Abstract
Reduced nitric oxide synthase activity in rats with chronic renal disease due to glomerulonephritis.
Background
Animal studies with systemic nitric oxide synthase (NOS) inhibition and renal ablation, suggest that NO deficiency is both a cause and a consequence of chronic renal disease (CRD).
Methods
This study examined a glomerulonephritis (GN) model of CRD to determine if NO is deficient. In addition to measuring indices of renal function (proteinuria, creatinine clearance, structural damage), indices of total and renal nitric oxide production also were assessed (total NOX excretion, renal NOS activity, renal NOS protein abundance, plasma levels of NOS substrate and endogenous inhibitor).
Results
Rats developed increasing proteinuria 12 to 20 weeks after induction of GN (with anti-glomerular basement membrane, GBM, antibody) and at 20 weeks exhibited reduced creatinine clearances and increased glomerulosclerosis relative to age-matched controls. Total NOX excretion was reduced and the renal cortical NOS activity and neuronal NOS (nNOS) abundance was decreased relative to controls. There was no impact on renal or aortic endothelial NOS expression or cerebellar nNOS. The plasma l-arginine (Arg) concentration was well maintained but plasma asymmetric dimethylarginine (ADMA) concentration increased in GN versus control animals.
Conclusions
Total and renal NOS activity is reduced in the GN model of CRD due to increased circulating endogenous NOS inhibitors and decreased renal nNOS abundance.
Keywords: neuronal NOS, endothelial NOS, arginine, asymetric dimethylarginine, nitrate + nitrite, ESRD, nitric oxide, progressive renal disease
There is clinical and experimental evidence that chronic progressive renal disease (CRD) and end-stage renal disease (ESRD) is associated with nitric oxide (NO) deficiency [1–6]. In addition, experimentally-induced NO deficiency causes CRD [7]. These associations suggest a possible vicious cycle that could contribute to progression of renal disease. The mechanistic insights possible from clinical studies are limited, and the animal work, while informative, has been largely focused on the CRD model produced by reduction of functional renal mass [4–6]. We therefore sought to investigate a different model of primary renal disease, leading to CRD. Since approximately 15% of the CRD in humans result from immune-mediated renal disease [8], we chose an anti-glomerular basement membrane glomerulonephritis (anti-GBM GN) model for study. In this rat experimentally induced anti-GBM GN can result in CRD with glomerulosclerosis after the acute inflammatory response has abated [9].
In the present study the two specific questions were (1) does systemic and/or renal NO deficiency develop in a GN model of CRD, and (2) if so, what mechanisms are involved? Rats were maintained for 20 weeks after administration of anti-rat GBM antibody and then compared to time controls. Periodic 24-hour urines were collected to monitor proteinuria and total NO production (from the stable NO oxidation products, NO2 + NO3 = NOX). Afterwards, the rats were sacrificed and tissues harvested for measurement of NOS protein abundance and NOS activity as well as for histologic assessment of injury. A blood sample was obtained for determination of levels of creatinine, BUN, arginine (Arg) and the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA).
METHODS
Male Sprague-Dawley rats (N = 14) were maintained on a nutritionally complete, low NOX diet (ICN AIN 76C; ICN Biomedicals, Cleveland, OH, USA) and tap water ad libitum. After baseline measurements in metabolic cages, experimental rats (N = 6) were pre-immunized (1 mg sheep IgG in 1 mL Freund's adjuvant, 6 sites intradermally), and five days later received sheep anti-rat anti-GBM antibody (1.5 to 3.0 mL/kg) by tail vein. Twenty-four hour urine was collected in metabolic cages periodically and assayed for total urinary protein (Bradford assay) and NOx by the Greiss reaction. After 20 weeks, GN rats and age-matched controls (N = 8) were sacrificed. Kidneys were prepared for histologic evaluation of glomerular injury (in a blinded fashion), NOS-activity measurement (determined by the conversion of [3H]-l-Arg to [3H]-l-citrulline) was measured in the soluble fraction of kidney cortex and medulla and expressed as the NG-monomethyl-l-arginine (L-NMMA; 10 mmol/L) and N-nitro-l-arginine methyl ester (L-NAME; 20 mmol/L) inhibitable conversion (~85% of total). Western blot analysis was conducted on kidney cortex [for endothelial (eNOS) and neuronal NOS (nNOS); 200 μg total protein), aorta (for eNOS; 100 μg total protein) and cerebellum (for nNOS; 10 μg total protein). NOS protein abundance was expressed as integrated optical density, IOD of nNOS or eNOS factored for Ponceau red stain (total protein loaded). Terminal blood was collected and assayed for NOX, creatinine (Cr), blood urea nitrogen (BUN), ADMA and Arg. Details of these various methods have been given previously [1, 3, 7, 10, 11].
