Abstract
Purpose
To develop a magnetic resonance histology (MRH) staining and fixation method by immersion to enhance the signal-to-noise ratio (SNR) with a paramagnetic contrast agent permitting microscopic acquisition within a 3-hour scan time.
Materials and Methods
Methods were optimized for gestational day 18.5 rat fetuses and for imaging at 9.4T with an RF refocused spin-echo pulse sequence (TR/TE = 75/5.2 msec). Fixation/staining was performed by immersion in Bouin’s fixative containing varied concentrations of ProHance (from 10:1 to 500:1 Bouin’s:ProHance) and for varied immersion durations (up to 24 hours).
Results
The results showed a significant change in T1 and T2 relaxation times as a function of concentration of contrast agent and immersion duration—as contrast agent penetrates the tissues, T1 was reduced as desired (typically by 10X), but at the same time, T2 was profoundly reduced (typically by 3X) due to both protein cross-linking from the fixative and the high concentration of contrast agent. A systematic assessment of this staining protocol showed increased SNR by 5 times over that in unstained specimens.
Conclusion
This staining protocol reduced scan time for very high-resolution images (19.5 microns) to only 3 hours, making MRH a routine tool for evaluating fetal development.
Keywords: MR microscopy, histology, staining, rat fetus
INTRODUCTION
The increasing use of genetically modified models of both mice and rats in a wide range of basic sciences has highlighted the need for rapid morphologic screening methods of rodent embryos and fetuses. The potential for MR microscopy was clear in the seminal papers of Lauterbur (1) and Mansfield (2). The technology was first applied to study embryonic development in 1986 (3). Subsequently, MR microscopy has been used in numerous studies of developing embryos and fetuses (4). Most conventional histology studies of embryos use paraformaldehyde for fixation because of its good tissue preservation (5,6). Fixation in formalin causes protein cross-linking (7,8), which results in rigid-like structures of very short T2 (9). This is not a problem for conventional histology because the tissue is processed for complete dehydration and hardening prior to staining and mounting. However, in proton MR imaging, the reduction of T2 due to binding of macromolecules can have a major impact on the signal.
Contrast agents were first used in embryo studies to highlight the vasculature (10,11). This use of contrast agents required tedious perfusion, which is not readily scaled to high throughput studies. More recently, the concept of active staining has been introduced where specimens are prepared in a mixture of fixative and MR contrast agent (gadolinium chelate) to infiltrate all of the tissues, which provides both good fixation and reduction of spin lattice relaxation time (12). A combined perfusion and immersion method was suggested by Zhang et al. (13) for studies of the chick embryo. But, all of these methods require considerable care in perfusion and cannot be used for rapid screening. We describe here an immersion technique that can be scaled to high throughput. The method is designed to a) provide high-quality fixation for conventional histopathology; b) produce a reduction of T1 to improve the signal-to-noise ratio (SNR); and c) minimize signal losses from reduced T2. The rat was the primary focus in this study for teratology applications, but all results should be readily translated to the mouse.
MATERIALS AND METHODS
Animal Preparation
All animal experiments were performed according to procedures approved by the local Institutional Animal Care and Use Committee.
Embryonic day 0.5 (E0.5) was defined as noon of the day a vaginal plug was detected after overnight mating of Sprague Dawley rats (Charles River Laboratories, Inc., Raleigh, NC). Forty-five E18.5 specimens were obtained after laparotomy and hysterectomy of female rats under general anesthesia (pentobarbital sodium, 100 mg/kg, intraperitoneally; butorphanol, 2 mg/kg, intraperitoneally). After the surgical procedure, the rats were humanely euthanized by an overdose of pentobarbital sodium.
The extracted specimens were placed in icy saline, and then quickly immersed in a mixture of Bouin’s fixative (LabChem Inc., Pittsburgh, PA) and a paramagnetic contrast agent (ProHance®, gadoteridol, Bracco Diagnostics, Inc., Princeton, NJ) for fixation and staining at room temperature for up to 24 hours. Bouin’s fixative is a mixture of three reagents—picric acid, formalin, and acetic acid. Bouin’s/ProHance concentrations ranged from 10:1 (v:v) to 500:1. After fixation, specimens were rinsed in phosphate buffered saline (PBS) and placed in an imaging tube filled with Fomblin® (perfluoro-polyether, Ausimont, Morristown, NJ) to reduce susceptibility artifacts at the surface of the specimen and avoid dehydration. Specimen tubes were designed to snuggly fit each specimen. The majority of our experiments were carried out within 6 hours of specimen removal from the fixative/stain.
