Abstract
Objective
Our earlier studies on Ugandan children surviving cerebral malaria showed cognitive deficits mainly in attention and memory. We now present the first study in sub-Saharan Africa to investigate the feasibility and potential benefits of computerized cognitive rehabilitation training on neuropsychological and behavioural functioning of children surviving cerebral malaria.
Methods
A randomized trial in which 65 children admitted 45 months earlier with cerebral malaria were recruited at Mulago Hospital, Kampala, Uganda. For eight weeks, 32 of the children received weekly training sessions using Captain’s Log cognitive training software and the other 33 were assigned to a non treatment condition. Pre- and post-intervention assessments were completed using CogState, a computerized neuropsychological battery, measuring Visuomotor Processing Speed, Working Memory, Learning, Attention and Psychomotor Speed and the Child Behavior Checklist measuring Internalising Problems, Externalising Problems and Total Problems.
Results
Pre-intervention scores were similar between both groups. Treatment effects were observed on Visual Spatial Processing Speed (group effect (standard error) 0.14 (0.03); p< 0.001); on a Working Memory and Learning task (0.08 (0.02); p< 0.001), Psychomotor Speed (0.14 (0.07); p= 0.04) and on Internalising Problems (−3.80 (1.56); p= 0.02) after controlling for age, sex, school grade, quality of the home environment and weight for age z scores. Similar treatment effects were observed when no adjustments for the above covariates were made.
Conclusions
Computerized cognitive training long after the cerebral malaria episode has immediate benefit on some neuropsychological and behavioral functions in African children. The long-term benefit of this intervention needs to be investigated.
Keywords: cognitive rehabilitation, Neuropsychology, behaviour, cerebral malaria, African children
INTRODUCTION
Cerebral malaria is the most severe form of malaria affecting 575,000 African children below five years of age of which 110,000 die [1]. Neuro-cognitive morbidity also presents a significant problem with more than 200,000 child survivors of cerebral malaria estimated to have long term cognitive impairment [2–4]. Retrospective studies show that cerebral malaria child survivors have cognitive deficits in several areas including language, memory, attention, visual spatial skills and somatosensory discrimination with some lasting up to nine years post illness [5–8].
We recently carried out the first two prospective studies to document the cognitive deficits after cerebral malaria in children [4,9]. Children surviving cerebral malaria were compared to those with uncomplicated malaria and healthy community controls on tests measuring memory, attention and learning. At six months, the cerebral group had 21.4% impaired in at least one of the three areas tested compared to 13.5% with uncomplicated malaria and 5.7% of the community controls [4]. At 24 months, the prevalence of impairment in the cerebral malaria group had increased to 26.3% compared to 7.6% of the community controls [9]. In addition to cognition, behavioral problems after malaria have also been documented in children and adults [10,11]. Cerebral malaria clearly has long term effects on a sizeable proportion of surviving children’s cognition and behaviour which may detrimentally affect achievement of their full potential if no interventions are carried out.
Interventions that have been proposed to deal with the impact of cerebral malaria include medication regimens, cognitive rehabilitation training, environmental enrichment, physical therapy, nutritional enrichment and early childhood education [3,12,13]. Bangirana and colleagues [12] have proposed that computerized cognitive rehabilitation training represents a suitable choice for cerebral malaria based on studies carried out in the Western world showing its effectiveness in pediatric traumatic brain injury and ADHD [14–17].
In their study on ADHD, Shalev and colleagues assigned thirty-six school-age Israeli children with ADHD to eight weeks of computerized attention training or to a control group [14]. Children in the control group played computer games - rather than receiving attention training - for the same amount of time. Parents of children in the attention training group reported a significant decline in their child’s inattentive symptoms compared to parents of children in the control group. After controlling for academic performance before training, children who received attention training did significantly better than controls in reading comprehension and in their speed of copying passages. Math performance did not significantly improve.
Klingberg and colleagues had previously concluded that visual-spatial working memory is a very sensitive measure of cognitive deficits in children with ADHD [15]. In subsequent work, they evaluated the cognitive benefits of 25 daily 30 minute sessions of computerized working memory training with children having ADHD [16]. They observed significant improvements on visual-spatial working memory and response inhibition. There were also improvements on parental ratings of inattention, hyperactivity, and impulsivity; though not in tests of distractibility or reasoning.
In a case study of three children with acquired brain injuries, computerised training of memory and attention was done daily for 20 weeks with pre- and post neuropsychological and behavioural assessments [17]. Improvements were seen in measures of attention, memory and behaviour.
These studies above show that the benefits from computerized cognitive rehabilitation training are not limited to trained tasks but also improve non trained tasks like behaviour and academic skills. Recent systematic reviews on the effectiveness of computerized cognitive rehabilitation training in brain injury all show its benefits in improving cognition and other functional abilities [18–20]. Though no such studies have been done in African children, the above studies are evidence enough that children who have survived cerebral malaria and other brain injuries may benefit from computerized cognitive rehabilitation training.
