Abstract
Phosphatidylglycerol (PG) is the major phospholipid of plant chloroplasts. PG from Arabidopsis thaliana has an unusual fatty acyl chain, 3-trans-hexadecenoyl (Δ316:1) in the sn-2 position of the major 18:3/Δ316:1-PG species, as well as in 18:2/Δ316:1-PG and 16:0/Δ316:1-PG. Upon low-energy collisionally activated dissociation (CAD) in a tandem quadrupole or in an ion-trap mass spectrometer, the [M – H]− ions of the PG molecules containing Δ316:1 give product-ion spectra that are readily distinguishable from those arising from PGs without the Δ316:1 species. The Δ316:1-fatty acyl-containing PGs are characterized by MS2 product-ion mass spectra that contain predominant [M – H – 236]− ions arising from loss of the Δ316:1-fatty acyl substituent as a ketene. This is attributable to the fact that the α-hydrogen of the Δ316:1-fatty acid substituent involved in the ketene loss is an allylic hydrogen, which is very labile. This leads to preferential neutral loss of 236 and drastic decline in the neutral loss of 254 (i.e., loss as a fatty acid), the unique features that signify the presence of Δ316:1-fatty acyl containing PGs. The neutral loss scan of 236, thus provides a sensitive tandem quadrupole mass spectrometric means to identify Δ316:1-containing PG species in lipid mixtures. This low-energy tandem mass spectrometric approach also permits the structures of the Arabidopsis PGs that consist of two isomeric structures to be unveiled.
Keywords: Phosphatidylglycerol, plant chloroplast phosphatidylglycerol, 3-trans-hexdecenoic acid, tandem mass spectrometry, electrospray ionization
Introduction
Phosphatidylglycerol (PG) is the major phospholipid of plant chloroplasts and about 85% of PG in Arabidopsis thaliana leaves is localized in the chloroplast. PG is thought to play an important role in the ordered assembly and structural maintenance of the photosynthetic apparatus in thylakoid membranes [1–4]. It is required in photosystem II of photosynthesis [5,6]. The chloroplast membranes of all photosynthetic eukaryotes contain a high proportion of the unusual fatty 3-trans-hexadecenoic acid (Δ316:1 or C16:1(3t)) which is always found esterified to the sn-2 of glycerol backbone of PG [7–10]. The Δ316:1 acid is the product of the activity of a phosphatidylglycerol fatty acid desaturase, FAD4 (EC 1.14.99.*), which acts exclusively on fatty acyl chains in the sn-2 position of PG in plant plastids, probably in the thylakoid membranes [11–14]. Indeed, Δ316:1 is believed to be found only in the photosynthetic membranes of eukaryotes [15].
The biological relevance of 3-trans-hexadecenoic acid in PG has been studied. For examples, loss of Δ316:1 fatty acid in mutants of Chlamydomonas reinharditii resulted in lack of proper assembly of the light-harvesting complexes associated with photosystem II [6,15]. Removal of Δ316:1 fatty acid from PG by phospholipase-A2 treatment of isolated thylakoids was reported to alter the efficiency of light capture and to change the kinetics of fluorescence induction (16). In addition, Chapman et al. reported that triazine-resistant plants have a higher level of PG-containing Δ316:1 fatty acid in membrane fractions enriched in photosystem II [17]. Low temperature treatments also induce an increase in the relative content of both linolenic and 3-trans-hexadecenoic acids in thylakoid membrane PG of squash cotyledons [18].
The 3-trans-hexadecenoic acid is atypical because of the trans configuration, and because of the position of the double bond near the carboxyl (at C3) rather than the methyl end of the fatty acid. The 3-trans-hexadecenoic acid was also found in seaweed [19], in which the structure was established by GC as its fatty acid methyl ester, as well as by GC/MS of the dimethyl-oxazoline derivative [19,20]. Although structural studies on PG using tandem quadrupole mass spectrometry have been previously reported [21,22], plant PG species containing 3-trans-hexadecenoic acid at sn-2 have been identified at the true molecular species level only by a derivatization/HPLC method [23]. Herein, we describe the utilization of tandem quadrupole and multiple-stage quadrupole ion-trap mass spectrometry to discern the fragmentation mechanism underlying the unique pattern of product ion formation by PGs containing Δ316:1. The presence of the Δ316:1-fatty acid at the sn-2 position in PG results in a product-ion spectrum readily distinguishable from that obtained from PG that does not contain 3-trans-hexadecenoic acid.