Data Acquisition and Measurements
All data were acquired at 9.4 Tesla (400 MHz), using a GE EXCITE console (Epic 11.0) modified for MR microscopy. A solenoid radiofrequency (RF) coil was used for transmit and receive, and its inner diameter x length was 14 x 37 mm. Two-dimensional images were acquired to measure T1 and T2 relaxation times. The matrix size was 256 × 256 (1-mm slice thickness), and the field-of-view (FOV) was 20 × 20 mm2, yielding in-plane resolution of 78 microns. The T1 measurements were obtained by acquiring series of 9 images, using a standard RF refocused spin echo sequence, with TR incremented logarithmically from 10–2560 msec (TE = 6.9 msec), for a total scan time of about 43 minutes (2 averages). Spin lattice relaxation times (T1) were calculated on a pixel-by-pixel basis from the series of 2D images by performing a 2-parameter least squares fit to the ln (STR/ S2560), where STR and S2560 are the signal in a given pixel at given TR and at TR = 2560 msec, respectively. Since the T1s of stained tissues are all less than 500 msec, S2560 represents the equilibrium magnetization at 5*T1. The signal recovery is described by Equation 1:
| [1] |
Histograms of the resulting calculated T1 images were derived to demonstrate the distribution of relaxation times. Histograms of the effective proton density were obtained from the 2D images acquired with TR = 2560 msec and TE = 6.9 msec. This effective proton density includes the true proton density, i.e. the number of spins per cc, modulated by the spin-spin relaxation time and diffusion.
The T2 measurements were obtained by acquiring series of 16 images, using a Carr-Purcell-Meiboom-Gill (CPMG) multi-echo sequence (total of 16 echoes) with TE ranging from 5.2–83.2 msec or 4.47–71.52 msec, inter-echo spacing of 5.2 msec or 4.47 msec, and TR = 2000 msec or 1000 msec, respectively. The total acquisition time with 2 averages was about 17 minutes or 8.5 min, respectively. The T2 images were calculated similarly to the T1 maps, using a least squares fit to Equation 2:
| [2] |
where STEmin was the signal at minimum TE.
Three-dimensional images were acquired using a 3D RF refocused spin warp encoding sequence with an initial non-selective π /2 pulse and a non-selective π refocusing pulse to generate the spin echo. Array sizes were 512 × 256 × 256 or 768 × 384 × 384 (partial Fourier encoding), covering a 20 × 10 × 10 mm FOV. The 768 × 384 × 384 volume was sampled asymmetrically, such that from the full sampling window [−kmax, +kmax], only [−kmax, −kmax+50%kmax] was covered along the frequency axis and both phase-encoding axes. The gain of the spectrometer was increased for the higher order encodings in the periphery of k-space, thereby increasing the effective dynamic range of the digitizer (14). This volume was then zero-filled to 1024 × 512 × 512 for an isotropic resolution of 19.5 microns (G. A. Johnson et al., unpublished data). This protocol effectively reduced TE and the total acquisition time. TR was 100 msec (for the lower resolution volume acquisitions at 39 microns) and 75 msec (for the higher resolution volume acquisitions at 19.5 microns), and TE was 5.5 msec and 5.2 msec, respectively.