This study presents a randomized trial examining the neuropsychological and behavioural benefits of computerized cognitive rehabilitation training in a cohort of children who had displayed cognitive deficits after cerebral malaria.
METHOD
Study population and recruitment
The present study was conducted at Mulago Hospital, Kampala, Uganda from November 2007 to April 2008. Study children had participated in an earlier study examining the cognitive and neurological outcomes of cerebral malaria with testing at 0, 3, 6, and 24 months [4,9]. Children were initially recruited into this earlier study if they were admitted to Mulago Hospital and met the World Health Organization criteria for cerebral malaria, namely, coma (Blantyre Coma Scale score of ≤2 or Glasgow Coma Scale score of ≤8), Plasmodium falciparum on blood smears, and no other cause of coma. Lumbar punctures were performed to rule out meningitis and encephalitis. Ugandan Ministry of Health national guidelines for drug treatment of cerebral malaria (quinine for 7 days, with a loading dose of 20 mg/kg administered intravenously and then 10 mg/kg every 8 hours) were followed. Additional treatment guidelines included an infusion of 25% dextrose (2 mL/kg) for children with blood glucose levels of <2.2 mmol/L (<40 mg/dL), treatment of acute seizures with intravenously or rectally administered diazepam (with the addition of phenobarbital for refractory seizures), and blood transfusions for children with hemoglobin levels of <5 g/dL.
Ninety two children were recruited; six were dropped because they did not meet the inclusion criteria. Of the 86 remaining children, 4 died during admission and 38 were below five years old leaving 44 to under go cognitive testing at baseline (0 months). At three and six months follow ups, 42 were traced for testing [4]. At the 24 months follow up, 38 of the 42 were traced and tested [9]. Of the 38 children who were below five years at baseline, 30 were traced at 24 months after they reached the age of five giving a total of 68.
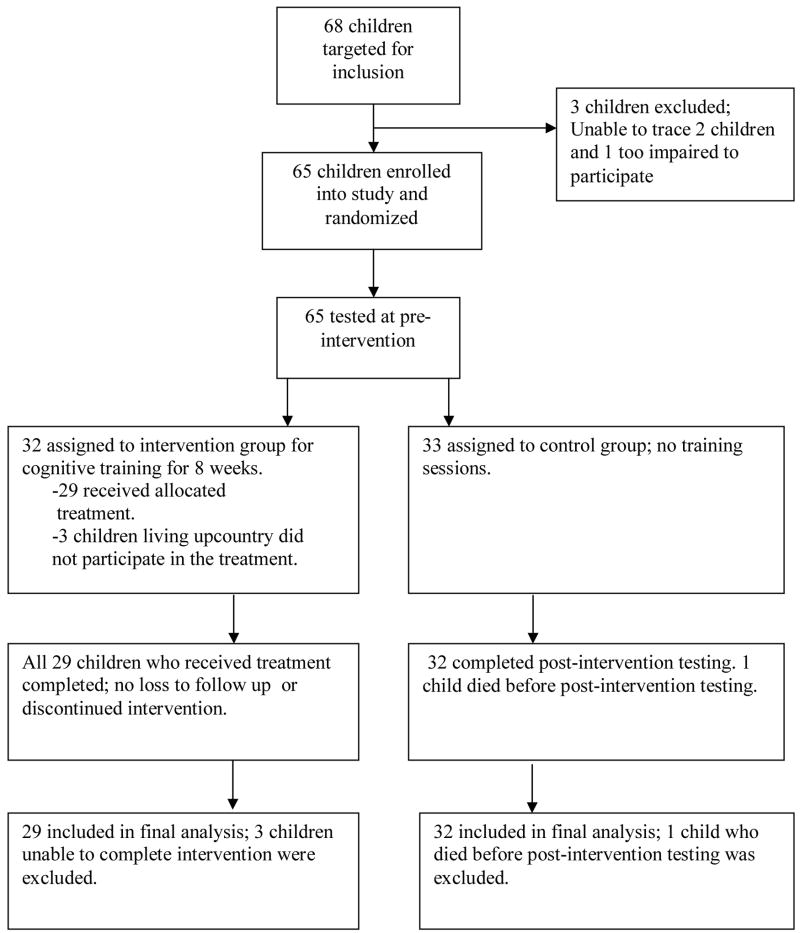
Approximately 18 months after the 24 months testing, children were asked to return for the intervention study. Of the 68 children seen at the 24 months assessment, 65 were traced and included in this study. Two children could not be traced while another had severe neurological complications resulting from cerebral malaria and could not participate in the study. Thirty seven of the 38 children tested at 24 months [9] were included in this study. The additional 28 were part of the 30 assessed at 24 months but were not included in the published studies [4,9] as they were below 5 years at baseline. Thirty two of the 65 children were assigned to the intervention and the other 33 to the control arm. One child in the control arm died before completing post-intervention assessment. Three children in the intervention living up country were unable to travel to and fro for the cognitive training and only did the pre- and post-intervention assessments. All 65 children were included in the pre-intervention analyses while 29/32 of the intervention group and 32/33 of the control group were included in the post-intervention analyses. See Figure 1.
