Abstract
Rationale
We investigated the molecular mechanism(s) that play a role in leptin signaling during the development of left ventricular hypertrophy (LVH) due to pressure overload. To this end, ob/ob leptin deficient and C57BL/6J control mice were subjected transverse aortic constriction (TAC).
Methods
Control sham C57BL/6J and ob/ob mice, along with C57BL/6J and ob/ob leptin deficient mice were subjected transverse aortic constriction (TAC) for 15 days and then evaluated for morphological, physiological, and molecular changes associated with pressure overload hypertrophy.
Results
Evaluation by echocardiography revealed a significant increase in left ventricular mass (LVmass) and wall thickness in ob/ob mice subjected to transverse aortic constriction (TAC) as compared to C57BL/6J. Analysis of the expression of molecular markers of LVH, such as atrial natriuretic peptide (ANP), revealed a blunted increase in the level of ANP in ob/ob mice as compared to C57BL/6J mice. We observed that leptin plays a role in modulating the transcriptional activity of the promoter of the ANP gene. Leptin acts by regulating NFATc4, a member of the nuclear factor activated T cell (NFAT) family of transcription factors in cardiomyocytes. Our in vivo studies revealed that ob/ob mice subjected to TAC failed to activate the NFATc4 in the heart, however, intraperitoneal injection of leptin in ob/ob mice restored the NFATc4 DNA-binding activity and induced expression of the ANP gene.
Conclusion
This study establishes the role of leptin as an anti-hypertrophic agent during pressure overload hypertrophy, and suggests that a key molecular event is the leptin mediated activation of NFATc4 that regulates the transcriptional activation of the ANP gene promoter.
Keywords: Leptin, ob/ob mice, Pressure overload hypertrophy, Atrial Natriuretic peptide
1. Introduction
Left ventricular hypertrophy (LVH) and the ensuing heart failure (HF) are among the most significant cardiovascular pathologies that account for a high percentage of morbidity and mortality in western countries. The causes of LVH are diverse, obesity among them, is increasingly becoming a significant contributor factor (de Simone, 2007). Several studies have reported a direct correlation between obesity and the development of LVH, but efforts to understand the precise role of obesity in LVH has been masked by the diverse clinical pathologies associated with obesity. Human obesity is characterized by an increase in the production of the adipocyte-derived, 16-kDa peptide, leptin (Zhang et al., 1994). Previous reports have suggested a physiological effect of leptin in the human heart based on a direct correlation between the plasma leptin levels and the degree of LVH, with an increase in wall thickness and left ventricular mass (Paolisso et al., 1999; Perego et al., 2005). Other failed to observe the correlation between leptin level and left ventricular remodeling (Pladevall et al., 2003).
Sustained chronic stress to the heart induces structural and functional remodeling giving rise to compensatory and non-compensatory hypertrophy (Swynghedauw, 2006; Ritter et al., 2003). The compensatory response during LVH is mediated by the atrial natriuretic peptide (ANP) and the brain natriuretic peptide (BNP) (Nishikimi et al., 2006; London, 2006). There is substantial information on the transcriptional control of the ANP encoding gene (Nppa) during cardiac development but less is known about the transcriptional mechanisms of ANP expression in the left ventricle during disease (Houweling et al., 2005).
The availability of an animal model of obesity, such as the leptin deficient mice (ob/ob), provides a significant tool to decipher the role of obesity in LVH (Barouch et al., 2003). In this report, we attempt to understand the role of obesity during the development of LVH, we subjected the ob/ob mice to transverse aortic constriction (TAC), an established model of pressure overload hypertrophy (Beckles et al., 2006). Based on M-mode echocardiography measurements, we found a significant increase in LVmass and wall thickness in ob/ob hearts. The expression of hypertrophic gene markers in the left ventricle, such as ANP was blunted in ob/ob as compared with wild type mice. Interestingly, the ANP expression was restored in ob/ob mice after chronic administration of leptin.