RESULTS
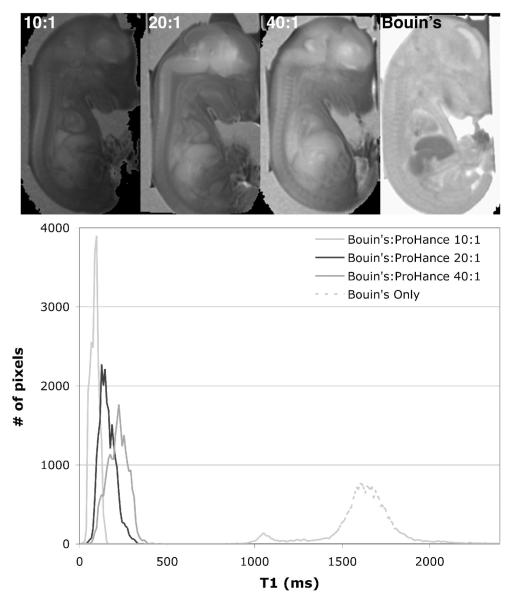
To assess the effect of concentration of contrast agent on T1, E18.5 fetuses were split into 4 groups of 3 specimens and immersed for fixation/staining for 3.5 h in the following mixtures: group 1 was soaked in Bouin’s only (no ProHance); group 2 was soaked in a mixture of Bouin’s and ProHance at a volume concentration of 40:1; group 3 was soaked in Bouin’s:ProHance at 20:1; and group 4 was soaked in Bouin’s:ProHance at 10:1. Figure 1 shows a representative T1 map (Fig. 1a) and its corresponding histogram (Fig. 1b) for each of the 4 groups. A region of interest (ROI) covering the whole specimens was used to derive the histograms, and the values given (mean and spread of histograms) are those averaged over the 3 specimens with their corresponding inter-specimen standard deviation. Without ProHance, the mean of the distribution was about 1570±160 msec with a spread of 411±145 msec. This spread is the standard deviation of the histogram, and therefore serves as another measure of the contrast agent penetration (i.e. high penetration corresponds to small spread). With addition of ProHance, T1 dropped to about 266±77 msec with a spread of 84±42 msec for the 40:1 dilution; 115±41 msec with a spread of 78±23 msec for the 20:1 dilution; and 74±22 msec with a spread of 42±17 msec for the 10:1 dilution. Those results showed that as the concentration of the contrast agent increased, T1 decreased and the distribution became narrower. This corresponded to a more complete and uniform penetration of the ProHance, therefore making the tissue T1s more homogeneous. Also note that the inter-specimen standard deviations decreased with increasing concentration: the initial variation from fetus to fetus, due to size differences (± 0.5 days in a given litter), became less significant as contrast agent was added.
Figure 1.
T1 maps (top) and corresponding histograms (bottom) of E18.5 fetuses immersed in Bouin’s:ProHance at (left to right) 10:1, 20:1, 40:1, and Bouin’s only. As the concentration of ProHance increases, T1 averaged over the whole body is reduced from ~1600 msec (Bouin’s only), to ~270 msec (40:1 Bouin’s:ProHance), ~160 msec (20:1), and ~90 msec (40:1).
The effect of immersion duration was assessed by measuring T1 and T2 for the following 5 groups, each containing 3 fetuses: fresh specimens, specimens bathed in Bouin’s:ProHance @ 20:1 for 3.5 h, 6 h, 12 h, and 24 h. For clarity, only data from fresh, 3.5 h and 24 h are reported here.
In a similar fashion to the concentration experiment, T1 and T2 histograms were plotted from an ROI covering the whole body in the T1 and T2 maps, respectively. The values were averaged over the 3 specimens, and the means and spreads were recorded with their inter-specimen standard deviation. T1 values are given in Table 1, which also reports values for selected organs (in which case the ROI was covering the most part of the organ of interest). For a fixed concentration of 20:1 Bouin’s:ProHance, as the time of immersion increased, the mean T1 was reduced from 3140 msec for fresh tissue, to 293 msec for an immersion of 3.5 h, and to 14 msec for a 24-h immersion. In a comparable way to the concentration experiment, increasing immersion duration caused the spreads and inter-specimen standard deviations to decrease, denoting a more homogeneous T1 distribution. In addition, at prolonged immersion times, signal loss from T2 reduction became significant.
Table 1.