Materials and Methods
Materials
Leaves of Arabidopsis thaliana, ecotype Columbia, were placed in 1 volume isopropanol with 0.01% butylated hydroxytoluene (as an anti-oxidant) at 75°C (approximately 1 leaf per mL isopropanol). After 15 min in hot isopropanol, 0.5 volumes chloroform and 0.2 volumes water were added. The tubes were agitated for 1 h, followed by removal of the extract. The plants were re-extracted with 1 volume chloroform:methanol (2:1) with 0.01 % butylated hydroxytoluene 5 times with appropriately 30 min shaking each time. Solvent was evaporated from the combined extracts, and the residue was dissolved in chloroform. Activated silicic acid (Unisil, Clarkson Chemical Co., Williamsport, PA) was mixed with chloroform and packed into a column. The leaf extract was applied to the column and eluted in five fractions: Fraction I, chloroform:acetone (1:1, v/v; 5 column volumes); Fraction II, acetone (10 column volumes); Fraction III, chloroform:methanol (19:1, v/v; 10 column volumes); Fraction IV, chloroform:methanol (4:1, v/v; 10 column volumes); Fraction V, chloroform:methanol (1:1, v/v; 20 column volumes) [modified from 24]. Fraction V, which was enriched in PG, phosphatidylcholine and phosphatidylinositol, and also containing phosphatidylethanolmine and phosphatidylserine (results not shown), was used in all experiments. PG standards were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA).
Mass spectrometry
Low-energy CAD tandem mass spectrometry experiments were conducted both on a Finnigan (San Jose, CA) LCQ DECA ion-trap (IT) mass spectrometer (MS) with the Xcalibur operating system and on a Finnigan TSQ-7000 triple-stage quadrupole (TSQ) mass spectrometer with ICIS operating system. Fraction V of the plant lipid extract and methanolic PG standard solution were infused (3 μL/min) to the ESI source, where the skimmer was set at ground potential, the electrospray needle was set at 4.5 kV, and temperature of the heated capillary was 260°C. The automatic gain control of the ion trap was set to 5×107, with a maximum injection time of 400 ms. Helium was used as the buffer and collision gas at a pressure of 0.75 mTorr. The MSn experiments were carried out with a relative collision energy ranging from 30–40% and with an activation q value at 0.25. The activation time was set at 100 ms. Mass spectra were accumulated in the profile mode, typically for 3–5 min for MS2- and MS3-spectra. The mass resolution of the instrument was tuned to 0.6 Da at half peak height.
For product-ion spectra obtained with a TSQ instrument, the precursor ions were selected in the first quadrupole (Q1), collided with Ar (2.3 mTorr) in the rf-only second quadrupole (Q2) and analyzed in the third quadrupole (Q3). The collision energies were set at 32 eV. Both Q1 and Q3 were tuned to unit mass resolution and scanned at a rate of 3 sec/scan. The mass spectra were accumulated in the profile mode, typically for 5–10 min for a tandem mass spectrum.
Results and Discussion
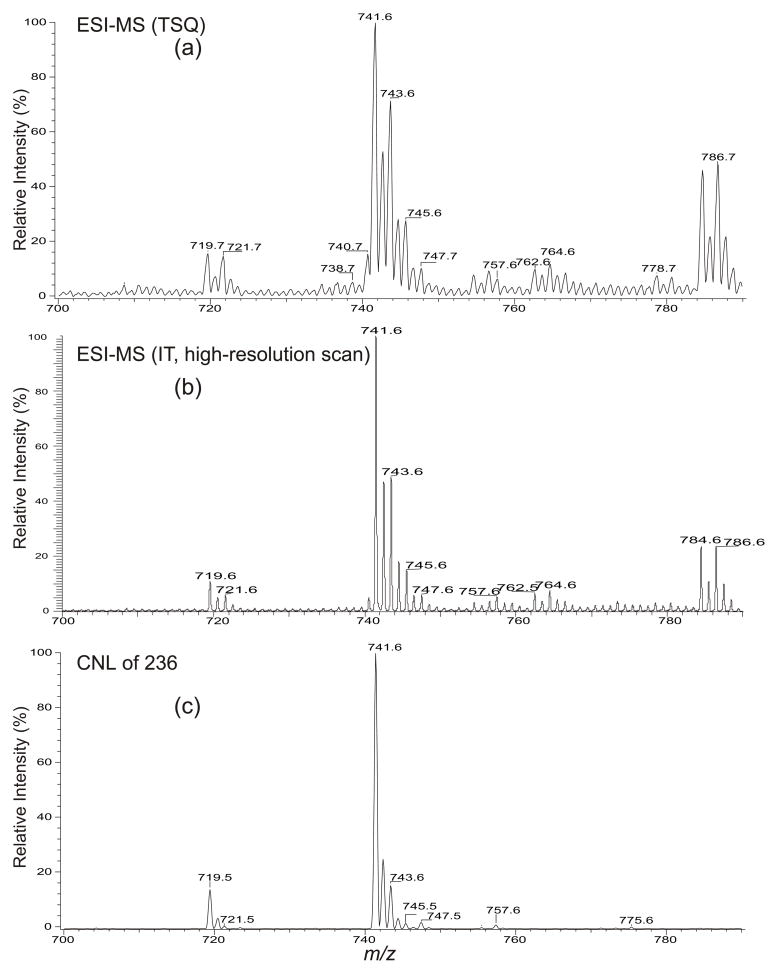
The ESI full scan mass spectrum of the lipid extract obtained with a TSQ instrument in the negative-ion mode contains several deprotonated ([M – H]−) PG species at m/z 719, 721, 741, 743, 745, 747 and 757 (Figure 1a). The spectrum is identical to that obtained with an IT instrument in the enhanced scan mode (zoom scan) (Figure 1b), which shows a low baseline and a well-resolved spectrum. The profile of the tandem mass spectrum obtained by neutral loss scan of 236 (Figure 1c) is also similar but this scan is selective, as will be discussed below. PG species consisting of two isomeric structures were observed (Table 1). The structural characterization and the mechanism(s) underlying the fragmentation processes under low-energy CAD are described below.
