most studies provided APACHE II score data which can be compared to PROWESS. As noted above, approval of rhAPC was based on its apparent effectiveness in patients in PROWESS with APACHE II scores ≥ 25. The mean (± standard deviation [SD]) APACHE II score in these patients was 31 (± 5) and 28-day and hospital mortality rates were 30.9% and 36.7%, respectively. It is therefore noteworthy that while the reported mean APACHE II scores in subsequent uncontrolled trials have been consistently less than 31, mortality rate (28 day or ICU or hospital) has been the same or greater than in PROWESS in most studies (Table 2) (Figure 2). It is possible that the APACHE II score does not accurately reflect underlying severity of disease. Frequently, the mean number of organs injured was higher in uncontrolled trials compared to patients with APACHE II scores ≥ 25 in PROWESS. However, even if one further controls for this variable by stratifying patients based on number of injured organs, mortality rates with rhAPC in those studies presenting such data have in almost all subgroups been greater than in PROWESS with the exception of those from ENHANCE14,73,88,99,102 (Figure 3). It should be noted that some uncontrolled studies reported that rhAPC use was associated with mortality rates, which although higher than in PROWESS, was less than predicted rates or rates in matched nonrandomized untreated patients.88,90
Assessment of rhAPC’s bleeding risk since the PROWESS trial
While the benefit reported with rhAPC in PROWESS has not appeared as great during subsequent clinical experience, the bleeding risk has persisted and possibly increased. As with benefit, one can examine later placebo-controlled trials as well as uncontrolled studies to assess this potential problem with rhAPC, keeping in mind the caution necessary with such comparisons.
Placebo-controlled trials
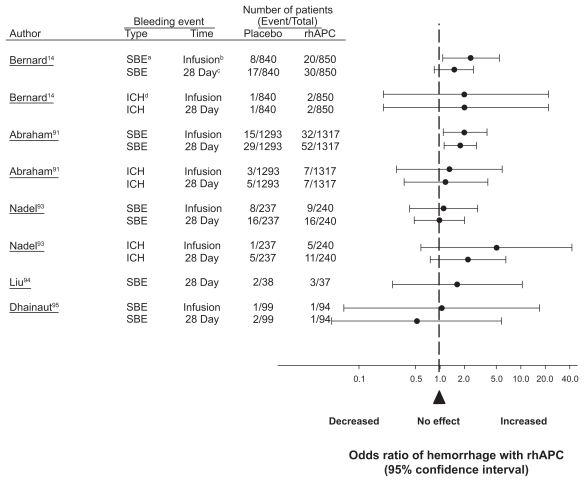
Despite exclusion criteria in PROWESS designed to reduce the risk of hemorrhage with rhAPC, compared to placebo, treatment was associated with increases in serious bleeding both during infusion (2.4% vs 1.0%; p = 0.02) and over the 28-day follow up period (3.5% vs 2.0%; p = 0.06) (Table 3 and Figure 3). Intracerebral hemorrhage (ICH) was not significantly different with treatment as compared to placebo (0.2% vs 0.1%, respectively; p = 0.6). Employing similar exclusion criteria to PROWESS, the incidence of serious bleeding in ADDRESS was increased with rhAPC compared to control in patterns very similar to PROWESS (2.4% vs 1.2% during infusion [p = 0.02] and 3.9% vs 2.2% over the 28-day follow-up [p = 0.01]) (Figure 4). The incidence of ICH however, was not statistically different (0.5% vs 0.4%, respectively; p = 0.79). Post-hoc analysis from ADDRESS data found that for patients who had undergone surgery within the past 30 days and had single organ dysfunction, compared to placebo, rhAPC was associated with higher 28-day (20.7% vs 14.1%; p = 0.03) and in-hospital (23.4% vs 19.8%; p = 0.26) mortality rates. This group also had a higher rate of bleeding than placebo during infusion (10.3% vs 5.1%; p = 0.01) and the 28-day follow-up period (10.9% vs 6.1%; p = 0.03). Based on a similar trend in 28-day mortality rate in this same subgroup in PROWESS (24% vs 16%, respectively; p = 0.60), the FDA issued a drug warning for patients with single organ dysfunction and recent surgery.105
Table 3.