Calculated T1s for different organs of E18.5 fetuses (N=3-4)
| Fresh | 3.5 h | 24 h | ||||
|---|---|---|---|---|---|---|
| Mean (msec) |
Spread (msec) |
Mean (msec) |
Spread (msec) |
Mean (msec) |
Spread (msec) |
|
| Whole | 3140 ± 347 | 1500 ± 436 | 293 ± 65 | 355 ± 57 | 14 ± 3 | 13 ± 3 |
| Brain | 4436 ± 1122 | 684 ± 212 | 1523 ± 166 | 244 ± 119 | 44 ± 15 | 7 ± 3 |
| Heart | 3936 ± 809 | 755 ± 303 | 531 ± 173 | 78 ± 34 | 20 ± 2 | 5 ± 2 |
| Liver | 2121 ± 322 | 394 ± 177 | 577 ± 71 | 81 ± 9 | 34 ± 9 | 7 ± 1 |
Representative T2 maps (Fig. 2a) and corresponding histograms (Fig. 2b) showed a significant decrease of T2 with increasing immersion duration. For fresh tissue, T2 was about 58±2.4 msec with a spread of 35±2.4 msec. After a 3.5-h immersion in Bouin’s:ProHance, T2 dropped to 30±1.5 msec with a spread of 31±4.9 msec, and after 24 h, it was about 17±2.6 msec with a spread of 49±13.8 msec (this large spread includes the non-significant residues of the higher valued tail of the distribution).
Figure 2.
T2 maps (top) and corresponding histograms (bottom) of E18.5 fetuses stained for different durations (left to right): fresh tissue, 3.5 h, and 24 h. As the immersion duration increases, T2 average over the whole body is reduced from ~60 msec (fresh tissue), to ~30 msec (3.5 h) and to ~17 msec (24 h).
The cause of this dramatic reduction between short and long immersion times is further explained by the histograms of the effective proton density shown in Figure 3. Care was taken to maintain the relative spin density between the specimens by fixing the spectrometer gain and scaling in the reconstruction. Note that the units of the abscissa in Figure 3 are the effective proton density, which includes spin-spin relaxation time and diffusion together with the true proton density. At 9.4 T with FOV < 25 mm, the consequences of T2 and diffusion are significant. For fresh tissues (t = 0, no fixation, no staining) the histogram of the effective proton density ranged from 5000 to 30000. After 3.5 hours of immersion, the histogram ranged from 5000 to 25000, and at 24 hours, the peak of the distribution was shifted to < 15000.
Figure 3.
Histograms of effective proton density (PD) are the highest for fresh specimens at PD(t=0) up to 25000, shifting to PD(t=3.5 hours) ranging from 8000–22000, and finally PD(24 hours) < 8000. As the immersion time increases, the effective proton density decreases due to reduction in T2.
Figure 4a-b demonstrates qualitatively the impact that the immersion time had on the SNR. Figure 4a shows a single coronal slice of an E18.5 specimen immersed for 3.5 hours in the 20:1 Bouin’s:ProHance mixture and scanned using the lower resolution (39 microns) 3D protocol (spin echo, TR/TE = 100/5.5 msec, FOV = 20 × 10 × 10 mm, matrix size = 512 × 265 × 256). Figure 4b shows a comparable slice from a specimen immersed in the same fixative/contrast agent mixture for 24 hours and scanned using the same protocol. Notice the drastic loss of signal from 3.5-hour to 24-hour immersion times due to reduction of T2. For 24 hours of immersion, the SNR was reduced by a factor of 5 over the whole specimen, by more than a factor of 2 in the brain, by a factor of 8 in the liver, and nearly a factor of 10 in the heart over the same organs in the specimen immersed for 3.5 hours.
Figure 4.
Coronal slices from 3D acquisitions (3D spin warp, TR/TE = 100 msec/5.5 msec, FOV = 20×10×10 mm, matrix size = 1024×512×512) of E18.5 specimens immersed for (a) 3.5 hours and (b) 24 hours.
As shown in the studies of the effect of immersion time, T1 and T2 continued to decrease when tissues remained in the fixative mixture [also shown by Hsu et al. (15)]. This suggested that a storage method was necessary to establish equilibrium between the tissues and the medium, and to maintain the desirable relaxation times achieved by the staining in the long-term. E18.5 specimens were immersed for 5 hours in 20:1 Bouin’s:ProHance, then split into 5 groups of 3. Specimens were stored in solutions of phosphate buffered saline (PBS) and ProHance with the following dilutions; a) PBS only; b) PBS:ProHance @ 500:1; c) PBS:ProHance @ 160:1; d) PBS:ProHance @ 80:1; e) PBS:ProHance @ 40:1. All the specimens were stored at 4°C to allow imaging on a weekly basis over a period of 4 weeks.
