Abstract
There is an emerging understanding of the importance of the vascular system within stem cell niches. Here we examine whether neural stem cells (NSCs) in the adult subventricular zone (SVZ) lie close to blood vessels, using 3-dimensional wholemounts, confocal microscopy and automated computer-based image quantification. We found that the SVZ contains a rich plexus of blood vessels that snake along and within neuroblast chains. Cells expressing stem cell markers, including GFAP, and proliferation markers, are closely apposed to the laminin-containing extracellular matrix (ECM) surrounding vascular endothelial cells. Apical GFAP+ cells are admixed within the ependymal layer and some span between the ventricle and blood vessels, occupying a specialized microenvironment. Adult SVZ progenitor cells express the laminin receptor alpha6beta1 integrin, and blocking this inhibits their adhesion to endothelial cells, altering their position and proliferation in vivo, indicating it plays a functional role in binding SVZ stem cells within the vascular niche.
Introduction
The microenvironment or niche is a key regulator of stem cell behavior in vivo (Fuchs et al., 2004). Adult NSCs generate neurons throughout life in the murine forebrain SVZ and the hippocampal dentate gyrus, unique stem cell niches that instruct neurogenesis (Alvarez-Buylla and Lim, 2004). An important goal of adult NSC studies is to understand the nature of the adult neurogenic niche, in order to facilitate NSC self-renewal and neural cell generation in vitro and in vivo.
Previous studies have identified the major neural cell types and their lineal relationships in the adult SVZ: Type B stem cells give rise to Type C transit amplifying cells, which in turn produce the Type A neuroblasts (Doetsch, 2003). Type B and Type C cells form a tubular network through which Type A neuroblasts migrate in the rostral migratory stream (RMS) towards the olfactory bulbs. These neurogenic tubes lie on the striatal wall of the lateral ventricle, directly beneath the ependymal layer (Doetsch et al., 1997). The neural cells are exposed to an ECM that is thought to trap niche growth factors; this matrix includes ‘fractones’: slender extravascular basal lamina structures that contain laminin (Kerever et al., 2007; Mercier et al., 2002).
Vascular cells are key elements of other stem cell niches, for example in the adult hippocampus (Palmer et al., 2000), the songbird ventricular zone (Louissaint et al., 2002), the bone marrow (Kiel et al., 2005), the intestine and skin (Fuchs et al., 2004). Moreover, brain cancer stem cells have an affinity for blood vessels, migrating along them during tumor spread, and stimulating their growth through VEGF secretion (Gilbertson and Rich, 2007). The SVZ of the MRL mouse, which has enhanced regenerative wound healing, exhibits increased proliferation associated with blood vessels (Baker et al., 2006). However the relationship of normal NSCs to blood vessels in the largest adult CNS germinal niche, the SVZ, is unknown. We have shown previously that endothelial cells release soluble factors that stimulate embryonic and adult SVZ NSC self-renewal and neurogenesis (Shen et al., 2004). However whether endothelial cells similarly influence NSCs in vivo is unclear.
Here we examine the relationship of adult SVZ NSC lineage cells to blood vessels using confocal imaging of SVZ wholemounts in which the normal 3-D relationships of cells are preserved. We quantified the cell-cell relationships in the niche using computational image analysis, building on software developed for studies of the parenchymal neuro-vascular niche (Lin et al., 2005). This allowed objective and quantitative description of the spatial relationships of large numbers of specified germinal niche elements. A quantitative description of the structure of the normal SVZ niche is valuable, as it provides a mathematical basis to understand how the niche is unique, and how it changes in aging or pathological situations.
This analysis of the 3D tissue revealed a prominent network of blood vessels running within the SVZ, and showed that NSCs, which express GFAP, lie intimately close to the vascular surface. It also revealed distinct layers of SVZ GFAP-GFP+ cells: The most apical (ventricular) layer is actually incorporated within the ependymal layer, and these cells sometimes contact both the ventricle and the vascular surface. Beneath this is a layer of tangential GFAP+ cells with long processes oriented along neuroblast chains and sometimes along co-aligned blood vessels. Moreover, we found that adult NSCs express the laminin receptor α6β1 integrin (VLA6), which is lost as they differentiate, and we demonstrate that this receptor plays a critical role in NSC adhesion to vascular cells and in regulating the SVZ lineage proliferation in vivo. Given the presence of blood vessels in other stem cell niches and the prevalence of α6 integrin expression on other stem cell types (Fortunel et al., 2003), it is possible that this molecular interaction may prove to be generally significant.
This study provides a new perspective of the vascularization of the SVZ and the importance of blood vessels to the SVZ niche.
Results
The adult mouse SVZ contains a dense network of blood vessels
Prior studies of transverse sections revealed blood vessels in the adult SVZ (Baker et al., 2006; Mercier et al., 2002). To see an overview of vascularization, we examined SVZ wholemounts (Figure. 1A). The central SVZ has large blood vessels originating from the ventral aspect (Figure 1B, arrowhead), as described previously (Dorr et al., 2007). The dorsal SVZ, which flows into the RMS, has a network of small vessels (Figure 1B, arrows) that run mainly in the anterior-posterior direction. Because of the high density of blood vessels in the dorsal aspect, we focused our studies there.
Figure 1. A dense plexus of blood vessels and associated Laminin+ structures in the SVZ.
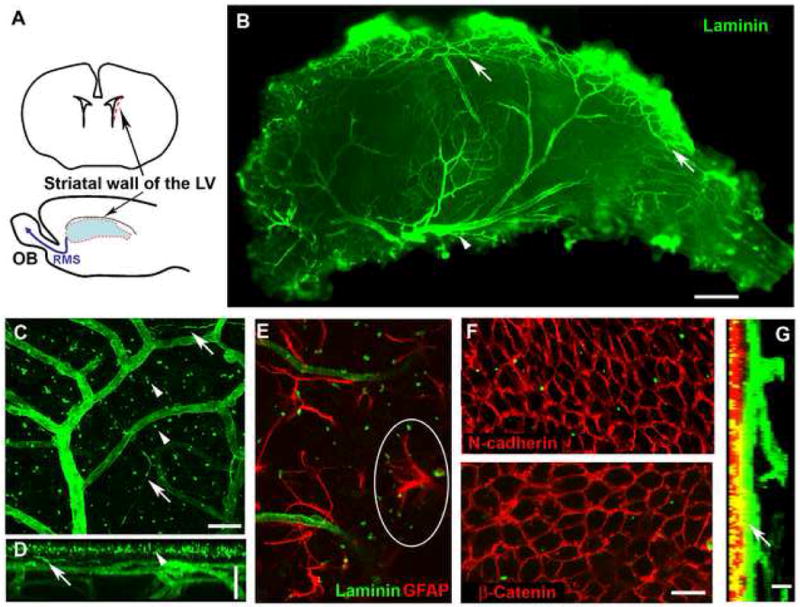
(A). SVZ wholemounts were dissected from the striatal wall of the lateral ventricle (red outline). OB, olfactory bulb; RMS, rostral migratory stream; LV, lateral ventricle.
(B). Laminin staining shows the dense network of blood vessels in the SVZ wholemount (viewed from the ventricular surface; anterior, left and dorsal, top. Arrows: vessels running along the dorsal border of the SVZ. Arrowhead: a large vessel in the ventral aspect.