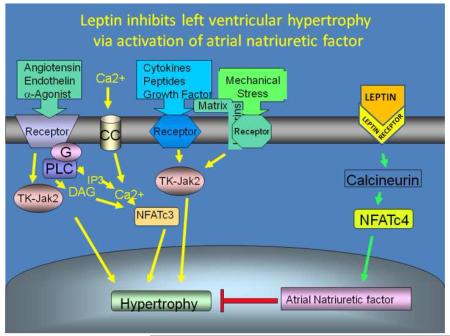
ANP is a direct moderator of cellular growth, and along with the natriuretic peptide receptor A (NPRA), plays an important autocrine role in the heart as an inhibitor of cardiac hypertrophy (Knowles et al., 2001; Oliver et al., 1997). Indeed, impaired expression or partial deficiency of the atrial natriuretic peptide gene results in exaggerated cardiac hypertrophy (Franco et al., 2004). These observations suggest that understanding the nature of the impaired ANP expression in ob/ob mouse hearts may provide important insite into the increase incidence of LVH among obese people. Several transcription factors have been associated with transcriptional control of the ANP gene promoter, including members of the GATA family, the myocyte enhancer factor (MEF2), Nkx2.5, members of the MADS box protein family, serum response factors (SRF) (Temsah et al., 2005), and dHAND (Zang et al., 2004). The activation of these transcription factors during LVH is the results of the induction of upstream signal transduction pathways, include the Jak/Stat pathway, Ca++-calmodulin dependent calcineurin pathway, the extracellular mitogen activated protein kinases (MAPK), p44/p42, p38, and the stress-activated protein kinase c-jun N-terminal kinase (JNK) (Swynghedauw, 2006; Ritter et al., 2003; Beckles et al., 2006). Our analysis of the ANP promoter revealed a conserved NFAT binding site. The NFAT family of transcription factors is Ca++-Calmodulin dependent, and are members of a well characterized signal transduction pathway involved in pathological hypertrophy (Wilkins et al., 2004). However, the genes targeted by this pathway during the compensatory and non-compensatory phases of left ventricular hypertrophy, such as in pressure overload hypertrophy are poorly understood (Clerk et al., 2007). Although most of the signal transduction pathways associated with LVH are known be activated by leptin (Yang et al., 2007), no reports have yet identified that Ca++-calmodulin dependent calcineurin pathway is modulated by leptin in the heart. An initial report showed the activation of NFAT3 by the calcineurin-dependent pathway during hypertrophy and consequently resulting in the transcriptional activation of the brain natriuretic peptide promoter (Molkentin et al., 1998). As described earlier, both ANP and BNP play a role in the compensatory response during LVH. Thus, the initial evidence of NFAT3 modulating the BNP promoter activity suggests a compensatory role for the NFAT family of transcription factors during pressure overload hypertrophy.
Here, we provide evidence suggesting that leptin plays a inhibitory role during the growth of the left ventricular remodeling undergoing LVH by modulating the expression of the ANP gene via activation of the NFAT transcription factors.
2. Methods
2.1 Animals
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996). Male mice C57/BL6 and ob/ob of 7 to 8 weeks old from Jackson’s Laboratory matched for sex and age were used in this study. Mice were subjected to TAC for 15 days as previously described (Beckles et al., 2006). Some ob/ob mice subjected to TAC received daily intraperitoneal injection with leptin (Sigma-Aldrich) in phosphate saline buffer (PBS) 0.3 mg/kg/day, based on average body weight of 40 g.
2.1.2 Antibodies and Chemicals
Polyclonal antibodies against NFATc3, and NFATc4 were purchased from Santa Cruz Biotechnology. Leptin was obtained from Sigma-Aldrich.
2.1.3 Histology
Myocyte cross-sectional areas and collagen staining were performed as previously described (Beckles et al., 2006).
2.1.4 Hypertrophic gene marker analysis
The Northern blot was performed as described in Beckles et al., 2006. The ANF and β-actin probes were obtained by RT—PCR using the following primers ANF (sense) 5′-atgggctccttctccatcac-3′, ANF (antisense) 5′-tcttcggtaccggaagct-3′, and β-actin (sense) 5′- ggtgacgaggcccagagcaagaga-3′ and β-actin (antisense) 5′-accgctcgttgccaatagtgatg-3′.
2.1.5 Echocardiographic measurement
M-mode and Two-dimensional echocardiography were performed as described earlier (Beckles et al., 2006).
2.1.6 Transient Transfection and Luciferase Assay
The transfection assay was previously described (Mascareno et al., 2005) The rat atrial natriuretic peptide promoter with the luciferase gene as reporter, and 10 ng of thymidine kinase promoter-driven Renilla luciferase-thymidine kinase vector, which was used to normalize the transfection efficiency. The promoter-less reporter vector, pGL2- Basic, was used as control. Luciferase assays were performed with the dual luciferase assay kit (Promega, Madison, WI). After 24 hr the transfected cells were maintained for 8 h in serum free medium, then leptin (100 ng/ml) was added and the cells collected after 6 hr. Cell treated with KN93 inhibitor (1 μM ) and leptin were also harvested after 6 hr.
2.1.7 Immunohistochemistry
Rat cardiac cells (H9C2) were cultured for 48 hr, and then incubated in serum free medium for 12 hr, followed by leptin treatment (100 ng/ml) for 6 hr. Cells were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature. Cell were washed with PBS containing 100 mM glycine, and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Blocking was performed with 1 % BSA, 1% horse serum in PBS for 1 hr at room temperature. NFATc4 antibody was incubated in 1% BSA in PBS overnight at 4 °C. Cells were washed in PBS followed by incubation with conjugated anti-rabbit Alexa fluor 594 for 1 hr at room temperature. Cells washed in PBS were incubated with Alexa fluor phalloidin 488 according to manufacturer (Invitrogen), followed by incubation with DAPI.
2.1.8 Preparation of Nuclear Extracts and Gel Mobility-Shift Assay (GMSA)
The gel shift assay was done essentially as previously described (Mascareno et al., 1998). The NFAT-domain DNA probe for protein binding was a double-stranded oligonucleotide containing the sequence 5′-cagggagaaggaatcctgaggc-3′ and complementary strand 5′-gcctgaggattccttgtccctg-3′, respectively. The oligonucleotide sequences containing a mutation substitution of the NFAT-domain was 5′- cagggataactaatcctgaggc -3′ (top strand). Polyclonal antibodies (4 μg) against NFATc3 and NFATc4 were added and incubated for 4 hr before addition of the DNA probe.