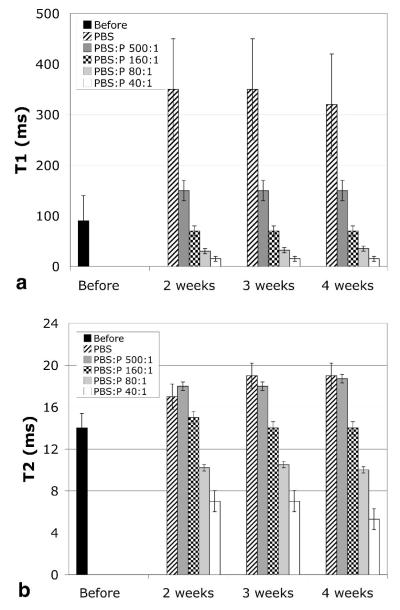
Figure 5a shows the quantitative shift in T1. The mean T1 measurements shown were derived from the T1 histograms of each specimen. Before storage, i.e. immediately after the 5-hour fixation, the peak of the T1 histogram was at 90 msec and the histogram spread (for 4 specimens) was from ~ 10 to 150 msec. Storage in buffered saline allowed the ProHance to diffuse out, which resulted in a shift of the T1 histogram such that the peak was at 340 msec and the spread in T1 values increased to 170–550 msec. Increasing the concentration of the ProHance in the storage solution resulted in a systematic shift of the peak of the T1 histogram to shorter values (< 50 msec for concentrations greater than 80:1). At the same time, the histogram became much more narrow. The accuracy of these measurements may be suspect since the T1s were so short that the sampling intervals for TR were probably not sufficient for accurate measure. Nevertheless, it is clear that one can reduce the T1 of the entire specimen to < 100 msec, which is, of course, the goal of the whole exercise in active staining. But the desirable reduction of T1 is not without penalty as shown in Figure 5b. Reduction in T2 also occurred from increased concentration of contrast agent.
Figure 5.
a) The peak of the T1 histogram of stained E18.5 specimens (N = 3-4) increases during storage for lower concentrations of ProHance (P) and decreases for higher concentrations: T1 (before storage, right after immersion in Bouins:ProHance 20:1 for 5 h) = 90 msec, T1(PBS) = 340 msec, T1(PBS:P 500:1) = 150 msec, T1(PBS:P 160:1) = 70 msec, T1(PBS:P 80:1) = 32 msec, T1(PBS:P 40:1) = 15 msec. T1 remains stable for at least 4 weeks. b) The concentration of ProHance in the storage solution also affects T2: T2(before) = 14 msec, T2(PBS) = 18.3 msec, T2(PBS:P 500:1) = 18.2 msec, T2(PBS:P 160:1) = 14.3 msec, T2(PBS:P 80:1) = 10.2 msec, T2(PBS:P 40:1) = 6.4 msec. T2 remains stable for at least 4 weeks. The bar height (mean) shown in those graphs is the peak in each histogram. The error bars shown are the width of the histograms at half maximum, averaged over the 3–4 histograms, and thus show the spread of the histograms. As the peak of the histograms is reduced, the spread decreases, indicating homogeneous reduction of T1/T2 in all tissues.
Since the T2s of the specimens were quite homogenous, the values shown in Figure 5b were determined from the 16-echo sequence by fitting the mean signal intensity of the entire specimen to the exponential decay (according to Equation 2). The standard deviation was that of the mean T2 of each of the 3 specimens in each group (i.e. inter-specimen deviation). Storage in most of the saline solutions resulted in a desirable increase in T2 as tissues were rehydrated after fixation. But with increasing concentration of ProHance, the T2 of the tissues was steadily reduced. As with the T1s, the equilibrium was reached within two weeks of storage resulting in values that were reasonably constant over the longer periods (3–4 weeks). When one considers Figure 5a and 5b together, it seems clear that the optimal storage solution was PBS:ProHance @ 160:1—this particular dilution established an equilibrium between the tissues and the medium such that T1 and T2 remained stable throughout 4 weeks.
