Abstract
In pregnant women infected with Plasmodium falciparum, the infected red blood cells (IRBCs) sequester in placenta by binding to the chondroitin 4-sulfate (C4S) chains of low sulfated chondroitin sulfate proteoglycan (CSPG). Placental CSPG, the natural receptor for IRBC adherence in the placenta, is the ideal molecule for studying structural interactions in IRBC adhesion to C4S, adhesion inhibitory antibody responses, and identification of parasite adhesive protein(s). However, because of difficulty involved in purifying placental CSPG, the commercially available bovine tracheal chondroitin sulfate A (bCSA), a copolymer having structural features of both C4S and C6S, has been widely used. To determine the validity of bCSA for C4S-IRBC interaction studies, we comparatively evaluated the characteristics of IRBC binding to placental CSPG and bCSA using three commonly used parasite strains. The results indicate that, in all three parasites studied, the characteristics of IRBC binding to placental CSPG and bCSA are qualitatively similar, but the binding capacity with respect to both the number IRBCs bound per unit area of coated surface and binding strength is significantly higher for CSPG than bCSA regardless of whether parasites were selected on CSPG or bCSA. These results demonstrate that placental CSPG is best suited for studying interactions between parasite adhesive protein(s) and C4S, and have implications in understanding IRBC-C4S structural interactions.
Keywords: Plasmodium falciparum, malaria parasite, pregnant women, infected erythrocytes, placental adherence, chondroitin 4-sulfate, placental chondroitin sulfate proteoglycan, bovine tracheal chondroitin sulfate A, binding characteristics
1. Introduction
In endemic areas where people are constantly infected with Plasmodium falciparum, immunity to clinical malaria is acquired during childhood years and therefore people, irrespective of gender, are resistant to malaria pathogenesis by their adolescent years (Trape et al., 1994). However, while men are protected by acquired immunity throughout their lives, women become susceptible to malaria during pregnancy (Brabin 1983; Brabin and Rogerson, 2001; Steketee et al., 2001; Gamain et al., 2007; Hviid and Salanti, 2007). This is because, in pregnant women, P. falciparum infected erythrocytes sequester in the placenta by binding to chondroitin 4-sulfate (C4S) (Fried and Duffy, 1996; Achur et al., 2000; Fried et al., 2006), resulting in the selection of an antigenically distinct variant form of parasite to which the individuals have no immunity. Multiplication of the selected parasites leads to accumulation of IRBCs at high density in the placenta, initiating infiltration of inflammatory cells and inflammatory responses (Ismail et al., 2000; Miller et al., 2002; Rogerson et al., 2007). These processes affect placental physiology, resulting in poor pregnancy outcomes, including low birth weight, abortion, and anemia and death in the mother and fetuses (McGregor et al., 1983; Duffy and Fried, 2003). Since the first discovery by Fried and Duffy (Fried et al., 1996), a number of studies have shown that C4S mediates adherence of IRBCs in the placenta (Rogerson and Brown 1997; Gysin et al., 1999; Achur et al., 2000; Maubert et al., 2000).
Previous studies from our laboratory have shown that a very low sulfated chondroitin sulfate proteoglycans (CSPGs) present predominantly in the intervillous space and at lower levels on the syncytiotrophoblast surface is the receptor of IRBC adherence (Achur et al., 2000; Muthusamy et al., 2004a). The CSPG purified from human placental intervillous space has been used for studying C4S-IRBC interactions (Achur et al., 2000). Alternatively, the commercially available bovine tracheal cartilage chondroitin sulfate A (bCSA) has been used in many studies for studying C4S-IRBC adhesion interactions and adhesion inhibitory immune responses (Chai et al., 2000; Lekana et al., 2002; Muthusamy et al., 2004b; Salanti et al., 2004; Oleinikov et al., 2008). bCSA is a copolymer consisting of 4- and 6-sulfated chondroitin disaccharide moieties (Alkhalil et al., 2000; Muthusamy et al., 2004b). Since bCSA isolation procedure involves extensive proteolysis of cartilage tissue, the core protein of bovine cartilage proteoglycan has been lost and bCSA thus prepared contains short peptides consisting of only a few amino acids residues to which the CSA chains are attached. Moreover, bCSA, in addition to 4-sulfate, contains 39% of 6-sulfate, which interferes with IRBC binding (Alkhalil et al., 2000; Fried et al., 2000; Muthusamy et al., 2004b).
