Abstract
The objective of this work was to determine the interactive effects between soy bioactive components and tamoxifen (TAM) on prevention of estrogen-dependent breast cancer (BRCA). We initially investigated the effects of soy isoflavone genistein and TAM on the growth and cell cycle progression of estrogen-dependent MCF-7 human BRCA cells, and on the expression of ERα, pS2 and EGFR genes in vitro. Genistein or TAM alone inhibited the growth of MCF-7 cells in part via G1 phase arrest, but their combinations showed suggestive antagonistic effects. We further evaluated the effects of bioactive soy components and TAM on the growth inhibition of MCF-7 tumors in a clinically relevant breast tumor model. TAM and bioactive soy components, genistein and soy phytochemical concentrate (SPC), delayed the growth of MCF-7 tumors. The combination of TAM with genistein or SPC, especially at the lower dose of TAM, had synergistic effects on delaying the growth of MCF-7 tumors. Biomarker determination suggests that the combination of TAM and soy components may synergistically delay the growth of MCF-7 tumors via their combined effects on induction of tumor cell apoptosis and inhibition of tumor cell proliferation. In addition, genistein and TAM combination synergistically delayed the growth of breast tumor via decreased estrogen level and activity, and down-regulation of EGFR expression. The results from our studies suggest that further investigations may be warranted to determine if the combination of TAM and bioactive soy components may be used for prevention and/or treatment of estrogen-dependent BRCA.
Introduction
Breast cancer (BRCA) is the most common cancer and the second leading cause of cancer death among women in USA (1). Multiple factors contribute to the development and progression of BRCA. Among these factors, estrogens play a crucial role. Estrogens bind to estrogen receptors (ERs), resulting in an activated complex that acts as a transcription factor through binding to target genes, promoting cell proliferation. Anti-estrogens have been widely studied for their roles in the prevention and treatment of breast tumors, especially on estrogen-dependent breast tumors. Tamoxifen (TAM) is an estrogen antagonist that blocks activity of estrogens in most tissues that are sensitive to estrogens. TAM has become the standard treatment for BRCA at all stages of the disease in both pre-menopausal and postmenopausal women (2). Its potential benefits for the prevention has also been investigated, and it has been found that TAM reduced BRCA risk by 50% among women who had a high risk of BRCA (3).
The risk of BRCA varies substantially throughout the world. Rates tend to be the highest among women in USA and Western Europe, whereas Japanese and Chinese women have the lowest rates (4). This ~6-fold difference in BRCA risk is not due to underlying genetic difference, because the incidence of BRCA in Asian women approaches that of American women after several generations of residence in USA (5). Asian women who were born in USA or in the West had similar incidence rates of BRCA to those women residing in the same areas (5). These findings suggest environmental influences, such as diet and nutrition, as the causal factors for BRCA risk.
The Asian diet is characterized by high content of soy products (6). Efforts are being made to identify the bioactive components in soybeans and soy products that may play a role in the prevention of BRCA. Much of the attention has focused upon genistein that is the predominant isoflavone found in soy. In addition, protease inhibitors, the Bowman–Birk inhibitor, inositol hexaphosphate (phytic acid), lignans, phytosterols and saponins found in soy products may also have bioactivities relevant to the inhibition of carcinogenesis (6–9).
Consumption of soy products and/or use of soy supplements have been increased by post-menopausal women as an alternative for alleviating menopausal related symptoms, particularly as the result of failed hormone replacement therapy trial. For BRCA survivors, the need for an alternative is even more urgent. More than 50% of BRCA survivors use alternative medicine for hormone replacement therapy (10), and isoflavones are one of their favorable choices (11–13). On the other hand, the interactive effect of soy components and TAM on BRCA has not been well defined experimentally, and the results have been conflicting. Whereas some in vitro and in vivo studies have shown potential antagonistic effects between soy active components and TAM on the growth inhibition of estrogen-dependent BRCA cells (14–17), the others have shown potential additive or synergistic effects (18–21).
With TAM being widely used for BRCA prevention and treatment and increased consumption of soy products and soy supplements by women with increased BRCA risk, it is imperative to define the interactive effect between soy components and TAM on BRCA prevention. This study was to evaluate the interactive effects between TAM and bioactive soy components on preventing the growth of estrogen-dependent MCF-7 human BRCA cells in vitro and in a clinically relevant tumor model.
Materials and methods
Soy phytochemicals and TAM
Genistein and daidzein were purchased from LC Laboratories (Woburn, MA). A soy phytochemical extract that represents soy phytochemicals commonly consumed in soy products, soy phytochemical concentrate (SPC), was provided by Archer Daniels Midland Company (Decatur, IL). It contained 51.9% soy isoflavones by weight (50.8% genistein aglycone equivalents, 40.5% daidzein aglycone equivalents and 8.7% glycitein aglycone equivalents). Other components were not quantified. TAM (Sigma, St Louis, MO) was used in cell growth inhibition assays, and TAM pellets (25 or 50 mg per pellet, 90-day release, Innovative Research of America, Sarasota, FL) were used in the animal study.
Cell culture and growth inhibition assay
MCF-7 human BRCA cells were purchased from the American Type Culture Collection (Bethesda, MD). Cells were routinely cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum plus antibiotics of tetracycline and penicillin at 37°C in an atmosphere of 5% CO2 in air.