Summary of adverse events in uncontrolled studies
| Patient with SBE (%)
|
Patients with ICH (%)
|
||||
|---|---|---|---|---|---|
| Author | Total number of patients | Infusion | Full study period | Infusion | Full study period |
| Tanzi102 | 599 | NAa | 28 (4.7) | NA | 3 (0.5) |
| Vincent73b | 2378 | 85 (3.6) | 165 (6.5) | 14 (0.6) | 33 (1.4) |
| Kübler100 | 302 | NA | 5 (1.7) | NA | NA |
| Spriet101 | 23 | 1 (4.3) | 1 (4.3) | NA | NA |
| Levi87 | 1935 | 202 (2.3) | 88 (4.6) | 6 (0.3) | 17 (0.9) |
| Kanji99 | 261 | 19 (7.3) | 25 (10) | 10 (0.4) | NA |
| Bertolini97 | 668 | 15 (4.6) | NA | NA | 6 (0.9) |
| Wheeler104 | 274 | 11 (4) | 12 (4.4) | 1 (0.4) | 1 (0.4) |
| Ridley88 | 351 | NA | 8 (2.3) | NA | 2 (0.6) |
| Vincent90 | 436 | NA | NA | NA | NA |
| Rowan89 | 1079 | NA | 80 (6) | NA | 7 (0.5) |
| Taylor103 | 99 | NA | 7 (7) | 0 (0) | 0 (0) |
| Gentry86 | 73 | 7 (10) | 9 (12.3) | NA | 2 (2.7) |
| Decruyenaere98 | 97 | 5 (5.2) | NA | 1 (1%) | NA |
Notes:
Not available;
Bolded studies were provided support by the manufacturer of rhAPC (Eli Lilly and Company).
Abbreviations: ICH, intracerebral hemorrhage; SBE, serious bleeding events. Means ± standard deviation.
Figure 4.
The effect of rhAPC on the odds ratio of a serious bleeding event or an intracerebral hemorrhage during drug infusion or over 28 days in controlled trials comparing this agent to placebo. Similar to PROWESS, in almost all cases, rhAPC was associated with a significant increase in the risk of hemorrhage or had an effect on the side of harm.
Notes: aSBE, serious bleeding event; bDuring infusion of rhAPC or placebo; cDuring the 28-day study period; d ICH, intracerebral hemorrhage.
The RESOLVE trial also employed exclusion criteria similar to PROWESS to reduce the risk of hemorrhage93 (Table 3). Although there was no difference in overall serious bleeding events comparing placebo and rhAPC treatment during the 28-day study period (6.8% vs 6.7%, respectively; p = 0.97), there were numerically more instances of ICH bleeding with rhAPC during infusion (5 [2.1%] vs 1 [0.4%]; p = 0.22) and over the 28-day study period (11 [4.6%] vs 5 [2.1%]; p = 0.13) (Figure 4). Based on this finding in combination with the lack of efficacy of treatment in RESOLVE, the FDA issued a warning about the use of rhAPC in children.106
In the trial testing the effects of rhAPC in patients with ALI, compared to placebo treatment, rhAPC was associated with an increase in the incidence of serious bleeding over the 28-day study period but this was not significant (8.1% vs 3.5%; p = 0.58)94 (Table 3). In the trial testing extended use of rhAPC, bleeding during the initial 96 h of rhAPC treatment was not reported. Following this initial infusion period, compared to placebo, rhAPC was associated with increases in total (3.2% vs 1.0%; p = 0.3) and serious bleeding events (1.1% vs 1.0%; p = 1.0), although not significantly. The incidence of treatment discontinuation due to bleeding was not reported.
Uncontrolled phase IIIB or IV studies
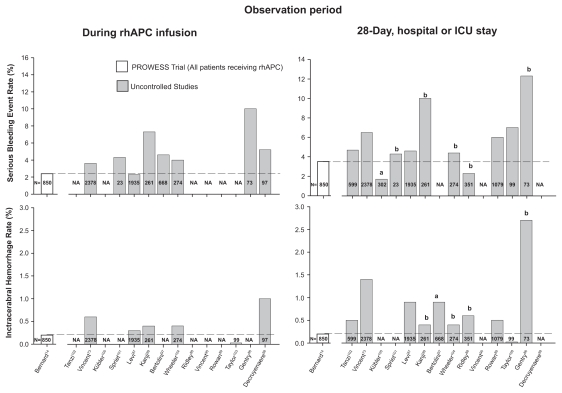
The ENHANCE trial employed exclusion criteria similar to PROWESS.73 It was therefore concerning that the incidence of serious bleeding and ICH during rhAPC infusion (3.6% and 0.6%, respectively) and over the 28-day study period (6.5% and 1.4%, respectively) in ENHANCE was substantially higher than in PROWESS (Table 3) (Figure 5).
Figure 5.
Comparison of the incidence of serious bleeding events (upper panel) and intracerebral hemorrhage (lower panel) during drug infusion or up until intensive care unit or hospital discharge in patients receiving rhAPC in PROWESS to those in subsequent uncontrolled trials. in almost all uncontrolled studies, these incidences were greater than in the PROWESS trial.
Notes: aICU stay; bHospital stay.
Abbreviation: ICU, intensive care unit; rhAPC, recombinant human activated protein C.