Figure 1.
The full scan mass spectra of the lipid extract from plant chloroplasts obtained with (a) a tandem quadrupole, and (b) an IT instrument. Panel (c) shows the tandem mass spectrum of the same mixture acquired by CNL of 236, exhibiting the PG species that contain a 16:1-fatty acid substituent, mainly a Δ316:1-fatty acid substituent at sn-2 in the mixture.
Table 1.
Composition of PG from plant chloroplasts.*
| [M – H]− | Structure | Relative abundance (% of base peak) |
|---|---|---|
| 719.6 | 16:0/Δ316:1-PG | 15 |
| 721.6 | 16:0/16:0-PG | 5 |
| 741.6 | 18:3/Δ316:1)-PG | 100 |
| 743.6 | 18:2/Δ316:1-PG & 18:3/16:0-PG | 50 |
| 745.6 | 18:2/16:0-PG & 18:1/Δ316:1-PG | 10 |
| 747.6 | 18:1/16:0-PG & 18:0/Δ316:1-PG | 8 |
| 757.6 | ** h18:3/Δ316:1-PG | 6 |
Data are derived from product-ion analysis with IT and TSQ mass spectrometers.
h denotes hydroxylated
The fragmentation processes revealed by MSn (n=2,3) tandem mass spectrometry
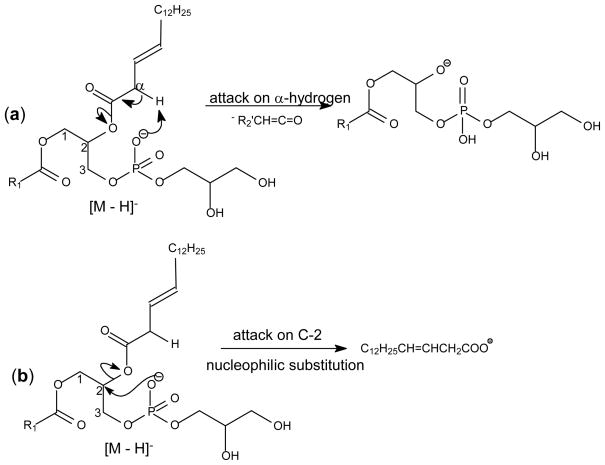
The formation of an ion corresponding to loss of a fatty acid substituent as a ketene (i.e., [M – H – R′xCH=CO]−) from a phospholipid is thought to be a charge-driven fragmentation process (CDF) involving participation of the α-hydrogen of the fatty acid substituent [15]. The anionic charge site at the phosphate abstracts the α-hydrogen of the fatty acid substituent, leading to the ketene loss (Scheme 1a). This fragmentation process probably is sterically more favorable with the fatty acid substituent at the sn-2 than with that at the sn-1 position, resulting in the preferential formation of the ion at m/z 483 ([M – H – R′2CH=CO]−) over the ion at m/z 481 ([M – H – R′1CH=CO]−) in the MS2 product-ion spectrum of the [M – H]− ion of 1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphoglycerol (16:0/Δ916:1-PG) at m/z 719 (Figure 2a). The phosphate charge site also renders nucleophilic attack more favorable on C2 than C1 of the glycerol backbone (Scheme 1b), resulting in the m/z 253 ion (R2CO2−) being more abundant than m/z 255 (R1CO2−). These features in the product-ion spectra are readily applicable for determination of the fatty acid substituent identities and their positions on the glycerol backbone [22,25]. The spectrum also contains ions at m/z 465 and 463, arising from losses of the fatty acid substituents as acids. The former ion is more abundant than the latter, consistent with the notion that the fatty acyl species in the sn-2 position (Δ916:1) is more readily lost.
Scheme 1.
The CDF processes leading to (A) ketene loss, and (B) formation of carboxylate anion. The schemes show the fragmentation processes occurred at sn-2, which are sterically more favorable.
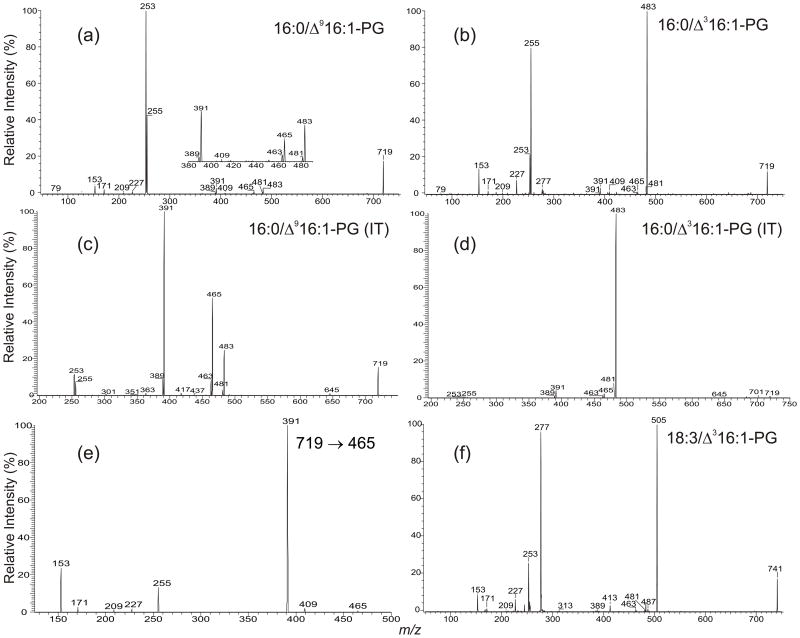
Figure 2.