For the cell growth assay, MCF-7 cells (106 cells per flask) were seeded into 25 cm2 cell culture flasks and settled overnight. The cells were then treated with the indicated concentrations of TAM and/or genistein/daidzein dissolved in 1:1 of dimethyl sulfoxide and ethanol. After treatment for 3 days, cells were trypsinized and counted with Z1 Coulter Particle Counter (Beckman Coulter Company, Miami, FL). The experiments were duplicated and repeated at least twice.
Cell cycle progression assays
Cells were grown and treated under the conditions as described above, harvested by trypsinization and centrifugation, washed with phosphate-buffered saline and fixed with 80% ethanol. Cells were then washed with phosphate-buffered saline, re-suspended, stained by adding propidium iodide (final concentration of 50 µg/ml) and RNase (final concentration of 50 µg/ml) and incubated at 37°C for 30 min. Stained cells were analyzed by flow cytometry (Becton Dickinson, Immunocytometry Systems, Mountview, CA) for fragmented DNA and cell cycle distribution using programs provided by Becton Dickinson.
Animal study
The animal study was conducted by using our previous animal model of orthotopic MCF-7 tumor (22). In brief, female severe combined immune-deficient mice (5- to 8-weeks old) were purchased from Taconic (Germantown, NY), and housed at the animal facility of Beth Israel Deaconess Medical Center in a pathogen-free environment equipped with laminar flow hoods and standard vinyl cages with air filters. After 1 week of acclimatization, intact mice were randomized into one of nine experimental groups (in each group, n = 12) and received corresponding dietary treatments 2 weeks before subcutaneous implantation of 17β-estradiol pellets (0.72 mg 17β-estradiol, 90-day release, Innovative Research of America). MCF-7 human BRCA cells (2 × 106 cells) were then implanted orthotopically into a mammary fat pad of each mouse, and the mice continued to receive the corresponding experimental diets throughout the study.
The experimental groups were as follows: (i) control: AIN-93M as the control diet; (ii) 0.07% genistein: AIN-93M with the addition of 0.07% genistein; (iii) 0.5% SPC: AIN-93M with the addition of 0.5% SPC providing the same amount of genistein aglycone equivalent as that in the diet (ii); (iv) 0.2% SPC: AIN-93M with the addition of 0.2% SPC; (v) 25 mg TAM: AIN-93M with subcutaneous implantation of 25 mg TAM (90-day release, Innovative Research of America) per mouse; (vi) 50 mg TAM: AIN-93M with subcutaneous implantation of 50 mg TAM (90-day release, Innovative Research of America) per mouse; (vii) 0.07% genistein and 25 mg TAM: AIN-93 with the addition of 0.07% genistein plus implantation of 25 mg TAM per mouse; (viii) 0.2% SPC and 25 mg TAM: AIN-93 with the addition of 0.2% SPC plus implantation of 25 mg TAM per mouse and (ix) 0.5% SPC and 50 mg TAM: AIN-93 with the addition of 0.5% SPC plus implantation of 50 mg TAM per mouse.
Food intake and body weight were measured weekly. Tumor diameters were measured twice weekly by a caliper, and tumor volumes were estimated using the following formula: volume (cm3) = (length × width2) ×0.523 (23). The experiment was finished when mean tumor volume in the control mice exceeded 2.0 cm3. At the end of the experiment, the mice were killed, primary tumors were excised and weighed and blood samples were collected. A tumor slice from each primary tumor tissue was carefully dissected and fixed in 10% buffer-neutralized formalin, paraffin embedded and sectioned at 4 µm thickness for histology and immunohistochemistry. Tumor specimens were snap frozen in liquid nitrogen and saved at −8°C for real-time polymerase chain reaction (PCR) quantitation of gene expression. All procedures with animals were reviewed and approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center.
In situ detection of apoptotic index
Apoptotic cells were determined by a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate–biotin nick end labeling assay using the ApopTag in situ Apoptosis Detection System (Oncor, Gaithersburg, MD) according to our previous procedures (22,24). The apoptotic index was expressed as the percentage of positive apoptotic tumor cells to total tumor cells.
Immunohistochemical determination of tumor cell proliferation
Proliferating cell nuclear antigen in tumor was determined by immunohistochemical staining to quantify proliferation index, as described previously (22,24). Both proliferating cell nuclear antigen-positive proliferating cells and total tumor cells were counted in six non-necrotic areas of each section using light microscopy at 400-fold magnification. The proliferation index was calculated as the percentage of proliferating cell nuclear antigen-positive tumor cells to total tumor cells.
Immunohistochemical determination of microvessel density
Microvessel density (MVD) was used as a marker for tumor angiogenesis and detected by immunohistochemical staining of Factor VIII following a method described previously (22,24). MVD was calculated by counting microvessels on ×200 fields under light microscopy at six representative non-necrotic sites of each section.
Real-time quantitative PCR analysis
Real-time PCR primers were designed using Beacon Designer 2.0 software (Palo Alto, CA). Total RNA was isolated by using Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). First-strand cDNA synthesis used 1 mM oligo dT of 18mer (Invitrogen, Carlsbad, CA) and 1.0 µg of total RNA per 30 µl reaction with Ready-To-Go You-Prime First-Strand Beads (Amersham Biosciences, Piscataway, NJ). For validation of the efficiency of the real-time PCR assay, 4-fold dilution series was generated using the cDNA. For the relative quantitation using the delta-delta Ct method, the equivalent of 0.15 µl of the cDNA synthesis step was used per reaction. The sequences of primers used in this study are listed in Table I.