The XPRESS trial employed package labeling to define patients eligible for enrollment. Compared to PROWESS, in both groups in XPRESS (rhAPC with either placebo or prophylactic heparin), the incidence of ICH during infusion was higher (0.3%), as was the incidence of serious bleeding (4.6%) and ICH (0.9%) over the 28-day study period (Table 3) (Figure 5). Considering those patients in XPRESS not receiving heparin (ie, rhAPC and placebo, n = 955), the incidence of serious bleeding and ICH were higher than in PROWESS both during infusion (2.5% serious bleeding and 0.3% ICH) and over the 28-day study period (5.2% serious bleeding and 0.7% ICH).
Other uncontrolled studies
In most other uncontrolled studies assessing the use of rhAPC in sepsis, patients were reported to have been treated based on package labeling.73,87,91,93–95 Despite the potential for bleeding with the agent, one of these studies provided no information on the incidence of this complication.90 In the majority of the remaining studies, the incidence of serious bleeding events and of ICH in particular were higher than in PROWESS either during infusion or the entire period of follow-up Table 3) (Figure 5).73,86–89,97–99,101–104
Efficacy vs bleeding risk with rhAPC
The efficacy noted with rhAPC in PROWESS has not been reproduced in subsequent studies. Neither of the later controlled trials in septic patients that employed the same regimen of rhAPC as PROWESS (ie, ADDRESS and RESOLVE) noted any significant benefit with treatment regardless of the underlying severity of disease. Across these three trials (enrolling approximately 5,000 patients), only one subgroup from one study demonstrated benefit (APACHE II scores ≥ 25 from the PROWESS trial). This subgroup included only 414 patients receiving rhAPC (17% of all patients receiving the agent in controlled trials). Notably, the later ADDRESS trial (n = 2,639 patients) also enrolled patients with APACHE II scores ≥ 25 (where investigators deemed them to be at low risk of death) but failed to reproduce the finding of efficacy. In fact, the treatment effect in ADDRESS was on the side of harm, opposite and different from the effect of rhAPC in PROWESS (Figure 1). This is more concerning since, different from PROWESS, the decision to treat in ADDRESS was actually based on calculation of an APACHE II score as would be done clinically. Prompting further questions about the efficacy of rhAPC in clinical practice, mortality rates from repeated uncontrolled studies have been consistently higher and APACHE II scores lower than in the PROWESS subgroup providing the basis for the agent’s original approval. Furthermore, although the mean number of injured organs have frequently been lower in these uncontrolled trials than in the PROWESS subgroup, after stratification, across this variable when this has been possible, mortality has tended to be higher. While this difference may relate to variation in administration of rhAPC (eg, increased treatment time or patients with bleeding precautions during clinical use) and does not constitute proof of harm, it is worrisome.
While the apparent efficacy of rhAPC in PROWESS has not been reproduced, an increased risk of bleeding has been confirmed in controlled trials and documented at even higher rates in clinical use. Why this risk appears more pronounced than during the original PROWESS trial is likely multifactorial. For example, investigating rhAPC in a larger number of control patients in the ADDRESS trial was necessary to document the agent’s risk in surgical patients. Also, during controlled trials that are designed primarily to show the efficacy of an agent, exclusion criteria are frequently applied that minimize the occurrence of adverse events. During clinical use of an agent, such exclusion criteria may not be applied as rigorously and the incidence of adverse events may be expected to increase.107 Members of the FDA advisory panel considering rhAPC were concerned that unless the strict exclusion criteria related to bleeding were rigorously applied in clinical use, there would be a greater incidence of bleeding with this therapy. Yet when rhAPC was approved by the FDA, many of the exclusion criteria related to baseline bleeding risks in the PROWESS trial were labeled as “warnings” in the package insert. This labeling has allowed physicians to weigh the risks vs benefits of administering rhAPC to patients at high risk for bleeding. However, if these bleeding risks had instead been categorized as “contraindications” such consideration would have been discouraged based on the package insert.
The possible problem associated with the present labeling of rhAPC was demonstrated in a recently published study, the results of which are summarized along with other studies above.86 In this particular study, 73 patients received rhAPC at two university hospitals. Twenty of these 73 patients (27%) met package criteria for having either a “warning” (n = 19) or a “contraindication” (n = 1) for use based on bleeding risk. Overall, nine of the 73 patients (12%) experienced a serious bleeding event. However, seven of these events occurred in patients identified as having a baseline bleeding risk vs just two in patients without such a risk. In other words, seven of the 20 patients with a baseline bleeding risk (35%) had a serious bleeding event when treated with rhAPC. Multivariate analysis also revealed an association between bleeding warnings and increased mortality with rhAPC therapy (odds ratio 5.2; p = 0.0098). The small size of this study does raise concern regarding possible sampling error. However, these findings agree with those from another study also summarized above, in which the risk of bleeding with rhAPC increased in patients with multiple organ failure and a relative contraindication to rhAPC therapy.99 Perhaps more striking, these and other trials found that use of rhAPC in the presence of bleeding precautions was quite prevalent.86,97,99
The FDA has issued several warning about bleeding risk in particular subgroups. Ongoing concerns regarding such risks have prompted the FDA to initiate an additional safety review of clinical use of rhAPC. This review is ongoing.108 The EMEA has also requested that an additional controlled trial of rhAPC be conducted in the patient group for which it is now prescribed. As a result of these concerns, two additional randomized controlled trials are underway in septic patients with persistent shock. Until the results from these trials are available for guidance, physicians must still decide which patients should be considered for treatment with rhAPC.109–111 In light of data from studies such as the ones outlined here, one approach for increasing the safety of rhAPC without compromising any potential efficacy is not to administer it to any septic patients with baseline bleeding risks, which effectively changes the labeled warnings to contraindications.
