Abstract
Background. There is growing evidence of genetic risk for susceptibility to IgA nephropathy. Among several candidate genes related to immunological regulation in renal tissue, TGFB1 is known to be a contributor to proliferation and the development of fibrosis.
Methods. We analysed several SNPs in a region of this gene using 212 DNA samples from biopsy-proven IgA nephropathy patients, 146 men and 66 women and 477 healthy age-matched controls (321 men and 156 women) from the same population in Sweden.
Results. Frequencies of four out of five selected SNPs (rs6957, rs2241715, rs1800471, rs1982073 and rs1800469) were found to significantly differ between male patients and male controls in a co-dominant model (corrected P ≤ 0.05) and of two SNPs (rs1982073 and rs1800469) in the allelic model (P ≤ 0.05 in 100 000 permutation test). Haplotype analysis for five selected SNPs revealed a significant association of TGGCG with protective effect (P = 0.0012, empirical P = 0.006, 100 000 permutations) and of CTGTA with susceptibility effect (P = 0.0018, empirical P = 0.008, 100 000 permutations). In our study, no association with TGFB1 variations was found when comparing female patients and female controls. No association was found for TGFB1 markers with disease progression for selected individuals from the patient's group. In addition, meta-analysis performed for SNP rs1982073 for combined patients and controls from our study together with published data from two independent studies showed a significant association.
Conclusions. Our experimental data together with the meta-analysis suggest TGFB1 as an important candidate gene for further biological studies of IgA nephropathy and as a possible target for therapy. Our data also indicate a possibility of a gender effect in the genetic background of IgA nephropathy.
Keywords: IgA nephropathy, polymorphism, SNP, TGFB1
Introduction
IgA nephropathy is the most common form of glomerulonephritis identified in all regions of the world where renal biopsy is widely practiced. Evidence derived from experimental models of spontaneous IgA nephropathy and studies of the genetics, familial occurrence and racial differences in prevalence support the influence of genetic factors in the development and progression of IgA nephropathy [1–3]. IgA nephropathy in Caucasian populations was shown to be more prevalent in men; the ratio of males to females with IgA nephropathy is around 6:1 in northern Europe and the United States [4]. Poor prognostic factors for progression of renal damage in patients with IgA nephropathy are male gender, older age, presence of hypertension, moderate or severe proteinuria and severe renal lesions [1].
Transforming growth factor-β1 (TGFβ1) is a multifunctional cytokine that mediates cell proliferation, differentiation and other functions in different cell types. TGFβ1 signalling is an essential mechanism in isotype switching to immunoglobulin A in B lymphocytes and up-regulates IgA production in general [5]. TGFβ1 contributes to the expansion of mesangial matrix, development of fibrosis and cell proliferation both in animal models and in human diseases [6,7]. The kidney seems to be particularly sensitive to the powerful fibrogenic actions of TGFβ1 that may contribute to fibrosis in most forms of renal diseases, leading to renal impairment and decreased renal function [8,9].
The TGFβ1 gene, TGFB1, is located at the human chromosome 19q13.1-3 (omim 190180). There are several indications that genetic polymorphism of this gene is involved in the development of fibrotic diseases [8,10,11] and may regulate TGFβ1 production [12]. Four previous studies of TGFB1 polymorphisms have demonstrated possible associations between susceptibility and/or severity of IgA nephropathy, but the results have so far been inconsistent [9,10,13,14].
In the present study, 212 unrelated patients with biopsy-proven IgA nephropathy and 477 healthy subjects were selected for studies of five various polymorphisms in the TGFB1 gene with consideration to gender. In addition, a meta-analysis including previous studies was performed in order to clarify the role of TGFβ1 as a possible susceptibility factor in IgA nephropathy.
Materials and methods
Subjects
A total of 212 unrelated patients (146 males and 66 females), mean age 38.5 ± 14.4 (range 17–77 years) with biopsy-proven IgA nephropathy, all self-reported Caucasians, and 477 individually sex- and age-matched healthy Caucasians from a Swedish population (321 males and 156 females), mean age 44.8 ± 13.0 (range 18–80 years), were included in the present investigation. The patients were recruited from the Department of Nephrology at the Karolinska University Hospital (n = 117), Danderyd Hospital (n = 31), Skövde Hospital (n = 36) and Linköping Hospital (n = 28), representing a population from the central part of Sweden. Patients with Henoch-Schönlein purpura and other forms of glomerulonephritis were not included in the study. For known information about kidney function in the patients at the time of diagnosis, see Table 1.
Table 1.