Table I.
Sequences of oligonucleotide primers used for real-time reverse transcription–PCR
| Genes | Sequence | Orientation | Amplification size (bp) |
|---|---|---|---|
| ERα (XM045967)a | 901AGGAGACTCGCTACTGTGC919 | Sense | 141 |
| 1041ACTGGTTGGTGGCTGGAC924 | Anti-sense | ||
| pS2 (X00474) | 144CCCGTGAAAGACAGAATTG162 | Sense | 120 |
| 243CGATGGTATTAGGATAGAAGC263 | Anti-sense | ||
| EGFR (NM005228) | 3404GACAGCATAGACGACACCTTC3424 | Sense | 142 |
| 3526CCTGGTAGTGTGGGTCTCTG3545 | Anti-sense | ||
| GAPDH (M33197) | 349GAGTCCACTGGCGTCTTC366 | Sense | 165 |
| 513GGAGGCATTGCTGATGATC595 | Anti-sense |
GenBank accession number.
PCRs were performed in a 25 µl final volume containing 20 mM Tris–HCl, pH 8.4, 50 mM KCl, 200 µM each deoxynucleoside triphosphate, 0.5 µM each primer, 3.5 mM MgCl2 and 1× SYBR Green (Bio-Rad, Hercules, CA). Three replicates were used. PCR was carried out with an initial 3 min denature at 95°C, followed by 40 cycles of a combined annealing and extension step at 60°C for 30 s and denaturation at 95°C for 10 s. Following the completion of the PCR amplification reaction, a melting curve analysis was performed by heating the sample to 95°C programed for 0 s followed by cooling down to 50°C for 1 min and inclemently heating the samples to 95°C with each step of increasing 1°C and a duration of 10 s while the fluorescence was measured continuously. Glyceraldehydes-3-phosphate dehydrogenase was used as a reference housekeeping gene for internal control. All real-time reverse transcription– PCRs were run on the iCycler iQ single color system (Bio-Rad). To check the amplification specificity, real-time PCR products were subjected to electrophoresis on a 1% agarose gel, stained with ethidium bromide, visualized and photographed under ultraviolet illumination.
Serum levels of 17β-estradiol and insulin-like growth factor-I
Serum levels of 17β-estradiol and insulin-like growth factor-I (IGF-I) were measured by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Diagnostic Systems Laboratories, Webster, TX).
Data statistical analyses
Results were expressed as means ± SDs and analyzed for statistical significance by analysis of variance followed by Fisher’s protected least-significant difference (25) based on two-sided comparisons among experimental groups. A P value of <0.05 was considered statistically significant.
Results
Effects of soy components and TAM on MCF-7 cell growth
We first evaluated the effects of the TAM, genistein or daidzein on the growth of MCF-7 cells. A dose-dependent growth inhibition was observed when MCF-7 cells were treated with genistein (0–50 µM) or TAM (0–6 µM) (Figure 1A). In particular, 50 µM genistein and 6 µM TAM inhibited the growth of MCF-7 cells by 86 and 61%, respectively. Daidzein had the less potent growth inhibition to MCF-7 cells than genistein. Daidzein at 50 µM inhibited MCF-7 cell growth by 36% (other data not shown).
Fig. 1.
Effects of TAM and genistein on the growth and G1 phase arrest of MCF-7 cells. (A) Cells were treated with the indicated concentration of tested components dissolved in 1:1 of dimethyl sulfoxide and ethanol. After treatment for 3 days, the cells were trypsinized and counted with Z1 Coulter Particle Counter. Theoretic additive inhibitory values in percentage of control = percentage of control for TAM treatment multiplied by percentage of control for genistein treatment. (B) Cell cycle progression was determined by flow cytometry. The results showed that both genistein and TAM arrested MCF-7 cells at G1 phase. Results are expressed as mean ± SD, n = 3.
Treatment of MCF-7 cells with TAM and genistein combinations at the higher doses (3 and 25 µM, respectively, and 6 and 50 µM, respectively) resulted in more potent growth inhibition, as compared with treatment with either compound alone (Figure 1A). The nature of the combined effect was estimated by using published methods (22,26), based on the principles described by Chouet al. (27). In brief, the expected value of combination effect between treatment 1 and treatment 2 is calculated as [(observed treatment 1 value)/(control value)] ×[(observed treatment 2 value)/(control value)] × (control value), and the combination index is calculated as the ratio of (expected value)/ (observed value). A ratio of >1 indicates a synergistic effect, and a ratio of <1 indicates a less than additive or an antagonistic effect (22). The theoretic additive inhibitory values for combinations of TAM and genistein were calculated and shown in Figure 1A. The growth inhibition rates of the genistein and TAM combinations were less than that of the theoretic additive combinations, suggesting that TAM and genistein combinations have suggestive antagonistic effects on inhibiting MCF-7 cell growth. Similarly, a suggestive antagonistic effect of daidzein and TAM combinations on MCF-7 cell growth inhibition was observed (data not shown).
Effects of TAM and genistein on MCF-7 cell cycle progression
The effects of TAM, genistein and TAM and genistein combinations on cell cycle progression of MCF-7 cells were further determined using flow cytometry. Genistein and TAM treatments resulted in a dose-dependent cell cycle arrest at G1 phase (Figure 1B). Consequently, the proportion of cells at S phase was decreased in a dose-dependent manner (data not shown), and no significant change of the distribution of cells at G2–M phases or sub-G0 phase (data not shown). Consistent with a suggestive antagonistic effect on the cell growth, the genistein and TAM combinations showed a suggestive antagonistic effect on G1 arrest.