In the present study, to determine the validity of bCSA as an alternative ligand for studying C4S-IRBC adhesion, we evaluated in parallel the characteristics of IRBC binding to the CSPG purified from human placenta and the commercial bCSA using adherent IRBCs selected from three commonly used parasite strains. The data demonstrate that the binding capacity of IRBCs to bCSA is significantly lower than that of placental CSPG. These results should be considered in interpreting data obtained from C4S-IRBC adhesion studies using bCSA.
2. Materials and methods
2.1. Placental CSPG and bovine tracheal CSA
The CSPG was isolated from the isotonic buffer extracts of freshly delivered placentas, collected from the Hershey Medical Center Maternity Ward. The placental extracts were applied on to DEAE-Sepharose columns and the bound CSPG was eluted using NaCl gradient as described previously (Achur et al., 2000). The CSPG was then purified by successive CsBr density gradient centrifugation and gel filtration on Sepharose CL-6B (Achur et al., 2000). The final purified CSPG preparation was dialyzed, lyophilized and weighed; the weight of CSPG correlates with the amount calculated based on glucuronic acid, N-acetylgalactosamine and protein compositions (Achur et al., 2000). The CS chains of the purified CSPG used in this study consist of ~8% 4-sulfated and ~92% non-sulfated disaccharides.
bCSA (catalog number C9819) was purchased from Sigma Chemical Co. As reported previously (Alkhalil et al., 2000; Muthusamy et al., 2004c), bCSA consists of 9% nonsulfated, 52% 4-sulfated and 39% 6-sulfated disaccharide moieties.
A partially sulfated C4S containing 36% 4-sulfated and 64% of nonsulfated disaccharide moieties was obtained by regioselective 6-O-desulfation of bCSA followed by fractionation on DEAE-Sephacel column as reported previously (Alkhalil et al., 2000; Muthusamy et al., 2004c).
2.2. Parasites and culturing
The cultured P. falciparum strains used in this study were FCR3, 3D7 and CS2. FCR3 is a Gambian isolate adopted for laboratory culturing (Jensen and Trager, 1978). 3D7 is a subpopulation of NF54 strain and was isolated in the laboratory of Dr. Christian F. Ockenhouse at the Walter Reed Army Institute of Research, Silver Spring. MD (Ockenhouse et al., 1991). This 3D7 subpopulation is different from the 3D7 clone used in malaria genome sequencing studies. The C4S-adherent 3D7 parasite used in this study exhibits a stable binding property similar to that of FCR3 (data not shown). The CS2 parasite was received from MR4 and is a CSA-binding parasite isolated from the E8B clone, which was derived from the Brazilian isolate ItG2 (Biggs et al., 1992; Rogerson et al., 1995). The C4S-adherent parasites were selected from the above parasites using placental CSPG-coated plates and were designated as FCR3-CSPG, 3D7-CSPG and CS2-CSPG. Parasites were also selected on bCSA-coated plates and named FCR3-bCSA and 3D7-bCSA. All parasites were freshly selected for maximal binding just prior to performing the assays. The parasites were cultured in RPMI 1640 medium supplemented with 25 mM HEPES, 29 mM sodium bicarbonate, 0.005% hypoxanthine, p-aminobenzoic acid (2 mg/liter), gentamicin sulfate (50 mg/liter), and 10% human plasma with O-positive human RBCs at 3% hematocrit. The cultures were incubated at 37°C in an atmosphere of 90% N2, 5% O2, and 5% CO2. The parasites were synchronized with 5% sorbitol as described previously (Lambros and Vanderberg 1979). IRBCs at the early trophozoite stage were used for the assays (Madhunapantula et al., 2007).
2.3. IRBC binding and inhibition assays
The solutions (10 to 15 μl) of the purified CSPGs or bovine tracheal C4S in PBS pH 7.2 were spotted on 150 × 15 mm plastic petri dishes and allowed to stand at 4 °C overnight. The nonspecific binding sites were blocked with 2% BSA at room temperature for 2 h and then overlaid with 2% suspensions of IRBCs (parasitemia was adjusted to 15–20% for each parasite) in RPMI 1640 medium or PBS, pH 7.2. Uninfected RBCs overlaid on CSPG-coated plates were used as negative controls. After incubation for 40 min, the Petri dishes were washed three times with PBS, pH 7.2. The bound IRBCs were fixed with 2% glutaraldehyde, stained with 1% Giemsa stain, and counted under a light microscope.