Statistical analysis was by one- and two-way ANOVA, unpaired t test and linear regression; P < 0.05 was considered statistically significant.
RESULTS
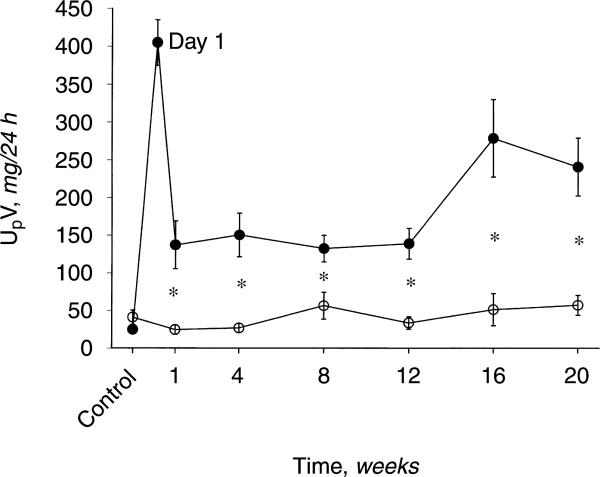
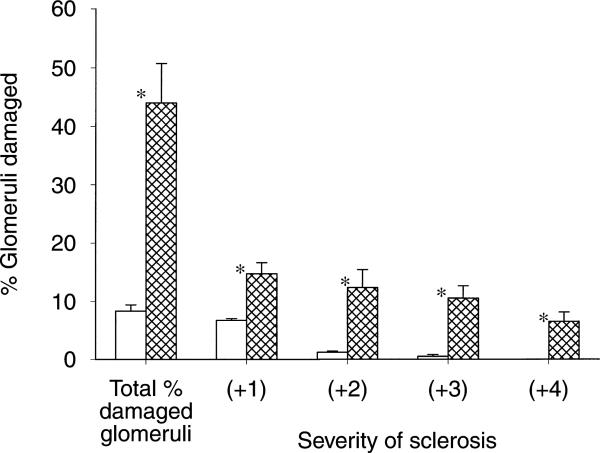
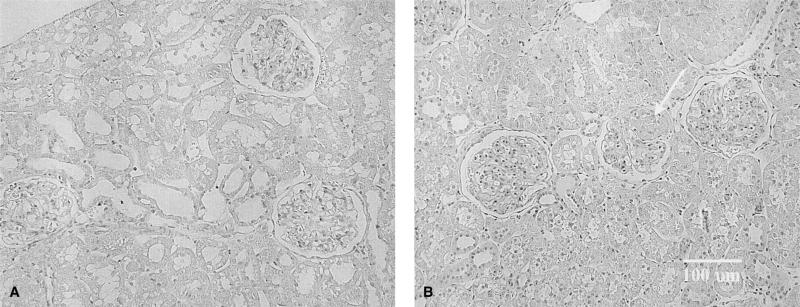
The 24-hour urine protein excretion (UpV) rose >10×on the day after anti-GBM antibody administration (Fig. 1). UpV then fell to a stable but elevated value over the next 12 weeks and then began to increase again in the GN rats. In contrast, controls showed only mild age-dependent increases in UpV over the 20 week observation. The heavy proteinuria at 20 weeks in GN rats correlated with significant glomerular sclerosis as well as an increase in the severity of injury versus time controls (Fig. 2). Representative photomicrographs of the renal histology are shown in Figure 3. In addition, BUN and plasma creatinine was elevated and 24-hour creatinine clearance was reduced versus controls, reflecting approximately 65% loss of renal function in GN rats (Table 1).
Fig. 1.
Twenty-four hour urinary protein excretion (UpV) versus time in rats with glomerulonephritis (GN, •) and controls (○). *P < 0.05, control vs. GN; whiskers denote difference vs. baseline value.
Fig. 2.
