Abstract
Xanthorhodopsin is a light-driven proton pump in the extremely halophilic bacterium Salinibacter ruber. Its unique feature is that besides retinal it has a carotenoid, salinixanthin, with a light harvesting function. Tight and specific binding of the carotenoid antenna is controlled by binding of the retinal. Addition of all-trans retinal to xanthorhodopsin bleached with hydroxylamine restores not only the retinal chromophore absorption band, but causes sharpening of the salinixanthin bands reflecting its rigid binding by the protein. In this report we examine the correlation of the changes in the two chromophores during bleaching and reconstitution with native all-trans retinal, artificial retinal analogues, and retinol. Bleaching and reconstitution both appear to be multi-stage processes. The carotenoid absorption changes during bleaching occurred not only uponhydrolysis of the Schiff base but continued while the retinal was leaving its binding site. In the case of reconstitution, the 13-desmethyl analogue formed the protonated Schiff base slower than retinal, and provided the opportunity to observe changes in carotenoid binding at various stages. The characteristic sharpening of the carotenoid bands, indicative of its reduced conformational heterogeneity in the binding site, occurs already when the retinal occupies the binding site but the covalent bond to Lys-227 via a Schiff base is not yet formed. This is confirmed by the results for retinol reconstitution, where the Schiff base does not form but the carotenoid exhibits its characteristic spectral change from the binding.
INTRODUCTION
Xanthorhodopsin (1) is a retinal-based proton pump in the cell membranes of the extremely halophilic eubacterium Salinibacter ruber (2). In addition to all-trans retinal, it contains one molecule of the C40-carotenoid acyl glycoside, salinixanthin (3), which functions as a light-harvesting antenna that transfers ca. 40% of the absorbed quanta to the retinal (1). Salinixanthin is the major carotenoid of this organism, comprising more than 90% of the total (3). A large fraction of salinixanthin in the cell membranes is not bound to xanthorhodopsin and may have a photoprotective role. The absorption spectrum of the carotenoid is remarkably different when in the bound state. The strong narrowing of the salinixanthin vibronic bands (1) suggests that in the binding site its conformational heterogeneity is restricted. This might be important for maximization of energy transfer efficiency. From analogy with other carotenoid binding proteins (4, 5), it is likely that immobilization of the salinixanthin ring through hydrogen bonding of its keto group to a protein residue involved in the binding (“locking” (5)), is responsible for the greater resolution of the vibronic bands. Upon binding, salinixanthin acquires asymmetric geometry or an asymmetric environment, and becomes chiral (6). Fluorescence studies have indicated that the carotenoid transition moment is oriented at 59° to the retinal, and thus the polyene chain probably lines up along the axes of the transmembrane helixes (7). Hydrolysis of the Schiff base linkage of the retinal to Lys-227 (the residue homologous to Lys-216 in bacteriorhodopsin) with hydroxylamine causes broadening of the carotenoid bands, and the spectrum becomes similar to that of the non-bound, excess carotenoid in the cell membranes (1). Reconstitution with all-trans retinal reverses this change. The spectral changes similar to these occur also transiently during the photocycle, apparently in response to electrostatic and conformational changes of the protein (1). On the other hand, protonation of the counter-ion to the Schiff base, Asp-83 (homologous to Asp-85 in bacteriorhodopsin), causes only a small absorption shift of the bound carotenoid, and does not affect the binding substantially, as one can conclude from lack of substantial changes in the extinction or resolution of the carotenoid bands when the pH is varied near the pKa of the counter-ion, ca. 6 (8).
An intriguing question is how retinal binding controls the carotenoid binding. In this paper we examine whether the fully formed retinal chromophore, with a covalent bond to Lys-227 and a protonated Schiff base, is necessary to induce the specific binding of the carotenoid. In addition to all-trans retinal, we used the all-trans 9-desmethyl and 13-desmethyl retinal analogues (9–11), as well as all-trans retinol that does not form a covalent bond with lysine. The bleaching kinetics and the analogues helped to dissect the stages of retinal-protein interaction and relate them to the carotenoid binding. Earlier studies on bacteriorhodopsin (12, 13) showed that reconstitution of this chromoprotein occurs in at least two major steps: i) rapid (seconds) incorporation of retinal into the binding site and formation of intermediates with modestly red-shifted absorption spectra (from 370 nm in retinal to 400 and 430 nm in these intermediates), and ii) a subsequent slow phase (minutes) corresponding to covalent binding of retinal and formation of characteristic band of the protonated Schiff base at 570 nm. The latter step was strongly slowed in some retinal analogues, particularly in the case of 13-desmethyl retinal (9). Our goal was to follow the bleaching and the reconstitution processes in xanthorhodopsin, and correlate the changes that take place in its retinal and salinixanthin chromophores.
MATERIALS AND METHODS
Cultures of Salinibacter ruber M31 were grown in SW medium with 0.2% of yeast extract as described earlier (14). The cells were broken by overnight dialysis against water, and the heavy fraction of cell membranes was obtained as before (6). Further purification included washing several times in 100 mM NaCl and then in water, and collecting the membrane fraction at 144,000 × g. The discarded supernatant contained small membrane fragments with substantial amounts of salinixanthin not bound to xanthorhodopsin, and cytochromes. This simple method did not involve any detergent. The final preparations contained highly purified xanthorhodopsin, in which the fraction of non-bound carotenoid was ca. 15%. The state of salinixanthin in xanthorhodopsin (and fraction of non-bound carotenoid) was determined from the resolution of its vibronic bands. In xanthorhodopsin a clear minimum is observed at 505 nm between the 521 nm and 486 nm carotenoid bands. The ratio of the amplitude of the 521 nm (measured from 505 nm minimum) divided by the absorbance at the 486 nm maximum is ca. 0.2 in the samples containing pure xanthorhodopsin. Presence of non-bound carotenoid decreases this ratio. The extinction coefficient of salinixanthin was not determined exactly (S. Liaaen-Jensen, personal communication), but from the values for the carotenoids with same number of conjugated bonds and containing 4-keto group, astaxanthin and canthaxanthin (15) it is predicted to be in the 125,000–140,000 M−1 cm−1 range.