Figure 1. Flow of participants in the study.
Sixty five of the 68 children targeted for inclusion were enrolled into the study, 32 were assigned to intervention group and 33 to the control group.
The control group had a lower proportion of males, but not statistically different (51.5% vs 71.9%; p=0.09) while age, school grade and anthropometric results were similar across the groups. For both groups, there was approximately 3.7 years between the acute cerebral malaria illness and commencement of this study. There was an average of 61.6 days between the pre- and post-intervention assessments for the control group compared to 80.9 days for the intervention (p=0.0001). Table 1 shows the demographic characteristics of the children.
Table 1.
Participants’ Demographic Characteristics
| Domain | Control N=33 | CCRT N=32 | P1 |
|---|---|---|---|
| Gender, male N (%) | 17 (51.5) | 23 (71.9) | 0.09 |
| Age (years) | 9.71 (2.28) | 10.09 (2.64) | 0.54 |
| School grade | 3.67 (2.30) | 4.09 (2.32) | 0.46 |
| Weight (kgs) | 28.55 (8.76) | 28.91 (9.14) | 0.87 |
| Height (cm) | 131.06 (14.35) | 131.68 (16.99) | 0.88 |
| Weight for age z score | −0.78 (0.85) | −1.01 (0.84) | 0.28 |
| Home environment score | 28.69 (6.95) | 27.72 (5.93) | 0.55 |
| Days between pre- and post-intervention testing | 61.64 (6.39) | 80.91 (17.21) | 0.0001 |
| Years between CM illness and CCRT | 3.71 (0.23) | 3.72 (0.25) | 0.90 |
| Missed CCRT sessions | - | 2.14 (2.89) | - |
CM=Cerebral Malaria, CCRT=Computerised Cognitive Rehabilitation Training, cm=centimetres, kgs=kilograms, N=Number.
All values are Mean (SD) unless otherwise stated.
Frequencies and means compared using Chi square and t tests respectively.
Written informed consent and assent was obtained from the parents/guardians and the children. Ethical approval for this study was granted by the Institutional Review Boards for Human Studies at Makerere University Faculty of Medicine, Michigan State University, University of Michigan and the Uganda National Council for Science and Technology.
Assessments
Quality of the home environment scores assessed in the earlier studies by a version of the Middle Childhood Home Observation for the Measurement of the Environment inventory [21] adapted for Uganda [4] were extracted from the database. The child’s weight and age were got prior to pre-intervention testing and nutrition was assessed by comparing weight for age to published norms [22] and obtaining a standardized z-score using Epi Info 3.3.2 (Centers for Disease Control and Prevention, Atlanta, GA). Level of education of the child was scored as: None = 0, Nursery = 1, Primary school grades 1–7 = 2–8, Secondary education = 9.
Cognitive Testing
Children were assessed using a computerized neuropsychological battery called CogState [23]. CogState consists of computerized cognitive tasks adapted from standard neuropsychological measures assessing a range of abilities, including learning, psychomotor speed, working memory and attention [24]. It is self-administered, automatically scored and requires about 15–20 minutes to complete. [23]. The use of playing cards (except for the maze tasks, see below) for stimuli and a game-like atmosphere improve attention and increase motivation in children completing the tasks and reduces any potential language demands. The CogState battery has proven its validity and reliability in assessing the cognitive function in ADHD, fetal alcohol syndrome, mild cognitive impairment, concussion, coronary artery bypass grafting and the effects of various medications [24]. Unpublished data on Ugandan children shows CogState to be sensitive in detecting mild cognitive impairment when compared to the Kaufmann Assessment Battery for Children Second Edition [25] and has moderate correlations with it.
The CogState maze tasks are the Groton Maze Chasing Task measuring visuomotor processing speed and the Groton Maze Learning Task measuring spatial working memory and learning which are presented on a 10 × 10 computer grid of grey tiles. In the maze chasing task the subject is required to follow a target moving along the grid by clicking the tiles it has passed through making sure not to skip tiles or move diagonally. In the maze learning task the subject is required to find a hidden pathway through the grid from the top left tile to the bottom right one by guessing his/her way through the grid while receiving feedback on each individual move.
The card tasks include Detection measuring psychomotor speed where the subject is required to press the YES button on the mouse as soon as a card turns face-up; Identification measuring visual attention where the subject is required to decide as quickly as possible whether the card that has turned face up is red (YES button) or not (NO button); One Card Learning measuring visual learning and memory where the subject decides whether the card has appeared before in the task by pressing the YES or NO buttons, and One Back Working Memory measuring working memory where the subject is required to immediately decide if the card is the same (YES button) as the previous one or not (NO button).
The primary outcome measures were the efficiency of performance (the number of correct moves divided by the time to complete the maze) for the maze tasks and the accuracy of performance (arcsine transformation of the proportion of correct responses) for the card tasks.