Glomerular filtration rate of the patients in the different stages of chronic kidney diseasea
| >90 ml/min | 90–60 ml/min | 30–59 ml/min | 15–29 ml/min | <15 ml/min | |
|---|---|---|---|---|---|
| Males, n = 77 (73.3%) | 19 (18.1%) | 28 (26.7%) | 20 (19.0%) | 6 (5.7%) | 4 (3.8%) |
| Females, n = 28 (26.7%) | 4 (3.8%) | 15 (14.3%) | 6 (5.7%) | 2 (1.9%) | 1 (1%) |
| Total, n = 105 (100%) | 23 (21.9%) | 43 (41.0%) | 26 (24.7%) | 8 (7.6%) | 5 (4.8%) |
aCalculated for individuals with available clinical data.
All patients gave informed consent, and the study was approved by the Ethics Committee of the Karolinska Hospital, Stockholm, Sweden.
Disease severity
One hundred and seventeen patients from the Karolinska University Hospital, who had been followed up for up to 12 years since renal biopsy, were investigated for the correlation between TGFB1 genotype and disease severity. The average age of these patients at the time of renal biopsy was 37.0 ± 13.2 years (range 17–77 years).
Glomerular filtration rate (GFR) was estimated from yearly serum creatinine measurements using the Modification of Diet in Renal Disease (MDRD) equation [15]. To investigate the correlation between genotype and disease severity, we used the following criteria: for benign disease, loss of GFR of <2 ml/min/year, for moderate progression loss of GFR of ≥2 to <5 ml/min/year or the progression to chronic kidney disease (CKD) stage 3 (GFR = 30–59 ml/min/1.73 m2), and for severe progression, loss of GFR of ≥5 ml/min/year or reaching CDK stage 4 or 5 (GFR = 15–29 ml/min/1.73 m2 and GFR<15 ml/min/1.73 m2).
Selection of markers
The TGFB1 gene in the HapMap database represents a sequence at chromosome 19q13.1 between two recombination blocks. We succeeded with five reproducible assays: in the promoter region at position −509, rs1800469 (C-509T), in the downstream 3′ genomic region, rs6957, in the intron, rs2241715, and two in the signal sequence of exon 1, rs1800471 (C915G or codon 25, arginine→proline) and rs1982073 (T869C or codon 10, leucine→proline). More detailed information of all selected SNPs with the minor allele frequencies is presented in Table 2.
Table 2.
Polymorphisms of the TGFB1 gene in IgA nephropathy patients
| Chromosome | Heterozygosity | |||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Position | Alternative name | position | Alleles | from NCBI | MAFa | ABI assay | Methods |
| rs6957 | Downstream 3′ genomic region | 46522446 | C/T | 0.414 | 0.167 | C___7818385_10 | TaqMan | |
| rs2241715 | Intron 1 | 46548726 | G/T | 0.467 | 0.289 | Assay by design | TaqMan | |
| rs1800471 | Signal sequence of exone 1 | Codon 25 or G915C (arginine→proline) | 46550716 | C/G | 0.112 | 0.081 | NA | REMb |
| rs1982073 | Signal sequence of exone 1 | Codon 10 or T869C (leucine→proline) | 46550761 | C/T | 0.397 | 0.378 | C___22272997_10 | TaqMan |
| rs1800469 | Promoter | C-509T | 46552136 | A/G | 0.485 | 0.295 | C___8708473_10 | TaqMan |
aMinor allele frequency from current study.
bRestriction Endonuclease Mapping method.
DNA and genotyping
DNA was extracted from EDTA blood samples (5–10 ml) by the ‘salting out’ method as described elsewhere [16]. To identify codon 25 allele polymorphism (rs1800471) in the TGFB1 gene, the restriction endonuclease mapping method (REM) was used as previously described [16]. To detect other SNPs (rs6957, rs2241715, rs1982073 and rs1800469) of the TGFB1 gene, the TaqMan allelic discrimination assay (Applied Biosystems, Foster City, CA, USA) was used. Three out of four assays were commercially available and one was designed for this project (Table 2). TaqMan allelic discrimination was performed according to the standard protocol in a 384-well plate with 10 ng of DNA per sample. PCR was run in GeneAmp PCR System 9700 (Applied Biosystems), and the fluorescent signals from the hybridization probes were detected by a 7900 Sequence Detector (Applied Biosystems).
To assess genotyping robustness, we re-genotyped a random subset of 191 cases and controls for rs1982073 SNP using the restriction endonuclease mapping method published previously [17], which resulted in a 99.5% match of genotyping calls with the TaqMan assay.