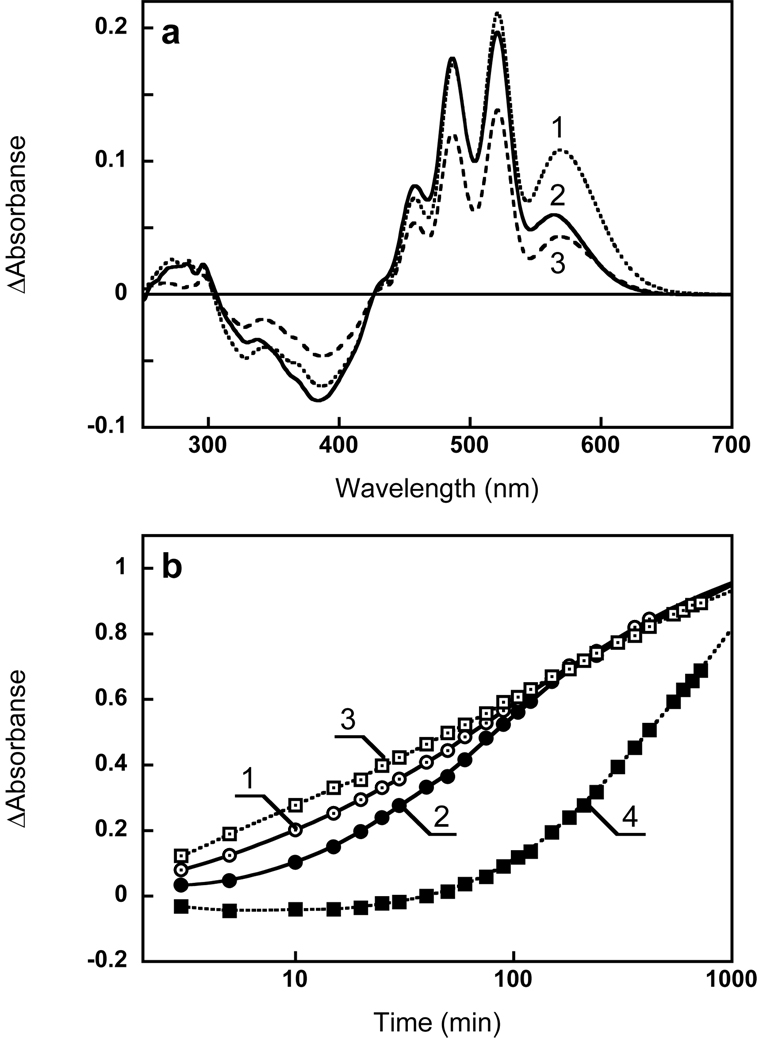
Xanthorhodopsin’s Schiff base was hydrolyzed by incubating in 200 mM hydroxylamine, pH 7, while illuminating at 550–600 nm with a 300 W tungsten lamp (200 mW/cm2). To avoid heating, a 5 cm layer of 4% CuSO4 solution was placed in the beam in front of the cuvette, which was kept at a constant temperature of 25°C with a water jacket. Since hydroxylamine is unstable at neutral pH, fresh solutions at pH 7.2 were made by mixing 2 M of hydroxylamine with 2 M NaOH (16). The retinal pigment bleached almost completely (>99%) in 3 hrs, as one can conclude from the kinetics of the absorption changes at 600 nm and 362 nm (Fig 1). The retinal oxime formed subsequently leaves the retinal binding site, and was removed by incubation with bovine serum albumin (overnight, 10 mg/ml) and washing with 100 mM NaCl (3 times) (Fig. 1a, spectra 2 and 3).
Figure 1.
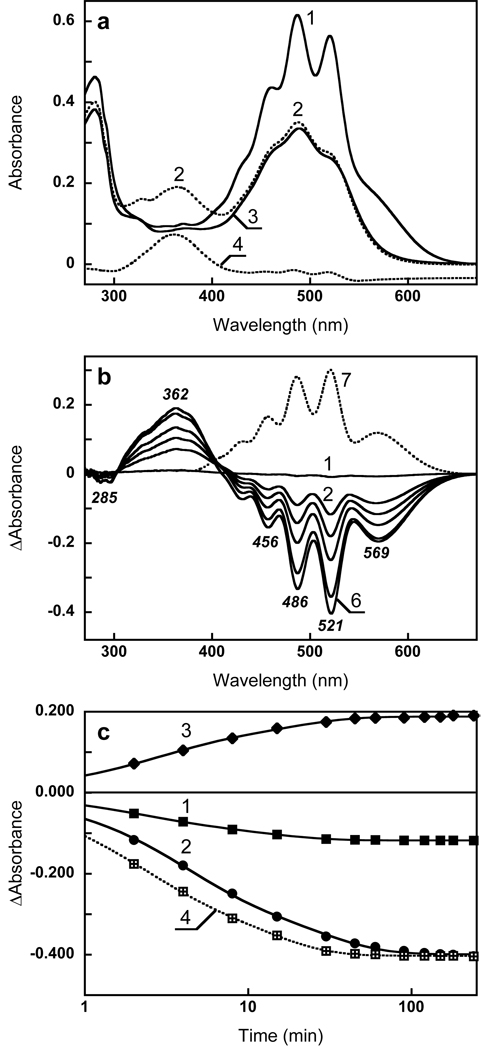
Absorption changes accompanying hydrolysis of xanthorhodopsin with hydroxylamine. (a) Spectrum 1, absorption spectrum of membranes in 100 mM NaCl; spectrum 2, after 6 hours incubation with 0.2 M hydroxylamine with illumination at 550–650 nm, pH 7.2; spectrum 3, after washing to remove retinal oxime (normalized with spectrum 2 at 533 nm, an isosbestic point between loosely bound and well bound carotenoid, see below); spectrum 4, difference between spectra 2 and 3. (b) Spectrum 1, absorption changes caused by incubation of the sample 2 min in the dark; spectra 2 through 6, difference spectra after illumination at 550–650 nm in the presence of 0.2 M hydroxylamine, pH 7.2, for: 2, 4, 8, 30, and 240 min, respectively; spectrum 7, 1 minus 3 in Fig. 1a. (c) Kinetics of absorption changes in the course of illumination in (b), followed at: 1, 600 nm (retinal chromophore); 2, 521 nm (carotenoid); 3, 362 nm (retinal oxime); 4, same as 1, but multiplied by 3.4 to normalize with 2.
All-trans retinal was from Sigma-Aldridge. The concentration of retinal was calculated from the extinction coefficient 42,800 M−1 cm−1 at 380 nm (17). 9-Desmethyl and 13-desmethyl analogs of all-trans retinal were synthesized as described earlier (18). They were added to well-washed bleached membranes containing xantho-opsin, in ethanol solution. The amount of the latter was less than 0.5% of the total volume of suspension in 100 mM NaCl, pH 8. The reconstitution was performed at room temperature (22°C). All-trans retinol (crystalline, synthetic, >95% pure) was purchased from Sigma-Aldridge. Its diluted ethanol solution exhibited a maximum at 325 nm.