2.1.9 Chromatin immunoprecipitation (ChIP) assay
ChIP was performed as described by the manufacturer (Upstate) with modifications. Left ventricle from control or leptin treated ob/ob mice were harvested after 24 hr of treatment. To amplify the region from nt –230 to –501 in the ANP promoter, PCR was performed with the forward 5′-gcccttatttggagcccctgac-3′ and the reverse primer 5′-cacagcccctttgccttgagc-3′. As internal control, we amplified a 320 bp fragment in the mouse GAPDH promoter, PCR was performed with the forward primer 5′-cccggcatcgaaggtggaagagt-3′ and the reverse primer 5′-ccctctggaaagctgtggcgtgat-3′.
2.1.10 Statistical Analysis
All results are presented as mean ± standard error of the mean (SEM). Multiple comparison was performed by one-way ANOVA with the Tukey- Kramer exact probability test or the Bonferroni test. P values < 0.05 were considered significant.
3. Results
3.1 Morphological and functional changes in the hearts in ob/ob mice subjected to TAC
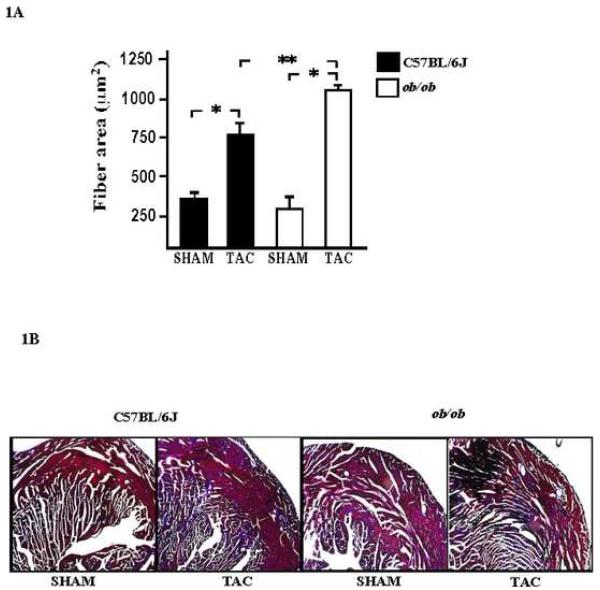
Male C57BL/6J and ob/ob mice hearts were subjected to pressure overload (TAC), and cardiovascular parameters were evaluated by echocardiography, the data from both group of mice are shown in Table 1. M-mode echocardiography revealed a statistically significant increase in the left ventricular mass, and in posterior wall thickness in wild type C57 BL/6J and ob/ob mice hearts as compared with sham mice. Additionally, ob/ob mice showed a significant increase in the left ventricular mass and in septal wall thickness as compared with C57BL/6J subjected to TAC. The left ventricular space diameter in systole (LVESD) and diastole (LVEDD) did not change significantly in either group subjected to TAC. Left ventricular systolic function as determined by fractional shortening appears to be normal in both animal models, suggesting that there is compensatory remodeling within the time-frame of the experimental protocol. The onset of cardiac hypertrophy in mice subjected to TAC was further evaluated by measuring myocyte cross sectional diameter and collagen deposition. The bar graph summarizes the data from left ventricular sections and shows that mice subjected to TAC have significantly enlarged myofibers as compared to sham in both, C57BL/6J and ob/ob mice. Furthermore, a significant increase was observed in myocyte cross sectional area of ob/ob mice subjected versus C57BL/6J mice to TAC (Figure 1A). A significant component of the left ventricle undergoing pressure overload hypertrophy is the increase accumulation of extracellular matrix. Masson Trichrome staining of left ventricular sections show more collagen deposition in ob/ob mice subjected to TAC as compared with C57BL6/J under similar stress (Figure 1B). These observations indicate that while cardiac hypertrophy occurred in TAC control animals as expected, the hypertrophic response and remodeling seems to be greater in ob/ob mice.
Table 1. Ventricular parameters in wild type and ob/ob mice hearts subjected to TAC.
Functional analysis obtained from M-mode echocardiograms. Measurement of the left ventricle, systolic (ESD) and diastolic (EDD) dimensions, intraventricular septum, wall thickness in each group revealed the development of left ventricular hypertrophy as determined by left ventricular mass (Lvmass) and posterior wall thickness (PWT). Values are means ± s.e.m. Sham vs TAC group
| C57BL/6J | ob/ob | |||
|---|---|---|---|---|
| Sham (n = 8) |
TAC (n = 9) |
Sham (n = 6) |
TAC (n = 6) |
|
| Body weight (g) | 28.4 ±0.4 | 29.3 ±0.5 | 46.2 ±0.7 | 49.1 ±0.3 |
| Heart rate (HR, beats/min) | 549 ±17 | 560 ±26 | 535 ±11 | 545 ±14 |
| Left Ventricular Mass (Lvmass, mg) | 71 ±7 | 111 ±4 ‡ | 85 ±3 | 139 ±8 ‡ # |
| Septal wall thickness diastole (mm) | 0.81 ±0.05 | 0.99 ±0.05 | 0.73 ±0.02 | 1.20 ±0.07 ‡ |
| Septal wall thickness systole (mm) | 1.55 ±0.01 | 1.61 ±0.04 | 1.35 ±0.04 | 1.82 ±0.02 ‡ |
| Posterior wall thickness diastole (mm) | 0.75 ±0.03 | 0.95 ±0.05 ‡ | 0.73 ±0.02 | 1.09 ±0.01 ‡ |
| LVEDD, (mm) | 3.20 ±0.06 | 3.10 ±0.04 | 3.55 ±0.04 | 3.46 ±0.06 |
| LVESD, (mm) | 1.56 ±0.04 | 1.61 ±0.03 | 1.75 ±0.01 | 1.56 ±0.01 |
| Fractional Shortening (%) | 53 ±3 | 48 ±5 | 50 ±2 | 54 ±3 |
P values < 0.05. ob/ob vs. C57BL/6J subjected to TAC
P values < 0.01 were considered statistically significant.