The TSQ product-ion spectra of (a) 16:0/Δ916:1-PG, (b) 16:0/Δ316:1-PG, and the corresponding IT MS2 spectra of (c) 16:0/Δ916:1-PG, (d) 16:0/Δ316:1-PG. The MS3-spectrum of the ion at m/z 465 (719 → 465) (e) illustrates that the ions at m/z 227, 171 and 153 that are characteristic ions of PG and the ion at m/z 391, which is a precursor ion of the carboxylate anion at m/z 255 arise from sequential fragmentation of m/z 465. Panel (f) is the product-ion spectrum of the ion at m/z 741, which is similar to the spectrum shown in Panel b, suggesting the presence of 18:3/Δ316:1-PG, the major species found in the PG extract.
The product-ion spectrum of the ion at m/z 719 from the lipid extract (Figure 2b) obtained with the same collision energy using a TSQ instrument is readily distinguishable from that arising from the 16:0/Δ916:1-PG standard (Figure 2a), consistent with the fact that the ion represents a 1-palmitoyl-2-(3-trans)-hexadecenoyl-sn-glycero-3-phosphoglycerol (16:0/Δ316:1-PG) [14]. Both the ions at m/z 253 and 255 are less prominent and the ion at m/z 253 is less abundant than the ion at m/z 255, a reversal in acyl anion abundances compared to those observed for 16:0/Δ916:1-PG. The ion at m/z 483 [M – H – R′2CH=CO]−, arising from loss of the Δ316:1-fatty acid as a ketene is the most prominent. The dominance of the ion at m/z 483 is in accord with the decline of the ion at m/z 253, and consistent with the notion that the α-hydrogen of the fatty acid substituent participates in the ketene loss [15]. The α-hydrogen of the Δ316:1-fatty acid substituent at sn-2 is situated between a carbonyl group and a carbon-carbon double bond. Thus, it is an allylic hydrogen and is very labile. Therefore, the fragmentation process leading to loss of the Δ316:1-fatty acyl ketene (Scheme 1a) is the most facile pathway.
The CDF process leading to ketene loss (Scheme 1a) competes with the CDF process that leads to acyl anions by nucleophilic substitution (Scheme 1b). Because loss of the Δ316:1-fatty acid at sn-2 as a ketene is the most facile pathway, the process leading to formation of R2CO2− at m/z 253 becomes less favorable. However, the process leading to m/z 481 due to loss of the 16:0-fatty acid substituent at sn-1 as a ketene (Scheme 1a) is less facile than that leading to the R1CO2− anion at m/z 255 (Scheme 1b), attributable to the fact that the α-hydrogen of the 16:0-fatty acid is less labile. The very facile ketene loss of the sn-2 substituent in the PG containing sn-2 Δ316:1 leads to a higher ratio of the R1CO2− ion to the R2CO2− ion than observed for phospholipids not containing an allylic α-hydrogen in the sn-2 fatty acid (Figure 2b vs. 2a).
The MS2 product-ion spectra of 16:0/Δ916:1-PG (Figure 2c) and 16:0/Δ316:1-PG (Figure 2d) obtained with an IT instrument are similar to those obtained with a TSQ. However, the carboxylate anions at m/z 253 and 255 are of low abundance, and the ions at m/z 463 and 465 arising from losses of the fatty acid substituents are more abundant, consistent with the notion that the fragmentation processes in an ion-trap are initiated by resonance excitation so that consecutive fragmentations induced by multiple collisions, as seen in an TSQ instrument, are minimal.
The ions at m/z 465 (719 - R2CO2H) and 463 (719 – R1CO2H) gives rise to ions at 391 and 389, respectively, by loss of a C3H6O2 residue. The fragmentation processes are supported by the IT MS3-spectra of the m/z 465 (719 → 465) (Figure 2e) and 463 (719 → 463) ions (not shown), which are dominated by the ions at m/z 391 (Figure 2e) and 389, respectively. The ions at m/z 391 (483 – 92) and 389 (481 – 92) can also arise from m/z 483 and 481, respectively, by elimination of a glycerol residue. However, these fragmentation pathways are less favorable, as revealed by the IT MS3-spectra of m/z 483 (719 → 483) (not shown). The ions at m/z 391 and 389 also give rise to the ions at m/z 255 and 253, respectively, by neutral loss of 136 [22,25,26]. These data suggest that, in the TSQ product-ion spectra, the carboxylate anions at m/z 255 and 253 of 16:0/Δ916:1-PG (Figure 2a) and 16:0/Δ316:1-PG (Figure 2b) can arise from consecutive fragmentations in addition to the nucleophilic substitution pathway shown in Scheme 1b. This is consistent with the low abundance of the carboxylate anions and the higher abundance of the ions at m/z 465/463 and 391/389 in the IT MS2 product-ion spectra (Figure 2c and 2d).