Absorption spectra were measured in 10×4 mm stoppered cuvettes with black walls on Shimadzu UV 1601 and UV 1700 spectrophotometers. The sample volumes were typically 0.9–1.0 ml, the path length, 1 cm. The spectral and kinetic data were fitted globally using FitExp program (19).
RESULTS
Kinetics of hydroxylamine bleaching
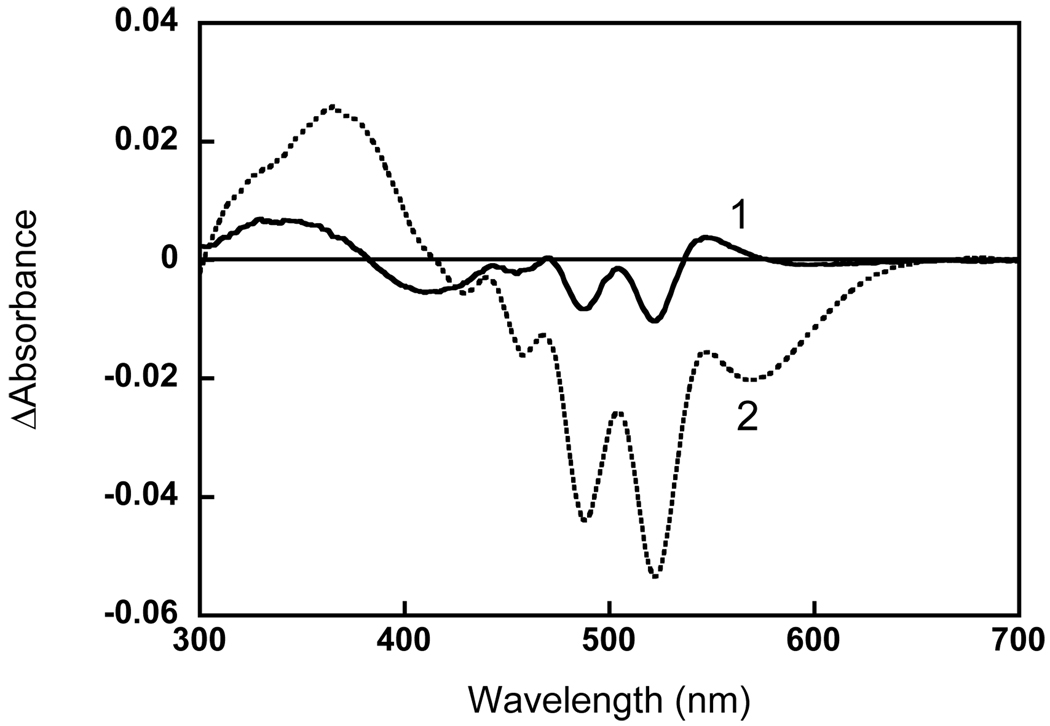
Fig. 1a shows the spectrum of Salinibacter ruber membranes at pH 8 before and after bleaching with hydroxylamine (spectra 1 and 2, respectively). As found earlier (1), hydrolysis of the Schiff base eliminates not only the retinal protein band (shoulder) at 560 nm but also broadens the sharp bands of the salinixanthin antenna at 521, 486, 456 nm, transforming its spectrum into a less structured one. The absorbance change in the 521 nm band was 2.2 times greater than at 569 nm in the final difference spectrum (Fig. 1b). These changes were accompanied by a decrease in the extinction of the aromatic amino acid residues at 297, 285 and 270 nm. The kinetics of absorption changes in the retinal chromophore band at 600 nm, carotenoid band at 521 nm and retinal oxime band at 362 nm are shown in Fig. 1c. The changes in the carotenoid band occur slightly slower than in the retinal and retinal oxime bands. As a result of this, the relative amplitude of the carotenoid peak at 521 nm, compared to the retinal band in the difference spectra (Fig. 1b), increases with time from 1.3 (after 2 min illumination) to 2.2 (after 6 hours). The possible reason for this is that the hydrolysis of the retinal Schiff base and the subsequent changes in the carotenoid binding site do not occur synchronously. This was demonstrated in the following experiment. The initial membranes were illuminated briefly (4 min) in the presence of 200 mM hydroxylamine. This bleached about 75% of the retinal chromophore and induced changes in the carotenoid bands. During subsequent incubation in the dark further absorption changes were observed mostly in the carotenoid bands with relatively smaller changes in the retinal bands. Global fit analysis revealed two components for the reaction in the dark (with time constants of 15 and 80 min), shown in Figure 2. The difference spectrum of the faster component (spectrum 1) showed the typical decrease of extinction in the carotenoid bands and no significant changes in the retinal band around 570 nm. A bilobe around 400 nm was apparently caused by a change in the environment of the retinal oxime. The second kinetic component showed both retinal and carotenoid bands, and was due to a slow hydrolysis of the Schiff base in the dark and subsequent changes in carotenoid binding.
Figure 2.
Amplitude spectra for the dark reaction of xanthorhodopsin with hydroxylamine. Two components obtained from the global fit of the difference spectra recorded in the dark after illumination of the sample for 4 min at 550–650 nm: 1, absorption changes with time constant 15 min; 2, with time constant 80 min.
The reason for a decoupling of the carotenoid changes from the retinal changes after the hydrolysis of the retinal Schiff base became clear from reconstitution experiments (see below), which showed that forming the well-resolved carotenoid bands (and tight binding of the carotenoid) do not require a covalent bond between retinal and the protein, only that the retinal binding site be occupied. The slower absorbance decrease at the carotenoid maxima corresponds to the diffusion of the retinal oxime from the binding site and the response of the carotenoid antenna to this event.
Washing the bleached membranes removes at least 90% of the retinal oxime but most of the carotenoid remains in the membrane (Fig. 1a, spectrum 3). The difference between spectra 2 and 3 in Fig. 1a (spectrum 4) is the retinal oxime band at 360 nm and minor changes of carotenoids caused mainly by removal of oxime from the retinal binding site. Suspensions of apo-membranes analogous to the one described above (Fig. 1a, spectrum 3) were used in the reconstitution experiments.