Fig. 1. Morphological changes in wild type and ob/ob mice hearts subjected to TAC.
A, light microscopic analysis was used to determine the myocyte cross sectional area in mice subjected to TAC as compared with those from sham animals in both groups. The increase in myocyte cross sectional area was significant in C57BL/6J and ob/ob mice subjected to TAC. Cross-sectional area was measured using the morphometric system NIH 1.63f. * P values < 0.05 versus sham C57BL6/J; **P < 0.01 versus TAC C57BL6/J (Magnification at 400x). B, collagen deposition in wild type and ob/ob mice subjected to TAC was obtained by the Masson trichrome stain in sections of the left ventricle (Magnification 200x).
3.2 The expression of ANP is blunted during LVH in ob/ob mice
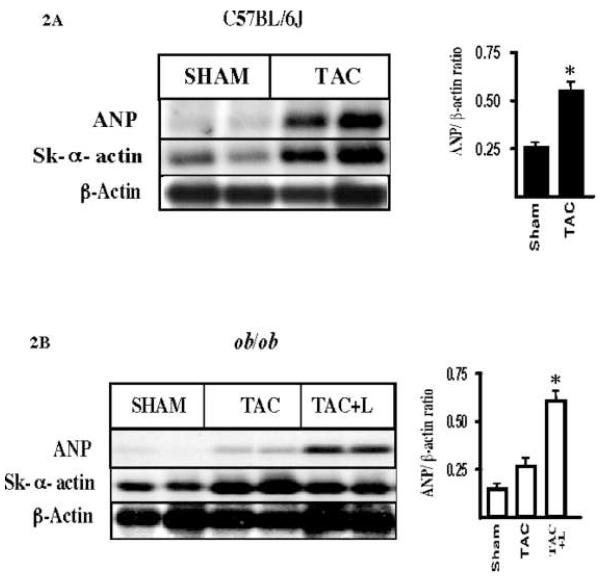
As a consequence of left ventricular remodeling, the expression of a well established set of genes are increased such as the ANP and the skeletal α-actin genes. A Northern blot analysis of total RNA obtained from the left ventricle of C57BL/6J sham or subjected to TAC shows a prominent increase in ANP and skeletal α-actin expression in the hypertrophied heart, as compared with sham C57BL/6J mice (Figure 2A). However, similar analysis in ob/ob mice revealed that pressure overload hypertrophy had little effect on the expression of ANP, while there was still an increase in skeletal α-actin expression (Figure 2B). To test the potential role of leptin as an inducer of ANP in vivo, we performed intraperitoneal injection of leptin (L) in ob/ob mice subjected to TAC. This resulted in a significant increase in the level of ANP expression in the heart of ob/ob mice, suggesting that leptin plays a significant role in the transcriptional induction of the ANP gene during TAC.
Fig. 2. Expression of molecular markers in the left ventricle of hearts subjected to pressure overload hypertrophy.
A, Northern blot hybridization of total RNA from the left ventricle of sham and TAC C57BL/6J mice shows an increase in expression of atrial natriuretic peptide (ANP) and skeletal α actin (Sk. α-actin). The bar graph shows the densitometric analysis of the relative ANP expression versus β-actin. Although the Northen blot shows samples from each group in duplicate, the relative values were obtained from experiments in triplicate, values are means ± s.e.m * p < 0.05 is statistical significant. B, as above, Northern blotting was done using the same DNA probes in RNA samples from left ventricle of ob/ob mice of sham and TAC groups. A blunted response of ANP expression during TAC is observed in ob/ob mice hearts. Chronic intraperitoneal administration of leptin triggered the expression of ANP in ob/ob mice subjected to TAC. Statistical analysis of the relative densitometric values for ANP expression versus β-actin was performed as before. The values were obtained from triplicate experiments. One way Anova and Bonferroni’s test were used. Values are means ± s.e.m * p < 0.05 is statistically significant. S, sham; TAC, trans-aortic constriction, TAC + L, trans-aortic constriction and intraperitoneal injection with leptin 0.3 mg/kg/ per day.
