Abstract
Whereas neural crest cells are the source of the peripheral nervous system in the trunk of vertebrates, the “ectodermal placodes,” together with neural crest, form the peripheral nervous system of the head. Cranial ectodermal placodes are thickenings in the ectoderm that subsequently ingress or invaginate to make important contributions to cranial ganglia, including epibranchial and trigeminal ganglia, and sensory structures, the ear, nose, lens, and adenohypophysis. Recent studies have uncovered a number of molecular signals mediating induction and differentiation of placodal cells. Here, we described recent advances in understanding the tissue interactions and signals underlying induction and neurogenesis of placodes, with emphasis on the trigeminal and epibranchial. Important roles of Fibroblast Growth Factors, Platelet Derived Growth Factors, Sonic Hedgehog, TGFβ superfamily members, and Wnts are discussed.
Keywords: placode induction, epibranchial, trigeminal, Shh, Wnt, TGF beta, platelet derived growth factor
INTRODUCTION
During development of vertebrate embryos, the peripheral nervous system arises from two cell types: neural crest cells and cranial ectodermal placodes. The term “placode” comes from the Greek, meaning “scale.” Accordingly, cranial placodes arise from regions of thickened ectoderm in the embryonic head that invaginate and/or delaminate to give rise to portions of the ear, lens, nose, as well as neurons in the trigeminal (Vth), facial (VIIth), glossopharyngeal (IXth), and vagal (Xth) cranial ganglia.
Placodal cells and neural crest share many properties including the ability to migrate and to delaminate from ectodermal tissue. In the case of neural crest, this occurs via an epithelial to mesenchymal transition (EMT), whereas for placodes, it is not yet clear if their ingression occurs by a classical EMT or some alternative process (Graham et al., 2007). The progeny of neural crest and placodes are similar: both can form sensory neurons, neuroendocrine cells, and cells that secrete special extracellular matrices (reviewed by Baker and Bronner-Fraser, 2001). While peripheral ganglia of the trunk are exclusively neural crest-derived, those arising at cranial levels have a dual origin from both neural crest and placodes. However, the glial components of the cranial ganglia are exclusively derived from neural crest.
In contrast to neural crest cells which are well-studied, comparatively little is known about the molecular events guiding formation and differentiation of ectodermal placodes and their derivatives. This review focuses on the events guiding development of the placodes that form cranial ganglia. We discuss known tissue interactions as well as newly discovered molecular processes that result in induction of placodes as well as how the different placodes emerge from initially intermixed cell populations. Traditionally, most placodes are classified as either neurogenic (trigeminal, epibranchial and lateral line) or sensory (lens, olfactory, and otic). This relates to their general function, since neurogenic placodes give rise to neurons in the Vth, VIIth, IXth, and Xth cranial ganglia, while sensory placodes give rise to portions of the sensory systems of the eye, nose, and ear. The adenohypophyseal placode, which gives rise to the anterior pituitary gland does not fit into either categories. Here, we emphasize development of two of neurogenic placodes (trigeminal and epibranchial placodes), while summarizing induction of the adenohypophyseal and sensory placodes, since this topic recently has been reviewed in depth elsewhere (Bhattacharyya and Bronner-Fraser, 2004; Lovicu and McAvoy, 2005; Ohyama et al., 2007; Schlosser, 2006; Streit, 2007).
Placode Induction from a Common Pre-placodal Domain
Induction toward specific placode fates is thought to be a multi-step process that involves multiple factors. The first step is establishment at gastrula stages of a pre-placodal domain, a horse-shoe shaped region around the prospective anterior neural plate, at the border of the neural plate and non-neural ectoderm. This domain arises at early neural plate stages and later generates all varieties of placodes (reviewed in Bailey and Streit, 2006). Molecularly, the pre-placodal domain is defined by its combinatorial expression of the transcription factors, Six, Eya, and Dach (Streit, 2002; McLarren et al., 2003; Bhattacharyya et al., 2004; Kozlowski et al., 2005; Litsiou et al., 2005). FGF, TGFβ and Wnt signaling pathways have been implicated in the induction of the preplacodal domain (i.e. Ahrens and Schlosser, 2005; Litsiou et al., 2005; Bailey et al., 2006; reviewed by Streit, 2007), as manipulation of these pathways changes the location of the neural plate border and affects the formation of the pre-placodal domain.
Placodes are distributed in an order that is relatively colinear with their final location (D’ Amico-Martel and Noden, 1983; ElAmraoui and Dubois, 1993; Kozlowski et al., 1997; Whitlock and Westerfield, 2000; Cobos et al., 2001; Streit, 2002; Bhattacharyya et al., 2004; Xu et al., 2008). Using focal dye labeling, recent fate maps of the olfactory and lens (Bhattacharyya et al., 2004) as well as trigeminal, epibranchial, and otic placodes (Streit, 2002; Xu et al., 2008) suggest that adjacent ectodermal cell populations can contribute to different placodes. Thus, there appears to be more overlap in the fate map than previously appreciated using lower resolution approaches (D’Amico and Noden,1983; Couly and Le Doaurin, 1985, 1987; ElAmraoui and Dubois, 1993; Cobos et al., 2001). For example, at St. 5, precursors of epibranchial and otic precursors placodes are distributed in overlapping domains in the posterior pre-placodal domain (Streit, 2002; Figure 1A). Similarly at St. 6–7, presumptive olfactory and lens precursors overlap in the anterior region (Bhattacharyya et al., 2004) as do otic and epibranchial precursors in the posterior region (Streit, 2002) (Figure 1B). At St. 8 and 10, the trigeminal placode domain has expanded rostrally into ectoderm adjacent to the presumptive forebrain to include progenitors that give rise to cells with the trigeminal nerve (McCabe et al., 2009), not reflected in the fate map of Xu et al. (2008) (Figure 1C, D).