Child Behaviour Checklist
Behaviour was assessed by the Child Behaviour Checklist [26,27], one of the most widely used instruments in child and adolescent psychiatry and paediatrics. It has been used in many African populations where it has shown good reliability and validity [28–30]. This checklist is a paper-pencil child behavioural assessment consisting of 113 items to which the parent/guardian responds. The secondary outcome measures chosen for this study were Internalising Behaviour, Externalising Behaviour and Total Problems.
Intervention
The computerized cognitive rehabilitation training package used was Captain’s Log software [31] consisting of 35 multi-level brain-training exercises designed to help develop and remediate a wide range of cognitive skills. Fifteen of the 35 possible brain-training exercises were chosen for this study. The criteria for deciding which exercises to include were: (1) having little or no verbal instructions so that children with poor grasp of English would benefit and (2) having simple or few movements with the track-ball. Pretesting demonstrated that Ugandan children who were, for the most part unfamiliar with computers, would be more comfortable using a track-ball than mouse, particularly if required movements were not large. The team that decided on these exercises was led by neuropsychologists who had been trained on using Captain’s Log (MJB and BG) who reviewed each of the possible training tasks with team members familiar with the children’s languages.
Four exercises were chosen from the ‘Attention Skills: Developmental’ module; 1) Scanning Reaction/inhibition (clicking the mouse if the colour of several varying images matches the color of the screen’s border), 2) Stimulus Reaction Time (the player is required to click the mouse once if the ‘target’ image appears), 3) Stimulus Reaction/Fields (the player is required to move the mouse and click it over the ‘target’ image) and 4) Stimulus Reaction/Inhibition (clicking the mouse if the colour of several random images appearing one at a time matches the color of the screen’s border). From the ‘Conceptual/Memory Skills’ module; 5) Conceptual (finding the missing part of a sequence from several choices), 6) Logical Sequences (finding and clicking on targets in the correct sequence), 7) Size Discrimination (clicking on target objects in order according to size) and 8) Symbolic Display Match (selecting and placing targets in the correct box based on various rules). Three exercises were chosen from ‘Visual Motor Skills’ module; 9) Visual Categorization (clicking on object that appears from behind a door according the category rule); 10) Visual Response Time (watching a grid of targets and clicking on any that changes), 11) Visuospatial Memory (searching for and find matching objects in a grid). From the ‘Logic Skills’ module; 12) Concept Logic (figuring out the secret rule in a number of images), 13) Match Logic (deciding whether images match or not), 14) Picture Logic (clicking on the target among foils) and 15) Sequential Logic (understanding the conceptual rules in respect to the logic of number/letter patterns).
Captain’s Log was programmed to run for 45 minutes with the first training session starting at the simplest level and the difficulty increased based on the child’s performance.
Procedures
Randomization to the different study arms was done prior to the start of the study by the first author in which 68 numbers (corresponding to the eligible 68 children) were all assigned to the two arms using random number generation. There was no blinding as the cognitive testing was done by the same research assistants carrying out the cognitive training.
At the study clinic, research assistants performed neuropsychological testing using CogState while at the same time the child behaviour checklist was administered to the mother. Children assigned to the intervention arm were given appointments to return the next week for computerized cognitive rehabilitation training while those in the control arm were given appointments to return for the second testing after eight weeks. Intervention children did 16 brain training sessions twice weekly for eight weeks from the clinic, home or school. Post-intervention testing was done one week after completion of the training to correspond with the post-intervention testing for the controls. The control group did not receive any intervention.
Statistical Analysis
Statistical Package for Social Sciences (SPSS) version 17.0 was used for the analyses. Separate univariate analyses for each of the nine outcome variables were performed since the outcome measures are typically not combined into other scales [24,28], and each outcome is a distinct cognitive/behavioral measurement.
The pre-intervention and post-intervention mean scores are reported by group. The unadjusted effect of the intervention is assessed using ANCOVA on the post-intervention scores with only the baseline pre-intervention score as covariate. To assess the effect of the intervention while controlling for other covariates, an ANCOVA is run on the post-intervention score with covariates age, grade, weight for age z scores, quality of the home environment, sex, and baseline pre-intervention score on the same outcome variable. Integrity of the model is checked by the Kolmogorov-Smirnov test for normality on the residuals, Levene’s test of equality of error variances, and boxplots for outliers. When the residuals show a long positive tail, ANCOVA is performed on the natural logarithm of the outcome variables to determine if changes in the significance occur. Finally, using the ANCOVA results, the adjusted effect of treatment is reported along with its P-value. To see if time between pre and post assessments could possibly be confounded with the intervention effect, another ANCOVA was run with time and baseline pre-intervention score as covariates.
Using the pre-intervention score as a baseline covariate is valid here due to the randomization of subjects to treatment [32] and should lead to more power for detecting intervention effects.
RESULTS
Demographic characteristic summaries by group, control and intervention, are reported in Table 1. The pre- and post-intervention scores for both groups on the CogState and CBCL are shown in Table 2.
Table 2.