3.3 Leptin triggers the transcriptional activation of NFATs
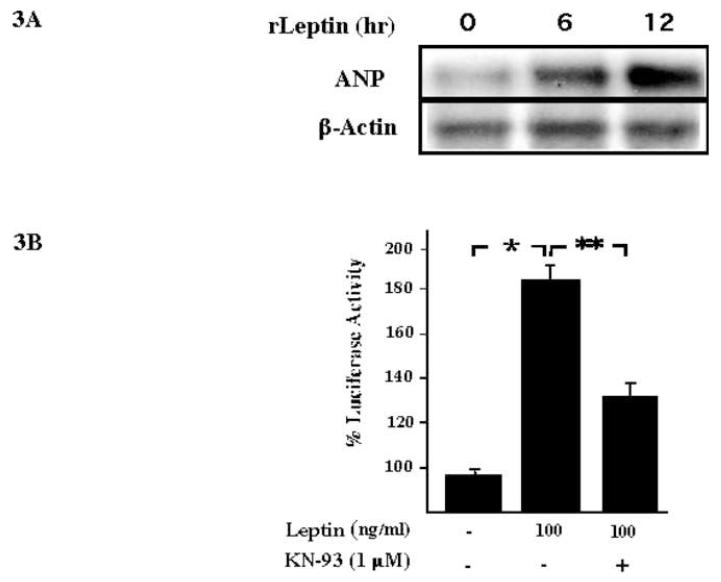
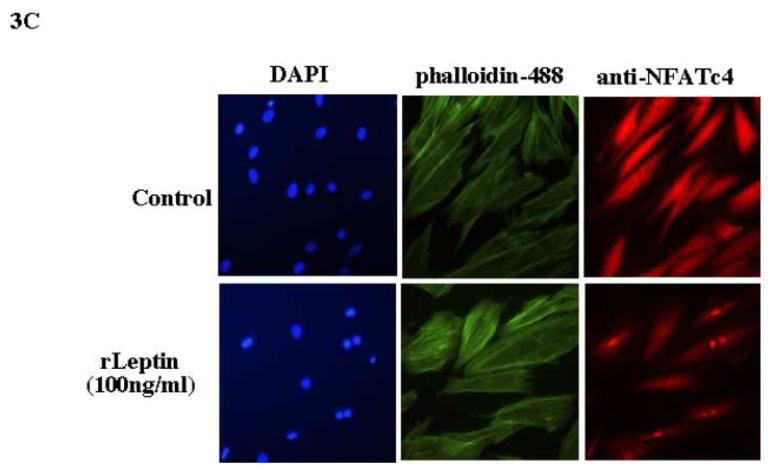
Our initial characterization of a leptin-NFAT signaling pathway was the observation that rat cardiac cells (H9C2) treated with leptin induced expression of ANP. We observed significant increase expression of ANP RNA after 6 hr of leptin treatment (Figure 3A). As mentioned earlier, sequence analysis of the promoter of the rat ANP gene revealed a conserved NFAT binding site located between nucleotides -314 to -300 (Seidman et al., 1988). A role for the Ca2+-Calcineurin-NFAT signaling pathway in ANP promoter activity was obtained by transient transfection of the rat ANP promoter into H9C2 cells. A significant increase in ANP-Luciferase reporter activity was observed after 6 hr of leptin treatment, while addition of KN-93, an inhibitor of calcineurin, decreased the leptin-mediated activation of the ANP promoter (Figure 3B). Further evidence of leptin mediated activation of the NFAT signaling pathway was obtained by immunofluorescence analysis. As seen in Figure 3C, leptin treatment triggered nuclear translocation of NFATc4 in H9C2 cells. The morphology of the cardiac cell was visualized by staining the actin filament and the nuclei.
Fig. 3. Leptin activates the rat Atrial natriuretic peptide promoter via the NFAT signaling pathway.
A, the Northern blot shows the transcriptional induction of ANP in leptin-treated (100 ng/ml) rat cardiac cell (H9C2). We determined the expression of β-actin as a loading control. B, transient transfection of the rat ANP promoter using luciferase expression as a reporter in H9C2 cells. A significant increase in luciferase activity was obtained after 6 h of leptin treatment. During leptin treatment, KN-93, an inhibitor of calcineurin activity shows a significant decrease in the transcriptional activity of the ANP promoter. The bar graph shows the results obtained from triplicate experiments. One way Anova and Bonferroni’s test were used. Values are means ± s.e.m * p < 0.05 is statistical significant. C, immunofluorescence analysis using rat H9C2 cell treated with leptin as before. Cardiomyocytes were fixed and incubated with polyclonal anti-NFATc4. Nuclear staining and cell morphology were obtained by staining with DAPI and Alexa fluor 488 phalloidin respectively.