All analysed markers were in Hardy–Weinberg equilibrium. Positive rates of genotype detection were 99.9% for rs2241715, 99.3% for rs6957 and 99.6% for the rest of the SNPs.
Statistical analysis
To assess genotype, allele and haplotype frequencies, Pearson's chi-square and/or Fisher's exact tests were performed when appropriate with SPSS 13.0 software. Haplotype analysis was carried out by HaploView [18], and the permutation test of this analysis was set to 100 000 permutations for single markers and haplotypes. For meta-analysis, the Mantel–Haenszel method was employed with a fixed effect and 95% confidence interval (95% CI) for odds ratio. Power calculation was performed for the two-tailed or one-tailed test when appropriate for 5% threshold of significance.
Results
Genotype frequencies of TGFB1 polymorphisms in male and female IgA nephropathy patients and healthy controls
We genotyped 212 unrelated IgA nephropathy patients, 146 men (68.9%) and 66 women (31.1%), and 477 healthy subjects, 321 men (67.3%) and 156 women (32.7%), for five various polymorphisms of the TGFB1 gene. The minor allelic frequencies (MAF) are presented in Table 2. A significant difference between the genotype distribution in male IgA nephropathy patients and male healthy controls was found in a co-dominant model for all five SNPs (Table 3a). After the Bonferroni correction for multiple comparisons, four out of five SNPs remained significantly associated with P ≤ 0.05. Genotype frequencies for studied SNPs in the female study group did not, however, show a significant difference (Table 3b).
Table 3.
Genotype frequencies of TGFB1 polymorphisms in (a) male and (b) female IgA nephropathy patients in a co-dominant model
| SNPs | Genotype frequency in co-dominant model | Chi-square | P-valuea | ||||
|---|---|---|---|---|---|---|---|
| (a) Male IgA nephropathy patients | |||||||
| TGFB1 rs6957 | Totalb | CC | CT | TT | |||
| Group | Control | 314 (100%) | 3 (1%) | 85 (27%) | 226 (72%) | 11.5 | 0.003 |
| Patient | 144 (100%) | 9 (6.3%) | 42 (29.1%) | 93 (64.6%) | |||
| TGFB1 rs2241715 | Total | GG | GT | TT | |||
| Group | Control | 315 (100%) | 171 (54.3%) | 120 (38.1%) | 24 (7.6%) | 7.4 | 0.02 |
| Patient | 145 (100%) | 59 (40.7%) | 73 (50.3%) | 13 (9%) | |||
| TGFB1 rs1800471 | Total | CC | CG | GG | |||
| Group | Control | 319 (100%) | 0 (0%) | 48 (15%) | 271 (85%) | 9.1 | 0.01 |
| Patient | 143 (100%) | 4 (2.8%) | 19 (13.3%) | 120 (83.9%) | |||
| TGFB1 rs1982073 | Total | CC | CT | TT | |||
| Group | Control | 314 (100%) | 132 (42%) | 147 (46.8%) | 35 (11.2%) | 9.9 | 0.007 |
| Patient | 144 (100%) | 40 (27.8%) | 78 (54.2%) | 26 (18%) | |||
| TGFB1 rs1800469 | Total | AA | AG | GG | |||
| Group | Control | 314 (100%) | 24 (7.6%) | 120 (38.2%) | 170 (54.2%) | 10.2 | 0.006 |
| Patient | 144 (100%) | 13 (9%) | 76 (52.8%) | 55 (38.2%) | |||
| (b) Female IgA nephropathy patients | |||||||
| TGFB1 rs6957 | Totalb | CC | CT | TT | |||
| Group | Control | 152 (100%) | 4 (2.6%) | 44 (28.9%) | 104 (68.5%) | 0.9 | 0.6 |
| Patient | 65 (100%) | 3 (4.6%) | 16 (24.6%) | 46 (70.8%) | |||
| TGFB1 rs2241715 | Total | GG | GT | TT | |||
| Group | Control | 153 (100%) | 75 (49%) | 64 (41.8%) | 14 (9.2%) | 1.4 | 0.5 |
| Patient | 66 (100%) | 35 (53%) | 28 (42.4%) | 3 (4.6%) | |||
| TGFB1 rs1800471 | Total | CC | CG | GG | |||
| Group | Control | 156 (100%) | 0 (0%) | 23 (14.7%) | 133 (85.3%) | 2.4 | 0.3 |
| Patient | 66 (100%) | 1 (1.5%) | 10 (15.2%) | 55 (83.3%) | |||
| TGFB1 rs1982073 | Total | CC | CT | TT | |||
| Group | Control | 153 (100%) | 56 (36.6%) | 78 (51%) | 19 (12.4%) | 0.3 | 0.8 |
| Patient | 66 (100%) | 26 (39.4%) | 31 (47%) | 9 (13.6%) | |||
| TGFB1 rs1800469 | Total | AA | AG | GG | |||
| Group | Control | 154 (100%) | 14 (9.1%) | 65 (42.2%) | 75 (48.7%) | 0.5 | 0.8 |
| Patient | 65 (100%) | 4 (6.2%) | 28 (43%) | 33 (50.8%) | |||
aUncorrected.