For adhesion-inhibition assays, 4% suspensions of IRBCs were preincubated with equal volume of C4S containing 36% 4-sulfate in PBS, pH 7.2, at room temperature for 30 min with intermittent mixing. The IRBC suspensions were layered on CSPG or bCSA coated spots on Petri dishes, and the assay was carried out as described.
2.4. Statistical analysis
The data were analyzed using GraphPad Prism version 3.0. Variations between two groups were determined by Student ‘t’ test. Two-way ANOVA was used to analyze differences among groups. P values of ≤0.05 were considered significant.
3. Results
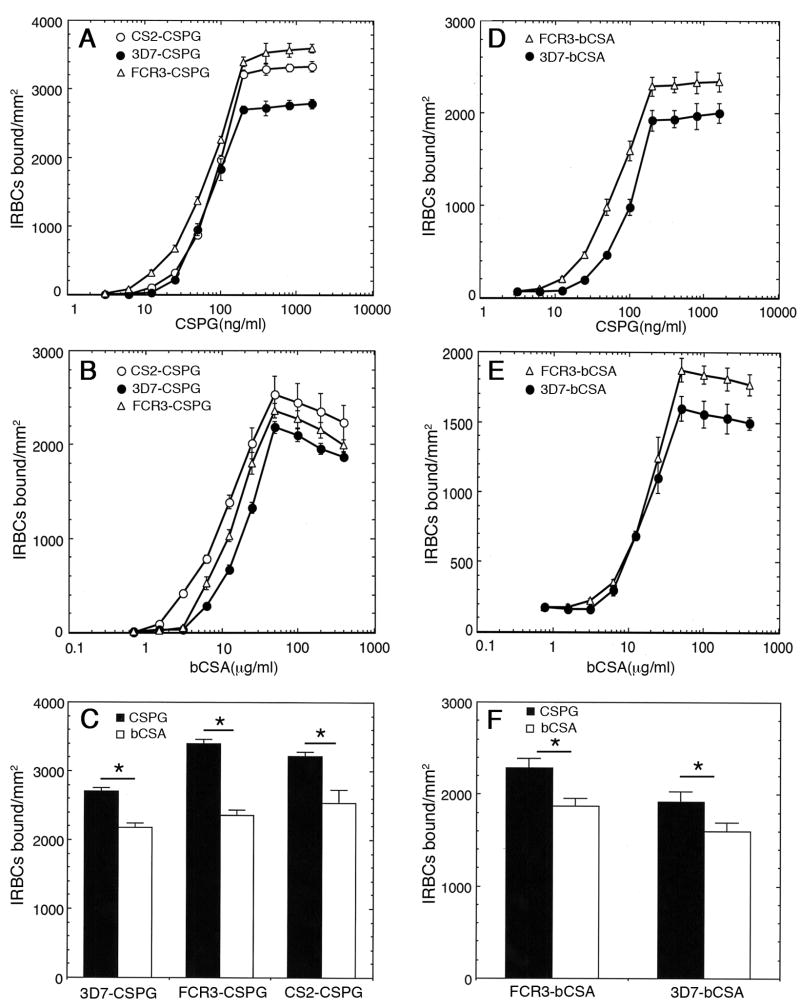
To determine the concentration required for effective coating of bCSA onto plastic plates for maximal IRBC binding, we coated plates with bCSA at concentrations ranging from 0.75 μg/ml to 400 μg/ml, and measured the IRBC binding using three C4S-adherent parasite strains, FCR3-CSPG, 3D7-CSPG and CS2-CSPG. In parallel, we also measured IRBC binding to plastic plates coated with 1.5 ng/ml to 1600 ng/ml solutions of CSPG purified from human placenta. The binding capacity of IRBCs freshly selected for maximal binding to placental CSPG was assessed. All three parasite strains could bind to immobilized placental CSPG and bCSA in a concentration dependent manner (Fig. 1A and 1B). Saturated levels of IRBC binding to CSPG and bCSA were found to be at coating concentrations of 50 μg/ml and 200 ng/ml, respectively. A comparison of IRBC binding to placental CSPG and bCSA showed that the number of IRBCs bound per unit area of coated plates was significantly higher for CSPG than that for bCSA, despite the fact that the coating concentration of CSPG was 250-fold lower than that of bCSA (Fig. 1C). Thus, in all parasite strains studied, the number of IRBCs bound per unit area on the CSPG-coated plates was higher than that on the bCSA-coated plates.