Conclusions
Treatment with rhAPC may have several potential beneficial effects during sepsis and septic shock. In addition to the inhibition of injurious thrombotic events, increasing data suggests rhAPC could have important anti-inflammatory effects via its interaction with EPCR and PAR-1. Despite its potential benefit in sepsis, the apparent efficacy of rhAPC in PROWESS has not been reproduced in subsequent trials, while an increased risk of bleeding has been confirmed in controlled trials and documented at even higher rates in clinical use. Such a complication is of even greater concern for patients as fragile as those with sepsis and septic shock. Thus, whether the potential benefits of this treatment outweigh its risks in septic patients during clinical use is not clear. Comparing uncontrolled clinical experience to the original PROWESS trial is confounded however by differences in the way rhAPC may have been administered in the two settings (eg, delayed treatment in clinical use) and in the patients receiving rhAPC (eg, increased bleeding precautions during clinical use).
Preclinical studies now suggest that rhAPC, besides having anticoagulant effects, may have anti-inflammatory, antiapoptotic and endothelial effects which could also be beneficial during sepsis. Why such an agent has not shown more consistent benefit since the original PROWESS trial is not clear. One possibility as we have outlined is that the risks associated with the agent may outweigh its benefits in particular subgroups that still require clearer definition. In this respect, however, the experience with rhAPC is not dissimilar from other immunomodulators which showed benefit in repeated preclinical sepsis studies but not in clinical ones.112
The PROWESS trial and the experience with rhAPC in sepsis presents issues similar to ones presented by initial trials of early goal-directed therapy and intensive insulin therapy.113 In each case, despite the highly significant survival effects reported with treatment, questions arose about trial design and conduct that suggested confirmatory trials were warranted. Despite these questions and possibly because of the high mortality associated with sepsis, each of these therapies was rapidly introduced clinically. However, subsequent experience with each of these agents resulted in more rather than fewer questions. In fact subsequent controlled trials with intensive insulin have highlighted the potential harm associated with this type of therapy in sepsis and the critically ill.114 This experience emphasizes the weakness of a single RCT. This weakness may relate to the fact that patients with sepsis are a heterogeneous group with a high overall mortality rate. Furthermore, the pathogenesis of sepsis is poorly understood. While the RCT minimizes selection bias, it remains a single experiment and does not guarantee external validity for a syndrome as complex and lethal as sepsis. As such, reproducible and highly consistent evidence of benefit in a clearly defined and easily identifiable group of patients is important before conferring regulatory approval or changing clinical practice.
Controlled trials are presently underway to better define the benefit-to-risk ratio of rhAPC in sepsis. Until such trials are complete, physicians employing rhAPC must carefully select those patients receiving it. One potential way to optimize the benefit-to-risk ratio with rhAPC would be to administer treatment clinically employing similar inclusion and exclusion criteria to those used during the original PROWESS trial.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274(12):968–974. [PubMed] [Google Scholar]
- 3.Martin CM, Priestap F, Fisher H, et al. A prospective, observational registry of patients with severe sepsis: the Canadian Sepsis Treatment and Response Registry. Crit Care Med. 2009;37(1):81–88. doi: 10.1097/CCM.0b013e31819285f0. [DOI] [PubMed] [Google Scholar]
- 4.Salvo I, de Cian W, Musicco M, et al. The Italian SEPSIS study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med. 1995;21(Suppl 2):S244–S249. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6(11):813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27(7):1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Levi M, de Jonge E, van Der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001;29(7 Suppl):S90–S94. doi: 10.1097/00003246-200107001-00028. [DOI] [PubMed] [Google Scholar]
- 9.Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] Crit Care. 2004;8(2):R82–R90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheth SB, Carvalho AC. Protein S and C alterations in acutely ill patients. Am J Hematol. 1991;36(1):14–19. doi: 10.1002/ajh.2830360104. [DOI] [PubMed] [Google Scholar]
- 11.Shorr AF, Bernard GR, Dhainaut JF, et al. Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Crit Care. 2006;10(3):R82–R90. doi: 10.1186/cc4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shorr AF, Nelson DR, Wyncoll DL, et al. Protein C: a potential bio-marker in severe sepsis and a possible tool for monitoring treatment with drotrecogin alfa (activated) Crit Care. 2008;12(2):R45. doi: 10.1186/cc6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcome in severe sepsis. Chest. 2001;120(3):915–922. doi: 10.1378/chest.120.3.915. [DOI] [PubMed] [Google Scholar]
