Abstract
Background
Determining the safety and pharmacokinetics of antifungal agents in neonates is important. A previous single-dose pharmacokinetic study of micafungin in neonates demonstrated that doses of 0.75 to 3 mg/kg produced lower plasma micafungin concentrations than in older patients because of increased apparent plasma clearance of micafungin in neonates. The primary objective of this study was to assess the safety and pharmacokinetics of an increased (15 mg/kg/day) dose of micafungin.
Methods
A repeated dose, open-label pharmacokinetic and safety trial of intravenous micafungin in 12 preterm neonates > 48 hours of life with suspected systemic infections. Neonates received 15 mg/kg/day of micafungin for 5 days. Blood samples were drawn relative to either the fourth or fifth dose. Systemic exposure was assessed by examination of the plasma area under the curve.
Results
The median birth weight and gestational age of the neonates were 775 g and 27 weeks, respectively. No adverse events related to micafungin were detected. The mean area under the curve and clearance for the cohort was 437.5 μg · h/mL and 0.575 mL/min/kg, respectively. The calculated clearance and volume of distribution for neonates was greater than that observed in older children and adults.
Conclusions
These data suggest that 15 mg/kg dosing in premature neonates corresponds to an exposure of approximately 5 mg/kg in adults. No adverse events related to micafungin were observed.
Keywords: antifungal, Candida, dosing, micafungin, premature infants
INTRODUCTION
Candida species are a leading cause of infectious disease related morbidity and mortality in the Neonatal Intensive Care Unit.1, 2 The incidence of candidemia in extremely low birth weight neonates (ELBW, < 1000 g birth weight) is 7% and the incidence is 12% in neonates < 750 g birth weight.1 Candidemia has an attributable mortality of 20–30%1 and frequently results in severe morbidity, including neurodevelopmental impairment, among survivors2.
Amphotericin B deoxycholate is the most commonly used antifungal in this population.3 Although amphotericin B deoxycholate is better tolerated in neonates than in adult patients, side effects are common and include renal impairment and electrolyte imbalances. Micafungin (FK463; Astellas Pharma US, Inc, Deerfield, IL) is a cyclic lipopeptide antifungal of the echinocandin class and is approved in the United States for adult patients and in Europe for adults and children (including neonates) for treatment of invasive candidiasis. Echinocandins are noncompetitive inhibitors of 1,3-β-D-glucan synthase, a fungus specific enzyme crucial to the biosynthesis of the fungal cell wall.4 This enzyme is not found in mammalian cells, and thus echinocandins have proven to be safe in adults and children.5, 6
Determining the safety and pharmacokinetics (PK) of micafungin in neonates addresses an unmet medical need given that data for this and other antifungals are sparse in neonates. A previous single-dose micafungin trial demonstrated that doses of 0.75, 1.5, and 3 mg/kg produced low plasma micafungin concentrations in preterm neonates compared with older patients consequent to increased clearance (CL) of the drug.7 Increased CL of micafungin has also been observed in pediatric patients < 8 years of age compared with adults.5
MATERIALS AND METHODS
Study Design
This was a single-center, open-label, repeated dose study to determine the tolerability and PK of intravenous micafungin administered to critically ill preterm neonates at the time of suspected systemic infection. The inclusion criteria were: 1) neonates > 48 hours of age and infants <120 days of life; 2) presence of sufficient venous access for administration of micafungin; and 3) clinical suspicion of a serious systemic infection requiring intravenous antimicrobial therapy. Exclusion criteria included: 1) history of hypersensitivity to an echinocandin; 2) exposure to an echinocandin in the month before the study; and 3) any condition that in the opinion of the investigator would preclude the patient from participation. The Institutional Review Board at Duke University Medical Center approved this study. All study participants were enrolled after obtaining written permission (informed consent) from the parent or legal guardian.
Administration of study drug and procedures
The 15 mg/kg dose of micafungin studied was extrapolated from the results of the previous single-dose study in neonates.7, 8 Micafungin was instituted as part of the empirical therapy given to the neonates at the time of a sepsis evaluation. Each neonate received 15 mg/kg once daily of micafungin for 5 days. The drug was given via constant rate infusion over 60 minutes using a syringe pump attached to microbore tubing. Appropriate blood, urine, or cerebrospinal fluid cultures were obtained as part of standard of care. Empirical therapy for the suspected systemic infection included at least 2 broad spectrum antibiotics. Hematology and serum chemistry values obtained as part of standard of care in the 72 hours before the first dose of micafungin and 72 hours after the last dose of micafungin were documented. Tolerability and short term safety were assessed during the period of micafungin administration and for 7 days after the last dose. Causality of the adverse events (AEs) was reported as not related, unlikely related, possibly related, probably related, or definitely related in the opinion of the attending physician.