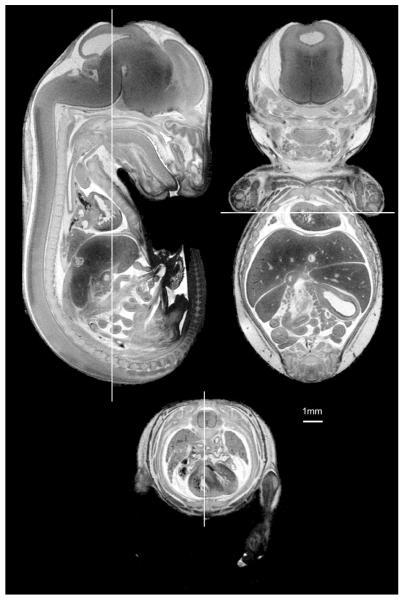
Figure 6 shows an example of the final result of our fixation/staining method: high-resolution 3D images can be acquired in short scan time. The E18.5 specimen was immersed in Bouin’s:ProHance at 20:1 for 6 hours. The images were extracted from a high-resolution 3D volume (spin warp, TR/TE = 75/5.2 msec, FOV = 20 × 10 × 10 mm, matrix size = 1024 × 512 × 512). The voxel size for these images is 19.5 × 19.5 × 19.5 microns and the total scan time was only 3 hours.
Figure 6.
Slices from a 3D dataset of an E18.5 fetus at 19.5-micron isotropic resolution: mid-coronal, mid-sagittal, and axial slices show SNR > 10:1 with an acquisition time of 3 hours (3D spin warp, TR/TE = 75msec/5.2msec, FOV = 20×10×10 mm, matrix size = 1024×512×512).
Good tissue preservation was confirmed with conventional histology on a representative specimen, and allowed us to implement this fixation and staining method to earlier stages of fetal development (E12.5 to E19.5 at 1-day intervals, data not shown). Since the permeability properties of tissues greatly change as the fetus grows, different immersion times were necessary.
DISCUSSION
This study has optimized a method for active staining in the rat fetus. The results first showed that the reduction of T1 depended on the concentration of contrast agent: ~90 msec for Bouin’s:ProHance 10:1; ~160 msec for Bouin’s:ProHance 20:1; and ~270 msec for Bouin’s:ProHance 40:1. Then, it was shown that T1/T2 and the quality of the image (SNR) were affected by the duration of fixation. At short immersion durations, SNR was increased by reduced T1. For longer immersion, T2 was drastically reduced by both high concentration of contrast agent and cross-linking induced by formalin. Thus the effective proton density was also reduced, which led to a significant loss of SNR. Throughout our analysis we have used the term “effective” proton density, which includes both T2 and diffusion decay. Since we did not measure changes in diffusion, we can only infer that the majority of the signal reduction arises from reduced T2 induced by cross-linking. Immersion for 3-6 h resulted in a reasonable tradeoff between the need for good fixation and staining, while limiting the reduction in T2 caused by cross-linking. These three competing phenomena—favorable T1 reduction; unfavorable T2 reduction due to high concentration of ProHance and to excess of fixation; and decrease in the effective proton density, determined the quality of the image. A compromise between a short T1 recovery time, a reasonable T2, and high proton density resulted in SNR increase that could be translated into shorter acquisition times at higher spatial resolution. Finally, a good storage method for equilibrium was found (PBS:ProHance 160:1) such that the contrast agent was retained in the tissues, and the signal loss caused by the fixing/staining solution was limited.
In conclusion, the use of the active stain and optimization of the 3D spin echo sequence has simultaneously allowed us to improve the spatial resolution and reduce the scan time. This reduction in scan time is critical for MR histology to advance from a laboratory curiosity to a mainstream application. The use of multiple coils (16) should permit the simultaneous acquisition of at least 4 specimens. It is relatively easy to envision an automated sample chamber, similar to that used by chemists for spectroscopy studies. These additional advances coupled with the major step forward here will permit routine application of 3D MR histology in the rat and mouse embryo of up to 32 specimens per day. We believe the resulting approach will present a practical method for screening in teratology studies, phenotyping in knockout mice, and further applications yet to be discovered.