- 14.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 15.Simmonds RE, Lane DA. Structural and functional implications of the intron/exon organization of the human endothelial cell protein C/ activated protein C receptor (EPCR) gene: comparison with the structure of CD1/major histocompatibility complex alpha1 and alpha2 domains. Blood. 1999;94(2):632–641. [PubMed] [Google Scholar]
- 16.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93(19):10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oganesyan V, Oganesyan N, Terzyan S, et al. The crystal structure of the endothelial protein C receptor and a bound phospholipid. J Biol Chem. 2002;277(28):24851–24854. doi: 10.1074/jbc.C200163200. [DOI] [PubMed] [Google Scholar]
- 18.Taylor FB, Peer GT, Lockhart MS, Ferrell G, Esmon CT. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001;97(6):1685–1688. doi: 10.1182/blood.v97.6.1685. [DOI] [PubMed] [Google Scholar]
- 19.Mosnier LO, Griffin JH. Protein C anticoagulant activity in relation to anti-inflammatory and anti-apoptotic activities. Front Biosci. 2006;11:2381–2399. doi: 10.2741/1977. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien PJ, Molino M, Kahn M, Brass LF. Protease activated receptors: theme and variations. Oncogene. 2001;20(13):1570–1581. doi: 10.1038/sj.onc.1204194. [DOI] [PubMed] [Google Scholar]
- 22.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 23.Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111(5):2667–2673. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280(20):19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 25.Esmon CT. Is APC activation of endothelial cell PAR1 important in severe sepsis?: No. J Thromb Haemost. 2005;3(9):1910–1911. doi: 10.1111/j.1538-7836.2005.01573.x. [DOI] [PubMed] [Google Scholar]
- 26.Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR. PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem. 2005;280(13):13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 27.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 28.Mather T, Oganessyan V, Hof P, et al. The 2.8 A crystal structure of Gladomainless activated protein C. EMBO J. 1996;15(24):6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 29.Gale AJ, Heeb MJ, Griffin JH. The autolysis loop of activated protein C interacts with factor Va and differentiates between the Arg506 and Arg306 cleavage sites. Blood. 2000;96(2):585–593. [PubMed] [Google Scholar]
- 30.Gale AJ, Griffin JH. Molecular characterization of an extended binding site for coagulation factor Va in the positive exosite of activated protein C. J Biol Chem. 2002;277(32):28836–28840. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 31.Gale AJ, Griffin JH. Characterization of a thrombomodulin binding site on protein C and its comparison to an activated protein C binding site for factor Va. Proteins. 2004;54(3):433–441. doi: 10.1002/prot.10627. [DOI] [PubMed] [Google Scholar]
- 32.Shen L, Villoutreix BO, Dahlbäck B. Involvement of Lys 62(217) and Lys 63(218) of human anticoagulant protein C in heparin stimulation of inhibition by the protein C inhibitor. Thromb Haemost. 1999;82(1):72–79. [PubMed] [Google Scholar]
- 33.Friedrich U, Nicolaes GA, Villoutreix BO, Dahlbäck B. Secondary substrate-binding exosite in the serine protease domain of activated protein C important for cleavage at Arg-506 but not at Arg-306 in factor Va. J Biol Chem. 2001;276(25):23105–23108. doi: 10.1074/jbc.M103138200. [DOI] [PubMed] [Google Scholar]
- 34.Yang XV, Banerjee Y, Fernández JA, et al. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci U S A. 2009;106(1):274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104(6):1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 36.Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J Biol Chem. 2007;282(12):9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 37.Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204(10):2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grignani G, Maiolo A. Cytokines and hemostasis. Haematologica. 2000;85(9):967–972. [PubMed] [Google Scholar]
- 39.Liaw PC, Esmon CT, Kahnamoui K, et al. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood. 2004;104(13):3958–3964. doi: 10.1182/blood-2004-03-1203. [DOI] [PubMed] [Google Scholar]
- 40.Mesters RM, Helterbrand J, Utterback BG, et al. Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit Care Med. 2000;28(7):2209–2216. doi: 10.1097/00003246-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Eckle I, Seitz R, Egbring R, Kolb G, Havemann K. Protein C degradation in vitro by neutrophil elastase. Biol Chem Hoppe Seyler. 1991;372(11):1007–1013. doi: 10.1515/bchm3.1991.372.2.1007. [DOI] [PubMed] [Google Scholar]
- 42.Borgel D, Bornstain C, Reitsma PH, et al. A comparative study of the protein C pathway in septic and nonseptic patients with organ failure. Am J Respir Crit Care Med. 2007;176(9):878–885. doi: 10.1164/rccm.200611-1692OC. [DOI] [PubMed] [Google Scholar]
- 43.Dhainaut JF, Yan SB, Margolis BD, et al. Drotrecogin alfa (activated) (recombinant human activated protein C) reduces host coagu-lopathy response in patients with severe sepsis. Thromb Haemost. 2003;90(4):642–653. doi: 10.1160/TH02-11-0270. [DOI] [PubMed] [Google Scholar]