bTotal number for different variations may be different due to genotyping failure.
The allele and haplotype frequencies with experimental chi-square P-values and empiric P-values after permutation test are presented in Tables 4 and 5. When comparing the frequency of alleles, we found significant differences for rs2241715, rs1982073 and rs1800469 comparing male patients and male controls (P < 0.02, Table 4a). To check for possible false positive associations, we performed a permutation test with 100 000 permutations simultaneously for single markers and haplotypes, which resulted in empiric P = 0.04 and P = 0.02 for rs1800469 and rs1982073, respectively. A relatively high degree of linkage disequilibrium (LD) was detected in this locus, which resulted in 5 observed out of 32 expected haplotypes, which made it relatively easy to reveal genetic variability with the selected number of SNPs. Two out of five marker haplotypes, TGGCG and CTGTA, showed a significant difference between male patients and male controls (P = 0.0012 and P = 0.0018, respectively, Table 5a), and these two haplotypes passed the permutation test with empiric P = 0.006 and 0.008, respectively. These haplotypes represent either protective (TGGCG) or susceptible (CTGTA) variants and are opposite in four out of five alleles. The protective TGGCG haplotype comprises 56.4% of all chromosomes in the control male population and the CTGTA represents only 5.7%. The former number corresponds to 90% and the latter to 86% power of analysis to detect significant difference at 0.05 threshold in the two-tailed test. We found no indication for an association of TGFB1 gene polymorphism with disease in females in the allelic model nor in the haplotype analysis for this study group (Tables 4b and 5b).
Table 4.
Allelic frequencies of TGFB1 polymorphisms in (a) male and (b) female IgA nephropathy patients and healthy controls
| Control, case | Control, case | 100 000 permutation, | |||
|---|---|---|---|---|---|
| SNPs | ratio counts | frequencies | Chi-square | P-valuea | P-value |
| (a) Male IgA nephropathy patients | |||||
| rs6957 | 537:91, 228:60 | 0.855, 0.792 | 5.77 | 0.016 | 0.08 |
| rs2241715 | 462:168, 191:99 | 0.733, 0.659 | 5.381 | 0.020 | 0.09 |
| rs1800471 | 581:47, 259:27 | 0.925, 0.906 | 1.011 | 0.314 | NS |
| rs1982073 | 411:217, 158:130 | 0.654, 0.549 | 9.401 | 0.002 | 0.02 |
| rs1800469 | 460:168, 186:102 | 0.732, 0.646 | 7.132 | 0.007 | 0.04 |
| (b) Female IgA nephropathy patients | |||||
| rs6957 | 52:252, 22:108 | 0.171, 0.169 | 0.002 | 0.9631 | NS |
| rs2241715 | 92:214, 34:98 | 0.301, 0.258 | 0.835 | 0.3608 | NS |
| rs1800471 | 285:23, 120:12 | 0.925, 0.909 | 0.333 | 0.5641 | NS |
| rs1982073 | 116:190, 49:83 | 0.379, 0.371 | 0.024 | 0.876 | NS |
| rs1800469 | 93:215, 36:94 | 0.302, 0.277 | 0.276 | 0.5996 | NS |
aUncorrected.
Table 5.