Unadjusted mean scores for the study participants
| Domain | Pre-intervention | Post-intervention | ||
|---|---|---|---|---|
| Control M (SD) | CCRT M (SD) | Control M (SD) | CCRT M (SD) | |
| Working memory | 0.63 (0.27) | 0.80 (0.26) | 0.69 (0.26) | 0.79 (0.24) |
| Detection | 0.97 (0.33) | 1.03 (0.30) | 1.04 (0.29) | 1.15 (0.24) |
| Identification | 0.93 (0.35) | 0.99 (0.29) | 0.98 (0.32) | 1.06 (0.25) |
| Card Learning | 0.61 (0.21) | 0.61 (0.15) | 0.62 (0.17) | 0.67 (0.20) |
| Maze Learning | 0.09 (0.06) | 0.10 (0.06) | 0.11 (0.07) | 0.20 (0.13) |
| Maze Chasing | 0.14 (0.10) | 0.13 (0.09) | 0.12 (0.06) | 0.26 (0.12) |
| Internalizing | 17.33 (6.73) | 18.48 (6.75) | 17.71 (7.49) | 15.52 (7.37) |
| Externalizing | 17.22 (7.27) | 19.59 (8.16) | 17.10 (8.03) | 16.66 (6.91) |
| Total Problems | 58.00 (17.97) | 61.97 (17.59) | 58.26 (23.29) | 55.41 (17.71) |
CCRT=Computerised Cognitive Rehabilitation Training
The unadjusted and adjusted ANCOVA results are reported in Table 3. Unadjusted results use only the baseline pre-intervention score as a covariate while the adjusted analyses use covariates age, grade, weight for age z scores, quality of the home environment, sex, and baseline pre-intervention score on the same outcome variable. In the adjusted model, strong intervention effects are found for Maze Learning (group effect (standard error) (0.08 (0.02); p<0.001), Maze Chasing (0.14 (0.03); p < 0.001) and for Internalizing Problems (−3.80 (1.56); p = 0.02). A significant intervention effect was found for Detection (0.14 (0.07); p= 0.04). Similar intervention effects are observed in the unadjusted model. For the significant intervention outcomes, adjusting for covariates increased the intervention effect on Detection by about 7.7% (from 0.13 up to 0.14), decreased the effect on Maze Learning by about 11% (from 0.09 down to 0.08), and increased the effect on Internalizing by about 16% (from −3.28 down to −3.80). The effect on Maze Chasing was unchanged.
Table 3.
| Domain | Unadjusted group effect M (SE) | P-value for unadjusted effect3 | Adjusted group effect M (SE) | P-value for adjusted effect3 |
|---|---|---|---|---|
| Working memory | 0.02 (0.06) | 0.76 | 0.03 (0.05) | 0.54 |
| Detection | 0.13 (0.06) | 0.04 | 0.14 (0.07) | 0.04 |
| Identification | 0.06 (0.07) | 0.38 | 0.08 (0.07) | 0.23 |
| Card Learning | 0.04 (0.04) | 0.34 | 0.02 (0.04) | 0.59 |
| Maze Learning | 0.09 (0.02) | <0.001 | 0.08 (0.02) | <0.001 |
| Maze Chasing | 0.14 (0.03) | < 0.001 | 0.14 (0.03) | <0.001 |
| Internalizing Problems | −3.28 (1.43) | 0.03 | −3.80 (1.56) | 0.02 |
| Externalizing Problems | −1.76 (1.52) | 0.25 | −1.62 (1.69) | 0.35 |
| Total Problems | −5.72 (4.20) | 0.18 | −5.65 (4.64) | 0.23 |
Effect from ANCOVA using only pre-intervention score as covariate.
Effect from ANCOVA using age, waz, MC-HOME score, sex, school grade and pre-intervention score as covariates.
Not adjusted for multiple outcomes.
WAZ=weight for age z score; MC-HOME=Middle Childhood Home Observation for the Measurement of the Environment; CCRT=Computerised Cognitive Rehabilitation Training.
Finally, to check whether the time between assessments significantly affected the outcomes, we ran ANCOVA with covariates time and baseline pre-intervention score. For all the outcome measures, time was never a significant factor (p-values between .199 and .837), and the trends of the intervention effects were similar with and without time. Significant intervention effects held up for Maze Learning (time adjusted P-value .001), Maze Chasing (time adjusted P-value < .001), and Detection (time adjusted P-value .04). The significance of Internalizing dropped (time adjusted P-value .144) while the significance of Total Problems increased (time adjusted P-value .065).
Because of the moderate sample sizes of about 30 in each group, the minimum detectable effect size (for 80% power) is slightly above 0.7. This is a strong effect, and the sample sizes in the study do not have sufficient power to reliably detect more modest effects which may be clinically significant.
The normality of the residuals was not a serious problem except for some high outliers in the three Child Behaviour Checklist variables. To see how inferences might be affected, we converted these three variables to the natural logarithm scale and ran the both ANCOVA models with natural log pre and post scores. For Internalizing, Externalizing, and Total Problems, the P-values for unadjusted intervention effects for mean log score were 0.005, 0.29, and 0.30, respectively and for adjusted intervention effects, the P-values were 0.002, 0.40, and 0.39, respectively. Thus, the P-values of Internalizing decreased in the log scale, while the P-values of the others increased. We suspect that the reported P-value of 0.02 for Internalizing is probably conservative.