Fig 1.
Analysis of P. falciparum IRBC binding to human placental CSPG and bCSA. Four mm-diameter circular spots on plastic Petri dishes were coated with solutions of low sulfated CSPG purified from human placenta or bCSA at the indicated concentrations. The spots were blocked with 2% BSA and overlaid with IRBCs. The unbound cells were washed off and the bound IRBCs were fixed with 2% glutaraldehyde, stained with Giemsa, counted under a light microscope at several fields. The number of cells bound per mm2 was calculated and means of two independent experiments performed in duplicates ± standard errors of the means plotted. (A) Binding of CSPG-selected IRBCs to placental CSPG; (B) binding of CSPG-selected IRBCs to bCSA; (C) comparison of CSPG-selected IRBCs binding to CSPG and bCSA coated at, respectively, 200 ng/ml and 50 μg/ml (saturated coating concentrations). (D) Binding of bCSA-selected IRBCs to placental CSPG; (E) binding of bCSA-selected IRBCs to bCSA; (F) comparison of bCSA-selected IRBCs binding to CSPG and bCSA coated at, respectively, 200 ng/ml and 50 μg/ml (saturated coating concentrations). Binding of IRBCs to CSPG is significantly higher for CSPG than bCSA irrespective of whether parasites were selected for CSPG or bCSA adherence. The p values for CSPG-selected parasites were <0.001 and that for bCSA-selected parasites were <0.05.
We also assessed the CSPG- and bCSA-binding capacity of FCR3-bCSA and 3D7-bCSA that were selected for bCSA adherence. As in the case of CSPG-selected parasites, these IRBCs could bind to both CSPG and bCSA in a dose-dependent manner (Fig. 1D and 1E). In both placental CSPG- and bCSA-selected parasites, the number of IRBCs bound to unit area of CSPG-coated plates was significantly higher than that of bCSA coated plates (compare Fig. 1E with 1F).
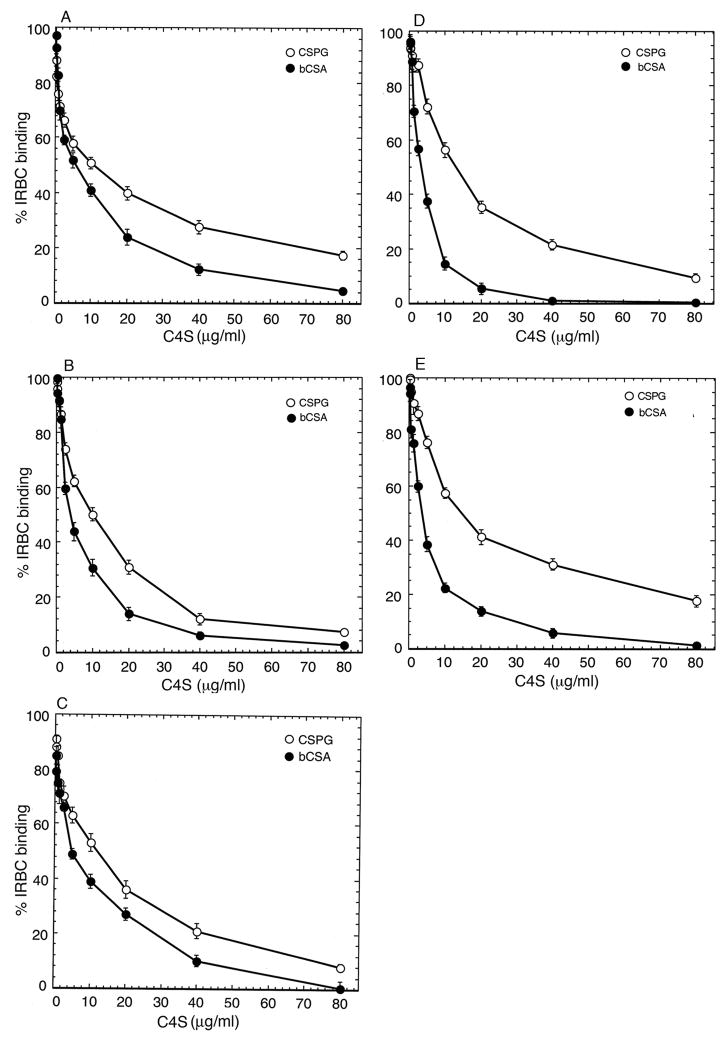
Next, we determined the binding strengths of IRBCs of all three parasite strains by measuring the concentration of a partially sulfated C4S polysaccharide (consists of 36% 4-sulfate) required for inhibition of IRBC binding to placental CSPG or bCSA coated at saturated concentration. Studies have shown that IRBC interactions with C4S involve dodecasaccharide motifs containing two 4-sulfated disaccharide moieties (Alkhalil et al., 2000; Beeson et al., 2002). In addition, the chondroitin sulfate chains of placental CSPG are predominantly nonsulfated and that the low amount of sulfate groups (8–10%) present are clustered in motifs consisting of 6–14 disaccharides at a density of 20–28% 4-sulfate (Achur et al. 2003). Because, the net sulfate content is very low, a large amount of placental CSPG is required if this CSPG were to be used as an inhibitor. In view of this consideration and because of the practical difficulty in purifying large quantities of placental CSPG, we