Haplotype frequencies of TGFB1 polymorphisms in (a) male and (b) female IgA nephropathy patients and healthy controls
| Haplotype | Control/case | 100 000 permutation | ||||
|---|---|---|---|---|---|---|
| Block | frequencies | Control/case ratio counts | frequencies | Chi-square | P-valuea | P-value |
| (a) Male IgA nephropathy patients | ||||||
| TGGCG | 0.528 | 355.5: 274.5, 130.5: 159.5 | 0.564, 0.450 | 10.42 | 0.0012 | 0.006 |
| TTGTA | 0.214 | 131.1: 498.9, 65.5: 224.5 | 0.208, 0.226 | 0.374 | 0.5411 | NS |
| CGGCG | 0.089 | 55.5: 574.5, 26.5: 263.5 | 0.088, 0.091 | 0.026 | 0.8709 | NS |
| TGCTG | 0.080 | 47.0: 583.0, 27.0: 263.0 | 0.075, 0.093 | 0.919 | 0.3378 | NS |
| CTGTA | 0.075 | 35.9: 594.1, 33.5: 256.5 | 0.057, 0.116 | 9.749 | 0.0018 | 0.008 |
| (b) Female IgA nephropathy patients | ||||||
| TGGCG | 0.526 | 161.8: 146.2, 69.6: 62.4 | 0.525, 0.527 | 0.001 | 0.9709 | NS |
| TTGTA | 0.220 | 69.4: 238.6, 27.2: 104.8 | 0.225, 0.206 | 0.194 | 0.6599 | NS |
| CGGCG | 0.092 | 27.2: 280.8, 13.4: 118.6 | 0.088, 0.101 | 0.194 | 0.6595 | NS |
| TGCTG | 0.070 | 19.8: 288.2, 10.9: 121.1 | 0.064, 0.082 | 0.455 | 0.4998 | NS |
| CTGTA | 0.064 | 21.6: 286.4, 6.8: 125.2 | 0.070, 0.051 | 0.545 | 0.4602 | NS |
aUncorrected.
Correlations between genotypes and progression of IgA nephropathy
Using the disease severity criteria defined in the ‘Materials and methods’ section in 95 patients with available clinical data, 52 (54.7%) patients were classified as having benign disease, 19 (20%) patients had moderate renal disease progression and 24 (25.3%) patients had severe renal disease progression. No significant differences in genotype frequencies in either the co-dominant model or the dominant/recessive model were observed among the different severity groups with or without stratification for gender (data not shown).
Meta-analysis
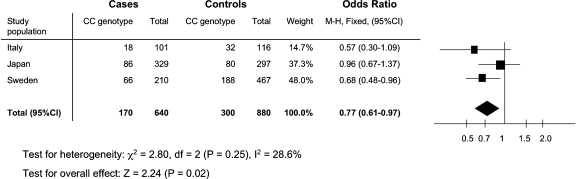
By searching information from PubMed and ISI Web of Knowledge, we found four published articles related to TGFB1 gene polymorphisms in IgA nephropathy in four different populations: Korean, Japanese, Italian and German [9,10,13,14]. No additional data were found in abstracts or proceedings. All papers had used similar selection criteria for IgA nephropathy patients compared to our study; however, selection of control groups differed from our study and stratification for gender was not done. Only in the study by Carturan et al., the control group was age- and gender-matched. In the study of Bantis et al., controls were matched by age, but not by gender. In the studies by Sato et al. and Lim et al., the control groups were discordant for age and for gender ratio. All studies except that by Carturan et al. reported that individuals in the control groups were of the same ethnicity as the patient groups. At least two TGFB1 polymorphisms (rs1982073 and rs1800469) were investigated in three studies [9,10,13]. Two studies [10,13] were included in meta-analysis together with our data for these TGFB1 markers. In the remaining study [9], the frequency of the genotype distribution for the rs1982073 significantly deviated from Hardy–Weinberg equilibrium, which questions the validity of the data. For this reason, we decided to exclude this particular study from the meta-analysis. As we could only use the frequency of variations for the combined group of both genders in the previously published studies, our own data were also not stratified by gender for the meta-analysis. The Mantel–Haenszel method with fixed effect demonstrated a significant cumulative odds ratio for rs1982073 (0.77, 95% CI 0.61–0.97) (Figure 1), suggesting that the TGFB1 gene polymorphism is associated with IgA nephropathy in the studied populations. In addition, rs1800469 showed a trend for association with disease susceptibility, which did not reach statistical significance in the meta-analysis (0.86, 95% CI 0.69–1.07). Both genetic markers displayed a protective effect for the common genotype (CC and GG respectively) and passed the test for heterogeneity between the studies.
Fig. 1.
Meta-analysis with rs1800469 in two different populations and our own data; the genetic marker displayed a protective effect for the common genotype with CC and passed test for heterogeneity between the studies.
Discussion
In the present study, we demonstrate an association of the TGFB1 gene polymorphism with IgA nephropathy. Our own data, together with the meta-analysis of previously published data, provide a strong indication of the importance of TGFβ1 in the development of IgA nephropathy.