The ion at m/z 741 (Figure 1) undergoes fragmentation processes similar to 16:0/Δ316:1-PG and gives rise to a product-ion spectrum similar to that shown in Figure 2b. The spectrum (Figure 2f) is dominated by the ion at m/z 505 (741 – 236) corresponding to loss of a 16:1-fatty acid substituent as a ketene and analogous to the m/z 483 ion in Figure 2b. The profile of the MS2-spectrum obtained with an IT instrument (data not shown) is similar to that shown in Figure 2d. The results indicate that the compound consists of a Δ316:1-fatty acid substituent, most likely at sn-2. The ions at m/z 481, arising from loss of 18:3-fatty acid as a ketene and at m/z 277, corresponding to an 18:3-fatty acid anion are also present in the spectrum, suggesting that an 18:3 fatty acid substituent resides at sn-1. The spectrum also contains ions at m/z 227, 209, 171 and 153, ions commonly observed for PG [15], confirming that the ion represents 18:3/Δ316:1-PG.
Characterization of PG consisting of isomeric structures
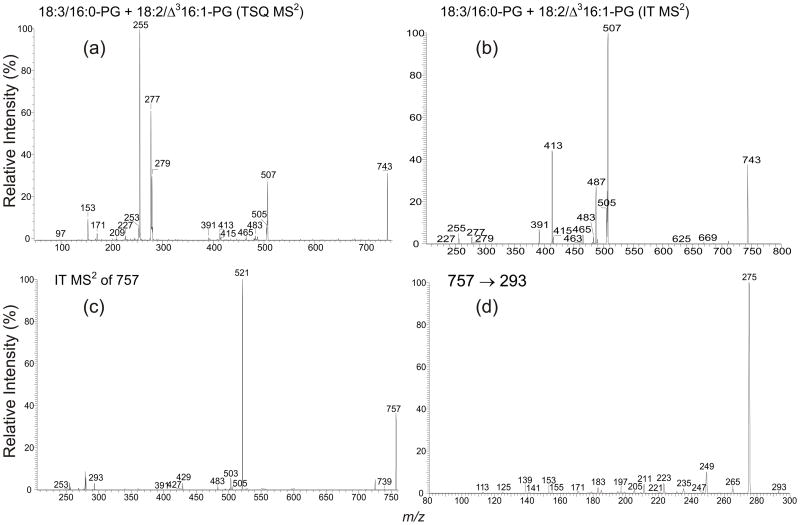
Product-ion analysis revealed two isomeric structures for the ions at m/z 743, 745 and 747 (Table 1). Determination of the structures is exemplified by the ion at m/z 743. The MS2 product-ion spectrum obtained with a TSQ instrument (Figure 3a) contains ions at m/z 227, 209, 171 and 153 that are diagnostic ions for PG, along with two pairs of the carboxylate anions at m/z 255/277 and 279/253, indicating that the ion may consist of 18:3/16:0-PG and 18:2/16:1-PG isomers. The 18:3/16:0-PG structure is recognized by the observation of the ions at m/z 505 and 487, arising from loss of a 16:0-fatty acid substituent as a ketene and as an acid respectively, and the ions at m/z 483 and 465, arising from the analogous losses of an 18:3-fatty acid substituent. The ion at m/z 505 is more abundant than m/z 483, and the ion at m/z 487 is also more abundant than the ion at m/z 465, indicating that the 16:0- and 18:3-fatty acid substituents are located at sn-2 and sn-1, respectively, consistent with the observation of a greater abundance of the ion at m/z 255 than the ion at m/z 277. The structural assignment is further supported by the MS2 product-ion spectrum obtained with an IT MS (Figure 3b), in which the differential formation of the above fragment ions also can be seen. The spectrum also features the greater prominence of the ion at m/z 413 (487 – 74), arising from loss of C3H6O2 from m/z 487, as compared to the ion at m/z 391 (465 – 74), arising from further loss of C3H6O2 from m/z 465.
Figure 3.
The product-ion spectrum of the ion at m/z 743 obtained with (a) a TSQ, and (b) an IT instrument. Panel (c) is the IT MS2-spectrum of the ion at m/z 757, which may consist of a h18:3/Δ316:1-PG structure. The presence of the hydroxylated 18:3-fatty acid substituent is consistent with the presence of a prominent ion at m/z 275, corresponding to loss of H2O from m/z 293, as shown in the MS3-spectrum of m/z 293 (757 → 293) (d).