rationalized that use of a C4S consisting of ~2 sulfate groups per dodecasaccharide binding motif is suitable for inhibition studies. Accordingly, the inhibition of IRBC binding to placental CSPG and bCSA was assessed for parasites selected on either CSPG (3D7-CSPG, FCR3-CSPG and CS2-CSPG) or bCSA (3D7-bCSA and FCR3-bCSA). In all parasite strains analyzed, the partially sulfated C4S could inhibit IRBC binding to either CSPG or bCSA in a dose-dependent manner (Fig. 2). Since the inhibitory ability of C4S is directly related to the strength with which IRBCs bind to CSPG or bCSA, the concentration of C4S required for inhibition of IRBC binding by 50% (IC50) should be a measure of the IRBC binding strength. For each parasite strain analyzed, the IC50 inhibitory concentration of C4S was about 2- to 3.5-fold higher for placental CSPG than that for bCSA (Table I). Thus, these results demonstrate that the strength of IRBCs binding to the CSPG is substantially higher than that of IRBC binding to bCSA, irrespective of whether parasites were selected on CSPG or bCSA.
Fig. 2.
Assessment of the strength of P. falciparum IRBC binding to placental CSPG and bCSA. The strength of IRBC binding to human placental CSPG or bCSA was determined by measuring the dose-dependent inhibition of IRBC binding by 36% C4S. The plastic Petri plates were coated with 200 ng/ml solution of CSPG or 50μg/ml solution of bCSA and blocked with 2% BSA. IRBCs (4% suspension in PBS) were incubated with 2 times the indicated concentrations of partially sulfated C4S and then overlaid onto the coated spots. The unbound cells were washed off and the bound cells were fixed, stained with Giemsa, and counted. Assays were carried out two times each in duplicate and percent binding (means ± standard errors of the means) with respect to control IRBC binding is plotted. A, 3D7-CSPG; B, FCR3-CSPG; C, CS2-CSPG; D, 3D7-bCSA; E, FCR3-bCSA. The concentrations of soluble C4S required for 50% inhibition of IRBC binding was considered as the direct measure of the binding strength of IRBCs. For each parasite studied, the strength of IRBC binding was ~2-fold higher for CSPG than bCSA.
Table 1.
Affinity of P. falciparum IRBC binding to human placental CSPG and bCSA
| Parasites | IC50 of C4S (μg/ml)a |
|
|---|---|---|
| CSPG | bCSA | |
| 3D7-CSPG | 10.9 ± 2.0 | 5.9 ± 0.5 |
| FCR3-CSPG | 10.2 ± 1.7 | 4.5 ± 0.2 |
| CS2-CSPG | 12.0 ± 0.5 | 5.2 ± 0.7 |
| 3D7-bCSA | 13.0 ± 0.4 | 3.5 ± 0.5 |
| FCR3-bCSA | 14.5 ± 1.4 | 4.0 ± 0.4 |
The concentration of partially sulfated C4S, consisting of 36% 4-sulfated and 64% nonsulfated disaccharide moieties, required for 50% inhibition of IRBC binding to either placental CSPG or bCSA. For each parasite strain studied, the values reported here for IRBC binding to CSPG and bCSA is based on the assay performed on same plastic plates.