(C-D). Laminin staining reveals blood vessels, Laminin specks (arrowheads) and fractones (arrows) (C, a projection image of confocal Z-stacks).
A projection image generated with the z-axis as the turning axis (D) shows the superficial Laminin specks, the SVZ vessel network parallel to the ventricular surface, and the deeper vessel branches that delve down into the striatum. Top: LV surface, bottom: striatal side. (Arrowheads indicate Laminin specks near the surface; arrows indicate fractones).
(E). Laminin specks were frequently contacted by GFAP+ processes, as circled.
(F). N-cadherin (upper, red) or β-catenin (lower, red) shows the pavemented ependymal cell layer. Some Laminin specks (green) are seen in the ependymal layer.
(G). Z-stacks of confocal images of an SVZ wholemount stained for N-cadherin (red) and Laminin (green) viewed from the z-axis. Left: ventricular surface, right: the striatal side. Note a section of vessel (arrow) immediately beneath the ependymal layer. Scale bars: B: 300 μm; C-D: 25 μm; F: 20 μm; G: 10 μm.
3D confocal laser scanning of an SVZ wholemount stained for Laminin revealed a superficial layer of Laminin specks, approximately 1 μm in diameter (Figure 1C, D, arrowheads) often associated with the tips of GFAP+ processes (Figure 1E). These specks were positive for Laminin α5 and β1 chains (not shown), which are components of Laminin 10, an isoform primarily expressed in endothelial cells in the adult (Hallmann et al., 2005), and positive for Nidogen (not shown), suggesting that they are part of the endothelial basal lamina. Beneath this layer is a dense network of vessels running parallel to the ventricular surface that has frequent perpendicular branches delving down into the striatal tissue. Laminin+ fractones were visible as elongated, slender structures sprouting from blood vessels, some reaching 100 μm in length (Figure 1C, D, arrows). We then examined how close the vessels were to the ventricular surface, using the ependymal layer as a reference point.
Ependymal cells are the most superficial cell layer lining the ventricle. Their pavemented pattern was seen in wholemounts after staining for β-catenin, N-cadherin (Figure 1F) or Vimentin (Figure S1A) and the cilia marker acetylated α-tubulin confirmed their multiciliate nature (Figure S1B). We found that the SVZ blood vessel plexus was generally very superficial, typically just 10-20 μm beneath the ependymal layer (Figure 1G), with the closest vessels being just 4.06 ± 0.50 μm (n=13 images from 4 mice) from the nearest ependymal cell nucleus.
Three layers of SVZ Type B cells: Apical, Tangential and Deep
SVZ stem cells are a sub-population of the Type B cells, which express the astrocyte marker glial fibrillary acidic protein (GFAP) (Doetsch et al., 1999a; Doetsch et al., 1999b). These cells can be visualized by GFP-driven expression from the GFAP promoter (Zhuo et al., 1997), as the GFP fills the cytoplasm. Here we examined their appearance in SVZ wholemounts using confocal imaging, which provides a unique perspective. We discovered three distinct layers of GFAP+ cells (Supplemental Movie).
A sub-population of GFAP+ Type B cells found at the apical edge (closest to the ventricle) of the SVZ have been reported to occasionally send a process through the ependymal layer to contact the ventricle, which might indicate an activated stem cell (Conover et al., 2000; Doetsch et al., 1999b). In the aged mouse, some astrocytes lie within the ependymal layer where they aid in tissue repair (Luo et al., 2008). Here we found that the apical GFAP-GFP+ cells closest to the ventricle frequently penetrate the ependymal layer, outlining the pavement pattern of ependymal cells (Figure 2A-C). Moreover, the GFP+ process often includes a portion of the cell nucleus (Figure 2B), in which case the Type B cell is actually intercalated into the ependymal layer. Thus in the adult mouse SVZ, which is not aged and has a normal level of regeneration, numerous Apical Type B cells are mixed with ependymal cells in the most superficial ventricular layer.
Figure 2. Three layers of distinct types of GFAP-GFP cells in the SVZ and their apposition to blood vessels.
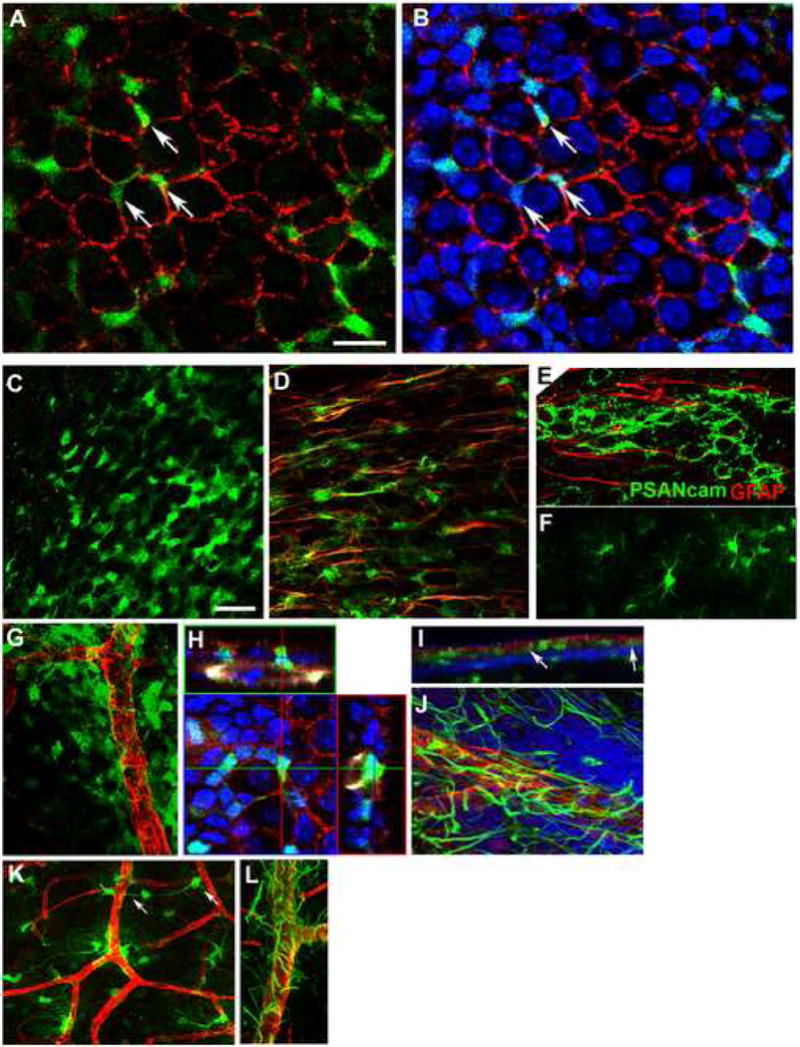
(A-B). Apical Type B cells are intercalated into the ependymal layer. (A) A single confocal scan of the SVZ wholemount from a GFAP-GFP mouse brain stained for N-cadherin (red) and GFP (green). (B) The same image shown in (A) with DAPI staining (blue) to show the inclusion of the nuclei of GFAP-GFP+ cells in the ependymal layer (3 examples indicated by arrows).