Figure 1.
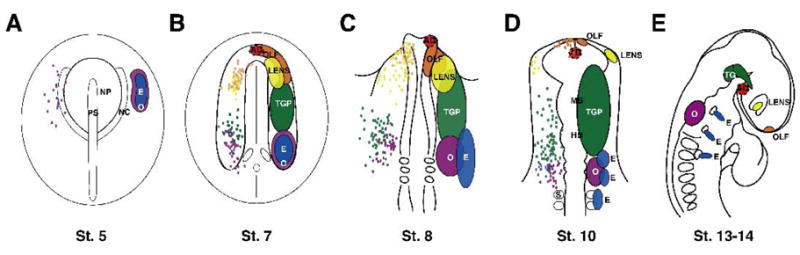
Locations of placodal regions in chicken embryos. For St. 5–10, merged fate maps are shown on the left hand side of the embryo as colored dots, with general placodal regions on right. A. At St. 5, fate mapping shows a broad overlap of presumptive otic and epibranchial precursors. B. At St. 7, the olfactory and lens precursor domains extensively overlap as well as trigeminal, epibranchial and otic precursor domains. C. At St. 8, the general trigeminal placode domain has been expanded rostrally to include placodal cells that give rise to trigeminal nerve cell bodies. D. Placodal domains continue to separate over time (St. 10). E. Placodes are found in distinct morphologically identifiable regions by St. 13–14. Adenohypophseal placode is surrounded by a dashed line to indicate the fate mapping was done at a lower resolution and not done at all time points. AD=adenohypophysis (red), E=epibranchial (blue), Lens (yellow), OLF=olfactory (orange), O=otic (purple), TGP=trigeminal placode (green), TG=trigeminal ganglion, MB=midbrain, HB=hindbrain, NP=neural plate, PS=primitive streak, NC=neural crest.
At stages which the earliest fate mapping experiments were performed, the presumptive placode cells may not yet have received their inductive signals. However, at St. 8, when otic and trigeminal placodes are undergoing induction, there is still extensive overlap of otic, epibranchial and trigeminal precursors (St. 8–9: Baker et al., 1999; Groves and Bronner-Fraser, 2000; Streit, 2002; Freter et al., 2008; McCabe et al., 2008; Xu et al., 2008). Similarly, since olfactory placode, induction is thought to occur between St. 8–10 (Sjodal et al., 2007; Bhattacharyya et al., 2008), progenitors may not have received an inductive signal before they have separated from the presumptive lens precursors.
The extensive overlap of precursors fated to give rise to different placodes (e.g. olfactory/lens; otic/trigeminal/epibranchial) complicates the question of what factors may mediate induction of different placodes. The shared domains suggest that placodal precursors can receive similar external stimuli but still adopt different fates. This raises the intriguing question of what processes determine the prospective fates of initially adjacent cell populations. Such is the case for trigeminal and epibranchial placodes, which lie adjacent to one another in the preplacodal domain, but subsequently differentiate into different types of neurons in distinct locations. One possibility is that placodal progenitors are multipotent and can give rise to multiple types of placodes. Alternatively, the pre-placodal domain may be comprised of committed precursors that are intermingled in a common domain. This question only will be resolved by performance of single cell lineage experiments at the pre-placodal stages.
A likely scenario is that after establishment of the pre-placodal domain, a second set of signals may specify subsequent steps that lead to acquisition of distinct placodal fates. Recent evidence suggests that cells within the pre-placodal domain may represent an equivalence group. Rather than being naive cells with general placode character, they appear to have acquired a “ground state” as prospective lens, and must receive further instructions, including promotion of alternative fates and repression of lens character, in order to differentiate otherwise (Bailey et al., 2006). For example, Bailey and colleagues find for the olfactory placode that FGF from the anterior neural ridge in conjunction with an unidentified inhibitory factor from the neural crest is required to suppress lens fate and then to induce olfactory placode cells. Consistent with the idea that there is a multiple step process of induction beginning at the pre-placodal stage, Martin and Groves (2006) find that generation of otic placode cells first requires acquisition of generic placode fate in the pre-placodal domain followed by FGF signaling to specify cells toward an otic fate. When they expose lateral epiblast explants to signals within the pre-placodal domain, then the application of FGF in vitro activates the full complement of otic markers. In contrast, addition of FGF without prior exposure to signals from the pre-placodal domain results in only partial otic induction. Similarly, zebrafish embryos exposed to ectopic FGF produce more epibranchial placode cells at the expense of lens cells (Nechiporuk et al., 2007). However, Sjodal et al. (2007) failed to detect bias of earlier pre-placodal cells toward a lens fate. Instead, they argue that the length of exposure to BMP signals dictates lens versus olfactory placode. These differences may reflect differences in location and age of embryos utilized, suggesting that further studies are required to understand the timing and factors involved in the refinement of the pre-placodal domain.
Individualization of Placodes
Of the six families of known placodal inducers (FGF, PDGF, Retinoic Acid, Shh, TGFβ superfamily, and Wnt) two stand out (summarized Table 2) for having roles in development of multiple placodes: FGF and the TGFβ superfamily (e.g. Faber et al., 2001; Leger and Brand, 2002; Nechiporuk et al., 2007; Sjodal et al., 2007). This may not be surprising since the placode fields, such as epibranchial and otic placodes, are adjacent to one another and both require FGF signaling. How then do the placodal precursors that are either physically intermixed and/or multipotent, requiring the same inductive cues, separate and eventually segregate to form distinct placodes?