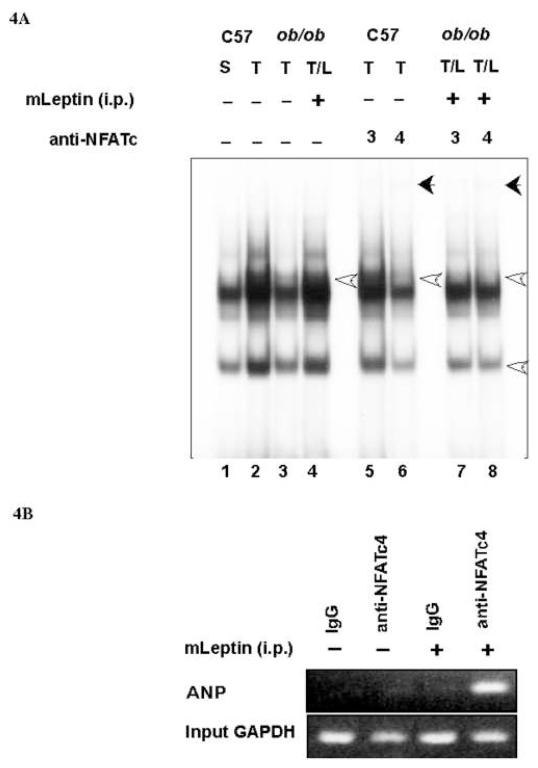
Characterization of NFAT activation by leptin in the left ventricle was obtained by gel mobility shift assay (GMSA). We use an oligonucleotide containing the conserved NFAT binding site present in the promoter of the ANP gene (-317 to -295) and nuclear extracts from the left ventricle of sham (S), and TAC (T) of both C57BL6/J and ob/ob mice. As seen in Figure 4A, there was an increase in protein-DNA interaction (see open arrow) in nuclear extracts of wild type mice subjected to TAC (lane 2) as compared with sham (lane 1). However, nuclear extracts from left ventricle of ob/ob mice subjected to TAC showed no significant change in protein-DNA interaction (lane 1 vs lane 3). Chronic treatment of ob/ob mice subjected to TAC with leptin resulted in an increase in protein-DNA complex formation (lane 1 vs lane 4) similar to those observed in extracts from wild type mice subjected to TAC (lane 2). Pre-incubation of the nuclear extracts of C57BL6/J mice heart subjected to TAC with antibodies against NFATc3 and NFATc4 shows that wild type mice subjected to TAC activates primarily NFATc4, as a decrease in protein-DNA complex was observed when the nuclear extracts were pre-incubated with anti-NFATc4 (lane 6 vs. lane 2), as well as a supershift (see closed arrow). Similar extracts pre-incubated with anti-NFATc3 antibodies did not alter the protein-DNA interaction (lane 5). However, nuclear extracts from ob/ob mice subjected to TAC and chronically treated with leptin revealed the presence of both NFATc3 and NFATc4 in the protein-DNA complexes, as the antibodies appear to reduce the major complexes (compare lane 7 or 8 vs lane 4), and to generate a supershift (closed arrow). In order to establish the transcriptional role of the NFATc4 on the ANP promoter in vivo, we performed chromatin immunoprecipitation (ChIP) assays with tissue extracts from left ventricle of ob/ob mice control or treated with leptin. As observed in Figure 4B, using primer specific for the promoter of the ANP gene, a PCR product was observed in tissue extracts treated with leptin and immunoprecipitated with anti-NFATc4. As an internal negative control we performed the immunoprecipitation with rabbit IgG which failed to amplify the ANP promoter, and as positive control, we used input DNA from each group to amplify a region encompassing the mouse GAPDH promoter.
Fig. 4. NFATc4-DNA interaction on the ANP promoter is regulated by leptin.
A, GMSA was done using the conserved NFAT-binding site and nuclear extracts obtained from sham and TAC mice of C57BL/6J and ob/ob background. Transverse aortic constriction (T) shows a significant increase in protein-DNA interaction in nuclear extracts from C57BL/6J mice subjected to TAC (lane 2 vs lane 1). However, the increase in binding activity was absent in nuclear extract of ob/ob mice subjected to TAC (Lane 3 vs lane 1). Increase in NFAT-binding activity was restored after intraperitoneal administration of leptin in ob/ob mice (lane 4 vs lane 3). The identity of one of the proteins in the protein-DNA complexes was obtained by preincubating the nuclear extracts with anti-NFATc3 or NFATc4 antibodies. Nuclear extracts from wild type mice subjected to TAC shows NFATc4 binding (lane 6), while, intraperitoneal injection in ob/ob TAC mice triggers NFATc3 and NFATc4 binding activities (lane 7 and lane 8). B, ChIP assay revealed the PCR amplification of the ANP promoter region after immunoprecipitation with anti-NFATc4 antibody of protein extracts from the left ventricle of ob/ob mice treated with leptin. Equal input DNA was used to amplify the promoter of the GAPDH gene as control for loading.