(C-F). Imaging GFAP-GFP+ cells from the ventricular surface towards the striatum, single z-stack images at 5 μm (C), 10 μm (D) and 20 μm (F) from the ependymal layer. (C). Apical GFAP-GFP+ cells have a neuroepithelial-like morphology and form a layer mixed within and just beneath the ependymal layer. (D). Tangential GFAP-GFP+ cells with one or two long processes (stained for GFAP, red) are prevalent immediately beneath the apical layer; the aligned processes run anterior-posterior, in the direction of neuroblast migration (stained for PSA-NCAM, green in E). (F). Near the striatal border, GFAP-GFP cells are typical of more mature astrocytes with multiple processes.
(G-L). GFAP-GFP+ cells and processes are closely apposed to blood vessels.
(G). Numerous epithelial-like GFAP-GFP+ cells (green) wrap around blood vessels (Laminin, red). Many GFAP-GFP+ cell somas sit on the vessel walls.
(H). Orthogonal sections of a confocal image showing an apical GFAP-GFP+ cell intercalated into the ependymal layer and contacting a blood vessel. The GFAP-GFP SVZ wholemount was stained for GFP (green), N-cadherin (red), Laminin (pseudocolored white), and DAPI (blue).
(I). A projection image of Z-stacks (viewed from the z-axis) of a GFAP-GFP SVZ wholemount stained for GFP (green), N-cadherin (red), Laminin (blue) shows a layer of GFP+ cells between the ependymal layer and the SVZ blood vessels. Note that some GFP+ cells contact both the ventricle surface and the vessels (arrows).
(J). Tangential SVZ astrocytes have long processes running along or between the blood vessels (GFAP, green, Laminin, red).
(K). Deep GFAP-GFP+ cells are close to vessels with frequent endfeet touching blood vessels. Arrows indicate a cell with its soma on a vessel and its process touching another branch.
(L). Deep astrocytic processes (GFAP, green) wrap around large vessels (Laminin, red). Scale bars: A-B: 20 μm; C, D, F: 50 μm.
Beneath the ventricular layer is a population of GFAP-GFP+ cells with one or more long processes running parallel to the surface. These cells, which we call Tangential Type B cells, resemble radial glia or translocating astrocytes in the embryonic forebrain (Jacobson, 1991). In the dorsal SVZ, the tangential processes run anterior-posterior, in the direction of neuroblast migration (Figure 2D, E).
Deeper, a third type of GFAP-GFP+ cell was observed with a small soma and numerous processes, typical of mature astrocytes (Figure 2F). These cells are probably differentiated astrocytes near the striatal parenchyma.
Cells with Stem cell markers lie close to the vasculature
We next examined the relationship of cells with NSC characteristics to the vasculature. SVZ wholemounts from GFAP-GFP mice were stained for ependymal markers, Laminin and the nuclear marker DAPI and in some cases for the surface marker LeX which labels the neurosphere-generating cells in the adult SVZ, the Type B and Type C cells (Capela and Temple, 2002).
Apical Type B cells are intercalated between ependymal cells in the ventricular layer and also form loose sheets of cells directly beneath that can be numerous around blood vessels (Figure 2C, G). Some contact both the ventricle and a blood vessel beneath (Figure 2H, I); these cells are in a unique microenvironment, with access to cerebrospinal fluid/ependymal factors at one end and blood/vascular endothelial factors at the other. The tangential SVZ astrocytes often have somas on blood vessels, and long processes running between or along the SVZ vessels (Figure 2J). The process-bearing, differentiated astrocytes deeper in the wholemount contact or wrap blood vessels with their processes, but have distant somas (Figure 2K, L), typical of parenchymal astrocytes (Simard et al., 2003).
To quantify the relationship of SVZ astrocytes to blood vessels, we examined 20 randomly chosen images from 4 mice in detail. The components of each image were segmented to define each essential cellular element: the nuclei, the vessels and the processes. The nuclei were defined by cell type specific markers, and the distance of each identified nucleus from the nearest blood vessel surface was calculated (Supplementary methods). An example of processing and quantification is shown in Figure 3, with a 3D rendering. We found that cells that were double-labeled with the progenitor markers LeX and GFAP lie significantly closer to the vasculature than other cell populations in the SVZ: 69.23% of LeX+GFAP+ cells lie within 5 μm versus only 26.3% of the cells lacking both markers (total 3806 cells, Kruskal-Wallis test, p<0.0001).
Figure 3. GFAP+ and LeX+ cells near vessel walls in 3-D images of SVZ wholemounts.
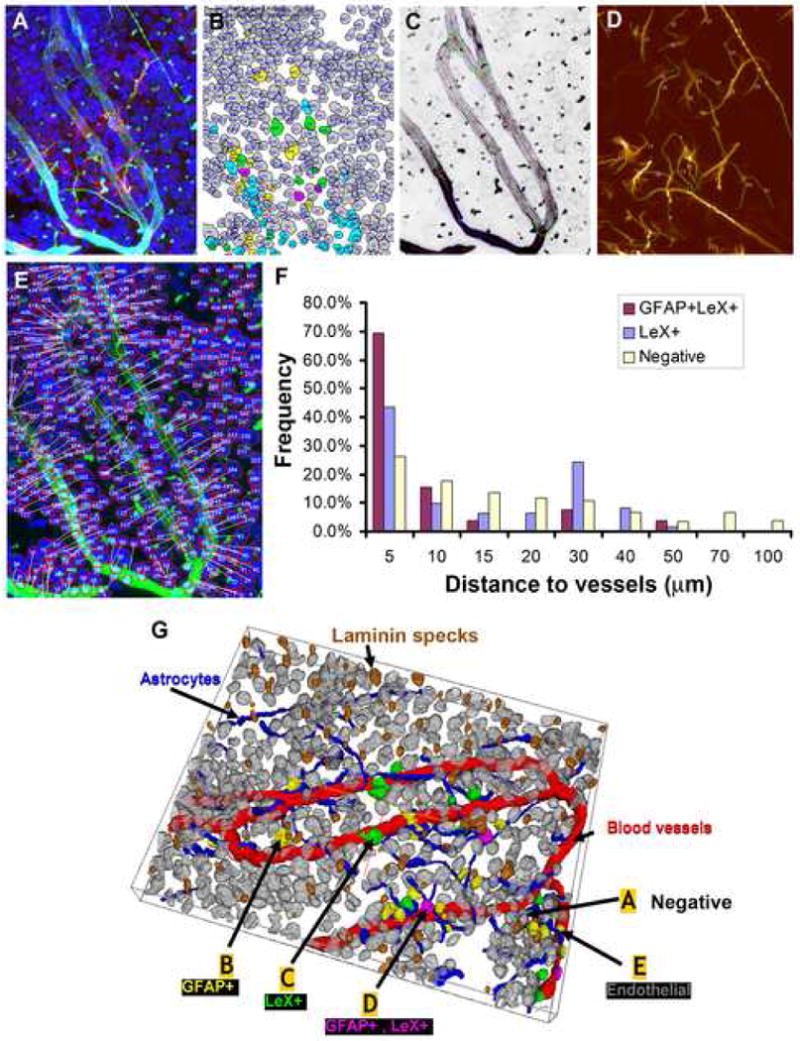
(A). 4-channel confocal image of SVZ wholemount stained for DAPI (blue), GFAP (green), Laminin (aqua), and LeX (red).
(B). Results of automated delineation of cell nuclei in the DAPI channel, and automated cell classification indicated by color labels of nuclei (green, LeX+; yellow, GFAP+; purple, GFAP+ and LeX+; gray, all other nuclei (negative).