- 44.Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290(2):238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 45.Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 46.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J. 2003;373(Pt 1):65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephenson DA, Toltl LJ, Beaudin S, Liaw PC. Modulation of monocyte function by activated protein C, a natural anticoagulant. J Immunol. 2006;177(4):2115–2122. doi: 10.4049/jimmunol.177.4.2115. [DOI] [PubMed] [Google Scholar]
- 48.White B, Schmidt M, Murphy C, et al. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol. 2000;110(1):130–134. doi: 10.1046/j.1365-2141.2000.02128.x. [DOI] [PubMed] [Google Scholar]
- 49.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276(14):11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 50.Hooper WC, Phillips DJ, Renshaw MA. Activated protein C induction of MCP-1 in human endothelial cells: a possible role for endothelial cell nitric oxide synthase. Thromb Res. 2001;103(3):209–219. doi: 10.1016/s0049-3848(01)00319-x. [DOI] [PubMed] [Google Scholar]
- 51.Toltl LJ, Beaudin S, Liaw PC, Group CCCTB. Activated protein C up-regulates IL-10 and inhibits tissue factor in blood monocytes. J Immunol. 2008;181(3):2165–2173. doi: 10.4049/jimmunol.181.3.2165. [DOI] [PubMed] [Google Scholar]
- 52.Franscini N, Bachli EB, Blau N, Leikauf MS, Schaffner A, Schoedon G. Gene expression profiling of inflamed human endothelial cells and influence of activated protein C. Circulation. 2004;110(18):2903–2909. doi: 10.1161/01.CIR.0000146344.49689.BB. [DOI] [PubMed] [Google Scholar]
- 53.Grinnell BW, Hermann RB, Yan SB. Human protein C inhibits selectin-mediated cell adhesion: role of unique fucosylated oligosaccharide. Glycobiology. 1994;4(2):221–225. doi: 10.1093/glycob/4.2.221. [DOI] [PubMed] [Google Scholar]
- 54.Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ. Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 2003;102(4):1499–1505. doi: 10.1182/blood-2002-12-3880. [DOI] [PubMed] [Google Scholar]
- 55.Taylor FB, Chang AY, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987;79(3):918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehmann C, Meissner K, Knöck A, et al. Activated protein C improves intestinal microcirculation in experimental endotoxaemia in the rat. Crit Care. 2006;10(6):R157. doi: 10.1186/cc5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nick JA, Coldren CD, Geraci MW, et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104(13):3878–3885. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- 58.Adrie C, Bachelet M, Vayssier-Taussat M, et al. Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Respir Crit Care Med. 2001;164(3):389–395. doi: 10.1164/ajrccm.164.3.2009088. [DOI] [PubMed] [Google Scholar]
- 59.Pinheiro da Silva F, Nizet V. Cell death during sepsis: integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis. 2009;14(4):509–521. doi: 10.1007/s10495-009-0320-3. [DOI] [PubMed] [Google Scholar]
- 60.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002;30(5 Suppl):S288–S293. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 61.Cheng T, Liu D, Griffin JH, et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9(3):338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 62.Sakar A, Vatansever S, Sepit L, Ozbilgin K, Yorgancioglu A. Effect of recombinant human activated protein C on apoptosis-related proteins. Eur J Histochem. 2007;51(2):103–109. [PubMed] [Google Scholar]
- 63.O’Brien LA, Richardson MA, Mehrbod SF, et al. Activated protein C decreases tumor necrosis factor related apoptosis-inducing ligand by an EPCR- independent mechanism involving Egr-1/Erk-1/2 activation. Arterioscler Thromb Vasc Biol. 2007;27(12):2634–2641. doi: 10.1161/ATVBAHA.107.153734. [DOI] [PubMed] [Google Scholar]
- 64.Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003;60(1):49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- 65.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105(8):3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 66.Finigan JH, Dudek SM, Singleton PA, et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingo-sine 1-phosphate receptor transactivation. J Biol Chem. 2005;280(17):17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 67.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92(6):1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 68.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 69.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005;19(12):1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 70.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170(9):987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 71.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169(11):1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 72.Food and Drug Administration. FDA Briefing Document: Anit-Infective Advisory Committee Drotrecogin Alfa (Activated) [Recombinant Human Activated Proetin C (rhAPC)] Sep 10, 2001. [Accessed March 10, 2009]. pp. 1–117. Available at: http://www.fda.gov/ohrms/dockets/AC/01/briefing/3797b1.htm.