The assignment of the 18:2/Δ316:1-PG structure is supported by the presence of the ion at m/z 507 (Figures 3a and 3b), corresponding to loss of the 16:1-fatty acid substituent as a ketene. This ion is most prominent in the product-ion spectrum obtained with an ion-trap (Figure 3b), but the ion at m/z 489, arising from loss of the 16:1-fatty acid substituent as an acid, is of low abundance, suggesting that the 16:1-fatty acid substituent is 3-trans-hexadecenoic acid, the unusual fatty acid attached to the sn-2 position of PG found in plant chloroplasts. The assignment of the 18:2/Δ316:1-PG structure is also consistent with the observation of a higher abundance of the ion at m/z 279 than the ion at m/z 253, similar to the profile seen for the 16:0/Δ316:1-PG (Figure 2b) and 18:3/Δ316:1-PG (Figure 2f), which contain the Δ316:1-fatty acid substituent at sn-2.
Identification of PG species containing Δ316:1-fatty acid substituent via constant neutral loss (CNL) scanning
As seen earlier, the product-ion spectra of PG containing the Δ316:1-fatty acid substituent at sn-2 are dominated by the [M – H – 236]− ion, arising from neutral loss of the Δ316:1-fatty acyl substituent as a ketene, and the [M – H – 254]− ion arising from neutral loss of the Δ316:1-fatty acyl substituent as an acid is of low abundance. As shown in Figure 1c, the spectrum acquired from CNL scanning of 236 of the lipid extract is similar to the full scan (Figures 1a and 1b), but only peaks from PGs that consist of Δ316:1-fatty acid are observed. The spectrum clearly demonstrates that the PG species from plant chloroplasts mainly contain a Δ316:1-fatty acid substituent, consistent with the results from the structural analysis of the individual ions from product-ion spectra (Table 1). In contrast, the signals of the similar ions in the spectrum acquired from CNL scanning of 254 (data not shown) are weak and the intensity of the ion at m/z 741, for example, is <2% than that seen in Figure 1c. This drastic change in the relative sensitivity between the spectra obtained from CNL scannings of 236 and of 254 provides distinction of the Δ316:1-fatty acid bearing PG from, for example, Δ916:1-bearing PGs, which also undergo similar neutral losses (i.e, CNL of 254 and 236), but in a different manner. Therefore, tandem quadrupole mass spectrometry with CNL scanning of 236, in combination with CNL scanning of 254 that confirms that the species indeed contains Δ316:1-fatty acid, provides a useful means for sensitive detection of PG species containing a Δ316:1-fatty acid substituent in mixtures.
In Figure 1c, an ion at m/z 757 is also present, suggesting that the ion may arise from a PG containing a Δ316:1-fatty acid substituent, probably a hydroxylated 18:3/Δ316:1-PG. This structural assignment is based on the finding that the profile of the IT MS2-spectrum of the ion at m/z 757 (Figure 3c), is similar to that shown in Figure 2d, supporting the presence of a Δ316:1-fatty acid substituent. The ion at m/z 293 may represent a hydroxylated 18:3-fatty acid (h18:3) substituent. This speculation is based on the notion that the ion at m/z 275 (293 – H2O), arising from loss of H2O is thepredominant ion in the IT MS3-spectrum of 293 (757 → 293) (Figure 3d) [27]. The presence of the ion at m/z 429 (503 – 74), arising from m/z 503 by loss C3H6O2 and the presence of the ion at m/z 739, arising from a water loss from 757 (Figure 3c) are also consistent with the presence of a hydroxylated 18:3-fatty acid residue in the molecule. However, further study is necessary to support the assigned structure and to confirm that the compound is indeed in the PG mixture.
Conclusions
The fragmentation processes observed for the PG species bearing a Δ316:1-fatty acid at sn-2 are consistent with the mechanisms previously proposed by us [22,25,26]. These PG species are readily distinguishable from other species in this phospholipid class by their unique low-energy tandem mass spectra from the [M – H]− ion, in which the fragment ion reflecting loss of the Δ316:1-fatty acid as a ketene (i.e., the [M – H - 236]−) is prominent due to the labile nature of the α-hydrogen of Δ316:1-fatty acid substituent. Recently, Moe et al. [28] and Thomas et al. [29] described ESI tandem mass spectrometric methods for location of the olefinic sites of phospholipids, following conversion to their 1,2-di-hydroxy derivatives by OsO4 [28] or on-line ozonolysis of double bonds to yield both a terminal oxoalkanoyl and a terminal C-hydroperoxy, C-methoxy alkanoyl radyl containing phospholipid anions [29]. These approaches are straightforward and should be useful, in particular, for locating the double bond(s) of the fatty acid substituents from various isomers.
Supplementary Material
Acknowledgments
The authors would like to thank Christen Buseman for preparation of the Arabidopsis lipid extract. Research at the Mass Spectrometry Resource of Washington University was supported by US Public Health Service grants P41-RR-00954, R37-DK-34388, P60-DK-20579, P01-HL-57278 and P30-DK56341. The Kansas Lipidomics Research Center’s research was supported by grants from NSF (MCB 0455318, DBI 0521587, and Kansas EPSCoR’s award, EPS-0236913), with support from the State of Kansas through Kansas Technology Enterprise Corporation and Kansas State University, as well as from US Public Health Service grant P20 RR016475 from the INBRE program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fong-Fu Hsu, Mass Spectrometry Resource, Division of Endocrinology, Diabetes, Metabolism, and Lipid research, Department of Internal Medicine Box 8127, Washington University, School of Medicine, St. Louis, MO 63110.