(C). Automated vessel tracing computed on the Laminin channel (vessel center lines are displayed in red and surfaces in blue).
(D). Automated tracing of astrocyte processes (green).
(E). Illustration of the method for computing distances of nuclear centroids to the nearest vessel segment.
(F). Histogram of pooled validated data from 6 images showing the distances of cells that were double LeX+GFAP+ or Lex+GFAP- to vasculature as compared to negative cells.
(G). 3D rendering of the segmentation and classification results. The cell nuclei are colored as in (B), vessel surfaces in red, astrocyte processes in blue, and Laminin specks brown. (labels A – E indicate cells that are negative, GFAP+, LeX+, GFAP+LeX+, and endothelial).
Stem cells in the adult SVZ are thought to be slowly-dividing, and have been identified histologically as label-retaining cells (LRCs) by BrdU labeling regimes (Johansson et al., 1999). To determine whether LRCs lie close to blood vessels, we injected adult mice with 50 mg/kg BrdU daily for 5 days to label even slowly-dividing cells, followed by a 24 day chase. The chase period allowed incorporated BrdU to be diluted in rapidly dividing cells, and for differentiated neural cells with label to migrate out of the SVZ. Cells remaining within the SVZ that retain BrdU are LRCs. We imaged the LRCs in relation to the Laminin-stained vasculature, and quantified 13 images from 4 SVZ wholemounts (74 LRCs). 40.54% of the LRCs were within 5 μm and 55.5% within 10 μm of the nearest blood vessel surface, in some cases within pockets formed by vessels (Figure 4A, arrows). The average distance of LRCs to blood vessels is significantly closer than non-LRCs (10.21 μm versus 23.84 μm; 1320 cells, Mann-Whitney test, P<0.0001). A similar close association was found for the sub-population of GFAP-expressing LRCs (Tavazoie et al, 2008). In contrast, only 25.30% of the total GFAP-GFP+ cells had somas within 5 μm of the blood vessel surface (Figure 4C), reflecting the fact that many ependymal layer-associated apical GFAP-GFP+ cells are not in direct contact with blood vessels. Hence cells with the proliferative phenotype attributed to SVZ stem cells are found in close apposition to blood vessels.
Figure 4. Neurogenesis occurs around the vessel network in the adult SVZ.
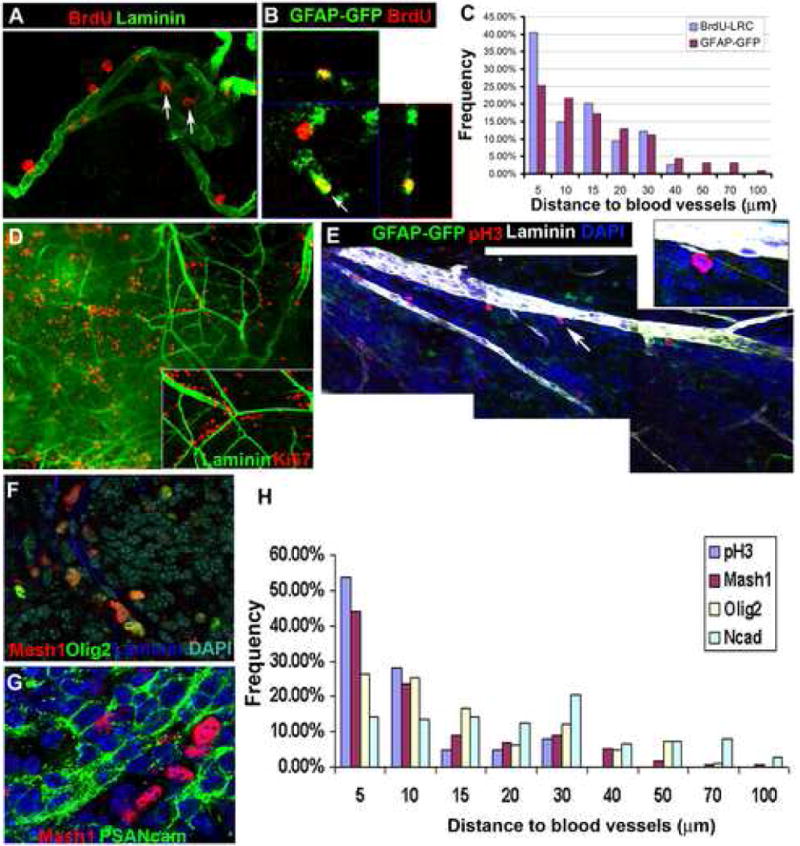
(A-C). Adult mice were given 5 daily BrdU injections then a 24 day chase to reveal LRCs.
(A). A confocal image of the SVZ showing BrdU LRC (red) proximity to blood vessel (Laminin+, green); some BrdU+ cells are in a niche formed by a knot of vessels (arrows).
(B). An orthogonal section of confocal z-stacks. Arrow: a BrdU-retaining cell (red) that is positive for GFAP-GFP. 25.0% of LRCs were found to be GFAP-GFP+ after GFP staining, similar to the 38% observed using a 6 week chase period (Tazavoie et al, 2008). The identity of GFAP-ve LRCs remains to be determined.
(C). Histogram of distance of LRCs and GFAP-GFP cells to vessels (Data from 13 images derived from 4 SVZ wholemounts).
(D). A low power image of an SVZ wholemount stained for the cell proliferation marker Ki67 (red) and Laminin (green). Ki67+ cells are frequently aligned next to blood vessels (arrow indicates the area shown inset in a higher magnification confocal image).
(E). A montage of single Z-sections of confocal images of a GFAP-GFP SVZ wholemount stained for pH3+ cells (red), Laminin (pseudocolored white), and DAPI (blue). PH3+ cells are adjacent to the vessels. Note one positive cell (arrow and inset) next to a Laminin+ fractone.
(F). Double-labeling of Mash1 (red) and Olig2 (green).
(G). Mash1+ cells (red) are near their lineal progeny PSA-NCAM+ Type A cells.
(H). A histogram of the distance to vessels of cells that are pH3+, Mash1+, Olig2+ and N-cadherin+. Most pH3+ cells are within 10 μm of the nearest vessel, Mash1+ cells are similarly close while Olig2+ cells are more widely distributed around the vessels. The distances of N-cadherin+ ependymal cells were plotted as a reference, and range from 5 μm-100 μm. Data pooled from 5 randomly chosen images.
Neurogenesis is associated with SVZ blood vessels
Slowly-dividing Type B SVZ stem cells produce rapidly dividing transit amplifying Type C cells, which express Olig2, Dlx2, Mash1 and LeX (Capela and Temple, 2002; Doetsch et al., 2002; Menn et al., 2006; Parras et al., 2004). These in turn generate Type A neuroblasts that express PSA-NCAM and continue to divide while migrating in the RMS towards the olfactory bulbs. We examined the relationship of these proliferative components of the SVZ lineage to the vasculature.
Numerous SVZ cells were labeled with the proliferative marker Ki67, and the more brightly stained cells were observed close to vessels (Figure 4D). Strikingly, phospho-Histone H3 (pH3)+ cells were intimately adjacent to blood vessels (average distance between nucleus and vessel surface, 5.43 ± 1.45 μm, n=4 images) (Figure 4E, H), and 82% were within 10 μm of the vessel surface. This suggests that most SVZ cells undergo mitosis near blood vessel walls. In contrast to the hippocampal dentate gyrus or songbird ventricular zone where endothelial cells proliferate (Louissaint et al., 2002; Palmer et al., 2000), we did not observe Ki67+CD31+ endothelial cells in the SVZ.