Kinetics of reconstitution with all-trans retinal: correlation with carotenoid binding
Addition of all-trans retinal to the Salinibacter cell membranes containing xantho-opsin initiates reconstitution of the retinal-protein, as indicated by formation of the 569 nm band, and sharpening of the vibronic bands of the carotenoid at 521, 486 and 457 nm (Figs. 3a and b). In the region where aromatic amino acids absorb, three sharp positive bands appear at 297, 285 and 270 nm (Fig. 3b), caused by increase in extinction of tryptophan and tyrosine residues. All-trans retinal also exhibits small and broad absorption bands in this wavelength range (20), which might contribute to the observed changes but they are not responsible for the 296 and 285 nm peaks of tryptophan. The minimum at 327 nm is assumed to be a decrease in the β-band of salinixanthin. The latter is usually absent or less pronounced in all-trans configurations of carotenoids with minimum distortions of the co-planarity of the double bonds in the conjugated chain, and has greater amplitude in cis isomers (15). The negative band in the difference spectra at 386 nm corresponds to depletion of the added retinal. The ratio of the absorption changes at 521 nm and 569 nm in the reconstitution was near the value upon bleaching (compare Fig. 3b and Fig. 1b), which implies that only a small fraction of carotenoid (if any) is lost during bleaching of the retinal band and the subsequent washing.
Figure 3.
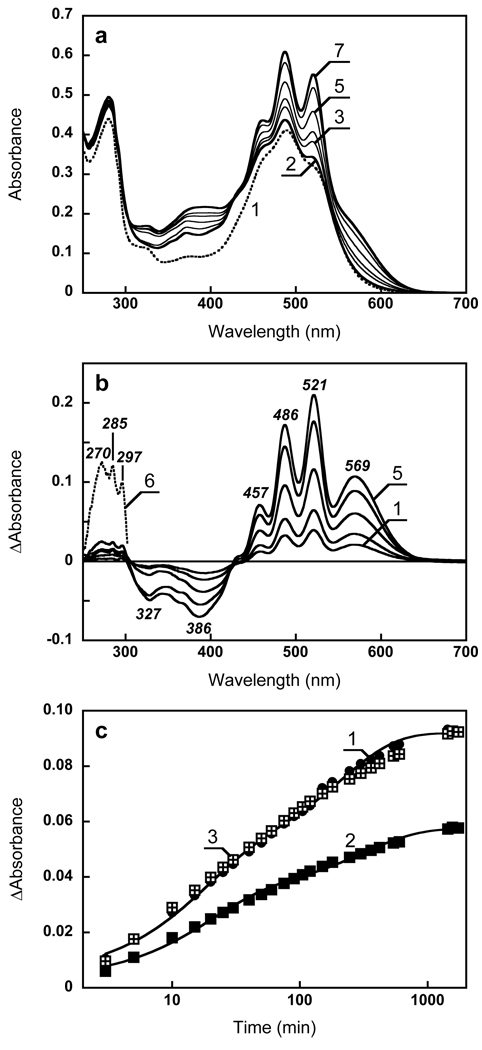
Reconstitution of xanthorhodopsin with retinal. (a) Spectrum 1, absorption spectrum of Salinibacter ruber cell membrane containing xantho-opsin; spectrum 2, 1 min after addition of 2 µl ethanol solution of all-trans retinal, final concentration, 3 µM; spectra 3 through 7, subsequent measurements at 5, 10, 40, 300 and 1560 min after addition of retinal. (b) Spectra 1 through 5, difference spectra between spectra 3 to 7 and spectrum 2 in Fig. 3a. Spectrum 6, same as 5 but multiplied by 7 to show the UV bands. (c) Kinetics of absorption changes. Curve 1, 600 nm, curve 2, 521 minus 533 nm. Curve 3, same as 2 but normalized at the maximum (at 26 hours). Note that the time scale is logarithmic.
The kinetics of the formation of xanthorhodopsin, assayed as absorption change at 600 nm (where virtually only the retinal chromophore absorbs), was biphasic with time-constants of 16 and 200 min, in Fig. 3c, curve 1). The amplitudes of the two phases are approximately equal. The changes in the carotenoid bands, sampled as a difference of absorption changes at 521 minus 533 nm, can be also fit with two components, with similar time constants (Fig. 3c, curve 2). The processes occur in parallel, and when the kinetics curves at retinal and carotenoid band are scaled together (Fig. 3c, curve 3) they coincide within experimental error. After 26 hours incubation with all-trans retinal (added in about 1.5 fold excess) the concentration of reconstituted xanthorhodopsin was at least 86% of that in the initial unbleached sample.
Reconstitution with desmethyl retinal analogues: effects on the kinetics of the retinal protein formation and carotenoid binding
Fig. 4 and Fig. 5 show spectral changes and kinetics that accompany reconstitution with 9-desmethyl and 13-desmethyl retinal. In case of the 9-desmethyl analogue, the band at 569 nm was relatively smaller (and the ΔA521/ΔA569 ratio larger) than for retinal, apparently due to the expected blue-shift of the chromophore band for the analogue pigment (Fig. 4a and Fig. 5a). For 9-desmethyl retinal, the kinetics of absorption changes in the retinal chromophore band (at 600 nm) and in the carotenoid 521 nm peak (assayed as a difference ΔA521-ΔA533) were similar, with the carotenoid 521 nm band rising slightly faster than the retinal band (curves 1 and 2 in Fig. 5b). It appears that removal of 9-methyl group did not significantly perturb the reconstitution. Approximately same amount of xanthorhodopsin was reconstituted after 26 hours of incubation with the analogue as with the native retinal, as judged from the amplitude of the carotenoid peaks (Fig. 5a, spectrum 2).
Figure 4.
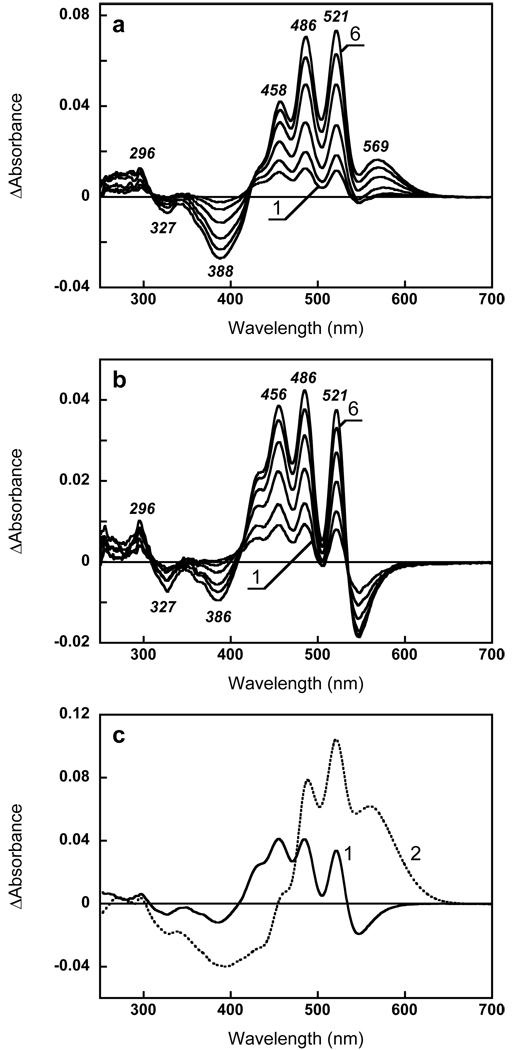
Reconstitution of xanthorhodopsin with retinal analogues. (a) Difference spectra accompanying reconstitution with all-trans 9-desmethyl retinal during the first 40 min after addition of retinal. Spectra 1 through 6, absorption changes at 3, 5, 10, 20, 30, 40, min after addition of 3 µM of all-trans 9-desmethyl retinal to xantho-opsin (the spectrum measured 1 min after addition was taken as a baseline). (b) Difference spectra accompanying reconstitution with 13-desmethyl retinal during the first 40 min after addition of retinal (same time intervals as for 9- desmethyl analogue in a). (c) Spectra 1 and 2, two components of spectral changes accompanying reconstitution with 13-desmethyl retinal corresponding to time constants 30 min and 550 min, respectively (from global fit).