The total number of damaged (sclerotic) glomeruli in control (□) and GN ( ) rats are given in the first columns. Next the severity of glomerular injury is given, with +1 representing mild damage, up to 25% of glomerulus involved; +2 representing moderate damage with 26 to 50% injury; +3 and +4 representing severe damage of 51 to 75% and 76% or more global sclerosis, respectively. *P < 0.05 control vs. GN.
) rats are given in the first columns. Next the severity of glomerular injury is given, with +1 representing mild damage, up to 25% of glomerulus involved; +2 representing moderate damage with 26 to 50% injury; +3 and +4 representing severe damage of 51 to 75% and 76% or more global sclerosis, respectively. *P < 0.05 control vs. GN.
Fig. 3.
(A) Section from a control rat with 3 normal glomeruli. (B) A section from a GN rat also contains 3 glomeruli. The middle glomerulus has ~50% sclerosis (arrow), while that on the right shows mesangial expansion and matrix accumulation. The glomerulus on the left also shows some increase mesangial matrix accumulation. Original magnification, ×300.
Table 1.
Blood and urine chemistry in rats after 20 weeks of CRD secondary to glomerulonephritis (post-GN) or in controls aged over a similar period
| Pcr |
BUN |
CCr mL/min/kg BW | PNOX μmol/L | PARG |
PADMA |
|||
|---|---|---|---|---|---|---|---|---|
| mg % | PNOX/PCr | μmol/L | PADMA/PARG | |||||
| Control | 0.41 ± 0.02 | 21 ± 1 | 6.3 ± 0.3 | 18 ± 2 | 0.51 ± 0.06 | 101 ± 8 | 1.08 ± 0.11 | 0.011 ± 0.001 |
| Post-GN | 0.98 ± 0.20 | 38 ± 10 | 2.4 ± 0.3 | 27 ± 4 | 0.35 ± 0.07 | 117 ± 13 | 3.81 ± 0.95 | 0.034 ± 0.008 |
| P value | <0.025 | <0.05 | <0.001 | <0.02 | NS | NS | <0.035 | <0.035 |
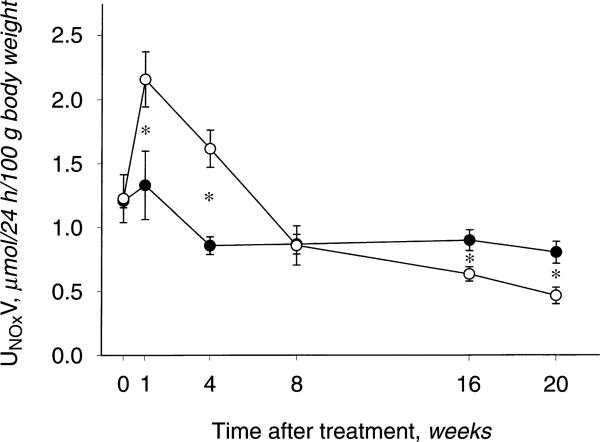
As shown in Figure 4, the UNOXV was similar at baseline and higher at weeks 1 and 4 in GN versus controls, coincident with the acute inflammatory response to anti-GBM antibody. By week 8, UNOXV in the GN group had normalized to control values and at 20 weeks was significantly reduced versus controls. Although plasma NOX concentration was higher in GN rats at 20 weeks versus controls (Table 1), the ratio of plasma NOX to plasma creatinine (to factor out the hemoconcentration of NOX secondary to reduced renal clearance) was not different between GN and controls. Together, the UNOXV and PNOX data suggest that total NO production is reduced in the GN rats with CRD versus controls.
Fig. 4.
Total nitric oxide (NO) production, indicated by 24-hour urinary NOX excretion (UNOXV) in control (○) and GN (•) rats. *P < 0.05, control vs. GN. Whiskers denote difference vs. baseline value.
The plasma L-Arg concentrations were similar between GN and controls whereas plasma ADMA was elevated by approximately 3.5× (Table 1). Of note, the plasma ADMA to Arg ratio was significantly elevated in GN versus controls. When PADMA was factored for plasma creatinine, the difference between groups was less (0.044 ± 0.005 vs. 0.030 ± 0.003, GN vs. control, P < 0.02), suggesting that most of the increase in PADMA was the result of loss of renal clearance.