Table 2.
Known secreted factors and receptors of placode induction. References are in Table 1 legend.
| Inducer Families | |||||||
|---|---|---|---|---|---|---|---|
| FGF | PDGF | Retinoic Acid | Sonic Hedgehog | TGFβ super family | Wnt | ||
| Sensory | Adenohypophysis | Shh25,50 | Nodal21 | ||||
| BMP410,49 | |||||||
| Lens | FGFR16 | BMP419,45 | |||||
| BMP711,54 | |||||||
| Olfactory | FGF83 | ||||||
| Otic | FGFR31,33,34,42 | RA23 | β-catenin40,42 | ||||
| FGF3, 8 zebrafish18,31,33,43,44 | |||||||
| FGF3, 10 mouse2,36,56 | |||||||
| FGF2 (1, 4), 3, 19 chick17,34 | |||||||
| Ectopic FGFs1,2,32,51 | |||||||
| Neurogenic | Epibranchial | FGFR38,39,48 | |||||
| FGF3, 8, 1917,38,39,48 | |||||||
| Trigeminal | PDGFRβ35 | Wnt3A6 | |||||
| PDGFD 35 | |||||||
There is evidence for intrinsic differences between the otic/epibranchial and olfactory/lens placodes which may account for their ability to differentially interpret the same cue. For example, both otic and epibranchial placode induction requires FGF signaling (reviewed by Ohyama et al., 2007; Nechiporuk et al., 2007; Nikaido et al., 2007; Sun et al., 2007). Consistent with the idea of distinct responses of otic versus epibranchial placodes, morpholinos to dlx3a and dlx4b or the B380 mutation in zebrafish which deletes dlx3a, dlx4b, and sox9a result in a loss of otic markers, but the epibranchial placode marker, sox3, is unaffected (Sun et al., 2007). Thus, although both initially require FGFs, other factors may later combine to confer unique fates for the adjacent placodes. One such factor is Wnt, which may be permissive or instructive for otic placode but repressive for epibranchial placodes (Ohyama et al., 2006; Freter et al., 2008). Similarly, olfactory and lens precursors are adjacent to one another in the St. 6 chicken embryo (Bhattacharyya et al., 2004), but their eventual fates can be biased by the length of exposure to BMPs, with continued exposure resulting in lens placodal cells (Sjodal et al., 2007). In a comprehensive review, Schlosser (2006) points out the similarities and differences of expression of transcription factors such as Otx, Emx, Six3/6, ANF, Pitx, Msx, Pax, Fox, and Tbox genes that show complexity and intrinsic differences between the presumptive placode cells in Xenopus. In this manner, the same molecules may induce a bi- or multi-potential precursor cell, but the specific fate of the cells will be dictated by a combination of factors that change with time and place as they find their final location.
A second example of a mechanism for segregating initially adjacent placodal precursors occurs in the adenohypophyseal versus lens decision. Shh is a positive mediator of adenohypophyseal, but not for lens or olfactory induction (Herzog et al., 2003; Dutta et al., 2005). When Shh is transiently reduced in the zebrafish mutant for gli2 (you-too), lenses are ectopically formed in the pituitary field (Karlstrom et al., 1999; Kondoh et al., 2000). Interestingly, ectopic olfactory placodes do not form in the mutant pituitary field. This result is consistent with the possibility that Shh is necessary to inhibit the placodal precursors in the pituitary field from retaining their ground state of lens placodal cells (Bailey et al., 2006). Thus, the inducer for one placode can become the inhibitor for another.
A third way to separate placodal precursors is to delay the induction of a population, allowing for changes in both intrinsic and extrinsic factors within precursor pool to bias the cells towards a particular fate. Examples of the importance of timing in placode formation are apparent during development of trigeminal and otic placodes. Otic placode cells are specified around St. 8 in the chicken (Groves and Bronner-Fraser, 2000), whereas the trigeminal placode cells are specified around St. 10. Transplantation studies illustrate the flexibility of the presumptive placode cells, such that if the ectodermal cells are transplanted sufficiently early, they will assume the fate of the new location. Perhaps the timing can be altered in these cells by the use of intrinsic factors such as geminin, which might inhibit the pro-neural bHLH transcription factors and maintain them as placodal precursors (Seo et al., 2005) until such time that the environment is no longer conducive to otic placode fate but inductive for trigeminal placode fate.
Finally, cell movements may play an important role in physically separating placode cells. The lens and olfactory precursors physically separate from one another sometime before specification of the olfactory placode in chick (Bhattacharyya et al., 2004), and the chick presumptive otic placode cells also undergo extensive movements (Streit, 2002). What drives these movements remains unknown. Cell-cell contact, changes in cell adhesion, as well as attractive and repulsive cues may promote separation of precursors to allow for differentiation into specific placodes.
One or more of the above-described scenarios are likely involved in allowing adjacent and intermixed populations of placodal precursors and/or multipotent progenitors to form different cell types in sensory and neurogenic placodes according to their stereotypic locations along the neuroaxis of the embryo.
Tissue Interactions and Inducers of Ectodermal Placodes
The currently known tissue interactions and molecular inducers of various placodes are summarized in Tables 1 and 2. These growth factors are used in many developmental processes and thus may also play later roles that are not listed in Table 2. An important caveat is that in some cases it is known that a member of a large family, like the TGFβ super family, is involved in placode induction, but the specific molecule has yet to be identified in vivo, and therefore is not included.
Table 1.