We then examined the location of the transit-amplifying Type C cells relative to SVZ blood vessels, and found that in general their somas were very close to the vessel surface. 43.55% of LeX+GFAP- cells, characteristic of Type C cells, had nuclei within 5 μm of the vessel surface (Figure 3F,G). Mash1+ cells, also putative Type C cells, were frequent directly beneath the apical type B layer and Mash1+ cell clusters were often near blood vessels with a similar proportion, 44.05%, being within 5 μm and 67.40% within 10 μm (Figure 4H, total 227 Mash1+ cells, Figure S2A). In contrast, Olig2+ cells were distributed evenly throughout the SVZ rather than being clustered and those found in deeper parts of the wholemount are probably mature oligodendrocytes (Figure S2B). Reflecting this distribution, just 26.51% of Olig2+ nuclei were within 5 μm (Figure 4H). Mash1 and Olig2 staining in the SVZ did not always overlap, suggesting that they label different subpopulations of type C cells (Figure 4F). PSA-NCAM+ cells were observed close to Mash1+ Type C cells, consistent with their lineal relationship (Figure 4G). Quantification of SVZ progenitor cell nuclei distance to nearest vessel shows their close association to the SVZ vasculature, in contrast to Ncadherin+ ependymal cells, most of which (as expected) do not show a close association to the vasculature (Figure 4H).
Type A neuroblasts are typically organized into chains of cells migrating towards the olfactory bulbs (Doetsch and Alvarez-Buylla, 1996; Lois et al., 1996). By co-staining for the Type A marker PSA-NCAM and Laminin, we observed that the chains had blood vessels running along and within them in the direction of migration towards the RMS (Figure 5A-D and montage, E), seen commonly in the dorsal SVZ and less frequently in the central SVZ. Thus the neuroblast chains in the dorsal SVZ have an intimately associated vasculature that is likely providing growth factors to this highly active cell population. Consistent with this, there is a notably higher SVZ cell density close to blood vessels: more than 50% of total SVZ cell nuclei are situated within 20 μm of blood vessel surfaces (Figure 5F).
Figure 5. Neuroblasts migrate in a chain along blood vessels.
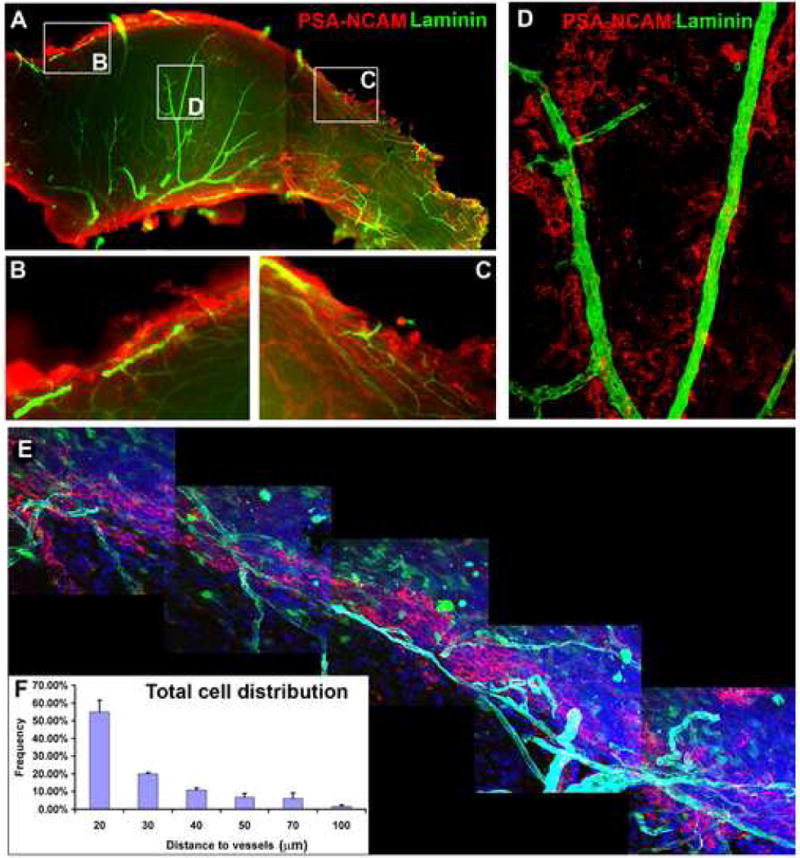
(A-D). Low power image of SVZ wholemount stained for PSA-NCAM (red) and Laminin (green); Left, anterior and top, dorsal. High magnification images of boxed areas are shown in (B-D). Chains of migrating PSA-NCAM+ neuroblasts are aligned along blood vessels in the anterior dorsal region (B), the posterior region (C) and some occur in the central SVZ (D).
(E). A montage of confocal images showing approximately 1 mm of the dorsal region of an SVZ wholemount derived from a GFAP-GFP brain stained for PSA-NCAM (red), Laminin (cyan) and DAPI (blue). A vessel complex travels along and within the neuroblast chain.
(F). Histogram showing the relationship between SVZ cell distribution and blood vessels. More than 50% of cells are within 20 μm of blood vessels. Data represents mean ± SEM from 3 SVZ wholemounts (3-5 images per wholemount).
α6β1 integrin on adult NSCs regulates endothelial binding
We asked what molecules might hold SVZ NSCs in the vascular niche. Laminin is expressed largely by vascular cells in the SVZ and is highly abundant and localized around blood vessels. Given this, we examined whether adult SVZ NSCs express the laminin receptor α6β1 integrin. The GoH3 antibody that recognizes the α-chain of α6β1 integrin, co-labeled with most Laminin+ elements in the SVZ and also labeled a sub-population of SVZ cells lying near vascular cells (Figure 6A). α6β1 integrin was expressed by cultured SVZ neurospheres, frequently by LeX+ cells (Figure 6B). LeX+ cells in acutely isolated, dissociated SVZ populations expressed α6 integrin, while the more differentiated PSA-NCAM+ cells, were largely negative or expressed significantly lower levels (Figure 6C). In 3-day clonal SVZ cell cultures, α6 integrin was strongly expressed by LeX+GFAP+ cells (Figure 6D). Hence the sub-population of cells that is found closest to blood vessels in vivo expresses high levels of this Laminin receptor, while neuroblasts, which are more distant from the surface of blood vessels, express lower levels.
Figure 6. α6β1 integrin is expressed in adult SVZ cells and required for SVZ cells to adhere to endothelial cells.