Previously, both linkage and association studies have been utilized to study the susceptibility risk factors for IgA nephropathy. Using genome-wide linkage microsatellite analysis, a linkage of IgA nephropathy to 2q36, 6q22–23, 4q26–31 and 17q12–22 has been suggested [3,19–22]. However, no obvious candidate genes within these linkage regions have so far been identified. Several candidate gene polymorphism association studies have previously been performed to explore the role of several genes that could be related to the susceptibility to and the progression of IgA nephropathy, including MHC class II [23], T-cell receptor α and β [24–26], uteroglobin [27,28], angiotensin-converting enzyme (ACE) [29,30], other renin–angiotensin system genes [31,32], as well as several cytokine genes [33]. However, some of these studies have shown contradictory results, and larger patient populations with an optimal study design are required to establish the role of specific genes in the susceptibility to IgA nephropathy. Although most studies used ethnically homogeneous study populations, the gender effect was not assessed in any of those investigations.
In our analysis of five TGFB1 gene polymorphisms in a cohort of 212 patients with IgA nephropathy and 477 age-, gender- and ethnicity-matched controls, we were able to demonstrate, with reasonable statistical power, an association between genetic variations in the TGFB1 gene and the susceptibility to IgA nephropathy in males. A meta-analysis of previously published data from two independent studies together with our results strengthens this conclusion.
There are four previous studies addressing the importance of the TGFB1 gene in IgA nephropathy in four different populations [9,10,13,14]. In the study performed in 329 Japanese patients with IgA nephropathy, two TGFB1 gene polymorphisms (rs1982073 and rs1800469) were shown to associate with heavy proteinuria and mesangial glomerular proliferation. This is at the moment the largest published genetic study of IgA nephropathy. However, no association with susceptibility to IgA nephropathy was demonstrated [10]. In the study from South Korea, analysing two SNPs, C-509T and T869C (rs1982073 and rs1800469), a significant difference in the genotype frequency was found comparing 108 patients with IgA nephropathy and healthy controls [9]. Since the reported frequencies of the rs1800469 genotypes significantly deviated from Hardy–Weinberg equilibrium, these data have to be taken with some caution. Two other studies were done in Europe in Caucasians with IgA nephropathy. In the study performed in Germany, one TGFB1 SNP, rs1800471 (C915G or codon 25, arginine→proline), showed the similar genotype distribution between IgA nephropathy patients and controls as well as between the progression and non-progression groups [14]. Finally, in an Italian study, three TGFB1 SNPs, Leu10→Pro (rs1982073), C-509T (rs1800469) and G-800A (rs1800468), were investigated in 101 patients with IgA nephropathy and 118 healthy controls [13]. A significant association was observed in the haplotype analysis, but genotype frequencies did not reach significant statistical difference.
Three previous studies have demonstrated either a tendency for an association or a significant association with at least some genetic markers in the TGFB1 gene. We performed a meta-analysis in order to further test the role of TGFB1 in the susceptibility to IgA nephropathy. In the meta-analysis, we combined our new findings and data with those obtained in two of the previously published studies [10,13] and included two genetic markers (rs1982073 and rs1800469) that were investigated in all of the studies. Due to the fact that data in previous studies could not be stratified by gender, we combined the female and male groups in our study into one group for this specific test. This meta-analysis supported the role of the TGFB1 gene in the development of IgA nephropathy in a combined group of 640 IgA nephropathy patients and 880 controls with a post hoc power estimate of 96% for the one-tailed test. However, this must be taken with caution due to a possible bias towards publishing of positive associations. It is also important to perform further extension of the study to get better statistical power to detect an association. Another way of addressing a lack of power could be replication of the association in independent cohorts.
However, it is possibly premature to claim functional consequences from any of the analysed variations, due to a strong pattern of LD in this region. In fact, three markers from our study (rs1800469, rs1982073 and rs1800471) belong to the recombination block with high LD (D′≈1.0), which means that any variation in this region may be in association with a certain phenotype, because of its close position to the mutual causal allele. This is a strong indication of either a true importance of the combination of common alleles, which were selected for our study, or for an unknown causal variation, which is in high LD with the analysed single-nucleotide polymorphisms (SNPs). In both cases, it is evident that genetic variations in the TGFB1 locus could be important contributors to disease development. It is also noteworthy to mention that the genetic landscape of variations in this locus seems to be very different in Caucasian and Chinese or Japanese populations and allelic frequencies are significantly different [34,35]. This must be taken into account in future studies of different patient populations. We excluded non-Caucasians from the current study to avoid extensive population admixture, but a proper genomic control in our study was not possible due to the limited number of included SNPs. Another potential difficulty is the failure in genotyping of some variations in this locus: out of nine tested SNP assays only four demonstrated a robust performance and were selected for our genetic study, which may illustrate either a high homology of sequences flanking these variations with other genetic regions or a high frequency of copy number polymorphisms in this locus.