- 73.Vincent J, Bernard GR, Beale R, et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005;33(10):2266–2277. doi: 10.1097/01.ccm.0000181729.46010.83. [DOI] [PubMed] [Google Scholar]
- 74.Warren HS, Suffredini AF, Eichacker PQ, Munford RS. Risks and benefits of activated protein C treatment for severe sepsis. N Engl J Med. 2002;347(13):1027–1030. doi: 10.1056/NEJMsb020574. [DOI] [PubMed] [Google Scholar]
- 75.Baillie JK, Murray G. Drotrecogin alfa (activated) in severe sepsis. N Engl J Med. 2006;354(1):94–96. author reply 94–96. [PubMed] [Google Scholar]
- 76.Carlet J. Looking at subgroups in an inhomogeneous population does not make these subgroups more homogeneous. Intensive Care Med. 2004;30(7):1497. doi: 10.1007/s00134-004-2324-3. [DOI] [PubMed] [Google Scholar]
- 77.Carlet J. Prescribing indications based on successful clinical trials in sepsis: a difficult exercise. Crit Care Med. 2006;34(2):525–529. doi: 10.1097/01.ccm.0000198329.85851.8e. [DOI] [PubMed] [Google Scholar]
- 78.Deans KJ, Minneci PC, Banks SM, Natanson C, Eichacker PQ. Substantiating the concerns about recombinant human activated protein C use in sepsis. Crit Care Med. 2004;32(12):2542–2543. doi: 10.1097/01.ccm.0000148090.94378.6a. [DOI] [PubMed] [Google Scholar]
- 79.Eichacker PQ, Danner RL, Suffredini AF, Cui X, Natanson C. Reassessing recombinant human activated protein C for sepsis: time for a new randomized controlled trial. Crit Care Med. 2005;33(10):2426–2428. doi: 10.1097/01.ccm.0000183002.26587.ff. [DOI] [PubMed] [Google Scholar]
- 80.Eichacker PQ, Natanson C. Recombinant human activated protein C in sepsis: inconsistent trial results, an unclear mechanism of action, and safety concerns resulted in labeling restrictions and the need for phase IV trials. Crit Care Med. 2003;31(1 Suppl):S94–S96. doi: 10.1097/00003246-200301001-00013. [DOI] [PubMed] [Google Scholar]
- 81.Friedrich JO. Drotrecogin alfa (activated) in severe sepsis. N Engl J Med. 2006;354(1):94–96. doi: 10.1056/NEJMc052759. author reply 94–96. [DOI] [PubMed] [Google Scholar]
- 82.LaRosa SP. Drotrecogin alfa (activated) in severe sepsis. N Engl J Med. 2006;354(1):94–96. author reply 94–96. [PubMed] [Google Scholar]
- 83.Mackenzie AF. Activated protein C: do more survive? Intensive Care Med. 2005;31(12):1624–1626. doi: 10.1007/s00134-005-2829-4. [DOI] [PubMed] [Google Scholar]
- 84.Poole D, Bertolini G, Garattini S. Errors in the approval process and post-marketing evaluation of drotrecogin alfa (activated) for the treatment of severe sepsis. Lancet Infect Dis. 2009;9(1):67–72. doi: 10.1016/S1473-3099(08)70306-2. [DOI] [PubMed] [Google Scholar]
- 85.Sweeney DA, Natanson C, Eichacker PQ. Recombinant human activated protein C, package labeling, and hemorrhage risks. Crit Care Med. 2009;37(1):327–329. doi: 10.1097/CCM.0b013e3181935102. [DOI] [PubMed] [Google Scholar]
- 86.Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with the use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit Care Med. 2009;37(1):19–25. doi: 10.1097/CCM.0b013e318192843b. [DOI] [PubMed] [Google Scholar]
- 87.Levi M, Levy M, Williams MD, et al. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated) Am J Respir Crit Care Med. 2007;176(5):483–490. doi: 10.1164/rccm.200612-1803OC. [DOI] [PubMed] [Google Scholar]
- 88.Ridley S, Lwin A, Wyncoll D, et al. Drotrecogin alfa (activated): diffusion from clinical trials to clinical practice. Eur J Anaesthesiol. 2008;25(3):211–216. doi: 10.1017/S0265021507002992. [DOI] [PubMed] [Google Scholar]
- 89.Rowan KM, Welch CA, North E, Harrison DA. Drotrecogin alfa (activated): real-life use and outcomes for the UK. Crit Care. 2008;12(2):R58. doi: 10.1186/cc6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vincent JL, Laterre PF, Decruyenaere J, et al. A registry of patients treated with drotrecogin alfa (activated) in Belgian intensive care units – an observational study. Acta Clin Belg. 2008;63(1):25–30. doi: 10.1179/acb.2008.004. [DOI] [PubMed] [Google Scholar]
- 91.Abraham E, Laterre PF, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353(13):1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- 92.Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S3–S5. doi: 10.1097/01.PCC.0000161289.22464.C3. [DOI] [PubMed] [Google Scholar]
- 93.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369(9564):836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 94.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178(6):618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dhainaut JF, Antonelli M, Wright P, et al. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Med. 2009;35(7):1187–1195. doi: 10.1007/s00134-009-1436-1. [DOI] [PubMed] [Google Scholar]
- 96.European Medicines Agency. Final Scientific Discussion Xigris S13. Apr 21, 2005. [Accessed March 12, 2009]. EMEA/H/C/396/0013. Available from: http://www.emea.europa.eu/humandocs/Humans/EPAR/xigris/XigrisM2.htm.