Figure 5.
(a) Comparison of absorption changes observed after 26 hours of reconstitution with: 1, retinal, 2, 9-desmethyl analogue; 3, 13-desmethyl analogue. In all cases ca. 3 µM of retinal or its analog was added to the same amount of xantho-opsin (ca. 2 µM). (b) Comparison of the kinetics of absorption changes in the carotenoid 521 nm band (assayed as a difference ΔA521–ΔA533) and the retinal band (at 600 nm), normalized at 26 h for 9-desmethyl (1 and 2, respectively) and 13-desmethyl (3 and 4, respectively).
In the case of the 13-desmethyl analogue, however, several features stand out as different. Formation of the retinylidene chromophore band is slowed many fold compared to that for retinal and 9-desmethyl retinal (as followed by absorption changes at 600 nm, Fig. 5b, curve 4). This enabled us to detect the carotenoid changes at a stage preceding formation of the retinal-protein covalent bond. Importantly, the characteristic sharpening of the carotenoid bands begin to occur long before the retinal protonated Schiff base is formed (see the difference spectra in Fig. 4b and kinetic curves 3 and 4 in Fig. 5b). The kinetics of spectral changes accompanying reconstitution with the 13-desmethyl analogue were fit globally with two spectral components (time constants, 32 min and 550 min). Their amplitude spectra are shown in Fig. 4c. The first corresponds apparently to the entry of the retinal analogue into the retinal binding site, and the response of the opsin and thus the carotenoid antenna to the state when covalent bond with the protein has not yet been formed. The second step involves formation of the protonated Schiff base bond, and further changes in the carotenoid band shape as more sites are occupied by retinal. This is different from both retinal and 9-desmethyl retinal in which the amplitude spectra of the two kinetic components were similar (not shown).
These observations suggest that, removing of the methyl group on C13 does not substantially affect the rate of entry of the retinal into the binding site (as compared to 9-desmethyl retinal) but strongly hampers the rate of formation of the Schiff base between the retinal analogue and Lys-227. The formation of the Schiff base with this analogue was slowed many fold in bacteriorhodopsin also (9). Apparently, removal of the bulky methyl group at C13 upsets the alignment of the C15=O bond and the nitrogen of the lysine, and does not permit an orientation favorable for the Schiff base formation. A somewhat different interaction of 13-desmethyl retinal with the protein is reflected also in a different perturbation of the aromatic amino acids. The 285 nm band is missing in the difference spectra, both at early and later times (Fig. 4c).
The most interesting finding with respect to the carotenoid antenna is that it undergoes large changes in response to addition of 13-desmethyl retinal analogue even before the Schiff base is formed (Fig. 4b). We suggest that when retinal occupies the retinal binding pocket, it induces conformational changes in the protein that in turn affect the carotenoid binding, and restrict its conformational heterogeneity. This idea was tested in the experiments with retinol.
Carotenoid response to the addition of retinol
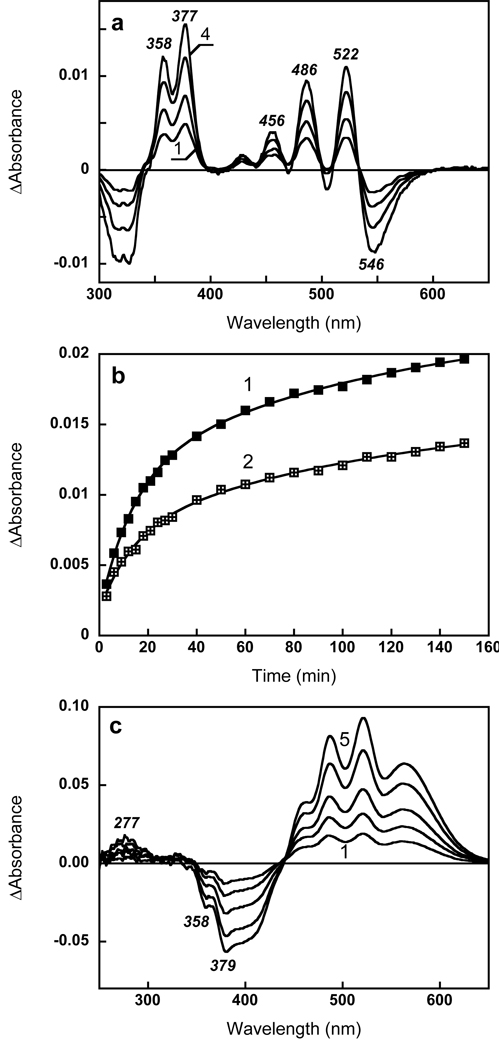
Retinol cannot form a Schiff base with a lysine, but in bacteriorhodopsin it can occupy the retinal binding site (12, 21). We found that it can fit into the retinal-binding site in xanthorhodopsin also, and induce binding changes of salinixanthin. The results of reconstitution with retinol are shown in Fig. 6. Addition of ca. 1 µM retinol (final concentration) to hydroxylamine-treated membranes containing about 2 µM xantho-opsin induced spectral changes that include numerous peaks. The difference spectra (Fig. 6a) contain two sharp bands at 377 and 358 nm, analogous to the maxima observed upon incorporation of retinol to bacteriorhodopsin (12). They are red-shifted from the maximum of free all-trans retinol (325 nm in ethanol) and much better resolved, indicating binding of retinol and immobilization of its cyclohexene ring, as observed in bacteriorhodopsin (12, 22), probably in the 6-s-trans conformation. The negative band at 330 nm corresponds to depletion of the added retinol. In parallel to the changes in the retinol bands, the characteristic carotenoid bands at 521, 486, 456 and 430 nm also appear (Fig. 6a), confirming that non-covalent binding of retinol can induce more rigid binding of salinixanthin. The latter is also the cause of the negative band at 546 nm, from decrease of the salinixanthin absorption bandwidth. No positive band around 570 nm appears, as expected. A very small band in this region appeared upon addition of large excess amounts of retinol (10 fold), perhaps from retinal, which might be present as a less than 1% impurity.