4. Discussion
Several reports suggest a correlation between the circulating levels of leptin, LVmass and myocardial wall thickness in non-obese hypertensive patients. Despite such a correlation, it is not clear whether an over-reactive or an impaired leptin signaling is involved. The data on mice lacking leptin (ob/ob) suggest that leptin plays an anti-hypertrophic role because these mice developed LVH readily and show an increase in LVmass and wall thickness. In vivo studies in ob/ob hearts undergoing obesity mediated hypertrophy that may explain the anti-hypertrophic role of leptin and the involvement of molecular signaling pathways have identified a decreased catalytic activity (p110α) of the phosphoinositide 3-kinase (PI3K) (Trivedi et al., 2008). Other reports showed depressed levels of β-adrenergic response, as well as changes in the expression of Ca2++ dependent excitation-contraction coupled components, such as SR Ca2++-ATPase and phosphorylation of phospholambam in ob/ob mice (Minhas et al., 2005), and a decrease in diastolic dysfunction (Christoffersen et al., 2003). Here, we report that ob/ob mice subjected to pressure overload hypertrophy developed a markedly enhanced LVH. These observations seem to correlate with the increase in LVmass reported in obese humans (Perego et al., 2005), and ob/ob mice (Barouch et al., 2003). Although our echocardiography data revealed a significant left ventricular remodeling, the fractional shortening remained unchanged suggesting a functional compensatory response during the time frame of our study. Similar observations have been reported previously in which 6 month old ob/ob mice hearts developed significant morphological changes while maintaining systolic function as measured by fractional shortening (Barouch et al., 2003). Other studies, however, reported a significant change in fractional shortening in ob/ob mice when subjected to acute myocardial infarction (MacGaffin et al., 2008). The severity in the reduction of fractional shortening during acute myocardial ischemia is likely due to the traumatic nature of the ischemic stress. We have reported that subjecting mice to pressure overload hypertrophy for 2 weeks allow the remodeling to occur as well as the known hypertrophic compensatory response to be established while maintaining normal systolic function (Beckles et al., 2006). We observed additional remodeling in ob/ob mice subjected to TAC that included the increased collagen deposition. Previous studies also described the increase in cardiac myocyte cross sectional area but did not observe collagen deposition (Minhas et al., 2005). Perhaps because that study used obesity as the stimulus for the trigger of LVH as compared with our study where the hearts were subjected to pressure overload hypertrophy.
The significant increase in LVmass as well as in collagen deposition in ob/ob mice subjected to TAC are also features observed in mice lacking ANP . Indeed, ANP null mice are characterized as being hypertensive (John et al., 1995), having left ventricular hypertrophy (Feng et al., 2003) and having increase extracellular matrix in the heart (Wang et al., 2003). The specific observation that the enhanced LVmass and extracellular deposition identified in ob/ob mice may share similar molecular pathways as the ANP null mice, was revealed by a blunted ANP expression in ob/ob mice subjected to TAC. The induction of ANP was restored in ob/ob mice subjected to TAC after intraperitoneal injection of leptin. Thus, it appeared that the molecular pathways eliciting the anti-hypertrophic response via ANP up-regulation are inhibited in ob/ob mice. The evidence in support of leptin as an inhibitor of left ventricular growth in animal models comes from direct infusion of leptin into ob/ob mice during obesity induced left ventricular hypertrophy that resulted in a decrease in LVmass (Minhas et al., 2005), and reversal of remodeling due to coronary artery ligation (McGaffin et al., 2008). Although both reports demonstrated the anti-hypertrophic role of leptin in vivo, neither reports addressed the molecular pathways involved.
Here, we provide evidence that leptin activates the transcription of the ANP gene via the NFATc4. Given the cardioprotective role of ANP during LVH, we suggest that the NFAT pathway is not only involved in pathological hypertrophy (Wilkins et al., 2004), but also compensatory hypertrophy. The lack of increase in NFATc4-DNA complex formation in ob/ob mice undergoing LVH was restored after intraperitoneal injection of leptin. Further supporting the transcriptional role of NFATc4 on the ANP promoter was the confirmation by ChIP assay that NFATc4 is activated by leptin and binds to the conserved NFAT domain in the ANP promoter in left ventricular tissue of leptin-treated ob/ob mice (Figure 4B). Perhaps, the NFAT transcription factors are induced in a temporal fashion during the transition from compensatory to pathological LVH, and it appears that the role of each NFAT protein during pressure overload hypertrophy is not interchangeable. Indeed, the essential role in the development of concentric hypertrophy due to pressure overload hypertrophy for NFATc3 but not NFATc4 was identified earlier, where the genetically modified mice lacking the NFATc4 gene developed LVH (Wilkins et al., 2002).
Other reports have suggested that leptin is pro-hypertrophic effect, and to activate well established hypertrophic signal transduction pathways in culture ventricular cardiomyocytes, however, this pro-hypertrophic role of leptin has been reported only in vitro (Rajapurohitam et al., 2003; Xu et al., 2004; Abe et al., 2007).
Taken together, we proposed that in ob/ob mice the impaired leptin signaling is unable to activate NFATc4 followed by a deficiency in up-regulation of the expression of natriuretic peptide that will facilitate the development of LVH. Thus, the anti-hypertrophic role of leptin in vivo requires the activation of NFATc4 followed by an increase in the expression of the atrial natriuretic peptide gene.