Note that nine individual ANCOVA’s were performed, one for each outcome variable. If the P-values are below 0.0055, then with nine variables, we can claim overall significance level 5% or better by Bonferroni. The P-values for the intervention effect on Maze Learning and on Maze Chasing are below 0.001, and for internalizing in the log scale, the P-value was 0.002. Thus, the multiple tests effect is not a problem for these 3 outcome variables. However, for Detection, the P-value is 0.04, which would only give overall significance of 37.8%. Thus, while we note that Detection shows a P-value below 0.05, it is not an extreme P-value and should be interpreted with caution due a possible multiple tests effect.
DISCUSSION
This study, the first of its kind in sub-Saharan Africa, was conducted to determine the potential benefits and feasibility of computerized cognitive rehabilitation training in children with a history of cerebral malaria. Intervention children had significant improvement in tests measuring visuomotor processing speed, working memory, learning and internalizing problems. Non-significant improvements also were seen in most of the other areas tested in the intervention group. In contrast, control group children who received no intervention had no significant improvement in any test.
Our earlier studies looking at the cognitive outcomes of cerebral malaria in this same cohort showed 21% impaired at six months and 26% impaired at 24 months follow up with memory and attention most affected [4,9]. The increasing frequency of impairment over time makes such interventions even more important for cerebral malaria survivors to prevent these cognitive deficits. The observed benefit of computerized cognitive rehabilitation training in these children at almost four years after the illness suggests the possibility of even better outcomes if these interventions are carried out early, as well as potentially reducing the later onset of cognitive deficits seen in a percentage of these cerebral malaria surviving children [4]. However, the present study addressed only short-term improvement in specific areas of cognition. Further prospective studies will be required to document if improvement is sustained long-term and if long-term improvement in other important areas known to be affected in cerebral malaria, particularly attention, can be achieved with other computerized cognitive rehabilitation training interventions.
After presenting cognitive exercises training attention, memory, psychomotor processing and reasoning skills, changes were seen in the two maze tasks and Detection showing improvement in visuomotor processing speed, working memory, psychomotor speed and learning. This suggests frontal lobe, parietal lobe and hippocampal involvement which are associated with these skills [33–35]. No improvements were seen in Identification measuring visual attention which could be attributed to the severity of attention deficits after cerebral malaria. Attention deficits after cerebral malaria are very common [4,6,7,9] and their frequency and severity increases over time [4] highlighting the need for early cognitive training after cerebral malaria to correct this trend.
Despite the severity of attention deficits after cerebral malaria, the lack of benefit in visual attention (Identification task) is surprising given that the greatest similarity between the training exercises and outcome measures was in the attention exercises and outcomes. CogState’s Identification tasks are similar to the Captain’s log’s Scanning Reaction/inhibition, Stimulus Reaction Time and Stimulus Reaction/Inhibition. There was no similarity in the other exercises and the maze tasks indicating that the observed benefits were not due to practice effect.
The potential for computerized cognitive rehabilitation training to alter brain circuitry as observed in this study is implied in adult studies that identify reduced schedules of brain activity, noisy processing, weakened neuromodulatory control, and negative learning as contributing to cognitive decline [36]. Regular computerized cognitive rehabilitation training can improve the cognitive deficits in the recovering cerebral malaria child by instituting a regular schedule of brain activity for these children. It can also strengthen neural network processes that undergird executive attention and working memory [37]. Finally, if designed properly computerized cognitive rehabilitation training can shape positive learning strategies that can extend to other functional domains and activities of daily living and learning for the child.
Other studies on children with ADHD that focused on training specific cognitive skills like working memory rather than a range of skills resulted in improvement in both trained and untrained tasks [15, 16]. This implies that dedicated training of specific abilities such as working memory that are foundational to other cognitive skills may result in improvement in both trained and untrained tasks. Future cognitive interventions for cerebral malaria may need to compare the benefits in training specific skills versus several skills.
Cerebral malaria in sub-Saharan Africa is most frequently seen in children below the age of five years [1], an age group where the resulting cognitive deficits have not been described prospectively though retrospective studies suggest that they do have cognitive deficits [38]. Unfortunately children below five years of age cannot comprehend and benefit from the training exercises presented in Captain’s Log. However, the observed benefits on neuropsychological test performance and behaviour in this study even when an intervention is carried out almost four years after the illness onset is promising for this younger age group who can be enrolled in such treatments when they become of age. Suitable, cost-effective and widely-distributable interventions for children younger than five with brain injury remain to be established. It will be important to define some of these interventions, as it is likely that early intervention will provide superior benefit to cognitive performance in children.