ACKNOWLEDGMENTS
All work was performed at the Duke Center for In Vivo Microscopy. We are grateful to Gary Cofer for his expertise in MRI and Boma Fubara for sharing her skills in embryo harvesting. Finally, we are grateful to Anjum Ali Sharief for assistance in performing the quantitative T2 measurements, and to Dr. Robert Maronpot for his histology analysis.
Grant support: NIH/NCRR (P41 RR005959) and NCI (R24 CA092656)
REFERENCES
- 1.Lauterbur PC. Image formation by induced local interactions - examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 2.Mansfield P, Grannell PK. Diffraction in microscopy in solids and liquids by NMR. Phys Rev B. 1975;12:3618. [Google Scholar]
- 3.Bone SN, Johnson GA, Thompson MB. MR microscopy of the developing chick embryo. Invest Radiol. 1986;21:782–787. doi: 10.1097/00004424-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Effmann EL, Johnson GA, Smith BR, Talbot GA, Cofer GP. Magnetic resonance microscopy of chick embryos in ovo. Teratology. 1988;38(1):59–65. doi: 10.1002/tera.1420380109. [DOI] [PubMed] [Google Scholar]
- 5.Schneider JE, Bamforth SD, Farthing CR, Clarke K, Neubauer S, Bhattacharya S. Rapid identification and 3D reconstruction of complex cardiac malformations in transgenic mouse embryos using fast gradient echo sequence magnetic resonance imaging. J Mol Cell Cardiol. 2003;35(2):217–222. doi: 10.1016/s0022-2828(02)00291-2. [DOI] [PubMed] [Google Scholar]
- 6.Nagara H, Inoue T, Koga T, Kitaguchi T, Tateishi J, Goto I. Formalin fixed brains are useful for magnetic resonance imaging (MRI) study. J Neurol Sci. 1987;81(1):67–77. doi: 10.1016/0022-510x(87)90184-5. [DOI] [PubMed] [Google Scholar]
- 7.Walker JF. Formaldehyde. Reinhold; New York: 1964. p. 701. [Google Scholar]
- 8.Pearse AGE. Histochemistry, theoretical and applied. Churchill-Livingstone; London: 1980. p. 624. [Google Scholar]
- 9.Maryanski MJ, Audet C, Gore JC. Effects of cross-linking and temperature on the dose response of a BANG polymer gel dosimeter. Phys Med Biol. 1997;42(2):303–311. doi: 10.1088/0031-9155/42/2/004. [DOI] [PubMed] [Google Scholar]
- 10.Smith BR, Effmann EL, Johnson GA. MR microscopy- chick embryo vasculature. JMRI. 1992;2:237–240. doi: 10.1002/jmri.1880020220. [DOI] [PubMed] [Google Scholar]
- 11.Smith BR, Johnson GA, Groman EV, Linney EA. Magnetic resonance microscopy of mouse embryos. Proc Natl Acad Sci U S A. 1994;91(9):3530–3533. doi: 10.1073/pnas.91.9.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson GA, Cofer GP, Gewalt SL, Hedlund LW. Morphologic phenotyping with magnetic resonance microscopy: the visible mouse. Radiology. 2002;222(3):789–793. doi: 10.1148/radiol.2223010531. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Yelbuz TM, Cofer GP, Choma MA, Kirby ML. Improved preparation of chick embryonic samples for magnetic resonance microscopy. Magn Reson Med. 2003;49(6):1192–1195. doi: 10.1002/mrm.10460. [DOI] [PubMed] [Google Scholar]
- 14.Maudsley AA. Dynamic range improvement in NMR imaging using phase scrambling. JMR. 1987;76:287–305. [Google Scholar]
- 15.Hsu JCM, Johnson GA, Smith WM, Reimer KA, Ideker RE. Magnetic resonance imaging of chronic myocardial infarcts in formalin fixed human autopsy specimens. Circulation. 1994;89(5):2133–2140. doi: 10.1161/01.cir.89.5.2133. [DOI] [PubMed] [Google Scholar]
- 16.Bock NA, Konyer NB, Henkelman RM. Multiple Mouse MRI. Magn Reson Med. 2003;49(1):158–167. doi: 10.1002/mrm.10326. [DOI] [PubMed] [Google Scholar]