4. Discussion
Subpopulations of P. falciparum parasites, expressing placental adherence-specific PfEMP1 called Var2CSA protein on the IRBC surface, have been shown to bind to C4S. The results presented here show that these C4S-adherent parasites bind well to placental CSPG, the natural receptor for IRBC adherence in human placenta. The results also show that parasites could bind to bCSA, even though it is substantially different in its structural features (consisting of 9% nonsulfated, 52% 4-sulfated and 39% 6-sulfated disaccharide moieties) compared to the C4S chains of placental CSPG that comprise ~8% 4-sulfated and ~92% nonsulfated disaccharide moieties. Previous studies have shown that, bCSA despite being markedly different in its structural features, could support the binding of IRBCs onto coated plates (Beeson et al., 2000; Fried et al., 2000; Ricke et al., 2000; Noviyanti et al., 2001; Salanti et al., 2003), and that it could also inhibit IRBC binding to CSPG (Alkhalil et al., 2000). The inhibitory capacity of intact bCSA was significantly increased when 6-sulfate groups were selectively removed (Alkhalil et al., 2000; Fried et al., 2000), suggesting that the presence of sulfate groups at the C-6 position of N-acetylgalactosamine affects the interaction of C4S. Nevertheless, bCSA has been extensively used for studying IRBC-C4S interactions. In view of the continued use of bCSA as a ligand for IRBC interaction studies and to determine its suitability as a ligand in studies that aimed to determine the structural interactions involved in IRBC binding to placental low sulfated C4S chains, we studied the relative binding characteristics of IRBCs from commonly used P. falciparum strains to bCSA and placental CSPG. The results show that IRBCs could bind to bCSA in a dose dependent manner, qualitatively similar to CSPG regardless of whether parasites were selected for binding to either placental CSPG or bCSA. However, in both cases, significant differences were observed in the number of IRBCs bound per unit area of plates coated with bCSA and CSPG. For all parasite strains studied, IRBCs could bind in significantly higher number to CSPG than to bCSA. The method used for the preparation of bCSA involves extensive proteolysis of tracheal CSPG core protein, resulting in the formation of CS chains attached to peptides with few amino acids. Considering that glycosaminoglycan chains in general are inherently highly hydrophilic and that the CSA chains in bCSA are attached to short peptide moieties, it is reasonable to presume that bCSA is not adsorbed efficiently onto plastic surface, leading to relatively low coating. In contrast, placental CSPG consisting of intact high molecular weight core protein is adsorbed well onto plastic surface through core proteins. Additionally, placental CSPG contains 6 to 8 chondroitin sulfate chains per molecule (Achur et al., 2000; 2003), and thus more number of low sulfated C4S tethered to each molecule, presenting high levels C4S chains per molecule of CSPG for IRBC binding. Therefore, a reason for the lower binding capacity of bCSA, as observed by the relatively lower number of IRBCs bound per unit area of the bCSA-coated plates compared to CSPG-coated plates, could be due to its poor coating efficiency. The other reason for the observed low level of IRBC binding to bCSA is likely due to the presence of a significant level (39%) of 6-sulfate groups, which are known to interfere with the binding (Alkhalil et al., 2000; Fried et al., 2000; Muthusamy et al., 2004b). However, because of the complexity of glycosaminoglycan structures it is not possible to assess the relative contribution of these two factors toward IRBC binding.