John Turk, Mass Spectrometry Resource, Division of Endocrinology, Diabetes, Metabolism, and Lipid research, Department of Internal Medicine Box 8127, Washington University, School of Medicine, St. Louis, MO 63110.
Todd D. Williams, University of Kansas Mass Spectrometry Laboratory, Malott Hall, University of Kansas, Lawrence, KS 66045
Ruth Welti, Kansas Lipidomics Research Center, Division of Biology, Kansas State University, Ackert Hall, Manhattan, KS 66506.
References
- 1.Sakurai I, Hagio M, Gombos Z, Tyystjarvi T, Paakkarinen V, Aro EM, Wada H. Requirement of Phosphatidylglycerol for Maintenance of Photosynthetic Machinery. Plant Physiol. 2003;133:1376–1384. doi: 10.1104/pp.103.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato N. Roles of the Acidic Lipids Sulfoquinovosyl Diacylglycerol and Phosphatidylglycerol in Photosynthesis: Their Specificity and Evolution. J Plant Res. 2004;117:495–505. doi: 10.1007/s10265-004-0183-1. [DOI] [PubMed] [Google Scholar]
- 3.Frentzen M. Phosphatidylglycerol and Sulfoquinovosyldiacylglycerol: Anionic Membrane Lipids and Phosphate Regulation. Curr Opin Plant Biol. 2004;7:270–276. doi: 10.1016/j.pbi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Joyard J, Maréchal E, Miège C, Block MA, Dorne A, Douce R. Structure, distribution and biosynthesis of glycerolipids from higher plant chloroplasts. In: Siegenthaler PA, Murata N, editors. Lipids in Photosynthesis: Structure, Function and Genetics. Kluwer Academic Publishers; Dordrecht, the Netherlands: 1998. pp. 21–52. [Google Scholar]
- 5.Hagio M, Gombos Z, Várkonyi Z, Masamoto K, Sato N, Tsuzuki M, Wada H. Direct Evidence for Requirement of Phosphatidylglycerol in Photosystem II of Photosynthesis. Plant Physiol. 2000;124:795–804. doi: 10.1104/pp.124.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineau B, Girard-Bascou J, Eberhard S, Choquet Y, Tremolieres A, Gerard-Hirne C, Bennardo-Connan A, Decottignies P, Gillet S, Wollman FA. A Single Mutation that Causes Phosphatidylglycerol Deficiency Impairs Synthesis of Photosystem II Cores in Chlamydomonas reinhardtii. Eur J Biochem. 2004;271:329–338. doi: 10.1046/j.1432-1033.2003.03931.x. [DOI] [PubMed] [Google Scholar]
- 7.Haverkate F, de Gier J van Deenen LL. The occurrence of delta 3-trans-hexadecenoic acid in phosphatidyl glycerol from spinach leaves. Experientia. 1964;20:511–512. doi: 10.1007/BF02154082. [DOI] [PubMed] [Google Scholar]
- 8.Dubacq JP, Trémoli’res A. Occurence and function of phosphatidylglycerol containing Δ3-trans-hexadecenoic acid in photosynthetic lamellae. Physiol Vég. 1983;21:293–312. [Google Scholar]
- 9.Selstam E. Development of thylakoid membranes with respect to lipids. In: Siegenthaler P-A, Murata N, editors. Advances in Photosynthesis, Vol 6, Lipids in Photosynthesis: Structure, Function and Genetics. Kluwer Academic Publishers; Dordrecht: 1998. pp. 209–224. [Google Scholar]
- 10.Browse J, Warwick N, Somerville CR, Slack CR. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ’16:3’ plant Arabidopsis thaliana. Biochem J. 1986;235:25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roughan PG. Phosphatidyl choline: Donor of 18-carbon Unsaturated Fatty Acids for Glycerolipid Biosynthesis. Lipids. 1975;10:609–614. doi: 10.1007/BF02532725. [DOI] [PubMed] [Google Scholar]
- 12.Mackender RO, Leech RM. The Galactolipid, Phospholipid, and Fatty Acid Composition of the Chloroplast Envelope Membranes of Vicia faba. L. Plant Physiol. 1974;53:496–502. doi: 10.1104/pp.53.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnishi M, Thompson GA., Jr Biosynthesis of the Unique trans-Delta 3-Hexadecenoic Acid Component of Chloroplast Phosphatidylglycerol: Evidence Concerning Its Site and Mechanism of Formation. Arch Biochem Biophys. 1991;288:591–599. doi: 10.1016/0003-9861(91)90241-a. [DOI] [PubMed] [Google Scholar]
- 14.Beisson F, Koo AJK, Ruuska S, Schwender J, Pollard M, Thelen J, Paddock T, Salas J, Savage L, Milcamps A, Mhaske VB, Cho Y, Ohlrogge JB. Arabidopsis thaliana Genes Involved in Acyl Lipid Metabolism. A 2003 Census of the Candidates, a Study of the Distribution of Expressed Sequence Tags in Organs, and a Web-Based Database. Plant Physiol. 2003;132:681–697. doi: 10.1104/pp.103.022988. URL: http://www.plantbiology.msu.edu/lipids/genesurvey/index.htm. [DOI] [PMC free article] [PubMed]