Effects of TAM, genistein and SPC on the growth of MCF-7 tumors in mice
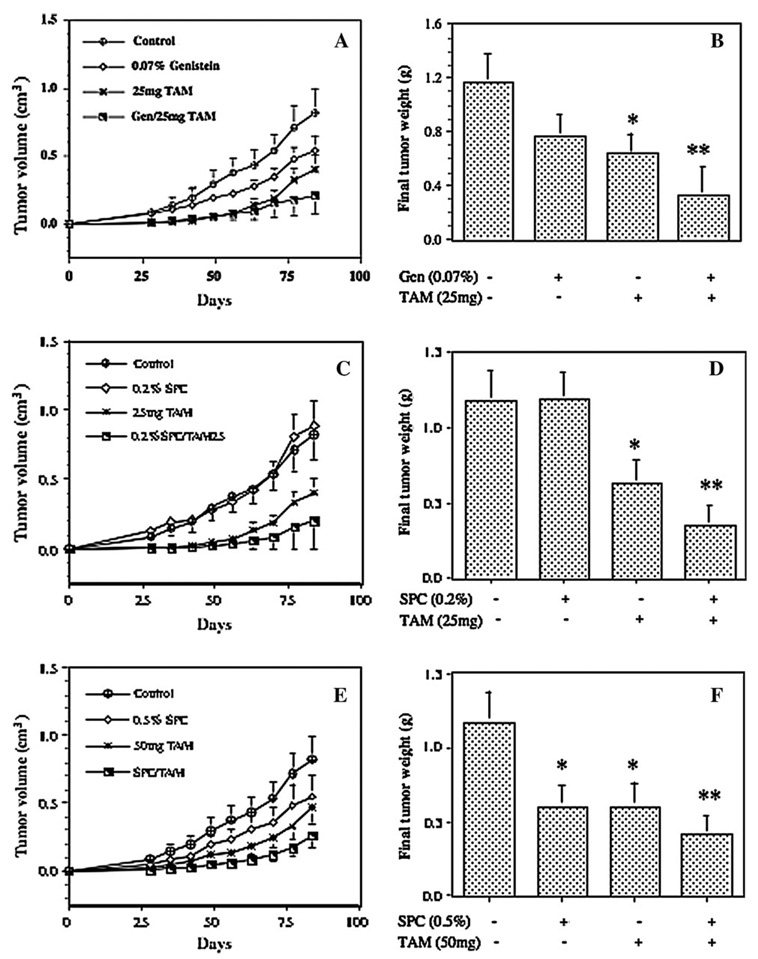
We used a clinically relevant animal model of orthotopic breast tumor to evaluate the effects of soy bioactive components and TAM, alone and in combinations, on prevention of estrogen-dependent breast tumor growth. Treatments with implantation of TAM or dietary supplementation of soy components did not significantly alter food intake or final body weight (data not shown). Figure 2 shows the time- and dose-dependent effects of genistein, SPC and TAM on tumor growth. To make results clearer to present, the standard error for each data point (<10% of the mean) was not included in Figure 2A, C and E. All treatments showed a time-dependent inhibition to MCF-7 tumor volume (Figure 2A, C and E) and final tumor weights (Figure 2B, D and F). SPC and TAM also delayed tumor growth in a dose-dependent manner. Although SPC at 0.2% of the diet did not significantly delay tumor growth, SPC at 0.5% significantly delayed tumor growth and reduced the final tumor weight by 49% (P < 0.05), and genistein at 0.07% of the diet reduced final tumor weight by 35% (P = 0.1). These data suggest that SPC may contain other bioactive components, other than genistein, that have additional anti-growth activities.
Fig. 2.
Effects of soy components and TAM combinations on tumor volume and final tumor weight in vivo. (A and B) Effects of genistein (0.07%) and TAM (25 mg), alone and in combination, on tumor volume and final tumor weight. (C and D) Effects of SPC (0.2%) and TAM (25 mg), alone and in combination, on tumor volume and final tumor weight. (E and F) Effects of SPC (0.5%) and TAM(50 mg), alone and in combination, on tumor volume and final tumor weight. Mice were treated with experimental diets for 2 weeks, supplemented with 17β-estradiol, implanted orthotopically with MCF-7 cells and continued on experimental diets throughout the study. Values are expressed as mean ± SEM. *P < 0.05 and **P < 0.01 (compared with the control).
Effects of soy phytochemicals and TAM combinations on the growth of MCF-7 tumor in mice
Several combinations of TAM and soy bioactive components were evaluated for their combined effects on tumor growth. The doses of TAM and active soy components genistein and SPC were selected so that the combination effects could be appropriately evaluated. All combination treatments showed more potent inhibition to tumor volume and final tumor weight than either treatment alone (Figure 2). Mice treated with genistein, TAM(25 mg) and genistein–TAM combination reduced final tumor weights by 35% (P = 0.1), 46% (P < 0.05) and 73% (P < 0.005), respectively (Figure 2B). The ratio of ‘observed value’ to ‘expected value’ was 1.12, suggesting that the combination of genistein and TAM have a synergistic effect on delaying the growth of MCF-7 tumor in vivo. Analyses of tumor volumes at other time points in general showed additive to synergistic effects (data not shown).