Given that the structural features of bCSA and chondroitin sulfate chains of placental CSPG are substantially different, it is likely that the strengths with which IRBCs adhere to the receptors are also different. Consistent with this prediction, the data presented here clearly show that, independent of whether parasites were selected on CSPG or bCSA, the strength of IRBC binding to bCSA as measured by the concentration of C4S required for inhibition of IRBC binding to CSPG by 50% (IC50; Table 1), is significantly lower than that for IRBC binding to placental CSPG. This interpretation is consistent with the fact that the presence of 6-sulfate groups interferes with IRBC binding (Alkhalil et al., 2000; Fried et al., 2000; Muthusamy et al., 2004b). Although the difference in IRBC binding strength to bCSA versus CSPG is not a concern for qualitative analysis regarding IRBC binding, it could be highly relevant for delineating C4S structural interactions involved in IRBC binding. For example, using CSPG as IRBC ligand, previous studies have shown that dodecasaccharide is the minimal chain length involved in IRBC binding (Alkhalil et al., 2000). In the case of oligosaccharides having 4,5-unsaturated glucuronic acid residue, the inhibitory activity of dodecasaccharides was found to be significantly lower than that of dodecasaccharides with unmodified glucuronic acid at the nonreducing end, and that tetradecasaccharide was required for maximun inhibition of IRBC binding (Achur et al., 2008). This is because in oligosaccharides having modified glucuronic acid at the nonreducing end, the disaccharide moiety does not interact with IRBCs (Achur et al., 2008). However, in studies that used bCSA as binding ligand, dodecasaccharides containing 4,5-unsaturated glucuronic acid could maximally inhibit IRBC binding to bCSA (Chai et al. 2002). The reason for the observed differential inhibition of IRBC binding to CSPG and bCSA by unsaturated dodecasaccharides remains unclear. Taken together our data argue that placental CSPG is more appropriate for studying C4S structural elements involved in IRBC binding, although the readily available bCSA can be used as a binding ligand for qualitative IRBC binding analysis.
Acknowledgments
This work is supported by grant AI45086 from National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achur RN, Valiyaveettil M, Alkhalil A, Ockenhouse CF, Gowda DC. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. Journal of Biological Chemistry. 2000;275:40344–40356. doi: 10.1074/jbc.M006398200. [DOI] [PubMed] [Google Scholar]
- Achur RN, Valiyaveettil M, Gowda DC. The low sulfated chondroitin sulfate proteoglycans of human placenta have sulfate group-clustered domains that can efficiently bind Plasmodium falciparum-infected erythrocytes. Journal of Biological Chemistry. 2003;278:11705–11713. doi: 10.1074/jbc.M211015200. [DOI] [PubMed] [Google Scholar]
- Achur RN, Kakizaki I, Goel S, Kojima K, Madhunapantula SV, Goyal A, Ohta M, Kumar S, Takagaki K, Gowda DC. Structural Interactions in Chondroitin 4-Sulfate Mediated Adherence of Plasmodium falciparum Infected Erythrocytes in Human Placenta during Pregnancy-Associated Malaria. Biochemistry. 2008 doi: 10.1021/bi801643m. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbor-Enoh ST, Achur RN, Valiyaveettil M, Leke R, Taylor DW, Gowda DC. Chondroitin sulfate proteoglycan expression and binding of Plasmodium falciparum-infected erythrocytes in the human placenta during pregnancy. Infection and Immunity. 2003;71(5):2455–2461. doi: 10.1128/IAI.71.5.2455-2461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. Journal of Biological Chemistry. 2000;275:40357–40364. doi: 10.1074/jbc.M006399200. [DOI] [PubMed] [Google Scholar]
- Beeson JG, Rogerson SJ, Cooke BM, Reeder JC, Chai W, Lawson AM, Molyneux ME, Brown GV. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nature Medicine. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs BA, Anders RF, Dillon HE, Davern KM, Martin M, Petersen C, Brown GV. Adherence of infected erythrocytes to venular endothelial selects for antigenic variants of Plasmodium falciparum. Journal of Immunology. 1992;149:2047–2054. [PubMed] [Google Scholar]
- Brabin BJ. An analysis of malaria in pregnancy in Africa. Bulletin World Health Organization. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- Brabin B, Rogerson S. The epidemiology and outcome of maternal malaria. In: Duffy PE, Fried M, editors. Malaria in Pregnancy: Deadly parasite, Susceptible Host. Taylor and Francis; New York, NY: 2001. pp. 27–51. [Google Scholar]
- Chai W, Beeson JG, Lawson AM. The Structural Motif in chondroitin Sulfate for Adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid. Journal of Biological Chemistry. 2002;277:22438–22446. doi: 10.1074/jbc.M111401200. [DOI] [PubMed] [Google Scholar]
- Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infection and Immunity. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- Fried M, Lauder RM, Duffy PE. Plasmodium falciparum: adhesion of placental isolates modulated by the sulfation characteristics of the glycosaminoglycan receptor. Experimental Parasitology. 2000;95:75–78. doi: 10.1006/expr.2000.4510. [DOI] [PubMed] [Google Scholar]