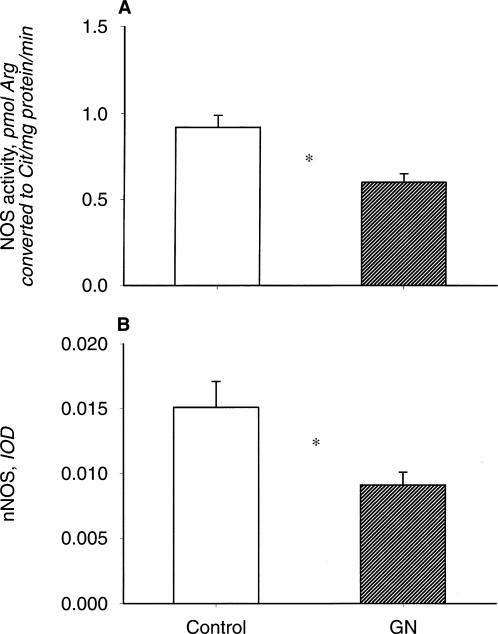
As shown in Figure 5, the NOS activity in the soluble fraction of renal cortex was reduced in the GN rats versus controls. The abundance of the nNOS was also lower in homogenates of renal cortex from GN (N = 5) versus controls. The nNOS protein abundance in cerebellum was similar in controls and rats with GN (data not shown). In contrast to the nNOS, the eNOS abundance in renal cortex was not altered in the CRD rats (data not shown). We also observed a similar eNOS abundance in homogenates of aorta (100 μg protein) between GN rats and controls (2.33 ± 0.17 and 2.63 ± 0.33 IOD units, respectively). Although there was inadequate protein available to perform Western blots on medulla, the NOS activity was markedly reduced in GN versus controls (0.80 ± 0.16 vs. 1.41 ± 0.17 pmol cit/min/mg protein, P < 0.02).
Fig. 5.
(A) Renal cortical nitric oxide synthase (NOS) activity in control and GN rat. (B) Relative abundance of the neuronal NOS isoform (nNOS; factored for total protein loaded, from Ponceau red stain) in control and GN rats. *P < 0.05.
DISCUSSION
The main novel finding in the present study is that in a rat model of chronic progressive renal disease secondary to GN, there are reductions in both total and renal NO generation. These findings agree with several reports in the 5/6th ablation or ablation/infarction models of CRD in the rat [4–6] and support the hypothesis that irrespective of etiology, CRD is a state of NO deficiency [1–6, 12].
To our knowledge, this study represents the first long-term evaluation of the NO system in experimentally-induced, immune-mediated renal disease. It is well known that early in GN, during the acute inflammatory response, increased renal NO production [from stimulated inducible NOS (iNOS)] occurs that contributes to the early pathology [13–15]. Indeed, since the severity of the acute inflammatory response is directly related to later development of CRD due to GN [9], the early increase in NO can be considered to contribute to the level of chronic injury. This iNOS induction and increased NO generation is relatively short lived [15], and as shown here, by eight weeks after immunization total NO generation has returned to normal, while by 20 weeks the total NO production is reduced. The reduction in total somatic NO generation was substantial (24-hour UNOXV was ~60% control) and since renal generation of NO contributes only a few % to the total [16], it is likely that NO generation also is reduced from non-renal sites in this GN model of CRD.
Total somatic NO production could be reduced via an increase in the level of endogenous inhibitors. An increase in the ratio of concentrations of ADMA (an Arg analog that functions as an endogenous NOS inhibitor) to Arg (native NOS substrate), as occurred in the GN rats, will reduce NO generation throughout the body. In humans with ESRD, plasma ADMA levels increase substantially [2, 3, 17], and according to Vallance and colleagues this is due to reduced renal clearance of the methylated Arg [17]. There is a good correlation between the plasma levels of creatinine and ADMA in the present study, suggesting that reduced renal clearance contributes to the elevated ADMA. Increased ADMA levels also can occur in humans with active renal disease [1], but in contrast to the ESRD patients not all CRD patients have elevated plasma ADMA despite a similar loss of renal clearance [11]. We have suggested that some individuals might be able to mobilize the enzyme dimethylarginine dimethylaminohydrolase (DDAH), which degrades ADMA, thus for a limited time maintaining a normal PADMA despite increased PCr.