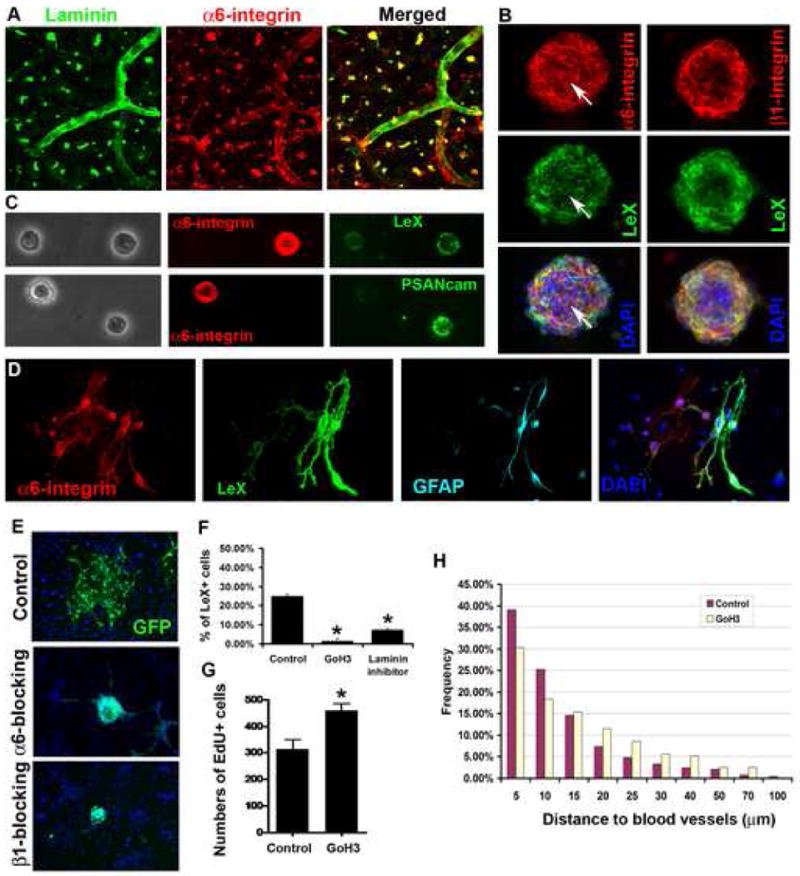
(A-D). α6 and β1 integrin expression in the SVZ and NSCs. (A). An SVZ wholemount stained for α6 integrin (red) and Laminin (green). α6 integrin staining is seen on laminin specks and blood vessels, as well as on SVZ cells. (B). α6 and β1 integrins detected in SVZ neurospheres and on LeX+ progenitor cells (green), DAPI in merged images. Arrows point to a dividing cell double-positive for α6 integrin and LeX. (C). Acutely fixed single SVZ cells show strong α6 integrin expression in a subpopulation of LeX positive cells, but weak or no staining in PSA-NCAM+ neuroblasts. (D). A 3-day SVZ clonal culture, the α6 integrin+ cells are LeX+GFAP+.
(E). After treatment with blocking antibodies to α6 or β1 integrin, SVZ neurospheres derived from adult GFP mice spread poorly on endothelial cells.
(F). Blocking with anti-α6 integrin antibody (GoH3) or laminin binding inhibitor peptide inhibits adhesion to endothelial monolayers. Data, Mean±SEM (n=2 experiments. ANOVA, Bonferroni test *P<0.005).
(G). Significantly increased proliferation (EdU-incorporated cells) in the dorsal SVZ after 6-day in vivo infusion of anti-α6 integrin. Data, Mean±SEM (n=3 SVZ wholemounts. *P<0.05, t-test).
(H). S-phase cells are significantly further from blood vessel surfaces after 6-day in vivo infusion of GoH3 antibody (Mann-Whitney test, P<0.001, n=234 cells for GoH3, n=246 cells for control group).
To test whether α6β1 integrin was important for SVZ NSCs to bind to endothelial cells, we plated SVZ neurospheres on top of an 80% confluent endothelial cell monolayer. These attached and spread in control antibody conditions (Figure 6), but after 1 hour incubation with the GoH3 antibody, which blocks α6β1 integrin binding to Laminin, 42.0% of neurospheres did not attach well and could be washed away after medium replacement. Most of those that attached did not spread as fully as those in control conditions (Figure 6E). Similarly, freshly dissociated LeX+ adult SVZ cells were largely washed off the endothelial layer after preincubation with the GoH3 antibody (Figure 6F, 1.3% ±0.03% of the cells remaining attached were LeX+, compared to 25.0%±0.02% in the control antibody treated group; ANOVA, Bonferroni test, P=0.0012). A β1 integrin blocking antibody or Laminin binding blocking peptide (Hunter et al., 1989) also impaired the ability of adult SVZ cells to bind and spread on vascular endothelial cell cultures (Figure S3, Figure 6E). Thus SVZ NSCs express a key receptor that enables them to bind to the Laminin-rich blood vessel surface and ECM.
Prior studies have shown that α6-integrin plays a role in guiding neuroblast migration in the RMS (Emsley and Hagg, 2003), but there are no reports examining its role in stem cell maintenance in the niche. To address this in vivo, α6-integrin blocking antibody (GoH3) was infused via minipumps into the lateral ventricle of adult mice. After 6 days of infusion, we gave an injection of EdU to label dividing progenitor cells 1 hour before sacrifice, then examined the position of SVZ progenitor cells in relation to the vasculature. Progenitor cells treated with GoH3 had moved away from the vascular surface a significant amount, with the proportion of EdU+ cells over 10 μm away from the surface increasing by 48.6% compared to control, consistent with the cells being released from close adhesion to the vessel surfaces (Figure 6G, H). Moreover, we found that the proliferation of SVZ lineage cells increased 33.6% after GoH3 treatment, indicating that the vascular niche is important for regulating proliferation of the closely apposed SVZ progenitor cells, including the slowly-dividing Type B population.
Discussion
Automated 3D analysis of confocal images derived from SVZ wholemounts has provided an extensive, quantitative description of the elements of the adult stem cell niche. The 3D viewpoint revealed important information about the structure of the adult SVZ, the arrangement of its constituent cells and their relationship to the vasculature and the ventricle, which we had not obtained from prior studies based on inspection of tissue sections. It would be valuable to confirm these findings in future studies and add ultrastructural information using serial reconstruction of EM sections. We have shown that there is a rich vascular plexus running within the striatal SVZ parallel to the ventricular surface. Cells with features of stem cells directly contact these blood vessels, and proliferating cells in the SVZ lineage are also closely apposed. When the interaction between SVZ progenitor cells and blood vessels was disturbed using an α6 integrin blocking antibody, proliferation was affected. Thus, SVZ blood vessels promote the development of the adult NSC lineage and are functionally important elements of the normal adult SVZ stem cell niche.
SVZ stem cells lie in a vascular-derived laminin-rich niche
Our data show that a sub-population of Type B and Type C cells reside close (within 5 μm) of the nearest blood vessel surface. We considered whether SVZ progenitor cells were found next to blood vessels because these provided a survival signal, so that stem cells excluded from this location would die. However staining adult SVZ wholemounts for markers of apoptosis revealed almost negligible cell death, using either TUNEL or Caspase 3 immunostaining (not shown), indicating that the association is via niche adhesion mechanisms, rather than survival selection.
Considering that endothelial cells are the greatest source of laminin in the SVZ, one simple model for NSC-vascular niche adhesion is via binding to laminin. α6β1 integrin is a laminin receptor, and interestingly, α6 integrin was identified as the sole common element of a variety of ‘stemness’ signatures on embryonic stem cells, embryonic NSCs and hematopoietic stem cells (Fortunel et al., 2003) and is expressed by other stem cell types, such as epidermis, prostate and mammary (Lawson et al., 2007; Li et al., 1998; Stingl et al., 2006). We show here that adult SVZ NSCs express α6 β1-integrin and that blocking this significantly impairs their ability to bind and spread on vascular endothelial cell cultures. Furthermore, blocking α6 β1 in vivo caused SVZ progenitor cells to move away from the vascular surface. These findings support our hypothesis that α6β1integrin-laminin interactions are critical to hold NSCs in a vascular niche, and that this interaction has functional consequences on lineage regulation, similar to integrin-dependent cell anchoring in invertebrate stem cell niches (Tanentzapf et al., 2007). The fact that the PSA-NCAM+ Type A cells express no or low levels of α6-integrin suggests that modulation of the integrin-laminin interaction enables more differentiated SVZ cells to leave the immediate vascular niche. Given that other types of stem cells reside close to blood vessels, and the expression of α6β1 expression by diverse stem cell types, this may prove to be a common mechanism for a stem cells to bind to the vascular niche.