- 15.Dubertret G, Mirshahi A, Mirshahi M, Gerard-Hirne C, Tremolieres A. Evidence from in Vivo Manipulations of Lipid Composition in Mutants that the Delta 3-trans-Hexadecenoic Acid-Containing Phosphatidylglycerol Is Involved in the Biogenesis of the Light-Harvesting Chlorophyll A/B-Protein Complex of Chlamydomonas reinhardtii. Eur J Biochem. 1994;226:473–482. doi: 10.1111/j.1432-1033.1994.tb20072.x. [DOI] [PubMed] [Google Scholar]
- 16.Krupa Z. The action of lipases on chloroplast membranes. III. The effect of lipid hydrolysis on chlorophyll-protein complexes in thylakoid membranes. Photosynthesis Res. 1984;5:177–184. doi: 10.1007/BF00028530. [DOI] [PubMed] [Google Scholar]
- 17.Chapman DJ, De-Felice J, Barber J. Characteristics of Chloroplast Thylakoid Lipid Composition Associated with Resistance to Triazine Herbicides. Planta. 1985;166:280–285. doi: 10.1007/BF00397361. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Siegenthaler PA. Low Temperature Treatments Induce an Increase in the Relative Content of Both Linolenic and Δ3-trans-Hexadecenoic Acids in Thylakoid Membrane Phosphatidylglycerol of Squash Cotyledons. Plant Cell Physiol. 1997;38:611–618. [Google Scholar]
- 19.Lamberto M, Ackman RG. Confirmation by gas chromatography/mass spectrometry of two unusual trans-3-monoethylenic fatty acids from the Nova Scotian seaweeds Palmaria palmata and Chondrus crispus. Lipids. 1994;29:441–444. doi: 10.1007/BF02537315. [DOI] [PubMed] [Google Scholar]
- 20.Lamberto M, Ackman RG. Positional isomerization of trans-3-hexadecenoic acid employing 2-amino-2-methyl-propanol as a derivatizing agent for ethylenic bond location by gas chromatography/mass spectrometry. Anal Biochem. 1995;230:224–228. doi: 10.1006/abio.1995.1467. [DOI] [PubMed] [Google Scholar]
- 21.Welti R, Wang X, Williams TD. Electrospray Ionization Tandem Mass Spectrometry Scan Modes for Plant Chloroplast Lipids. Anal Biochem. 2003;314:149–152. doi: 10.1016/s0003-2697(02)00623-1. [DOI] [PubMed] [Google Scholar]
- 22.Hsu FF, Turk J. Studies on Phosphatidylglycerol with Triple Quadrupole Tandem Mass Spectrometry with Electrospray Ionization: Fragmentation Processes and Structural Characterization. J Am Soc Mass Spectrom. 2001;12:1036–1043. [Google Scholar]
- 23.Xu Y, Siegenthaler PA. Phosphatidylglycerol Molecular Species of Photosynthetic Membranes Analyzed by High-Performance Liquid Chromatography: Theoretical Considerations. Lipids. 1996;31:223–229. doi: 10.1007/BF02522624. [DOI] [PubMed] [Google Scholar]
- 24.Christie WW. Lipid Analysis. 2. Pergamon Press; Oxford, England: 1982. pp. 109–110. [Google Scholar]
- 25.Hsu FF, Turk J. Electrospray Ionization with Low-Energy Collisionally Activated Dissociation Tandem Mass Spectrometry of Complex Lipids: Structural Characterization and Mechanisms of Fragmentation. In: Byrdwell WC, editor. Modern Methods for Lipid Analysis by Liquid Chromatography/Mass Spectrometry. AOCS Press; Champaign, IL: 2005. pp. 61–178. [Google Scholar]
- 26.Hsu FF, Turk J. Charge-driven Fragmentation Processes in Diacyl Glycerophosphatidic Acids upon Low-Energy Collisional Activation. A Mechanistic Proposal. J Am Soc Mass Spectrom. 2000;11:797–803. doi: 10.1016/S1044-0305(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 27.Hong S, Lu Y, Yanag R, Gotlinger KH, Petasis NP, Serhan CN. Resolvin D1, Protectin D1, and Related Docosahexaenoic Acid-Derived Products: Analysis via Electrospray/Low Energy Tandem Mass Spectrometry Based on Spectra and Fragmentation Mechanisms. J Am Soc Mass Spectrom. 2006 doi: 10.1016/j.jasms.2006.09.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moe MK, Anderssen T, Strøm MB, Jensen E. Total structure characterization of unsaturated acidic phospholipids provided by vicinal di-hydroxylation of fatty acid double bonds and negative electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2005;16:46–59. doi: 10.1016/j.jasms.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Thomas MC, Mitchell TW, Blanksby SJ. Ozonolysis of Phospholipid Double Bonds during Electrospray Ionization: A New Tool for Structure Determination. J Am Chem Soc. 2006;128:58–59. doi: 10.1021/ja056797h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.