The combinations of TAM with SPC also further potentiated the effect of either treatment alone on preventing the growth of MCF-7 tumors. Although SPC at 0.2% of the diet did not have a significant effect on final tumor weight, its combination with TAM at 25 mg significantly reduced final tumor weight by 70% (P < 0.005, Figure 2D). The ratio of observed value to expected value was 1.52, suggesting a strong synergistic effect of SPC (0.2%) and TAM (25 mg) combination on MCF-7 tumor growth. Analyses of tumor volumes at other time points also in general showed the synergistic results (data not shown). SPC at 0.5% of the diet, TAM at 50 mg and SPC (0.5%) and TAM (50 mg) combination delayed tumor growth and reduced final tumor weights by 49% (P < 0.05), 48% (P < 0.05) and 65% (P < 0.005), respectively (Figure 2F). Although the combination further reduced tumor growth, the ratio of observed value to expected value was 0.89, suggesting that the combination of SPC (0.5% of the diet) and TAM (50 mg) may have less than additive effect on tumor growth inhibition. Analyses of tumor volumes at other time points showed that the combination of SPC and TAM at the higher doses in general had an additive effect on tumor volume reduction (data not shown).
Effects of soy phytochemicals and TAM on proliferation and apoptosis of tumor cells and tumor angiogenesis
Table II shows the effects of treatments on modulation of tumor cell apoptosis and proliferation and tumor MVD. The samples from the genistein and TAM (25 mg) combination and the SPC (0.2%) and TAM (25 mg) were analyzed because of the suggestive additive to synergistic effects of these combinations on MCF-7 tumor growth inhibition. Apoptotic indices in MCF-7 tumors from mice treated with 0.07% genistein, 0.2% SPC, 0.5% SPC, TAM (25 mg), TAM (50 mg), genistein and TAM (25 mg) combination and SPC (0.2%) and TAM (25 mg) combination were increased by 2% (P > 0.05), 4% (P > 0.05), 114% (P < 0.005), 100% (P < 0.005), 148% (P < 0.005), 132% (P < 0.005) and 296% (P < 0.001), respectively, compared with that in the control tumors (Table II). The data suggest that the combinations between TAM (25 mg) with genistein or SPC have synergistic effects on inducing apoptosis of MCF-7 tumor cells in vivo.
Table II.
Effects of treatments with soy phytochemicals and TAM on tumor cell apoptosis, proliferation and angiogenesis and serum estrogen levels
| Apoptotic index (%) | Proliferation index (%) | MVD (no. of vessels/field) | 17β-estradiol (pg/ml) | |
|---|---|---|---|---|
| Control (n=8) | 2.22 ± 0.42a | 91.4 ± 4.5a | 4.00 ± 0.66a | 636.4 ± 113.8a |
| Genistein (0.07%) (n=8) | 2.26 ± 0.47a | 89.2 ± 3.8a | 3.36 ± 0.31bc | 887.5 ± 165.1a |
| SPC (0.2%) (n=8) | 2.30 ± 0.50a | 88.5 ± 2.2a | 3.83 ± 0.21ac | 859.9 ± 186.1a |
| SPC (0.5%) (n=7) | 4.74 ± 0.57b | 84.7 ± 3.3b | 3.50 ± 0.76ac | 699.2 ± 150.1a |
| TAM (25 mg) (n=8) | 4.44 ± 0.47b | 82.6 ± 5.9b | 3.33 ± 0.60bc | 713.3 ± 164.1a |
| TAM (50 mg) (n=7) | 5.50 ± 0.69b | 78.3 ± 6.5b | 2.89 ± 0.97bc | 693.7 ± 167.6a |
| Genistein–TAM (25 mg) (n=8) | 5.16 ± 0.60b | 81.0 ± 2.6b | 3.61 ± 0.69ac | 135.6 ± 33.3b |
| SPC (0.2%)–TAM (25 mg) (n=7) | 8.79 ± 0.40c | 72.0 ± 5.7c | 2.91 ± 0.53bc | 613.1 ± 141.2a |
Values are means ± SEMs. Within the column, the values not sharing a common superscript letter are significant (P < 0.05).
The proliferation indices in MCF-7 tumors from mice treated with the above diets were reduced by 2.4% (P > 0.05), 3.2% (P > 0.05), 7.3% (P < 0.05), 9.6% (P < 0.005), 14.3% (P < 0.005), 11.4% (P < 0.005) and 21.2% (P < 0.005), respectively, compared with that of the control (Table II). The results also suggest that the combinations have additive to synergistic effects on inhibiting MCF-7 cell proliferation in vivo. Although the treatments showed inhibition of angiogenesis of MCF-7 tumors, the results did not indicate apparent additive or synergistic effects between TAM and genistein, or SPC (Table II).
Effects of soy phytochemicals and TAM on serum levels of estrogen and IGF-I
As shown in TableII , the genistein and TAM (25 mg) combination treatment significantly reduced serum level of 17β-estradiol by 78.7% (P < 0.05), compared with the control. On the other hand, genistein or TAM (25 mg) alone, and other treatments did not significantly alter serum estrogens. Serum levels of IGF-I were not significantly altered by any treatments, alone or in combination (data not shown).