A limitation of the study was the reliance on computerised assessment as the cognitive outcome measures. This was necessary, however, due to logistics and availability of testing materials and personnel. The observed benefit in only three of the six CogState tests and the reported improvements in parent-rated child behaviour suggest that the neuropsychological benefits of computerized cognitive rehabilitation training may not be solely due to familiarity with computers. The improvement in these three tasks suggests that the intervention children might have learnt strategies and motor skills to perform learning, working memory and visuomotor tasks (more complex tasks) which had no effect on the basic neural connectivity responsible for the other four tasks. The acquisition of these new strategies is still a benefit for the children.
A second limitation was that the intervention lasted longer than two months because; children had to attend classes (on weekends at times), the intervention spanned the Christmas holiday where some parents take children upcountry and there was a general fuel crisis in the country at the time that made travel difficult, all of which caused some sessions to be missed. The 29 children that completed the intervention missed an average of only two sessions which were made up for. Having the control group not do any exercises was another limitation that could have biased responses to the behavioural assessment. Despite these shortcomings, we believe implementation of computerized cognitive rehabilitation training is still feasible because we were able to arrange and have training done from schools and to work around the holiday schedule to complete the scheduled training sessions.
The prolonged scheduling of times for the intervention resulted in a much shorter duration between pre- and post- testing for the control group as compared to the intervention group. With the shorter duration between testing for the control children, practise effects could have been more pronounced for this group – this would have biased in favour of improved performance in the control group, something we did not observe.
The present study was designed to assess the feasibility of computerized cognitive rehabilitation training in a group of Ugandan children with a history of cerebral malaria. The study demonstrated that such cognitive training is possible and the short-term improvement in specific outcomes highlights the potential benefit and feasibility of computerized cognitive rehabilitation training in this population. The computerized cognitive rehabilitation training approach allows multiple computers to be used with small groups of children simultaneously with only a single facilitator, providing a lower cost, but effective intervention.
The success of computerized cognitive rehabilitation training depends on the availability of computers and trainers to carry out these interventions. Computer usage in sub-Saharan Africa is on the increase as evidenced by the increasing access to the internet from 0.1% in 1998 to 0.4% in 2002 [39]. The actual computer usage is much higher than this because these numbers do not take into account the sharing of media that is common in many African countries [40]. The possibility of educational services such as computerized cognitive rehabilitation training being made available via mobile phones or personal digital assistants [41] is a further a boost to these computer based interventions. In addition, mobile phone usage in Africa is increasing: there were over 13 million new mobile subscribers in 2003 while the number of mobile phone users in Uganda has multiplied 131 times in the last few years [39]. Community development projects like the One Laptop Per Child where low cost laptops are to be given to children in developing countries [42] make computerized cognitive rehabilitation training services in the rural areas a real possibility in the near future.
In conclusion, computerized cognitive rehabilitation training interventions for cerebral malaria children can improve short-term neuropsychological test performance and behaviour almost four years after the illness. Similar interventions done earlier in the recovery course might have even better outcomes. Now that the feasibility and short–term benefits of computerized cognitive rehabilitation training have been demonstrated in older children with cerebral malaria, the long-term benefit of computerized cognitive rehabilitation training in these children and the provision of appropriate interventions for children below five years who bear the brunt of the cerebral malaria illness require further attention.
Acknowledgments
We thank the children and their families who participated in this study, the head teachers who allowed us to train the children from their schools and the dedicated research team. We are grateful to Professor Elsa Shapiro, Pediatric Neuropsychologist at the University of Minnesota for her useful comments in preparing this manuscript.
Funding for this study was provided by a University of Michigan Global Health Research Training (GHRT) award to BG and by faculty start-up funding through the Michigan State University Department of Neurology & Ophthalmology to MJB.