Origin and Inducing tissues for placodes. Subdivisions within a row indicate separate tissues are sufficient. ANR=anterior neural ridge. References are for Table 1 and Table 2.
| Placodes | ||||||
|---|---|---|---|---|---|---|
| Adenohypophysis | Lens | Olfactory | Otic | Epibranchial | Trigeminal | |
| Origin | ANR7,13 | ANR and Non-neural ectoderm5 | ANR7,55 | Neural folds and Non- neural ectoderm47 | Non-neural ectoderm8,9,52 | Non-neural ectoderm9,57 |
| Non-neural ectoderm12,14,15,27,28,41 | Non-neural ectoderm and ANR5 | |||||
| Inducing Tissue(s) | ANR and mesoderm22,49 | Neural Plate and Mesoderm24 | ANR and Neural Crest3 | Mesoderm26 | Mesoderm38 | Dorsal neural tube4,46 |
| Hindbrain29,42,53 | Hindbrain48 | |||||
| Mesoderm and Hindbrain20,30,31,37,43 | ||||||
Lombardo et al., 1998
Vogel and Davies, 1993
Epibranchial Placodes
For many developmental processes, the first step in changing from an undifferentiated cell to a differentiated cell type occurs in response to signals emanating from adjacent tissue. This process of “induction” can be mediated by cell-cell contact, secreted factors, or a combination thereof.
The epibranchial placodes, comprised of the geniculate, petrosal, and nodose placodes, contribute to the facial, glossopharyngeal, and vagal cranial nerves respectively. Induction of the epibranchial placodes has been ascribed to several tissues including the pharyngeal endoderm plus the underlying neural crest (Webb and Noden, 1993), the pharyngeal endoderm alone (Begbie et al., 1999), and most recently to the mesenchyme (Nechiporuk et al., 2007) and the hindbrain (Sun et al., 2007). However, the pharyngeal endoderm may promote neurogenesis rather than induction of the epibranchial placodes (Begbie et al., 1999; Nechiporuk et al., 2005).
The reasons for different proposed sources of inducer rests on the likelihood that placode induction is a multistep process, such that different inducers may function at different times. For this reason, it is critical to define the timing of inductive events in order to understand the roles of various growth factors in formation and differentiation into specific types of neurons. In the St. 10 in chick embryo, epibranchial placodes begin to be morphologically distinguishable from surrounding non-neural ectoderm as thickened epithelium in the hindbrain, rostral and caudal to the otic placode (Abu-Elmagd et al., 2001). This is well before onset of expression of neuronal markers, which begins at St. 16 (Begbie et al., 1999). This suggests that the placode is induced on or before St. 10. However, prospective molecular markers characteristic of particular placodes are often expressed prior to overt morphological manifestations. Thus, specification toward a particular fate may precede overt changes in morphology. “Specification” is here defined under experimental conditions such that when cells are specified, they maintain their fate in a neutral culture environment in the absence of additional factors. For example, cranial ectodermal explants cultured from the prospective epibranchial placode region of St. 9 chick generate neurons in the absence of growth factors (Begbie et al., 1999). Therefore, induction toward a neuronal fate has already occurred by St. 9 in the avian epibranchial placodes. Further evidence for this idea comes from experiments addressing the role of FGF signaling in the pre-placodal region. When signaling is blocked using short hairpin RNA to FGF3 and FGF19 at St. 4, epibranchial placode formation is greatly reduced by St. 13. Conversely, over-expression of FGF leads to an expansion of epibranchial placodes (Freter et al., 2008).
Freter et al. (2008) hypothesize that the specification response of epibranchial placode cells to FGF signaling from a common otic/epibranchial placode progenitor occurs between 5ss-7ss (St. 8–9). At least one additional signal, Wnt, helps sort the common otic/epibranchial progenitors into separate pools. Although Wnt signaling is inhibitory for epibranchial placode induction, it does not appear to effect otic placode induction directly, at least in the chick system. Rather, Wnt appears to play a later role in otic placode commitment (Freter et al., 2008). On the other hand, studies in the mouse argue that Wnt signaling is instructive rather than permissive for the specification of the otic placode (Ohyama et al., 2006). Several differences were noted between the chick and mouse studies upon activation of Wnt pathway by constitutive activation of β-catenin. In the chicken, constitutively active β-catenin constructs introduced at late gastrulation stages, caused no change in early otic placode markers (Soho1, Nkx5.1) (Freter et al., 2008). In contrast, the mouse Pax2-Cre line was expressed later, at the onset of neural crest migration (Ohyama et al., 2004), which caused an increase in otic placode markers (Dlx5, Pax8, Pax2) at the expense of surrounding ectoderm cells. The differences in results may relate to both the timing and levels of expression. Finally, there may be species differences in the roles of Wnt signaling in otic and epibranchial placodes since TOPgal reporting of Wnt signaling was not detected in the mouse epibranchial placode precursors (Ohyama et al., 2006). Therefore, when considering the process of induction of a placode, it is important to take into account a combination of positive influences, such as FGF signals, permissive and sometimes inhibitory factors, such as Wnts, as well as differences between species.