Figure 6.
Reconstitution of xanthorhodopsin with retinol. (a) Difference spectra upon addition of ca. 1 µM of retinol to ca. 2 µM xanto-opsin: Spectra 1 through 4, 10, 20, 60, 140 min after addition of retinol, respectively. (b) Kinetics of the absorption changes at, 1, 377 nm; 2, 521 nm. (c) Absorption changes upon addition of 3 µM all-trans retinal after incubation first with 3 µM retinol. Spectra 1 through 5, 3, 5, 10, 20, 40 min after addition of retinol. Note that the 358 and 379 nm bands caused by replacement of retinol by retinal in the binding site.
The kinetics of non-covalent binding of retinol and more rigid binding of carotenoid, were slower than the kinetics with retinal. When the amount of retinol added was half of that of xantho-opsin, the band of bound retinol at 377 nm appeared with time constant of 35 min while the difference band of salinixanthin at 521 nm had a similar time-constant. A slightly better fit of the data is achieved using two components, a fast phase of about 14 min and a slow phase of about 120 min (Fig. 6b). The ratio of absorption changes in carotenoid and retinol bands, ΔA522/ΔA358, is ca. 0.7. When retinol was added in 4 fold excess, the kinetics of fast phase was 6 min for retinol and still 13 min for the carotenoid band indicating that the latter time constant might be inherent for carotenoid binding.
It should be noted that only a small fraction of the retinal-binding sites (ca. 10%) were occupied by retinol even after prolonged (4 hours) incubation with 5 fold excess of retinol. This estimate is based on the amplitude of absorption changes at 377 and 521 nm, and from the fact that the main band of added retinol remains at 330 nm and the amount of bound retinol does not substantially increase after 3 hours of incubation (data not shown). Nevertheless some conclusions can be made about the strength of the carotenoid binding, even under conditions of partial occupancy of the retinal binding site.
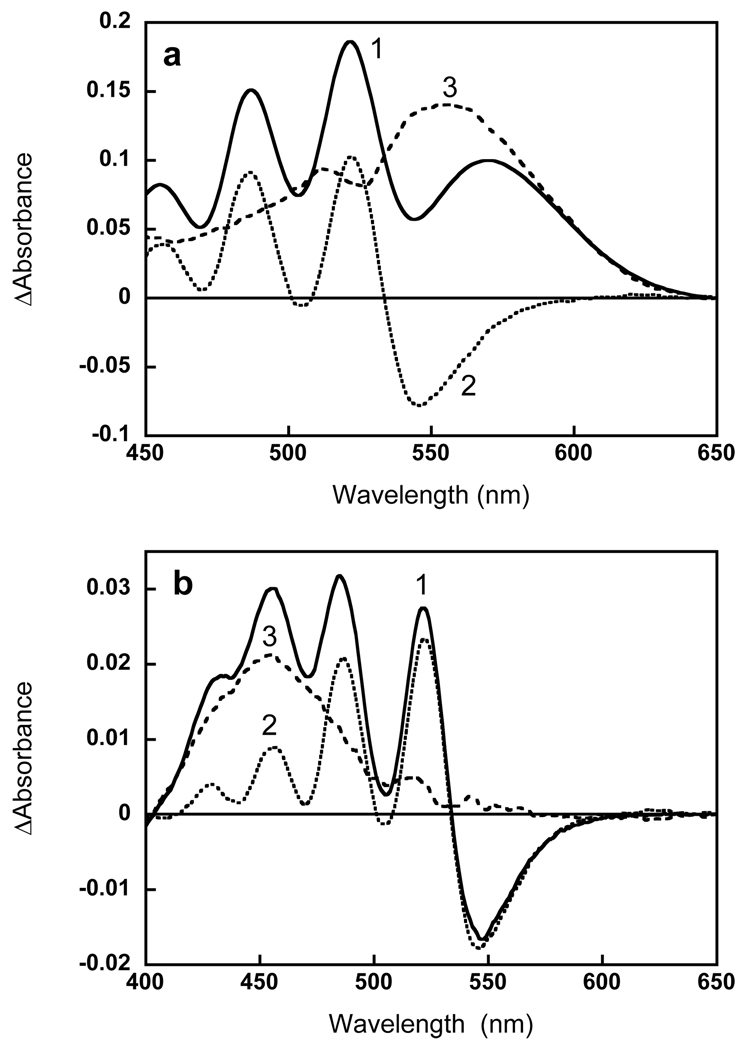
By comparing the shape of the difference spectra for retinol (Fig. 6a) and retinal (Fig. 3b) binding, we conclude that non-covalent binding of retinol induces a change in the 521 nm band (and other carotenoid bands) comparable in magnitude to that observed upon covalent binding of retinal and upon non-covalent binding of 13-desmethyl analog (which is observed during first 40 min incubation with this analog, Fig. 4b). This follows from the ratio of the absorption changes in the 521 nm carotenoid band and retinol (or retinal band). In the case of retinol this ratio, ΔA521/ΔA378, is about 0.7. In order to determine the analogous ratio for all-trans retinal the absorption changes observed upon reconstitution with all-trans retinal had to be deconvoluted for the carotenoid and retinal components. This is shown in Fig. 7a. The absorption changes observed upon addition of retinol, ΔAretinol, at wavelengths longer than 410 nm are caused exclusively by changes in carotenoid bands. These changes can be used to fit the absorption changes that occur upon retinal binding, ΔAretinal. After scaling the ΔAretinol to fit the amplitude of the carotenoid band at 521 nm and subtracting them from ΔAretinal, the amplitude and shape of the retinal band was obtained and the ratio of the retinal to carotenoid components, ΔA521/ΔA555 was found to be equal to 0.8. In this deconvolution, the retinal band exhibits a second shoulder at 512 nm and slightly shorter maximum 555 nm vs 558–559 nm from earlier estimates from action spectrum (1, 23).
Figure 7.
Separating the spectral changes of the carotenoid and the retinal. (a) Deconvolution of the absorption changes upon reconstitution with retinal (1) into carotenoid (2) and retinal (3) components, using the carotenoid spectrum obtained upon addition of retinol (spectrum 4 in Fig. 5a). (b) Deconvolution of absorption changes for non-covalent binding of 13-desmethyl retinal (spectrum 1, same as spectrum 6 in Fig. 4a) to carotenoid (2) and retinal (3) components, as explained in the text.