In our study, no significant differences in genotype frequencies between the non-progression and the progression groups of IgA nephropathy patients with impaired renal function could be demonstrated.
In summary, by using our own experimental data and a meta-analysis of previously published data, we propose that the TGFB1 gene is an important contributor for the susceptibility to IgA nephropathy. Additional replication for such association and further studies are warranted to investigate disease mechanisms and the physiological role of TGFB1 in the development of kidney diseases. Moreover, we suggest considering the effect of gender in such studies.
Acknowledgments
We would like to thank Eva Jemseby, Per-Anton Westerberg, Susanne Schepull, Micael Gylling and Per Eriksson for their helpful assistance. Dr Jarl Ahlmén's involvement at the initial stage of this study is highly appreciated. We are grateful for the support by the Sida/SAREC, Roche, and The Fund for Renal Research (Heart and Lung Foundation), Sweden.
Conflict of interest statement. None declared.
(See related article by M. Barua and Y. Pei. Identifying susceptibility genes of IgA nephropathy: research in progress. Nephrol Dial Transplant 2009; 24: 2957–2959.)
References
- 1.Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med. 1990;89:209–215. doi: 10.1016/0002-9343(90)90300-3. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 3.Julian BA, Quiggins PA, Thompson JS, et al. Familial IgA nephropathy. Evidence of an inherited mechanism of disease. N Engl J Med. 1985;312:202–208. doi: 10.1056/NEJM198501243120403. [DOI] [PubMed] [Google Scholar]
- 4.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–748. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 5.Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001;31:1706–1715. doi: 10.1002/1521-4141(200106)31:6<1706::aid-immu1706>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Border WA. Transforming growth factor-beta and the pathogenesis of glomerular diseases. Curr Opin Nephrol Hypertens. 1994;3:54–58. doi: 10.1097/00041552-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Coll E, Cormand B, Campos B, et al. Association of TGF-beta1 polymorphisms with chronic renal disease. J Nephrol. 2004;17:794–799. [PubMed] [Google Scholar]
- 9.Lim CS, Kim YS, Chae DW, et al. Association of C-509T and T869C polymorphisms of transforming growth factor-beta1 gene with susceptibility to and progression of IgA nephropathy. Clin Nephrol. 2005;63:61–67. doi: 10.5414/cnp63061. [DOI] [PubMed] [Google Scholar]
- 10.Sato F, Narita I, Goto S, et al. Transforming growth factor-beta1 gene polymorphism modifies the histological and clinical manifestations in Japanese patients with IgA nephropathy. Tissue Antigens. 2004;64:35–42. doi: 10.1111/j.1399-0039.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 11.Xaubet A, Marin-Arguedas A, Lario S, et al. Transforming growth factor-beta1 gene polymorphisms are associated with disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:431–435. doi: 10.1164/rccm.200210-1165OC. [DOI] [PubMed] [Google Scholar]
- 12.Awad MR, El-Gamel A, Hasleton P, et al. Genotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation. 1998;66:1014–1020. doi: 10.1097/00007890-199810270-00009. [DOI] [PubMed] [Google Scholar]
- 13.Carturan S, Roccatello D, Menegatti E, et al. Association between transforming growth factor beta1 gene polymorphisms and IgA nephropathy. J Nephrol. 2004;17:786–793. [PubMed] [Google Scholar]
- 14.Bantis C, Heering P, Aker S, et al. Influence of cytokine gene polymorphisms on IgA nephropathy. Ren Fail. 2008;30:135–140. doi: 10.1080/08860220701805182. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, et al. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Padyukov L, Hahn-Zoric M, Blomqvist SR, et al. Distribution of human kappa locus IGKV2-29 and IGKV2D-29 alleles in Swedish Caucasians and Hong Kong Chinese. Immunogenetics. 2001;53:22–30. doi: 10.1007/s002510000291. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Morinobu A, Kanagawa S, et al. Transforming growth factor beta 1 gene polymorphism in Japanese patients with systemic lupus erythematosus. Kobe J Med Sci. 2007;53:15–23. [PubMed] [Google Scholar]