Recent work in zebrafish has advanced understanding of the molecular nature of epibranchial placode induction. The epibranchial placode can be morphologically detected at 24 hours post fertilization (hpf), much later than the hypothesized time of induction (Nechiporuk et al., 2005). Several studies have implicated FGF signaling in the induction process (Nechiporuk et al., 2007; Nikaido et al., 2007; Sun et al., 2007). Nikaido et al. (2007) provide evidence of a role for FGF8 in epibranchial placode induction. Using FGF8 hypomorphant mutant embryos, acerebellar (ace), they find that ace mutant phenotype can be rescued with a FGF8 bead. They further illustrate a general role for FGF signaling using FGFR inhibitor treated embryos, which have a reduction in the epibranchial markers Sox3 and Phox2a. Their work suggests that FGF signaling may function before 10 hpf. In contrast, other experiments using morpholinos against FGF3 and FGF8, the FGFR inhibitor SU5402, as well as a dominant negative FGFR1 (dnFGFR1) driven by heat shock promoter, suggest a requirement for FGF3 in addition to FGF8, rather than FGF8 alone (Sun et al., 2007; Nechiporuk et al., 2007). In particular, a dnFGFR1 line makes it possible to define the temporal requirement for FGF, between 10 hpf and 16.5 hpf (Nechiporuk et al., 2007), and possibly earlier (Nikaido et al., 2007). When dnFGFR1 cells are transplanted into a wild-type host, they do not contribute to the forming wild-type epibranchial placodes, indicating that FGFs function cell autonomously (Nechiporuk et al., 2007). Importantly, these studies demonstrate that both FGF3 and FGF8 are necessary for epibranchial placode induction in zebrafish.
From where and at what time do factors involved in epibranchial placode induction originate? Work from both chicken and zebrafish have narrowed the source of the inducers to the mesenchyme and hindbrain. At the time of induction in chicken (St. 4–9) and zebrafish (~10–16.5 hpf), the presumptive epibranchial placodal ectoderm is in close proximity to the hindbrain and the mesenchyme, but the pharyngeal endoderm has not yet come into close contact (Quinlan et al., 2004; Holzschuh et al., 2005). Sun et al. (2007) postulate that FGF3 and FGF8 signals emanate from the hindbrain at these times. However, by using mesoderm mutants, endoderm mutants, and double mutants, in addition to transplantation of mesenchyme and hindbrain cells, Nechiporuk et al. (2007) provide convincing evidence that mesenchyme could also be a source of inducer. Interestingly, transient application of the FGFR inhibitor SU5402 followed by its removal reverses the effect of placodal inhibition such that the epibranchial placodes form, albeit in a delayed fashion (Sun et al., 2007). Given that FGF signaling is necessary for initiation as well as maintenance of the epibranchial placode phenotype, it is difficult to discriminate between inducing versus maintenance signals. Moreover, both FGF3 and FGF8 are expressed in the hindbrain and mesenchyme at 10 hpf (Reifers et al., 2000, Nechiporuk et al., 2007). However, hindbrain expression is earlier and more robust. Taken together, the combined experiments of Nikaido et al. (2007), Nechiporuk et al. (2007), and Sun et al. (2007) suggest that the pharyngeal endoderm is not necessary for the initial induction of epibranchial placodes. Factors such as BMP7 from the pharyngeal endoderm appear to be involved in neurogenesis (Begbie et al., 1999; Nechiporuk et al., 2005). Rather the hindbrain and/or underlying mesenchyme secrete FGF3 and FGF8 to induce the ectoderm to become epibranchial placodes.
Trigeminal Placode
The trigeminal placode arises from the non-neural ectoderm adjacent to the neural tube at the presumptive midbrain and caudal hindbrain (D’Amico-Martel and Noden, 1983; Xu et al., 2008). Here we will concentrate on induction of the ophthalmic lobe of the trigeminal placode. Less is known about the maxillomandubular lobe due to the paucity of markers and delay in its development. Previous studies have shown that a factor or factors secreted by the dorsal neural tube are required for trigeminal placode induction to occur sometime after HH8 in the chicken (Stark et al., 1997; Baker et al., 1999; McCabe et al., 2004; McCabe and Bronner-Fraser, 2008). However, the molecular nature of these factors has been unknown for some time. An RT-PCR screen identified receptors to various growth factors as possible candidates for factors involved in trigeminal placode induction. Members of the Fibroblast Growth Factors, Insulin-like growth factors, Platelet Derived Growth Factors, Sonic Hedgehog, Transforming Growth Factor super family, and Wnt families all are expressed in the neural fold at the right place and time to be involved in trigeminal placode induction (McCabe et al., 2007).
The role of one of these candidates, PDGF family, has been explored in induction of the ophthalmic trigeminal placode (McCabe and Bronner-Fraser, 2008). A combination of in vitro and in vivo approaches implicated PDGFD signaling through PDGFRβ as critical for ophthalmic trigeminal placode induction in the chicken. Addition of a pharmacological inhibitor to PDGFRα and PDGFRβ blocks trigeminal placode induction, as assayed by expression of Pax3 and CD151, two molecular markers of the trigeminal placode. Furthermore, blocking PDGF signaling also inhibits subsequent neurogenesis. The function of PDGF appears to occur early, at the induction phase, since addition of the PDGFR inhibitor after the majority of trigeminal placode cells are specified, fails to cause a significant loss of neurons. Therefore, PDGF signaling is necessary for ophthalmic trigeminal placode induction, and loss of neurogenesis appears to be secondary to blocking induction. Interestingly, injection of exogenous PDGFD (PDGFDD) into the embryos increases the size of the trigeminal placode and the number of neurons. However, PDGF ligands that occur in both homo- and heterodimeric forms (i.e, PDGFAA, AB, BB, CC, or DD) alone are unable to induce placode cells, suggesting that the ligand is necessary but not sufficient for induction. Thus, additional yet to be identified factors appear to be involved in trigeminal placode induction and may function in cooperation with PDGFs.