Subtraction of the carotenoid bands from the difference spectrum of reconstitution with 13-desmethyl after 40 min incubation shows that this analogue absorbs at 455 nm when incorporated into the binding site but before formation of the pigment (Fig. 7b).
Addition of retinal to the membranes reconstituted with retinol replaces the latter in the binding site, as one can conclude from the negative peaks at 377 and 358 nm in the difference spectra (Fig. 6c). It fully regenerates xanthorhodopsin, to the same extent as when retinal was added alone, indicating complete replacement of the bound retinol. The amplitude of the changes in the carotenoid bands upon addition of retinal after retinol is smaller by a fraction equal to the changes produced by retinol binding, thus providing direct evidence that non-covalent binding of retinol causes changes in the carotenoid of the same or similar magnitude as covalent binding of retinal.
DISCUSSION
Both removal and rebinding of the retinal to xanthorhodopsin occur in at least two steps, as in bacteriorhodopsin (12). In the experiments with hydroxylamine-induced bleaching, the first step is the hydrolysis of the Schiff base forming a retinal oxime, and second is the oxime leaving the binding site. The loss of resolution of the carotenoid bands occurs not only in the first step but continues during the second step, suggesting that carotenoid binding depends not only on a covalent bond of retinal and protein as might have been expected, but also on steric effects from occupancy of the retinal chain in its binding site. This idea was tested and confirmed in reconstitution experiments under conditions when the two steps could be well separated in time or when one of them was abolished.
In the reconstitution, the first step is the entry of the retinal into the binding site, and the second is the formation of the Schiff base. Time resolution of these steps is difficult in some cases but possible in others. They are not resolved for retinal and 9-desmethyl retinal, but can be observed for 13-desmethyl retinal. The first step is accompanied by a substantial red-shift of the retinal absorption maximum, apparently from planarization of the cyclohexene ring with the chain and interaction with the residues of the retinal binding pocket. This stage is clearly seen in recostitution with 13-desmethyl retinal where this intermediate absorbs at 453 nm (Fig. 7b), a 3632 cm−1 shift from absorption maximum of the13-desmethyl retinal at 389 nm. The incorporation of retinol into the retinal binding site is accompanied by a similar 53 nm shift (from 325 nm in ethanol to 378 nm, which corresponds to 3814 cm−1). This intermediate is not seen when reconstitution is performed with native retinal because apparently it does not accumulate in large amounts during the reconstitution. The second step is formation of a covalent bond with lysine and protonation of the Schiff base, accompanied by a larger spectral shift to 550–560 nm.
The results thus suggest the following basic scheme:
Where R stands for retinal or its analogue; XO, xantho-opsin containing salinixanthin; [R.X'O], the xantho-opsin with retinal (or the analogue) in the retinal binding site and salinixanthin “locked” in its site, and XR, the fully formed xanthorhodopsin. The second step is strongly delayed in the case of 13-desmethyl retinal and does not occur when reconstitution is with retinol. The carotenoid absorption changes observed upon reconstitution with these retinal analogues had the same spectral features as when retinal was used for reconstitution, providing evidence that similar molecular events underline these changes.
Most interesting is that the major changes in the carotenoid binding occur during the first step. This means that insertion of the ligand (the all-trans retinal or its analogues) in the binding site induces conformational changes in the protein, which result in a tight binding of the carotenoid so that its conformational heterogeneity is reduced. The changes in the carotenoid antenna caused by the subsequent formation of the protonated Schiff base are smaller. Thus, although an exact quantitative estimation could not be made at this time, the main influence of the retinal on the binding of salinixanthin appears to originate from the presence of the polyene chain in the retinal binding site.
It is likely that immobilization of the chain and the ring occurs by steric hindrance and strengthening of a hydrogen bond between the keto group of the ring and the protein. Since this ring is in conjugation with the main chain, its movements would strongly affect the resolution of the vibronic absorption bands (15). It has been shown that reduction of the 4-keto group in free salinixanthin to hydroxyl group greatly increases the resolution of the vibronic bands (3). Apparently hydrogen bonding of the keto group in the protein can produce a similar effect. The tight binding of salinixanthin most likely has direct implication both for the efficient energy transfer (6).
Earlier studies on bacteriorhodopsin indicated that internal, core domains of bacterio-opsin are much more accessible for H2O/D2O exchange after bleaching than in the native pigment with the chromophore in place, indicating a more open and hydrophilic structure (24). However, no changes in the protonation states of buried aspartates Asp96 and Asp115 were detected upon reconstitution, just an IR frequency shift indicating a more hydrophobic environment in the reconstituted state (25). The presence of retinal chromophore affects the folding and conformation of bacteriorhodopsin (26).
Reconstitution of xanthorhodopsin with retinal causes changes also in tryptophan absorption (increase in the 297 nm and 285 nm bands). The Trp residues at positions 86 and 182 (using bacteriorhodopsin nomenclature) most likely contribute to these changes. With the 13-desmethyl analogue the perturbation of Trp residue is much less pronounced, lending further support for this assignment, particularly with respect to the Trp182 homologue in the cytoplasmic domain which faces the 13-methyl group of retinal. The lack of perturbation of this residue in 13-desmethyl-reconstitution nevertheless does not lead to inhibition of the carotenoid changes. The changes from the retinal binding can be transmitted to the carotenoid binding site in form of conformational change or changes in the polarity of the environment. To “lock” the antenna in the binding site by a hydrogen bond, relatively small changes in the distance between the hydrogen donor group (such as tyrosine) and 4-keto group of 1–2 Å might be needed, which could be induced by the retinal entering its binding site. Ongoing experiments on crystallization of xanthorhodopsin and modeling of salinixanthin docking will provide further details on interaction of the two chromophores and linkage between the two binding sites.
Acknowledgments
This work has been supported in part by grants from the U.S. Army Research Office to S.P.B. (W911NF-06-1-0020), National Institutes of Health (GM29498) and the Department of Energy (DEFG03-86ER13525) to J.K.L, and the Helen and Milton A. Kimmelman Center for Biomolecular Structure and Assembly to M.S.