Besides its adhesion function, the blood vessel ECM can trap growth factors such as fibroblast growth factor that impact NSC function (Kerever et al., 2007). Thus the laminin specks, fractones and blood vessel surfaces are likely to be a source of regulatory growth factors that play a functional role in controlling SVZ lineage progression.
SVZ neurogenesis occurs around blood vessels
Endothelial cells from a variety of sources, including bovine pulmonary artery and a mouse brain cell line, release factors that stimulate NSC self-renewal and neurogenesis in vitro (Shen et al., 2004). A close association of proliferating cells with the vasculature was seen in the SVZ of MRL mice (Baker et al., 2006) and in forebrain cancer stem cells (Gilbertson and Rich, 2007). Here we found that normal SVZ cells in vivo tend to proliferate adjacent to blood vessels. Disruption of α6 integrin function in vivo causes SVZ progenitor cells to move away from blood vessels and unexpectedly stimulates proliferation. This suggests that α6 integrin signaling in neural-vascular interactions is complex and may have multiple effects on SVZ progenitors depending on their stage of development. The fact that both type B and type C cells are close to vessels suggests that direct contact with blood vessels may help maintain stem cell properties and regulate the proliferative state of Type B and C cells. One explanation for the increase in proliferation we observe after anti-α6 antibody treatment is that homeostatic control of stem cell activity due to contact with vessels is perturbed when SVZ cells lose direct contact, but progenitors are still in range of diffusible signals from vessels that stimulate proliferation.
In the dorsal SVZ, the neuroblast chains appear built around blood vessels that run with them in the orientation of migration, and an interesting question is whether the vessels guide construction of the chains and their orientation towards the olfactory bulbs, and help stimulate neuroblast migration, which is plausible given the commonalities between molecular mechanisms of cell guidance in these two systems (Weinstein, 2005). The Type A cells, even at a distance from the vessels, might well be influenced by blood-born factors derived from blood vessels (Tavazoie et al, 2008) or by secreted molecules diffusing from vascular associated cells that could impact their migration. It is also possible that the tangential Type B cells we observed with elongated processes oriented in the direction of migration (Figure 2) might also play a role in this process, analogous to the role of radial glia in the embryonic brain, along with the established mechanism of neuroblast chain migration, which occurs independently of glial guidance (Doetsch and Alvarez-Buylla, 1996; Lois et al., 1996; Wichterle et al., 1997).
Apical Type B cells lie within the ependymal layer
We were surprised to find by visualizing GFAP-GFP filled cells in SVZ wholemount preparations, that apical Type B cells frequently penetrate the ependymal layer. Importantly, the apical processes, often containing visible nuclei, are squeezed between the ependymal cells to contact the ventricle. This means that the ventricular surface is an admixture of ependymal cells and Apical Type B cells, more similar to the embryonic germinal zone, with primary progenitors touching the ventricular space. Apical Type B cells also formed loose sheets of cells just under the ependymal layer (Figure 2), where their flattened morphology is reminiscent of primitive neuroepithelial progenitor cells in endothelial co-culture (Shen et al., 2004). Some Apical Type B cells have nuclei close to the surface and others deeper in the SVZ. This raises the intriguing possibility of nuclear translocation related to the cell cycle, as occurs in the embryonic forebrain and the adult bird brain (Alvarez-Buylla et al., 1998; Jacobson, 1991), perhaps with M-phase next to blood vessels – explaining the preponderance of pH3+ nuclei adjacent to blood vessels.
Previous studies have suggested that the GFAP-expressing Type B cells that lie close to ependymal cells include the SVZ stem cell population. We now find that some of these cells span between the ventricle and the SVZ blood vessels and are therefore in a unique position to receive signals from both sources. As even large molecules can penetrate through the ependymal layer, which do not have tight junctions in the adult (Brightman, 1965), and given that blood-derived signals can enter the SVZ as shown in the accompanying paper (Tavazoie et al, 2008), our findings together indicate two important routes for soluble factors to impact the SVZ stem cell lineage.
The germinal vasculature is maintained throughout forebrain development
The arrangement of adult SVZ lineage cells to the SVZ vascular plexus revealed in this study bears remarkable similarity to the cellular arrangement of embryonic forebrain germinal zones (Figure 7). We suggest that this conserved arrangement of a germinal vascular plexus plays a critical role in maintaining the NSC lineage at all stages by providing an ECM-rich niche regulating the spatial arrangement of the cells, as well as growth factors and nutrients to stimulate proliferation, differentiation and migration. It will be important to determine whether the germinal zone blood vessel cells express unique molecules that distinguish them from other blood vessels in the CNS, accounting for some of the unique properties of the neurogenic niche.
Figure 7. Summary model comparing the vascularization of adult and embryonic germinal zones.
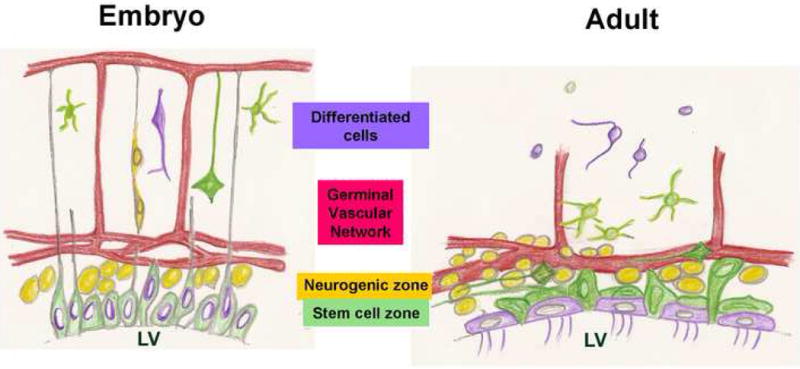
In the embryo, an apical layer of stem cells produces an actively proliferating SVZ which generates neurons that migrate towards the pia guided by radial glia. Coincident with neurogenesis, the vasculature grows from the pial surface towards the germinal cells, a source of VEGF (Breier et al., 1992) and form a plexus at the germinal zones, parallel to the ventricular surface (Shen et al., 2004; Strong, 1964).
The adult SVZ has an essentially similar structure: Apical Type B cells, believed to include the SVZ stem cells, are intercalated into the ependymal layer and directly contact the ventricle. Just subjacent is an SVZ of active proliferation, differentiation and migration, including Mash1+ and Olig2+ Type C progenitors, PSA-NCAM+ Type A neuroblasts and Tangential Type B cells. The adult germinal zone is intimately associated with an SVZ vascular plexus.