Wnts have been implicated in specification and maintenance of cell fate in the trigeminal placode (Lassiter et al., 2007) and more recently in neurogenesis in the trigeminal ganglion (Dude et al., 2009). In ovo overexpression of Wnt3a results in premature differentiation of trigeminal placode cells (Canning et al., 2008). Because the significance of Wnt versus FGF signaling in ovo is difficult to tease apart due to the apparent feed forward nature of each signals in the midbrain region, in vitro ectodermal explant experiments were performed such that Wnt3A and FGF8 could be tested independently. Interestingly, Wnt3A but not FGF8 was able to induce Pax3 mRNA expression in ectodermal explants, at least in the presence of complex tissue culture medium that contains other unidentified factors (Canning et al., 2008). Thus, the trigeminal placode appears to utilize at least two inducers, namely PDGF and Wnt3A (McCabe et al., 2008; Canning et al., 2008).
Adenohypophyseal placode
The adenohypophyseal placode gives rise to endocrine secretory cells of the anterior pituitary gland. The placode arises in amniotes from the anterior neural ridge (Couly and Le Douarin, 1985; Eagleson and Harris, 1990) and non-neural ectoderm (Knouff, 1935; Eagleson et al., 1995; ElAmraoui and Dubois, 1993; Osumi-Yamashita et al., 1994; Kouki et al., 2001; Dutta et al., 2005) which gives rise to Rathke’s pouch after being induced by the prospective diencephalon. In the anamniote zebrafish, the adenohypophyseal placode is formed from a solid structure in the anterior head (Gleiberman et al., 1999; Takuma et al., 1998). Of the several inducers of the adenohypophyseal placode, Shh is currently thought to be one of the first factors required for induction (Herzog et al., 2003; Treier et al., 2001), with members of the TGFβ superfamily such as Nodal (Glasgow et al., 1997) and BMP4 (Takuma et al., 1998; Davis and Camper, 2007) playing later roles.
Lens placode
The lens and adenohypophyseal placodes are the only ones that fail to form neurons. Rather, the lens placode generates lens fiber cells necessary for vision. It is derived from the non-neural ectoderm and anterior neural ridge (Bhattacharyya et al., 2004). Early experiments suggested that the lens was induced solely by the neural retina. However, it is now clear that signals from the mesoderm and the neural plate are required for induction of the lens placode (Henry and Grainger, 1990). These signals include FGF (Faber et al., 2001) and two TGFβ family members, BMP4 (Furuta and Hogan, 1998; Sjodal et al., 2007) and BMP7 (Dudley et al., 1995; Wawersik et al., 1999).
Olfactory placode
Of all the placodes, the olfactory placode gives rise to the most diverse cell populations, including secretory cells (support, mucosal, and endocrine), primary sensory cells, as well as stem cells (reviewed in Schlosser, 2006). Originally the olfactory placode was thought to originate solely from the anterior neural ridge (Couly and Le Douarin, 1985; Whitlock and Westerfield, 2000). However, recent work reveals a dual origin with contributions from the adjacent non-neural ectoderm and the anterior neural ridge (Bhattacharyya et al. 2004). Interestingly, blocking BMP signaling can promote olfactory placode cell production over lens placode cells at St. 4 in the chicken embryo (Sjodal et al., 2007). At a slightly older stage (St. 6), FGF8 has been shown to promote olfactory placode cells over lens cells around the pre-placodal stage (Bailey et al., 2006).
Otic placode
The otic placode arises from a broad area that includes non-neural ectoderm as well as neural folds (Streit, 2002). The order and importance of inducing tissues remains controversial, and may vary between species, especially given that many of the inducers are expressed in different patterns and therefore may be substituting and/or compensating for one another. Current evidence supports a role for mesoderm alone (Kil et al., 2005), hindbrain alone (Waskiewicz et al., 2001; Kwak et al., 2002), and mesoderm and hindbrain combined (Gallagher et al., 1996; Mendonsa and Riley, 1999; Ladher et al., 2000; Leger and Brand, 2002; Phillips et al., 2001) in the induction of the otic placode across several species, including Xenopus, zebrafish, and chicken. Accordingly, several inducers have been identified including members of the FGF family (Mahmood et al.,1995; McKay et al.,1996; Lombardo et al., 1998; Reifers et al., 1998; Vendrell et al., 2000; Adamska et al., 2001; Furthauer et al., 2001; Phillips et al., 2001; Leger and Brand, 2002; Maroon et al., 2002; Alvarez et al., 2003; Liu et al., 2003; Wright and Mansour, 2003; Martin and Groves, 2006; Freter et al., 2008), retinoic acid (Hans et al., 2007), and Wnt signaling (Ohyama et al., 2006; Park et al., 2008).
CONCLUSION
Understanding early steps of placode formation is important for understanding development of the peripheral nervous system of the head. Molecular players in the process of trigeminal and epibranchial placode induction are currently being identified. Not surprisingly, many of the same signals, such as FGFs and BMPs, that function at other times and places in development are also critical for formation of placodes and their derivatives. Some placodes such as lens and otic have been studied in depth, whereas others (e.g. trigeminal and epibranchial placodes) are less well-understood but the subject of ongoing investigations. Determining the time, location, and nature of placode inducing signals has greatly increased understanding of the fundamental concepts governing early development of the peripheral nervous system.
Acknowledgments
We would like to thank Drs. Sonja McKeown and Sujata Bhattacharyya for critical reading of the manuscript. This work was supported by NIH R01DE16459.