Acknowledgments
Funding This work was supported by Grant No. HL073399 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Ono K, Kawamura T, Wada H, Kita T, Shimatsu A, Hasegawa K. Leptin induces elongation of cardiac myocytes and causes left ventricular dilation with compensation. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2387–96. doi: 10.1152/ajpheart.00579.2006. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- Beckles DL, Mascareno E, Siddiqui MAQ. Inhibition of Jak2 phosphorylation attenuates pressure overload cardiac hypertrophy. Vascul. Pharmacol. 2006;45:350–357. doi: 10.1016/j.vph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–3490. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- Clerk A, Cullingford TE, Fuller SJ, Giraldo A, Markou T, Pikkarainen S, Sugden PH. Signaling pathways mediating cardiac myocite gene expression in physiological and stress responses. J. Cell. Physiol. 2007;212:311–322. doi: 10.1002/jcp.21094. [DOI] [PubMed] [Google Scholar]
- de Simone G. Morbid obesity and left ventricular geometry. Hypertension. 2007;49:7–9. doi: 10.1161/01.HYP.0000251714.60547.06. [DOI] [PubMed] [Google Scholar]
- Feng JA, Perry G, Mori T, Hayashi T, Oparil S, Chen YF. Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin. Exp. Pharmacol. Physiol. 2003;30:343–349. doi: 10.1046/j.1440-1681.2003.03836.x. [DOI] [PubMed] [Google Scholar]
- Franco V, Chen F, Oparil S, Feng JA, Wang D, Hage F, Perry G. Atrial natriuretic peptide dose-dependently inhibits pressure overload-induced cardiac remodeling. Hypertension. 2004;44:746–50. doi: 10.1161/01.HYP.0000144801.09557.4c. [DOI] [PubMed] [Google Scholar]
- Gardner DG, Chen S, Glenn DJ, Grisby CL. Molecular biology of natriuretic peptide system: implication for physiology and hypertension. Hypertension. 2007;49:419–426. doi: 10.1161/01.HYP.0000258532.07418.fa. [DOI] [PubMed] [Google Scholar]
- Houweling AC, van Borren MM, Moorman AF, Christoffels VM. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc. Res. 2005;67:583–593. doi: 10.1016/j.cardiores.2005.06.013. [DOI] [PubMed] [Google Scholar]
- John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, Rockman HA, Maeda N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J. Clin. Invest. 2001;107:975–84. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London B. Natriuretic peptides and cardiac hypertrophy. J. Am. Coll. Cardiol. 2006;48:506–507. doi: 10.1016/j.jacc.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Mascareno E, Dhar M, Siddiqui MAQ. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc. Natl. Acad. Sci. USA. 1988;95:5590–94. doi: 10.1073/pnas.95.10.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaffin KR, Sun CK, Rager JJ, Romano L, Zou B, Mathier MA, Doherthy RM, McTiernan CF, O’Donnell CP. Leptin signaling reduces the severity of cardiac dysfunction and remodeling after chronic ischaemic injury. Cardiovasc. Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023. [DOI] [PubMed] [Google Scholar]
- Minhas KM, Khan SA, Raju SV, Phan AC, Gonzalez DR, Skaf MW, Lee K, Tejani AD, Saliaris AP, Barouch LA, O’Donnell CP, Emala CW, Berkowitz DE, Hare JM. Leptin repletion restores depressed β-adrenergic contractility in ob/ob mice independently of cardiac hypertrophy. J. Physiol. 2005;565:463–474. doi: 10.1113/jphysiol.2005.084566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc. Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc. Natl. Acad. Sci. U S A. 1997;94:14730–5. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, de Divitiis O, Varricchio M. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34:1047–1052. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P, Barbieri M, Morabito A, Paolisso G, Folli F, Pontiroli AE. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J. Clin. Endocrinol. Metab. 2005;90:4087–4093. doi: 10.1210/jc.2004-1963. [DOI] [PubMed] [Google Scholar]
- Pladevall M, Williams K, Guyer H, Sadurni J, Falces C, Ribes A, Pare C, Brotons C, Gabriel R, Serrano-Rios M, Haffner S. The association between leptin and left ventricular hypertrophy: a population-based cross-sectional study. J. Hypertens. 2003;21:1467–1473. doi: 10.1097/00004872-200308000-00009. [DOI] [PubMed] [Google Scholar]
- Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ. Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- Ritter O, Neyses L. The molecular basis of myocardial hypertrophy and heart failure. Trends. Mol. Med. 2003;9:313–321. doi: 10.1016/s1471-4914(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Seidman CE, Wong DW, Jarcho JA, Bloch KD, Seidman JG. Cis-acting sequences that modulate atrial natriuretic factor gene expression. Proc. Natl. Acad. Sci. USA. 1988;85:4104–08. doi: 10.1073/pnas.85.11.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swynghedauw B. Phenotypic plasticity of adult myocardium: molecular mechanisms. J. Exp. Biol. 2006;209:2320–2327. doi: 10.1242/jeb.02084. [DOI] [PubMed] [Google Scholar]
- Temsah R, Nemer M. GATA factors and transcriptional regulation of cardiac natriuretic peptide genes. Regul. Pept. 2005;128:177–185. doi: 10.1016/j.regpep.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yang R, Barouch LA. Decreased p110 catalitic activity accompanies increased myocyte apoptosis and cardiac hypertrophy in leptin deficient ob/ob mice. Cell Cycle. 2008;7:560–565. doi: 10.4161/cc.7.5.5529. [DOI] [PubMed] [Google Scholar]
- Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SW, Chen YF. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- Wilkins B, De Windt L, Bueno O, Braz J, Glascock B, Kimball TR, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophy growth. Mol. Cell. Biol. 2002;22:7603–7613. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, Luo JD. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation. 2004;110:1269–1275. doi: 10.1161/01.CIR.0000140766.52771.6D. [DOI] [PubMed] [Google Scholar]
- Yang R, Barouch LA. Leptin signaling in obesity: cardiovascular consequences. Circ. Res. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- Zang MX, Li Y, Xue LX, Jia HT, Jing H. Cooperative activation of atrial natriuretic peptide promoter by dHAND and MEF2C. J. Cell. Biochem. 2004;93:1255–66. doi: 10.1002/jcb.20225. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]