Methods
SVZ wholemount dissection and staining
SVZ wholemounts of striatal lateral wall were dissected (Lois and Alvarez-Buylla, 1993) from 2-3 month old Swiss-Webster mice (Taconic) or heterozygous GFAP-GFP transgenic mice (FVB/N-Tg(GFAPGFP)14Mes/J, Jackson Laboratory) using heterozygous mice because of their high correspondence between GFP expression and GFAP immunostaining (Tavazoie et al, 2008). Briefly, a 2-4 mm-long strip of SVZ tissue covering the striatum was dissected from under the corpus callosum to the ventral tip of the lateral ventricle. The SVZ wholemounts were fixed in cold 4% paraformaldehyde in 0.1M PBS for 30 min, washed with PBS, blocked with 10% NGS in PBS/0.3% Triton X-100 for 30 min at RT then incubated with primary antibodies diluted in blocking buffer for 12-48hrs at 4°C. Tissues were washed 3X in PBS/0.3% Triton 15 min on a rocker, and incubated with appropriate secondary antibodies at RT for 2 hrs. After washing, DAPI was added and the wholemounts transferred to a glass slide and coverslipped with a 100μm adhesive spacer in Prolong Gold anti-fade reagent (Invitrogen) for confocal imaging.
Antibodies used: LeX, mouse IgM, 1:10 (ATCC #HB78); GFAP, Rabbit IgG, 1:1000 (Dako) or mouse IgG, 1:500 (Millipore); PSA-NCAM, Mouse IgM, 1:800 (Millipore); Laminin, rabbit IgG, 1:1000 (Sigma) or chicken IgY, 1:500 (Abcam); Laminin α5 chain specific antibody, rabbit IgG, 1:500 (a gift from Dr. Jeffery Miner); Laminin β1, rat IgG, 1:50 (Millipore); β-catenin and N-Cadherin, mouse IgG1, 1:200 (BD); Olig2, rabbit IgG 1:40000 (Dr. Charles Stiles); Mash1, mouse IgG, 1:2 (Dr. David Anderson); Alex488-GFP, rabbit IgG, 1:1000 (Invitrogen); phospho-Histone H3, rabbit IgG, 1:1000 (Upstate); BrdU, mouse IgG, 1:100 (BD); Ki67, rabbit IgG, 1:500 (Lab Vision). α6-integrin, rat IgG, 1:50 and β1-integrin, rat IgG, 1:25 (Millipore).
Primary antibodies were visualized using Alexa-conjugated (Invitrogen) and Cy5-conjugated (Jackson Laboratory) secondary antibodies.
BrdU-LRC assay
BrdU (Sigma)(10 mg/ml solution) was injected daily intraperitoneally (50 mg/kg) into GFAP-GFP mice for 5 days. Mice were sacrificed 24 days after the last injection. SVZ wholemounts were treated with 0.5N or 2N HCl at 37°C for 30 min before staining. GFAP-GFP cells were revealed with an antibody to GFP.
Image acquisition and quantification
The SVZ wholemounts were imaged with an inverted Zeiss LSM 510Meta-NLO confocal laser scanning microscope and images acquired using Zeiss LSM510 software. Areas for image selection were picked at random from the SVZ after general inspection of the wholemount for tissue integrity and staining quality. For some experiments we randomly selected areas from the dorsal or the central aspect of the wholemount. Three single photon lasers (Argon, HeNe 543, HeNe 633) were used to visualize Alexa488, Alexa 546 or 568, and Alexa 647 or Cy5 respectively. A two-photon Chameleon laser was tuned at 740nm for viewing DAPI. The SVZ wholemounts were scanned from the ventricular surface to the striatal side. 0.5 μm or 1 μm z-stacks were collected using 40X or 63X objectives and processed using the Zeiss software. The image quantification method is detailed in supplementary information.
Integrin-blocking co-culture assays
Endothelial cells (BPAE or MbEND, ATCC) maintained according to ATCC protocols were grown in 24-well plates in DMEM plus 10% FBS to 80% confluence. Medium was changed to serum-free DMEM NSC medium 2 days before co-culture. Freshly isolated SVZ cells or 7-day SVZ neurospheres from GFP mice were pre-incubated with GoH3 blocking antibody 1:50 (Beckman Coulter), β1 integrin blocking antibody (BD) or IgG control antibody (BD). To assess adhesion, 1 hour after plating the medium was changed, washing away non-adhered cells or neurospheres; the remaining attached cells or neurospheres were counted.
In vivo blocking of α6 integrin-laminin binding
Micro-osmotic pumps (model 1007D Alzet) were pre-loaded with GoH3 antibody or Rat IgG control at 4μg/ml in artificial CSF and implanted into adult female Swiss Webster mice anesthetized with 3% isoflurane. The cannula was stereotactically inserted into the lateral ventricle at 0.85mm medial of bregma. After 6 days of infusion, mice were injected intraperitoneally (100 mg/kg) with EdU (Invitrogen) (10 mg/ml solution), an alternative to BrdU, 1hr before sacrifice.
The SVZ wholemounts were dissected, EdU labeling for S-phase cells was detected using the Click-it Kit (Invitrogen), and were stained for laminin and DAPI. EdU+ cells in the dorsal SVZ were counted in low-power images and distance of EdU+ cells to blood vessels was quantified in high-power confocal images.
Supplementary Material
Acknowledgments
We thank Rebecca Stern for assistance with the summary figure, Patricia Lederman and Cindy Butler for technical help, and Adreinne Dorr and Michael Dipersio for discussions on the SVZ vasculature. We are grateful for antibodies from Drs. Charles Stiles, David Anderson and Jeffrey Miner. This project was supported by NINDS through NIH grant R01NS051531, NYS contract C#022044 and the Regenerative Research Foundation. E.K. was supported by NIDA grant 5T32DA007307. Portions of this work were supported by the Center for Subsurface Sensing and Imaging Systems, NSF Engineering Research Centers Program (Award Number EEC-9986821) and by Rensselaer Polytechnic Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Buylla A, Garcia-Verdugo JM, Mateo AS, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr, Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. doi: 10.1002/cne.21090. [DOI] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Brightman MW. The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. I. Ependymal distribution. J Cell Biol. 1965;26:99–123. doi: 10.1083/jcb.26.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Dorr A, Sled JG, Kabani N. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. Neuroimage. 2007;35:1409–1423. doi: 10.1016/j.neuroimage.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. alpha6beta1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- Fortunel NO, Otu HH, Ng HH, Chen J, Mu X, Chevassut T, Li X, Joseph M, Bailey C, Hatzfeld JA, et al. Comment on “‘Stemness’: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science. 2003;302:393. doi: 10.1126/science.1086384. author reply 393. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Developmental Neurobiology. New York: Plenum; 1991. [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Bjornsson CS, Smith KL, Abdul-Karim MA, Turner JN, Shain W, Roysam B. Automated image analysis methods for 3-D quantification of the neurovascular unit from multichannel confocal microscope images. Cytometry A. 2005;66:9–23. doi: 10.1002/cyto.a.20149. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Luo J, Shook BA, Daniels SB, Conover JC. Subventricular zone-mediated ependyma repair in the adult mammalian brain. J Neurosci. 2008;28:3804–3813. doi: 10.1523/JNEUROSCI.0224-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Strong LH. The Early Embryonic Pattern of Internal Vascularization of the Mammalian Cerebral Cortex. J Comp Neurol. 1964;123:121–138. doi: 10.1002/cne.901230111. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120:299–302. doi: 10.1016/j.cell.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