- 18.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 19.Bisceglia L, Cerullo G, Forabosco P, et al. Genetic heterogeneity in Italian families with IgA nephropathy: suggestive linkage for two novel IgA nephropathy loci. Am J Hum Genet. 2006;79:1130–1134. doi: 10.1086/510135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharavi AG, Yan Y, Scolari F, et al. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22–23. Nat Genet. 2000;26:354–357. doi: 10.1038/81677. [DOI] [PubMed] [Google Scholar]
- 21.Paterson AD, Liu XQ, Wang K, et al. Genome-wide linkage scan of a large family with IgA nephropathy localizes a novel susceptibility locus to chromosome 2q36. J Am Soc Nephrol. 2007;18:2408–2415. doi: 10.1681/ASN.2007020241. [DOI] [PubMed] [Google Scholar]
- 22.Julian BA, Wyatt RJ, Matousovic K, et al. IgA nephropathy: a clinical overview. Contrib Nephrol. 2007;157:19–26. doi: 10.1159/000102284. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama F, Tanaka T, Yamada R, et al. Single-nucleotide polymorphisms in the class II region of the major histocompatibility complex in Japanese patients with immunoglobulin a nephropathy. J Hum Genet. 2002;47:532–538. doi: 10.1007/s100380200080. [DOI] [PubMed] [Google Scholar]
- 24.Batra A, Smith AC, Feehally J, et al. T-cell homing receptor expression in IgA nephropathy. Nephrol Dial Transplant. 2007;22:2540–2548. doi: 10.1093/ndt/gfm228. [DOI] [PubMed] [Google Scholar]
- 25.Deenitchina SS, Shinozaki M, Hirano T, et al. Association of a T-cell receptor constant alpha chain gene polymorphism with progression of IgA nephropathy in Japanese patients. Am J Kidney Dis. 1999;34:279–288. doi: 10.1016/s0272-6386(99)70356-2. [DOI] [PubMed] [Google Scholar]
- 26.Li PK, Poon P, Phil M, et al. Association of IgA nephropathy with T-cell receptor constant alpha chain gene polymorphism. Am J Kidney Dis. 1997;30:260–264. doi: 10.1016/s0272-6386(97)90061-5. [DOI] [PubMed] [Google Scholar]
- 27.Matsunaga A, Numakura C, Kawakami T, et al. Association of the uteroglobin gene polymorphism with IgA nephropathy. Am J Kidney Dis. 2002;39:36–41. doi: 10.1053/ajkd.2002.29875. [DOI] [PubMed] [Google Scholar]
- 28.Shijubo N, Kawabata I, Sato N, et al. Clinical aspects of Clara cell 10-kDa protein/uteroglobin (secretoglobin 1A1) Curr Pharm Des. 2003;9:1139–1149. doi: 10.2174/1381612033455026. [DOI] [PubMed] [Google Scholar]
- 29.Coppo R, Amore A, Peruzzi L, et al. Angiotensin antagonists and fish oil for treating IgA nephropathy. Contrib Nephrol. 2007;157:27–36. doi: 10.1159/000102285. [DOI] [PubMed] [Google Scholar]
- 30.Locatelli F, Vecchio LD, Pozzi C. IgA glomerulonephritis: beyond angiotensin-converting enzyme inhibitors. Nat Clin Pract Nephrol. 2006;2:24–31. doi: 10.1038/ncpneph0055. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama K, Yoshida M, Nishio H, et al. Polymorphisms of renin-angiotensin system genes in childhood IgA nephropathy. Pediatr Nephrol. 2001;16:350–355. doi: 10.1007/s004670000555. [DOI] [PubMed] [Google Scholar]
- 32.Ong-Ajyooth S, Ong-Ajyooth L, Limmongkon A, et al. The renin–angiotensin system gene polymorphisms and clinicopathological correlations in IgA nephropathy. J Med Assoc Thai. 1999;82:681–689. [PubMed] [Google Scholar]
- 33.Wada J, Sugiyama H, Makino H. Pathogenesis of IgA nephropathy. Semin Nephrol. 2003;23:556–563. doi: 10.1053/s0270-9295(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe Y, Kinoshita A, Yamada T, et al. A catalog of 106 single-nucleotide polymorphisms (SNPs) and 11 other types of variations in genes for transforming growth factor-beta1 (TGF-beta1) and its signaling pathway. J Hum Genet. 2002;47:478–483. doi: 10.1007/s100380200069. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka T, Yamakage A, Yamazaki S. The polymorphism of transforming growth factor-beta1 gene in Japanese patients with systemic sclerosis. Br J Dermatol. 2002;147:458–463. doi: 10.1046/j.1365-2133.2002.04947.x. [DOI] [PubMed] [Google Scholar]