REFERENCES
- 1.Balashov SP, Imasheva ES, Boichenko VA, Antón J, Wang JM, Lanyi JK. Xanthorhodopsin: A proton pump with a light-harvesting carotenoid antenna. Science. 2005;309:2061–2064. doi: 10.1126/science.1118046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antón J, Oren A, Benlloch S, Rodríguez-Valera F, Amann R, Rosselló-Mora R. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int. J. Syst. Evol. Micrbiol. 2002;52:485–491. doi: 10.1099/00207713-52-2-485. [DOI] [PubMed] [Google Scholar]
- 3.Lutnaes BF, Oren A, Liaaen-Jensen S. New C40-carotenoid acyl glycoside as principal carotenoid in Salinibacter ruber, an extremely halophilic eubacterium. J. Nat. Prod. 2002;65:1340–1343. doi: 10.1021/np020125c. [DOI] [PubMed] [Google Scholar]
- 4.Kerfeld CA, Sawaya MR, Brahmandam V, Cascio D, Ho KK, Trevithick-Sutton CC, Krogmann DW, Yeates TO. The crystal structure of a cyanobacterial water-soluble carotenoid binding protein. Structure. 2003;11:55–65. doi: 10.1016/s0969-2126(02)00936-x. [DOI] [PubMed] [Google Scholar]
- 5.Roszak AW, McKendrick K, Gardiner AT, Mitchell IA, Isaacs NW, Cogdell RJ, Hashimoto H, Frank HA. Protein regulation of carotenoid binding: gatekeeper and locking amino acid residues in reaction centers of Rhodobacter sphaeroides. Structure. 2004;12:765–773. doi: 10.1016/j.str.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Balashov SP, Imasheva ES, Lanyi JK. Induced chirality of light-harvesting carotenoid salinixanthin and its interaction with the retinal of xanthorhodopsin. Biochemistry. 2006;45:10998–11004. doi: 10.1021/bi061098i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balashov SP, Imasheva ES, Wang JM, Lanyi JK. Carotenoid to retinal excited-state energy transfer in xanthorhodopsin. Submitted. [Google Scholar]
- 8.Imasheva ES, Balashov SP, Wang JM, Lanyi JK. pH-dependent transitions in xanthorhodopsin. Photochem. Photobiol. 2006;82:1406–1413. doi: 10.1562/2006-01-15-RA-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gartner W, Towner P, Hopf H, Oesterhelt D. Removal of methyl-groups from retinal controls the activity of bacteriorhodopsin. Biochemistry. 1983;22:2637–2644. [Google Scholar]
- 10.Nakanishi K, Crouch RK. Application of artificial pigments to structure determination and study of photoinduced transformations of retinal proteins. Isr. J. Chem. 1995;35:253–272. [Google Scholar]
- 11.Weidlich O, Schalt B, Friedman N, Sheves M, Lanyi JK, Brown LS, Siebert F. Steric interaction between the 9-methyl group of the retinal and tryptophan 182 controls 13-cis to all-trans reisomerization and proton uptake in the bacteriorhodopsin photocycle. Biochemistry. 1996;35:10807–10814. doi: 10.1021/bi960780h. [DOI] [PubMed] [Google Scholar]
- 12.Schreckenbach T, Walckhoff B, Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur. J. Biochem. 1977;76:499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- 13.Friedman N, Ottolenghi M, Sheves M. Heterogeneity effects in the binding of all-trans retinal to bacterio-opsin. Biochemistry. 2003;42:11281–11288. doi: 10.1021/bi035011u. [DOI] [PubMed] [Google Scholar]
- 14.Peña A, Valens M, Santos F, Buczolits S, Antón J, Kämpfer P, Busse H-J, Amann R, Rosselló-Mora R. Intraspecific comparative analysis of the species Salinibacter ruber. Extremophiles. 2005;9:151–161. doi: 10.1007/s00792-005-0430-y. [DOI] [PubMed] [Google Scholar]
- 15.Britton G. UV/Visible Spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids. Basel, Boston, Berlin: Birkhäuser Verlag; 1995. pp. 13–62. [Google Scholar]
- 16.Ebrey TG. Synthetic pigments of rhodopsin and bacteriorhodopsin. In: Packer L, editor. Methods Enzymol. 1982. pp. 516–521. [Google Scholar]
- 17.Rehorek M, Heyn MP. Binding of all-trans-retinal to the purple membrane. Evidence for cooperativity and determination of the extinction coefficient. Biochemistry. 1979;18:4977–4983. doi: 10.1021/bi00589a027. [DOI] [PubMed] [Google Scholar]
- 18.Blatz PE, Lin M, Balasubramaniyan P, Balasubramaniyan V, Dewhurst PB. Series of synthetic visual pigments from cattle opsin and homologs of retinal. J. Am. Chem. Soc. 1969;91 doi: 10.1021/ja01049a069. [DOI] [PubMed] [Google Scholar]
- 19.Dioumaev AK. Evaluation of intrinsic chemical kinetics and transient product spectra from time-resolved spectroscopic data. Biophys. Chem. 1997;67:1–25. doi: 10.1016/s0301-4622(96)02268-5. [DOI] [PubMed] [Google Scholar]
- 20.Sperling W, Rafferty CN. Relationship between absorption spectrum and molecular conformations of 11-cis-retinal. Nature. 1969;224:591–594. doi: 10.1038/224591a0. [DOI] [PubMed] [Google Scholar]
- 21.Ovchinnikov YA, Shkrob AM, Rodionov AV, Mitzner BI. An effective competitive inhibitor of bacterioopsin-retinal recombination. FEBS Lett. 1979;97:15–19. [Google Scholar]
- 22.Schreckenbach T, Walckhoff B, Oesterhelt D. Specificity of the retinal binding site of bacteriorhodopsin: chemical and stereochemical requirements for the binding of retinol and retinal. Biochemistry. 1978;17:5353–5359. doi: 10.1021/bi00618a005. [DOI] [PubMed] [Google Scholar]
- 23.Boichenko VA, Wang JM, Antón J, Lanyi JK, Balashov SP. Functions of carotenoids in xanthorhodopsin and archaerhodopsin, from action spectra of photoinhibition of cell respiration. Biochim. Biophys. Acta. 2006;1757:1649–1656. doi: 10.1016/j.bbabio.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kluge T, Olejnik J, Smilowitz L, Rothschild KJ. Conformational changes in the core structure of bacteriorhodopsin. Biochemistry. 1998;37:10279–10285. doi: 10.1021/bi9802465. [DOI] [PubMed] [Google Scholar]
- 25.Rüdiger M, Tittor J, Gerwert K, Oesterhelt D. Reconstitution of bacteriorhodopsin from the apoprotein and retinal studied by Fourier-transform infrared spectroscopy. Biochemistry. 1997;36:4867–4874. doi: 10.1021/bi962426p. [DOI] [PubMed] [Google Scholar]
- 26.Kollbach G, Steinmüller S, Berndsen T, Buss V, Gärtner W. The chromophore induces a correct folding of the polypeptide chain of bacteriorhodopsin. Biochemistry. 1998;37:8227–8232. doi: 10.1021/bi972268h. [DOI] [PubMed] [Google Scholar]