We also have reported that the increased PADMA in renal failure patients is associated with functional impairment of endothelial NO generation. When plasma from ESRD patients (with PADMA levels similar to those in the GN rat) was incubated with cultured vascular endothelial cells, a marked inhibition of eNOS activity occurred [18]. In the CRD patients, plasma from those with elevated PADMA always inhibited eNOS activity in cultured endothelial cells while those with normal PADMA had no inhibitory effect [11]. Furthermore, inhibition of eNOS activity in vitro was seen when endothelial cells were incubated with artificial solutions containing a similar amount of ADMA as found in the GN rat plasma [11, 18]. Therefore, the increased PADMA/PArg seen in GN rats probably contributed to the reduction in total NO generation (from 24-hour UNOXV).
We expect that the increased PADMA/PArg in GN rats also contributed to a reduced renal NOS activity. In addition, when measured in vitro after removal of endogenous ADMA and Arg, NOS activity remained significantly reduced in both cortex and medulla of kidneys from GN rats. The decline in renal cortical NOS activity is associated with a decline in renal cortical nNOS abundance. Since nNOS resides in the cytosol, and the in vitro NOS activity assay was conducted on the soluble fraction, it is likely that decreased renal nNOS abundance contributes to the reduced renal NOS activity in rats with CRD secondary to GN. Of course, we recognize that many factors other than protein abundance will contribute to the intrinsic activity of the NOS, and may also be altered in GN.
This study identifies two separate mechanisms by which renal NO production is reduced in the rat with CRD secondary to GN. Reductions in renal NOS activity and nNOS abundance also have been reported previously for the renal ablation models of CRD, although as yet there is no mechanistic explanation [4–6]. In the present study nNOS protein expression in cerebellum of GN rats is normal, suggesting that the nNOS down-regulation is not ubiquitous, although reduced nNOS expression in other non-renal tissue cannot be ruled out.
In this GN model of CRD there was preservation of the eNOS protein abundance in kidney cortex, as well as in aorta, with only the renal nNOS abundance being reduced. This contrasts with the more severe injury seen in the advanced 5/6th ablation model where decreases in both renal and aortic eNOS abundance were reported [5]. However, early (~2 to 3 weeks) after 5/6th renal ablation/infarction we found that the nNOS, but not eNOS protein is reduced (abstract; Wagner et al, J Am Soc Nephrol 12:828A, 2001), suggesting that the renal nNOS is most sensitive to developing renal damage. There are few clinical data on NOS protein abundance in renal disease. By immunohistochemistry, the glomerular expression of eNOS is reduced in patients with Wegener's granulomatosis [19] and also in GN, when there is severe renal injury [20]. To date there has been no assessment of the renal nNOS.
We do not yet understand the intermediate mechanisms that link developing CRD with falls in renal nNOS abundance. However, the functional implications are significant. The renal nNOS is most abundant in macula densa and plays an important “permissive” vasodilatory role in the tubuloglomerular feedback regulation of GFR [21]. Thus, a decline in renal cortical nNOS will cause renal vasoconstriction and falls in GFR, and this undoubtedly contributes to the overall fall in renal function seen in the GN rats. Loss of the “antigrowth, antifibrotic” influence of NO, irrespective of its source of origin, also will promote the development of structural injury.
In conclusion, CRD secondary to GN leads to somatic and renal NO deficiency. The increased circulating ADMA (secondary to loss of renal clearance) is a likely contributor to the decreased somatic NO production. High PADMA will inhibit renal NO production and the local decrease in nNOS abundance will exacerbate local renal NO deficiency, which is likely to contribute to the progression of renal disease as well as causing functional declines. This rat model of CRD is less aggressive than the ablation/infarction models usually studied and provides an animal model that is more representative of human renal disease.
ACKNOWLEDGMENTS
These studies were supported by NIH grants # R01 DK56843 and DK 45517 (CB) and DK 34198 and DK 47659 (WC). Part of this work was previously published in abstract form (Wagner et al, J Am Soc Nephrol 11:632A, 2000). We gratefully acknowledge the technical support of Ms. Lennie Samsell and Ms. Chris Stalnacker.