- Fried M, Domingo GJ, Gowda DC, Mutabingwa TK, Duffy PE. Plasmodium falciparum: chondroitin sulfate A is the major receptor for adhesion of parasitized erythrocytes in the placenta. Experimental Parasitology. 2006;113:36–42. doi: 10.1016/j.exppara.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Gamain B, Smith JD, Viebig NK, Gysin J, Scherf A. Pregnancy-associated malaria: parasite binding, natural immunity and vaccine development. International Journal of Parasitology. 2007;37:273–283. doi: 10.1016/j.ijpara.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Gysin J, Pouvelle B, Fievet N, Scherf A, Lepolard C. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infection and Immunity. 1999;67:6596–6602. doi: 10.1128/iai.67.12.6596-6602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L, Salanti A. VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria. Parasitology. 2007;134:1871–1876. doi: 10.1017/S0031182007000121. [DOI] [PubMed] [Google Scholar]
- Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Human Pathology. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Trager W. Plasmodium falciparum in culture: establishment of additional strains. American Journal of Tropical Medicine and Hygiene. 1978;27:743–746. doi: 10.4269/ajtmh.1978.27.743. [DOI] [PubMed] [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. Journal of Parasitology. 1979;65:418–420. [PubMed] [Google Scholar]
- Lekana Douki JB, Traore B, Costa FT, Fusai T, Pouvelle B, Sterkers Y, Scherf A, Gysin J. Sequestration of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A, a receptor for maternal malaria: monoclonal antibodies against the native parasite ligand reveal pan-reactive epitopes in placental isolates. Blood. 2002;100:1478–1483. doi: 10.1182/blood-2002-01-0315. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Achur RN, Gowda DC. Developmental stage- and cell cycle number-dependent changes in characteristics of Plasmodium falciparum-infected erythrocyte adherence to placental chondroitin-4-sulfate proteoglycan. Infection and Immunity. 2007;75:4409–4415. doi: 10.1128/IAI.00478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubert B, Fievet N, Tami G, Boudin C, Deloron P. Cytoadherence of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunology. 2000;22:191–199. doi: 10.1046/j.1365-3024.2000.00292.x. [DOI] [PubMed] [Google Scholar]
- McGregor IA, Wilson ME, Billewicz WZ. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Transaction of Royal Society of Tropical Medicine Hygiene. 1983;77:232–244. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Muthusamy A, Achur RN, Bhavanandan VP, Fouda GG, Taylor DW, Gowda DC. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. American Journal of Pathology. 2004a;164:2013–2025. doi: 10.1016/S0002-9440(10)63761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy A, Achur RN, Valiyaveettil M, Madhunapantula SV, Kakizaki I, Bhavanandan VP, Gowda DC. Structural characterization of the bovine tracheal chondroitin sulfate chains and binding of Plasmodium falciparum-infected erythrocytes. Glycobiology. 2004b;14:635–645. doi: 10.1093/glycob/cwh077. [DOI] [PubMed] [Google Scholar]
- Muthusamy A, Achur RN, Valiyaveettil M, Gowda DC. Plasmodium falciparum: adherence of the parasite-infected erythrocytes to chondroitin sulfate proteoglycans bearing structurally distinct chondroitin sulfate chains. Experimental Parasitology. 2004c;107:183–188. doi: 10.1016/j.exppara.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Ho M, Tandon NN, Van Seventor GA, Shaw S, White NJ, Jamieson GA, Chulay JD, Webster HK. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: Differential adhesion of infected erythrocytes. J Infect Dis. 1991;164:163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- Oleinikov AV, Francis SE, Dorfman JR, Rossnagle E, Balcaitis S, Getz T, Avril M, Gose S, Smith JD, Fried M, Duffy PE. VAR2CSA domains expressed in Escherichia coli induce cross-reactive antibodies to native protein. Journal of Infectious Diseases. 2008;197:1119–1123. doi: 10.1086/529526. [DOI] [PubMed] [Google Scholar]
- Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, Hviid L. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. Journal of Immunology. 2000;165:3309–16. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infectious Diseases. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Molecular Microbiology. 2003;49:179–91. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. Journal of Experimental Medicine. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. American Journal of Tropical Medicine and Hygiene. 2001;64 (1–2 Suppl):28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P, et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. American Journal of Tropical Medicine and Hygiene. 1994;51:123–137. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]