- 97.Bertolini G, Rossi C, Anghileri A, Livigni S, Addis A, Poole D. Use of Drotrecogin alfa (activated) in Italian intensive care units: the results of a nationwide survey. Intensive Care Med. 2007;33(3):426–434. doi: 10.1007/s00134-007-0554-x. [DOI] [PubMed] [Google Scholar]
- 98.Decruyenaere J, De Backer D, Spapen H, et al. 90-day follow-up of patients treated with Drotrecogin Alfa (activated) for severe sepsis: a Belgian open label study. Acta Clin Belg. 2009;64(1):16–22. doi: 10.1179/acb.2009.005. [DOI] [PubMed] [Google Scholar]
- 99.Kanji S, Perreault MM, Chant C, Williamson D, Burry L. Evaluating the use of Drotrecogin alfa (activated) in adult severe sepsis: a Canadian multicenter observational study. Intensive Care Med. 2007;33(3):517–523. doi: 10.1007/s00134-007-0555-9. [DOI] [PubMed] [Google Scholar]
- 100.Kübler A, Mayzner-Zawadzka E, Durek G, et al. Results of severe sepsis treatment program using recombinant human activated protein C in Poland. Med Sci Monit. 2006;12(3):CR107–CR112. [PubMed] [Google Scholar]
- 101.Spriet I, Meersseman W, Wilmer A, Meyfroidt G, Casteels M, Willems L. Evaluation of drotrecogin alpha use in a Belgian university hospital. Pharm World Sci. 2006;28(5):290–295. doi: 10.1007/s11096-006-9045-3. [DOI] [PubMed] [Google Scholar]
- 102.Tanzi M. Use of Drotrecogin alfa (activate) (Xigris) for the treatment of severe sepsis: Medical use evalaution by Novation, the supply company of VHA and UHC. Irving, TX: Novation; 2004. [Google Scholar]
- 103.Taylor BJ, Lee SJ, Waxman K. Bleeding complications with Drotrecogin alfa activated (Xigris): a retrospective review of 31 operative and 68 non-operative patients with severe sepsis. Am Surg. 2008;74(10):898–901. [PubMed] [Google Scholar]
- 104.Wheeler A, Steingrub J, Schmidt GA, et al. A retrospective observational study of drotrecogin alfa (activated) in adults with severe sepsis: comparison with a controlled clinical trial. Crit Care Med. 2008;36(1):14–23. doi: 10.1097/01.CCM.0000298309.73776.CB. [DOI] [PubMed] [Google Scholar]
- 105.Food and Drug Administration. FDA MedWatch – Xigris Safety Alert. Mar 17, 2005. [Accessed March 15, 2009]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm151239.htm.
- 106.Food and Drug Administration. FDA MedWatch – 2005 Safety Information Alerts. Apr 28, 2005. [Accessed March 15, 2009]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHuman-MedicalProducts/ucm151239.htm.
- 107.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 108.Food and Drug Administration. Early Communication about an Ongoing Safety Review Xigris (Drotrecogin alfa [activated]) Feb 4, 2009. [Accessed March 2009]. Available from: http://www.fda.gov/cder/drug/early_comm/drotrecogin_alfa.html.
- 109.Activated Protein C and Corticosteroids for Human Septic Shock (APROCCHS) [Accessed April 10, 2009]. NCT00625209. Available from: http://clinicaltrials.gov/ct2/show/NCT00625209.
- 110.Efficacy and Safety of Drotrecogin Alfa (Activated) in Adult Patients With Septic Shock. [Accessed April 10, 2009]. NCT00604214. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00604214?term=efficacy+and+safety+of+drotrecogin+alfa+in+severe+sepsis&rank=4.
- 111.Finfer S, Ranieri VM, Thompson BT, et al. Design, conduct, analysis and reporting of a multi-national placebo-controlled trial of activated protein C for persistent septic shock. Intensive Care Med. 2008;34(11):1935–1947. doi: 10.1007/s00134-008-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eichacker PQ, Parent C, Kalil A, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166(9):1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 113.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 114.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]