References
- Abu-Elmagd M, Ishii Y, Cheung M, Rex M, Le Rouedec D, Scotting PJ. cSox3 expression and neurogenesis in the epibranchial placodes. Dev Biol. 2001;237:258–69. doi: 10.1006/dbio.2001.0378. [DOI] [PubMed] [Google Scholar]
- Adamska M, Herbrand H, Adamski M, Kruger M, Braun T, Bober E. FGFs control the patterning of the inner ear but are not able to induce the full ear program. Mech Dev. 2001;109:303–13. doi: 10.1016/s0925-4773(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev Biol. 2005;288:40–59. doi: 10.1016/j.ydbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–38. doi: 10.1242/dev.00881. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–17. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Marcelle C, Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–56. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Begbie J, Brunet JF, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- Bell D, Streit A, Gorospe I, Varela-Nieto I, Alsina B, Giraldez F. Spatial and temporal segregation of auditory and vestibular neurons in the otic placode. Dev Biol. 2008;322:109–20. doi: 10.1016/j.ydbio.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev Biol. 2004;271:403–14. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Hierarchy of regulatory events in sensory placode development. Curr Opin Genet Dev. 2004;14:520–6. doi: 10.1016/j.gde.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135:4165–77. doi: 10.1242/dev.026633. [DOI] [PubMed] [Google Scholar]
- Canning CA, Lee L, Luo SX, Graham A, Jones CM. Neural tube derived Wnt signals cooperate with FGF signaling in the formation and differentiation of the trigeminal placodes. Neural Develop. 2008;3:35. doi: 10.1186/1749-8104-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Shimamura K, Rubenstein JL, Martinez S, Puelles L. Fate map of the avian anterior forebrain at the four-somite stage, based on the analysis of quail-chick chimeras. Dev Biol. 2001;239:46–67. doi: 10.1006/dbio.2001.0423. [DOI] [PubMed] [Google Scholar]
- Couly G, Le Douarin NM. Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development. 1990;108:543–58. doi: 10.1242/dev.108.4.543. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985;110:422–39. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–68. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–60. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dude CM, Kuan CY, Bradshaw JR, Greene ND, Relaix F, Stark MR, Baker CV. Activation of Pax3 target genes is necessary but not sufficient for neurogenesis in the ophthalmic trigeminal placode. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Aspock G, Burdine RD, Schier A, Westerfield M, Varga ZM. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132:1579–90. doi: 10.1242/dev.01723. [DOI] [PubMed] [Google Scholar]
- Eagleson G, Ferreiro B, Harris WA. Fate of the anterior neural ridge and the morphogenesis of the Xenopus forebrain. J Neurobiol. 1995;28:146–58. doi: 10.1002/neu.480280203. [DOI] [PubMed] [Google Scholar]
- Eagleson GW, Harris WA. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J Neurobiol. 1990;21:427–40. doi: 10.1002/neu.480210305. [DOI] [PubMed] [Google Scholar]
- Eagleson GW, Jenks BG, Van Overbeeke AP. The pituitary adrenocorticotropes originate from neural ridge tissue in Xenopus laevis. J Embryol Exp Morphol. 1986;95:1–14. [PubMed] [Google Scholar]
- elAmraoui A, Dubois PM. Experimental evidence for the early commitment of the presumptive adenohypophysis. Neuroendocrinology. 1993;58:609–15. doi: 10.1159/000126599. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–38. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–24. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Reifers F, Brand M, Thisse B, Thisse C. sprouty4 acts in vivo as a feedback- induced antagonist of FGF signaling in zebrafish. Development. 2001;128:2175–86. doi: 10.1242/dev.128.12.2175. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–75. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher BC, Henry JJ, Grainger RM. Inductive processes leading to inner ear formation during Xenopus development. Dev Biol. 1996;175:95–107. doi: 10.1006/dbio.1996.0098. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Karavanov AA, Dawid IB. Neuronal and neuroendocrine expression of lim3, a LIM class homeobox gene, is altered in mutant zebrafish with axial signaling defects. Dev Biol. 1997;192:405–19. doi: 10.1006/dbio.1997.8761. [DOI] [PubMed] [Google Scholar]
- Gleiberman AS, Fedtsova NG, Rosenfeld MG. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol. 1999;213:340–53. doi: 10.1006/dbio.1999.9386. [DOI] [PubMed] [Google Scholar]
- Graham A, Blentic A, Duque S, Begbie J. Delamination of cells from neurogenic placodes does not involve an epithelial-to-mesenchymal transition. Development. 2007;134:4141–5. doi: 10.1242/dev.02886. [DOI] [PubMed] [Google Scholar]
- Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–99. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev Biol. 1990;141:149–63. doi: 10.1016/0012-1606(90)90110-5. [DOI] [PubMed] [Google Scholar]
- Herzog W, Zeng X, Lele Z, Sonntag C, Ting JW, Chang CY, Hammerschmidt M. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol. 2003;254:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–42. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13:388–93. doi: 10.1101/gad.13.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil SH, Streit A, Brown ST, Agrawal N, Collazo A, Zile MH, Groves AK. Distinct roles for hindbrain and paraxial mesoderm in the induction and patterning of the inner ear revealed by a study of vitamin-A-deficient quail. Dev Biol. 2005;285:252–71. doi: 10.1016/j.ydbio.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Knouff RA. The developmental pattern of ectodermal placodes in rana pipiens. Journal of Comparative Neurology. 1935;62:17–71. [Google Scholar]
- Kondoh H, Uchikawa M, Yoda H, Takeda H, Furutani-Seiki M, Karlstrom RO. Zebrafish mutations in Gli-mediated hedgehog signaling lead to lens transdifferentiation from the adenohypophysis anlage. Mech Dev. 2000;96:165–74. doi: 10.1016/s0925-4773(00)00387-7. [DOI] [PubMed] [Google Scholar]
- Kouki T, Imai H, Aoto K, Eto K, Shioda S, Kawamura K, Kikuyama S. Developmental origin of the rat adenohypophysis prior to the formation of Rathke’s pouch. Development. 2001;128:959–63. doi: 10.1242/dev.128.6.959. [DOI] [PubMed] [Google Scholar]
- Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem Cell Biol. 1997;75:551–62. [PubMed] [Google Scholar]
- Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES. The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev Biol. 2005;277:27–41. doi: 10.1016/j.ydbio.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Kwak SJ, Phillips BT, Heck R, Riley BB. An expanded domain of fgf3 expression in the hindbrain of zebrafish valentino mutants results in mis-patterning of the otic vesicle. Development. 2002;129:5279–87. doi: 10.1242/dev.129.22.5279. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–7. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Lassiter RN, Dude CM, Reynolds SB, Winters NI, Baker CV, Stark MR. Canonical Wnt signaling is required for ophthalmic trigeminal placode cell fate determination and maintenance. Dev Biol. 2007;308:392–406. doi: 10.1016/j.ydbio.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–62. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–24. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Isaacs HV, Slack JM. Expression and functions of FGF-3 in Xenopus development. Int J Dev Biol. 1998;42:1101–7. [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Kiefer P, Guthrie S, Dickson C, Mason I. Multiple roles for FGF-3 during cranial neural development in the chicken. Development. 1995;121:1399–410. doi: 10.1242/dev.121.5.1399. [DOI] [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- Martin K, Groves AK. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development. 2006;133:877–87. doi: 10.1242/dev.02267. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Bronner-Fraser M. Essential role for PDGF signaling in ophthalmic trigeminal placode induction. Development. 2008;135:1863–74. doi: 10.1242/dev.017954. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Manzo A, Gammill LS, Bronner-Fraser M. Discovery of genes implicated in placode formation. Dev Biol. 2004;274:462–77. doi: 10.1016/j.ydbio.2004.07.012. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Sechrist JW, Bronner-Fraser M. Birth of ophthalmic trigeminal neurons initiates early in the placodal ectoderm. J Comp Neurol. 2009;514:161–173. doi: 10.1002/cne.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KL, Shiau CE, Bronner-Fraser M. Identification of candidate secreted factors involved in trigeminal placode induction. Dev Dyn. 2007;236:2925–35. doi: 10.1002/dvdy.21325. [DOI] [PubMed] [Google Scholar]
- McKay IJ, Lewis J, Lumsden A. The role of FGF-3 in early inner ear development: an analysis in normal and kreisler mutant mice. Dev Biol. 1996;174:370–8. doi: 10.1006/dbio.1996.0081. [DOI] [PubMed] [Google Scholar]
- McLarren KW, Litsiou A, Streit A. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev Biol. 2003;259:34–47. doi: 10.1016/s0012-1606(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Mendonsa ES, Riley BB. Genetic analysis of tissue interactions required for otic placode induction in the zebrafish. Dev Biol. 1999;206:100–12. doi: 10.1006/dbio.1998.9134. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–23. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–30. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2007;236:564–71. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Noden DM. Spatial integration among cells forming the cranial peripheral nervous system. J Neurobiol. 1993;24:248–61. doi: 10.1002/neu.480240210. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–9. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol. 2007;51:463–72. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Osumi-Yamashita N, Ninomiya Y, Doi H, Eto K. The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Dev Biol. 1994;164:409–19. doi: 10.1006/dbio.1994.1211. [DOI] [PubMed] [Google Scholar]
- Park BY, Saint-Jeannet JP. Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev Biol. 2008;324:108–21. doi: 10.1016/j.ydbio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol. 2001;235:351–65. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Quinlan R, Martin P, Graham A. The role of actin cables in directing the morphogenesis of the pharyngeal pouches. Development. 2004;131:593–9. doi: 10.1242/dev.00950. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–95. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Reifers F, Walsh EC, Leger S, Stainier DY, Brand M. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar) Development. 2000;127:225–35. doi: 10.1242/dev.127.2.225. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–51. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Seo S, Herr A, Lim JW, Richardson GA, Richardson H, Kroll KL. Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev. 2005;19:1723–34. doi: 10.1101/gad.1319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodal M, Edlund T, Gunhaga L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell. 2007;13:141–9. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural tube-ectoderm interactions are required for trigeminal placode formation. Development. 1997;124:4287–95. doi: 10.1242/dev.124.21.4287. [DOI] [PubMed] [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Dev Biol. 2002;249:237–54. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol. 2007;51:447–61. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–86. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–40. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Treier M, O’Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–86. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Vendrell V, Carnicero E, Giraldez F, Alonso MT, Schimmang T. Induction of inner ear fate by FGF3. Development. 2000;127:2011–9. doi: 10.1242/dev.127.10.2011. [DOI] [PubMed] [Google Scholar]
- Waskiewicz AJ, Rikhof HA, Hernandez RE, Moens CB. Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning. Development. 2001;128:4139–51. doi: 10.1242/dev.128.21.4139. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–88. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Webb JF, Noden DM. Ectodermal Placodes: Contributions to the Development of the Vertebrate Head. Amer Zool. 1993;33:434–447. [Google Scholar]
- Whitlock KE, Westerfield M. The olfactory placodes of the zebrafish form by convergence of cellular fields at the edge of the neural plate. Development. 2000;127:3645–53. doi: 10.1242/dev.127.17.3645. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–90. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Xu H, Dude CM, Baker CV. Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Dev Biol. 2008;317:174–86. doi: 10.1016/j.ydbio.2008.02.012. [DOI] [PubMed] [Google Scholar]