REFERENCES
- 1.Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58:1261–1266. doi: 10.1046/j.1523-1755.2000.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt RJ, Yokota S, Tracy TS. Nitric oxide production is low in end stage renal disease patients on peritoneal dialysis. Am J Physiol (Renal Physiol) 1999;276:F794–F797. doi: 10.1152/ajprenal.1999.276.5.F794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt R, Domico J, Samsell L, et al. Indices of activity of the nitric oxide system in patients on hemodialysis. Am J Kidney Dis. 1999;34:228–234. doi: 10.1053/AJKD03400228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri ND, Ni Z, Wang XQ, et al. Downregulation of nitric oxide synthase in chronic renal insufficiency: Role of excess PTH. Am J Physiol (Renal Physiol) 1998;274:F642–F649. doi: 10.1152/ajprenal.1998.274.4.F642. [DOI] [PubMed] [Google Scholar]
- 5.Aiello S, Noris M, Todeschini M, et al. Renal and systemic nitric oxide synthesis in rats with renal mass reduction. Kidney Int. 1997;52:171–181. doi: 10.1038/ki.1997.317. [DOI] [PubMed] [Google Scholar]
- 6.Roczniak A, Fryer JN, Levine DZ, Burns KD. Downregulation of neuronal nitric oxide synthase in the rat remnant kidney. J Am Soc Nephrol. 1999;10:704–713. doi: 10.1681/ASN.V104704. [DOI] [PubMed] [Google Scholar]
- 7.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Renal Data System . USRDS 1990 Annual Data Report. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 1990. pp. D.2–D.25. [Google Scholar]
- 9.Scandrett AL, Kissane J, Lefkowith JB. Acute inflammation is the harbinger of glomerulosclerosis in anti-glomerular basement membrane nephritis. Am J Physiol (Renal Physiol) 1995;268:F258–F265. doi: 10.1152/ajprenal.1995.268.2.F258. [DOI] [PubMed] [Google Scholar]
- 10.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol (Renal Physiol) 2001;49:F996–F1000. doi: 10.1152/ajprenal.2001.280.6.F996. [DOI] [PubMed] [Google Scholar]
- 11.Xiao S, Wagner L, Schmidt RJ, Baylis C. Circulating eNOS inhibitory factor in some patients with chronic renal disease. Kidney Int. 2001;59:1466–1472. doi: 10.1046/j.1523-1755.2001.0590041466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001;16(Suppl 1):60–62. doi: 10.1093/ndt/16.suppl_1.60. [DOI] [PubMed] [Google Scholar]
- 13.Jansen A, Cook T, Taylor GM, et al. Induction of nitric oxide synthase in rat immune complex glomerulonephritis. Kidney Int. 1994;45:1215–1219. doi: 10.1038/ki.1994.161. [DOI] [PubMed] [Google Scholar]
- 14.Narita I, Border WA, Ketteler M, Noble NA. Nitric oxide mediates immunologic injury to kidney mesangium in experimental glomerulonephritis. Lab Invest. 1995;72:17–24. [PubMed] [Google Scholar]
- 15.Lianos EA, Liu J. Changes in inducible nitric oxide synthase expression in experimental glomerulonephritis. Proc Soc Exp Biol Med. 1997;215:405–411. doi: 10.3181/00379727-215-44151. [DOI] [PubMed] [Google Scholar]
- 16.Baylis C, Vallance P. Editorial Review: Measurement of nitrite and nitrate (NOx) levels in plasma and urine; what does this measure tell us about the activity of the endogenous nitric oxide. Curr Opin Nephrol Hypertens. 1998;7:1–4. doi: 10.1097/00041552-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Vallance P, Leone A, Calver A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 18.Xiao S, Schmidt R, Baylis C. Plasma from ESRD patients inhibits nitric oxide synthase (NOS) activity in cultured human and bovine endothelial cells. Acta Phys Scand. 2000;168:175–179. doi: 10.1046/j.1365-201x.2000.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heeringa P, Bijl M, de Jager-Krijjen A, et al. Renal expression of endothelial and inducible nitric oxide synthase, and formation of peroxynitrite–modified proteins and reactive oxygen species in Wegener's granulomatosis. J Pathol. 2001;193:224–232. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH782>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Furusu A, Miyazaki M, Abe K, et al. Expression of endothelial and inducible nitric oxide synthase in human glomerulonephritis. Kidney Int. 1998;53:1760–1768. doi: 10.1046/j.1523-1755.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- 21.Welch WJ, Wilcox CS. What is brain nitric oxide synthase doing in the kidney? Curr Opin Nephrol Hypertens. 2002;11:109–115. doi: 10.1097/00041552-200201000-00016. [DOI] [PubMed] [Google Scholar]