Effects of soy phytochemicals and TAM on the expression of ERα, pS2 and EGFR in vitro and in vivo
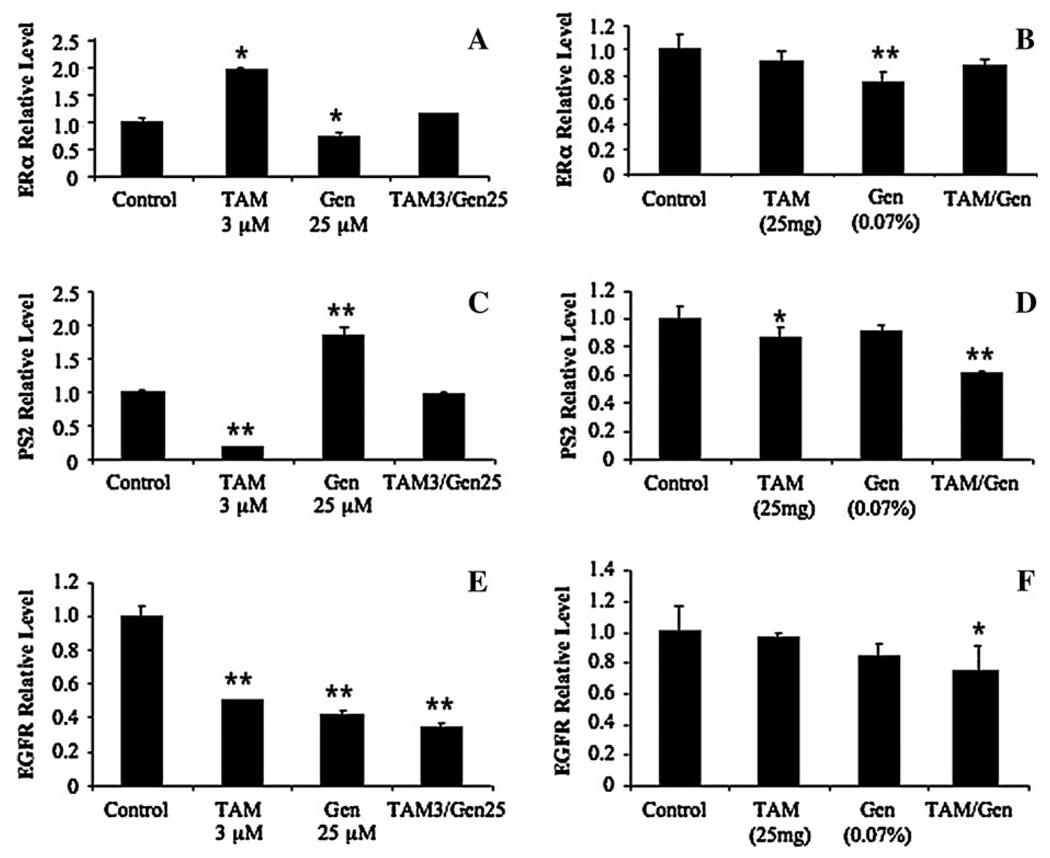
Efforts were made to compare the expression of certain genes, such as ERα, pS2 and EGFR, in the in vitro and in vivo samples. Real-time quantitative PCR analysis demonstrated that except for a few situations, expression patterns of ERα, pS2 and EGFR were similar in both cultured cancer cells and the xenograft tissues in mice (Figure 3). ERα content was significantly decreased by the genistein treatment both in vitro and in vivo, increased by the TAM treatment in vitro and not significantly changed by their combination treatment (Figure 3A and B). In contrast, the expression of pS2, an estrogen-responsive gene, was significantly increased by genistein treatment in vitro, but not in vivo, decreased by TAM treatment both in vitro and in vivo and significantly decreased by the combination treatment in vivo (Figure 3C and D). EGFR expression level was significantly down-regulated by genistein and TAM treatments in vitro (Figure 3E), but its down-regulation was not significant in vivo (Figure 3F). The down-regulation of EGFR expression by genistein and TAM was further significantly potentiated by their combination treatment both in vitro and in vivo (Figure 3E and F). We also determined the effects of treatments on ERβ expression, and the results showed that the experimental treatments, alone or in combinations, did not significantly alter ERβ expression (data not shown).
Fig. 3.
Effects of genistein and TAM, alone and in combination, on expression of ERα (A and B), pS2 (C and D) and EGFR (E and F) in vitro and in vivo. The mRNA contents were quantitated by real-time PCR approach. Results are expressed as mean ± SD, n = 3. *P < 0.05 and **P < 0.01, when compared with the control.
Discussion
The aim of this study was to evaluate the interactive effects between TAM and bioactive soy components on prevention of the growth of estrogen-dependent BRCA in a clinically relevant human BRCA animal model. We found that TAM and bioactive soy components inhibited the growth of MCF-7 tumors, and the combination of TAM with genistein or SPC, especially at the low doses of TAM and SPC, had synergistic effects on the growth inhibition of MCF-7 tumors. Biomarker determination suggests the mechanisms by which TAM and soy component combinations may synergistically inhibit the growth of MCF-7 tumors via their combined effects on induction of tumor cell apoptosis and inhibition of tumor cell proliferation. In particular, genistein may synergistically enhance the activity of TAM on breast tumor growth inhibition via decreased estrogen level and activity, and down-regulation of EGFR expression. It is the first report, to the best of our knowledge, to demonstrate that the genistein and TAM combination may synergistically inhibit the growth of estrogen-dependent breast tumors via significant modulation of circulating level of estrogen in vivo.
