Abstract
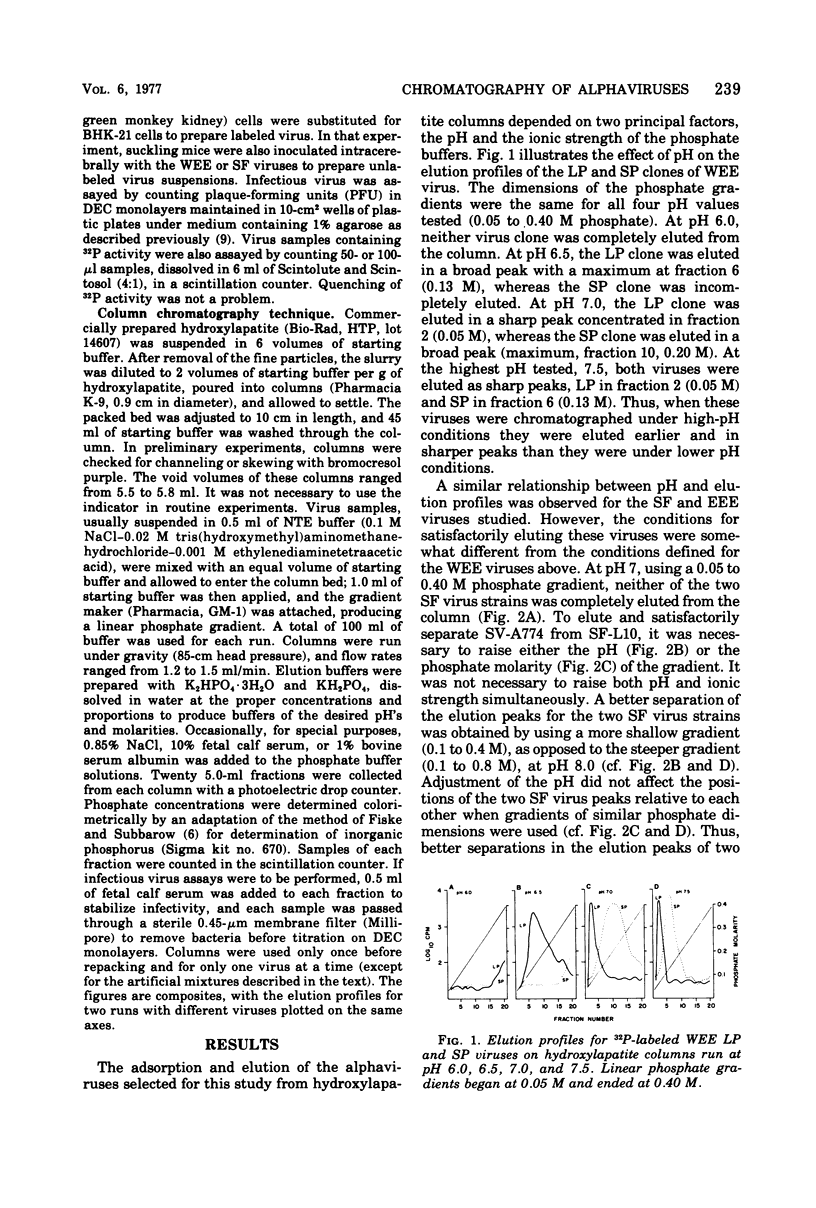
Hydroxylapatite column chromatography methods were developed to characterize selected alphavirus populations. Different conditions of pH and phosphate molarity were required to obtain satisfactory elution profiles and separations for Western equine encephalomyelitis virus strains, compared with Eastern equine encephalomyelitis virus and Semliki Forest virus strains. Raising the pH of the buffers effected earlier elutions of all viruses. Selection of phosphate gradients with more gentle slopes and adjustment to the proper pH effected better separations of virus subpopulations. Elution profiles were not affected by 0.85% NaCl, 10% fetal calf serum, or 1% bovine serum albumin, which are common constituents of virus-stabilizing diluents. Passage of Western equine encephalomyelitis and Semliki Forest viruses in BHK-21, Vero, or duck embryo cell cultures or in suckling mouse brains did not usually affect elution profiles, unless passage also resulted in a shift in the plaque size marker. Essentially all infectious virus applied to the column was recoverable in appropriate fractions. This permitted accurate determinations of heterogeneity within alphavirus populations. For Western equine encephalomyelitis large-plaque (LP) and small-plaque (SP) virus populations, it was possible to detect ratios of 1 LP in a population of 106 SP, and 1 SP in 103 LP by using linear phosphate gradients. When stepwise elution procedures were used, it was possible to detect ratios of 1 SP in a population of 105 LP. Hydroxylapatite column chromatography therefore appears to be a useful tool for characterizing alphaviruses and for isolating minority subpopulations of viruses of biological or epidemiological importance from apparently homogeneous virus stocks.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Burness A. T. Separation of plaque-type variants of encephalomyocarditis virus by chromatography on calcium phosphate. J Virol. 1967 Apr;1(2):308–316. doi: 10.1128/jvi.1.2.308-316.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming P. Semliki forest virus-chick embryo cell interactions. J Gen Virol. 1973 Jun;19(3):353–367. doi: 10.1099/0022-1317-19-3-353. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B., Dendy E., Eddy G. A. Correlates to increased lethality of attenuated Venezuelan encephalitis virus vaccine for immunosuppressed hamsters. Infect Immun. 1974 May;9(5):924–930. doi: 10.1128/iai.9.5.924-930.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Gorelkin L. Selective clearance of a benign clone of Venezuelan equine encephalitis virus from hamster plasma by hepatic reticuloendothelial cells. J Infect Dis. 1975 Dec;132(6):667–676. doi: 10.1093/infdis/132.6.667. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B. Virulence heterogeneity of a predominantly avirulent Western equine encephalitis virus population. J Gen Virol. 1976 Jul;32(1):121–128. doi: 10.1099/0022-1317-32-1-121. [DOI] [PubMed] [Google Scholar]
- OZAKI Y., DIWAN A. R., TAKIZAWA M., MELNICK J. L. CHROMATOGRAPHY OF POLIOVIRUS ON CALCIUM PHOSPHATE AND ITS APPLICATION TO THE IDENTIFICATION OF VACCINE PROGENY STRAINS. J Bacteriol. 1965 Mar;89:603–610. doi: 10.1128/jb.89.3.603-610.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen C. E., Jr, Slocum D. R., Levitt N. H. Chromatography of Venezuelan equine encephalomyelitis virus strains on calcium phosphate. Appl Microbiol. 1972 Jul;24(1):91–95. doi: 10.1128/am.24.1.91-95.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. A., Johnson K. M. Antigenic variants of Venezuelan equine encephalitis virus: their geographic distribution and epidemiologic significance. Am J Epidemiol. 1969 Mar;89(3):286–307. doi: 10.1093/oxfordjournals.aje.a120942. [DOI] [PubMed] [Google Scholar]