References
- 1.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64(1 2 suppl):57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 2.Carter JA, Neville BG, Newton CR. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res Rev. 2003;43(1):57–69. doi: 10.1016/s0165-0173(03)00192-9. [DOI] [PubMed] [Google Scholar]
- 3.Olness K. Effects on brain development leading to cognitive impairment: a worldwide epidemic. J Dev Behav Pediatr. 2003;24(2):120–130. doi: 10.1097/00004703-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 4.John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–e99. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter JA, Ross AJ, Neville BGR, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10(1):3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 6.Holding PA, Stevenson J, Peshu N, et al. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg. 1999;93(5):529–534. doi: 10.1016/s0035-9203(99)90368-1. [DOI] [PubMed] [Google Scholar]
- 7.Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr. 2002;23:353–364. doi: 10.1097/00004703-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Dugbartey AT, Spellacy FJ, Dugbartey MT. Somatosensory discrimination deficits following pediatric cerebral malaria. Am J Trop Med Hyg. 1998;59(3):393–396. doi: 10.4269/ajtmh.1998.59.393. [DOI] [PubMed] [Google Scholar]
- 9.Boivin MJ, Bangirana P, Byarugaba J, et al. Cognitive impairment following cerebral malaria in children: a prospective study. Pediatrics. 2007;119 (2):e360–e366. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dugbartey AT, Dugbartey MT, Apedo MY. Delayed neuropsychiatric effects of malaria in Ghana. J Nerv Ment Dis. 1998;186(3):183–6. doi: 10.1097/00005053-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Birbeck GL, Potchen MJ, Kaplan P, et al. EEG and neuroimaging findings in Malawian childhood cerebral malaria survivors. Neurology. 2007;12(supplement 1):A138. [Google Scholar]
- 12.Bangirana P, Idro R, John CC, et al. Rehabilitation for cognitive impairments following cerebral malaria in African children: Strategies and limitations. Trop Med Int Health. 2006;11:1341–1349. doi: 10.1111/j.1365-3156.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 13.Idro R, Jenkins NE, Newton CRJC. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 14.Shalev L, Tsal Y, Mevorach C. Computerized progressive attentional training (CPAT) program: effective direct intervention for children with ADHD. Child Neuropsychol. 2007;13(4):382–388. doi: 10.1080/09297040600770787. [DOI] [PubMed] [Google Scholar]
- 15.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 16.Klingberg T, Fernell E, Olesen PJ, et al. Computerized training of working memory in children With ADHD A Randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Van’t Hooft I, Andersson K, Sejersen T, et al. Attention and memory training in children with acquired brain injuries. Acta Paediatr. 2003;92:935–940. doi: 10.1080/08035250310004586. [DOI] [PubMed] [Google Scholar]
- 18.Cicerone KD, Dahlberg C, Kalmar K, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81:1596–615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- 19.Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86:1681–92. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Sitzer DI, Twamley EW, Jeste DV. Cognitive training in Alzheimer’s disease: a meta-analysis of the literature. Acta Psychiatr Scand. 2006;114:75–90. doi: 10.1111/j.1600-0447.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell BM, Bradley RH. Home Inventory Administration Manual. 3. Little Rock, AR: University of Arkansas; 2001. [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat. 2002;11:246. [PubMed] [Google Scholar]
- 23.Westerman R, Darby DG, Maruff P, et al. Computer-assisted cognitive function assessment in pilots. Australian Defence Force Health. 2001;2:29–36. [Google Scholar]
- 24.Pietrzak RH, Maruff P, Mayes LC, et al. An examination of the construct validity and factor structure of the Groton Maze Learning Test, a new measure of spatial working memory, learning efficiency, and error monitoring. Arch Clin Neuropsychol. 2008;23:433–445. doi: 10.1016/j.acn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children Manual. 2. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 26.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles: an integrated system of multi-informant assessment. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 27.Achenbach TM, Rescorla LA. Multicultural supplement to the manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2007. [Google Scholar]
- 28.Stevens GWJM, Vollebergh WAM, Pels TVM, et al. Predicting internalizing problems in Moroccan immigrant adolescents in the Netherlands. Soc Psychiatry Psychiatr Epidemiol. 2005;40:1003–1011. doi: 10.1007/s00127-005-0988-9. [DOI] [PubMed] [Google Scholar]
- 29.Weisz JR, Sigman M, Weiss B, et al. Parent reports of behavioral and emotional problems among children in Kenya, Thailand, and the United States. Child Dev. 1993;64(1):98–109. doi: 10.1111/j.1467-8624.1993.tb02897.x. [DOI] [PubMed] [Google Scholar]
- 30.Ivanova MY, Achenbach TM, Dumenci L, et al. Testing the 8-Syndrome Structure of the Child Behavior Checklist in 30 Societies. J Clin Child Adolesc Psychol. 2007;36(3):405–417. doi: 10.1080/15374410701444363. [DOI] [PubMed] [Google Scholar]
- 31.Sandford JA. Captain’s Log Computerized Cognitive Training System. Richmond, VA: Brain Train; 2007. [Google Scholar]
- 32.Breukelen GJP. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J Clin Epidemiol. 2006;59:920–925. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- 34.Nichols EA, Kao Y, Verfaellie M, et al. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolb B, Whishaw IQ. Fundamentals of Neuropsychology. 6. Madison Avenue, NY: Worth Publishers; 2009. [Google Scholar]
- 36.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 37.Zillmer EA, Spiers MV. Principles of Neuropsychology. Belmont, CA: Wadsworth; 2001. [Google Scholar]
- 38.Idro R, Carter JA, Fegan G, et al. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2006;91:142–148. doi: 10.1136/adc.2005.077784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanmugavelan M. Mobile Africa must not Leave its villages behind. Contemporary Review. 2004;285(July):33–34. [Google Scholar]
- 40.Jensen M. ICT in Africa: A Status Report. In: Dutta S, Lanvin B, Paua F, editors. The Global Information Technology Report 2002–2003: Readiness for the Networked World. New York: Oxford University Press; 2003. pp. 86–100. [Google Scholar]
- 41.Chen J, Kinshuk Mobile Technology in Educational Services. Journal of Educational Multimedia and Hypermedia. 2005;14(1):91–109. [Google Scholar]
- 42.Chapman G. One Laptop Per Child gets moving [The Australian Web site] 2007 November 12; [Google Scholar]