In this study, we used a clinically relevant animal model with maintained estrogen levels through implantation of 17β-estradiol pellets into severe combined immune-deficient mice (22). Ovariectomization is usually used to mimic estrogen status in post-menopausal women. However, this manipulation of estrogens may not be relevant for establishing the estrogen-dependent breast tumor animal model. We propose that an animal model with maintained estrogen levels would have greater clinical relevance than one with depleted estrogen levels for the estrogen-dependent MCF-7 breast tumors in vivo. Estrogen levels in intact female mice are not sufficient to support the growth of estrogen-dependent MCF-7 cells, and the growth of MCF-7 tumors in intact mouse requires estrogen supplements (28). Removal of estrogen pellets stops MCF-7 tumor growth (28). Conversely, estrogen-dependent breast tumors develop and progress in postmenopausal women, suggesting that estrogen levels in post-menopausal women are sufficient to support growth of estrogen-dependent breast tumors. Circulating estrogen levels in animals are higher than that in women. Therefore, clinically relevant animal models of estrogen-dependent BRCA should contain estrogen levels adequate to support tumor growth.
By using this relevant animal model, we found that the combinations of TAM with genistein or SPC had additive to synergistic effects on inhibiting the growth of MCF-7 tumors. Further biomarker analyses suggest that these combination regimens may exert their additive/ synergistic effects via differential cellular and molecular mechanisms. The TAM (25 mg) and SPC (0.2%) combination had significant potentiating effects on inducing tumor cell apoptosis, inhibiting tumor cell proliferation and inhibiting tumor angiogenesis (Table II). Although the TAM (25 mg) and genistein (0.07%) combination also had potentiating effects on induction of tumor cell apoptosis and inhibition of tumor cell proliferation, it dramatically decreased circulating estrogen levels by 79% (Table II).
Our finding that genistein and TAM combination significantly decreased circulating estrogen levels provides important experimental evidence to understand the mechanism by which the combination of TAM and genistein may synergistically inhibit the growth of MCF-7 tumors in vivo. Estrogen plays an essential role in the development and growth of estrogen-dependent BRCA. The action of estrogen is mediated via interaction with ERα and ERβ that initiates a series of events, leading to the modulation of hormone-responsive genes and cell proliferation. In this study, each mouse was implanted with the same dose of estrogen pellet; thus, the exogenous estrogen levels were similar in mice of all experimental groups. The dramatically decreased circulating levels of estrogen in the TAM and genistein combination group suggest that the genistein and TAM combination may significantly modulate estrogen metabolism in other organs, most probably in liver. Further investigation of the roles genistein and TAM may play in estrogen metabolism-related enzymes and measurement of certain estrogen metabolites may provide crucial information on how the combination synergistically decreases circulating estrogen levels. This finding may also explain in part why the genistein and TAM combination does not show synergistic growth inhibition in the in vitro system because this estrogen metabolism may not occur in vitro.
ERs, especially ERα, also play an important role in mediating estrogen function. We found in this report that genistein down-regulated ERα expression in vivo (Figure 3B), but TAM or the TAM and genistein combination did not significantly alter ERα expression in vivo. The expression of ERβ was not measured. Thus, the synergistic effect of genistein and TAM combination may not be explained by modulation on ERα expression/function. As a result, the observed synergistic down-regulation of pS2 (Figure 3D), an estrogen-responsive gene by the TAM and genistein combination may be primarily due to decreased estrogen levels. Our results support that alteration of estrogen level and activity may provide an effective approach for prevention and treatment of estrogen-dependent BRCA.
Our finding that genistein and TAM combination synergistically down-regulated the expression of EGFR may also provide a possible mechanism of synergistic effect between genistein and TAM on tumor growth inhibition. EGFR is involved in the pathogenesis of human breast carcinoma. Treatment of BRCA cells with EGFR inhibitors could significantly inhibit human BRCA cell growth (29). The effects of genistein on EGFR expression have not been well defined, ranging from significant inhibition (30), non-significant effect (31), to significant increase (32). TAM-resistant BRCA cells have higher expression of EGFR (33). Our in vitro studies showed that TAM (3 µM) and genistein (25 εM) significantly down-regulated EGFR expression (Figure 3E). Although TAM (25 mg) or genistein (0.07%) did not significantly down-regulate EGFR expression, the combination significantly down-regulated EGFR expression in vivo in a synergistic manner (Figure 3).
In conclusion, by using a clinically relevant breast tumor animal model, we found that bioactive soy components, such as genistein and SPC, enhanced the TAM activity in inhibiting the growth of estrogen-dependent breast tumors in an additive or synergistic manner via differential cellular and molecular mechanisms. In particular, we first reported that one of the mechanisms by which genistein and TAM combination had potentiating effects on tumor growth is via modulation of estrogen level and biological activity. Our results support further investigations on the application of soy components and TAM combination strategy for prevention and/or adjuvant therapy of hormone-dependent BRCA.
Acknowledgements
This study was supported in part by Susan Komen’s Breast Cancer Research Foundation (BCTR0402749) and the United States Public Health Service (RO1 AT00863).
Abbreviations
- BRCA
breast cancer
- ER
estrogen receptor
- IGF-I
insulinlike growth factor-I
- MVD
microvessel density
- PCR
polymerase chain reaction
- SPC
soy phytochemical concentrate
- TAM
tamoxifen
Footnotes
Conflict of Interest Statement: None declared.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2005. [Google Scholar]
- 2.Fisher B, et al. Five vs more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptorpositive tumors. J. Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 4.Henderson BE, et al. The international variation in breast cancer rates: an epidemiological assessment. Breast Cancer Res. Treat. 1991;18:S11–S17. doi: 10.1007/BF02633520. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler RG, et al. Migration patterns and breast cancer risk in Asian-American women. J. Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 6.Messina MJ, et al. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AR. The evidence for soybean products as cancer preventive agents. J. Nutr. 1995;125:733S–743S. doi: 10.1093/jn/125.3_Suppl.733S. [DOI] [PubMed] [Google Scholar]
- 8.Rao AV, et al. Saponins as anticarcinogens. J. Nutr. 1995;125:717s–724s. doi: 10.1093/jn/125.3_Suppl.717S. [DOI] [PubMed] [Google Scholar]
- 9.Shamsuddin AM. Inositol phosphates have novel anticancer function. J. Nutr. 1995;125:725s–732s. doi: 10.1093/jn/125.3_Suppl.725S. [DOI] [PubMed] [Google Scholar]
- 10.Newton KM, et al. Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet. Gynecol. 2002;100:18–25. doi: 10.1016/s0029-7844(02)02005-7. [DOI] [PubMed] [Google Scholar]
- 11.Messina MJ, et al. Soy for breast cancer survivors: a critical review of the literature. J. Nutr. 2001;131:3095S–3108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 12.Brzezinski A, et al. Phytoestrogens: the "natural" selective estrogen receptor modulators? Eur. J. Obstet. Gynecol. Reprod. Biol. 1999;85:47–51. doi: 10.1016/s0301-2115(98)00281-4. [DOI] [PubMed] [Google Scholar]
- 13.Carusi D. Phytoestrogens as hormone replacement therapy: an evidence-based approach. Prim. Care Update Ob. Gyns. 2000;7:253–259. doi: 10.1016/s1068-607x(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 14.Jones JL, et al. Genistein inhibits tamoxifen effects on cell proliferation and cell cycle arrest in T47D breast cancer cells. Am. Surg. 2002;68:575–578. [PubMed] [Google Scholar]
- 15.Ju YH, et al. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477. [PubMed] [Google Scholar]
- 16.Liu B, et al. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res. 2005;65:879–886. [PubMed] [Google Scholar]
- 17.Seo HS, et al. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res. Treat. 2006;99:121–134. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 18.Constantinou AI, et al. The soy isoflavone daidzein improves the capacity of tamoxifen to prevent mammary tumours. Eur. J. Cancer. 2005;41:647–654. doi: 10.1016/j.ejca.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Shen F, et al. Tamoxifen and genistein synergistically down-regulate signal transduction and proliferation in estrogen receptor-negative human breast carcinoma MDA-MB-435 cells. Anticancer Res. 1999;19:1657–1662. [PubMed] [Google Scholar]
- 20.Tanos V, et al. Synergistic inhibitory effects of genistein and tamoxifen on human dysplastic and malignant epithelial breast cells in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;102:188–194. doi: 10.1016/s0301-2115(01)00582-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, et al. Potential beneficial metabolic interactions between tamoxifen and isoflavones via cytochrome P450-mediated pathways in female rat liver microsomes. Pharm. Res. 2004;21:2095–2104. doi: 10.1023/b:pham.0000048202.92930.61. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J-R. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int. J. Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J-R, et al. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J. Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J-R, et al. The inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res. 1998;58:5231–5238. [PubMed] [Google Scholar]
- 25.Steel RGD, et al. Principles and Procedures of Statistics: A Biometrical Approach. New York, NY: McGraw-Hill book Company, Inc; 1980. [Google Scholar]
- 26.Yokoyama Y, et al. Synergy between angiostatin and endostatin: inhibition of ovarian cancer growth. Cancer Res. 2000;60:2190–2196. [PubMed] [Google Scholar]
- 27.Chou TC, et al. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Soule HD, et al. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- 29.Normanno N, et al. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with trastuzumab (Herceptin) on human breast cancer cell growth. Ann. Oncol. 2002;13:65–72. doi: 10.1093/annonc/mdf020. [DOI] [PubMed] [Google Scholar]
- 30.Lamartiniere CA, et al. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J. Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 31.El-Zarruk AA, et al. The anti-proliferative effects of tyrosine kinase inhibitors towards tamoxifen-sensitive and tamoxifen-resistant human breast cancer cell lines in relation to the expression of epidermal growth factor receptors (EGF-R) and the inhibition of EGF-R tyrosine kinase. Cancer Lett. 1999;142:185–193. doi: 10.1016/s0304-3835(99)00167-6. [DOI] [PubMed] [Google Scholar]
- 32.Cotroneo MS, et al. Genistein action in the prepubertal mammary gland in a chemoprevention model. Carcinogenesis. 2002;23:1467–1474. doi: 10.1093/carcin/23.9.1467. [DOI] [PubMed] [Google Scholar]
- 33.Hiscox S, et al. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib (‘Iressa’, ZD1839) Clin. Exp. Metastasis. 2004;21:201–212. doi: 10.1023/b:clin.0000037697.76011.1d. [DOI] [PubMed] [Google Scholar]