Pharmacokinetic sampling and assay
Up to five 100 µL PK blood samples were obtained from each neonate around the fourth or fifth micafungin dose. Samples were obtained 2 hours before the start of the dose and at 1, 3–6, 8–12, and 18–24 hours after the completion of the infusion.. Samples were collected in 1 mL sodium heparin tubes and centrifuged at 1,500 g for 10 minutes. The plasma was transferred to a polypropylene tube and stored at -70°C until the time at which the analysis of the samples was to be performed.
Plasma samples were analyzed using a validated liquid chromatography method with mass spectrometric detection7. The micafungin (FK463) and the internal standard (0.50 µg/mL FR195743) were extracted from the samples by protein precipitation. After the dilution of the supernatant with 10.0 mM ammonium acetate in water, the mixed solution was directly injected and analyzed using liquid chromatography (LC) with mass spectrometric detection. The peaks were identified on a Sciex API 4000 mass spectrometer equipped with electrospray (ESI) operating in negative ion mode. The masses monitored were 1268.4 for FK463 and 1313.5 for FR195743 (IS for FK463). Peak area ratios (compound/internal standard) were fitted to a linear weighted (1/concentration2) least squares linear regression analysis to calculate the line of best fit from the data. The equations of the calibration curves were then used to calculate the concentrations of FK463 and FR195743 in the samples from their measured peak area ratios. The limit of quantitation for micafungin was 0.05 µg/mL. The calibration range was 0.05 to 25.0 µg/mL. This LC/MS method for the determination of FK463 was validated and met the requirements for specificity, sensitivity, linearity, recovery, precision, accuracy, and dilution integrity.
Statistical and pharmacokinetic analysis
Data from all patients who provided blood samples were included in the summaries of the PK profiles. PK results were summarized by weight cohort (<1000 g or ≥ 1000 g). The peak plasma micafungin concentration (Cmax) was obtained directly from the data. Previous investigations of the continuous intravenous infusion have shown that micafungin PK fits a 2-compartment model assuming the same η for CL, central compartment volume of distribution (V) and steady state volume of distribution (Vss). Therefore, this type of model was selected for use in this analysis. ADVAN3/TRANS5 [with CL, V, inter-compartment clearance (Q) and Vss] was used by the PREDPP subroutine library. The population PK parameters were assumed to have log-normal distribution and the relative error model was also assumed for the residual error. Due to the sparse sampling per subject as well as a small number of subjects enrolled in this study, data from the study by Heresi et al6 were included in the analysis dataset. In that study, a single 0.75, 1.5, or 3.0 mg/kg dose of micafungin was administered to 18 premature neonates. Posthoc PK parameters such as CL, V, Vss, Q were obtained by using NONMEM (version VI, level 1.0). Secondary parameters were calculated as follows.
- weight-corrected total clearance (mL/min/kg)
- Vdβ(L/kg)
Pharmacokinetic evaluation was made using a sparse sampling approach in this study. Since a blood sample was not collected at the end of infusion for each subject, reliable estimates of tmax could not be made. The individual posthoc CL and steady state apparent Vdss were weight-normalized and individual area under the curve (AUC0–24) was obtained from dose and the posthoc estimate of CL.
After assessing that the PK data were normally distributed, PK parameters were compared between neonates weighing < 1000 and ≥ 1000 g using a two-tailed Student t-test. Laboratory values (serum chemistry and hematology) were compared before and after micafungin administration using the Wilcoxon signed-rank test. Statistical analysis was performed using STATA 9 (College Station, TX). The significance limit accepted for all statistical analyses was α = 0.05.
RESULTS
Patients
All 12 neonates received 5 doses of micafungin. The median birth weight was 775 g [interquartile range; 670, 925 g] (Table 1 online only). Ten of the neonates were ELBW, and 4 had a birth weight < 750 g. At enrollment, 7 of the neonates had a weight < 1000 g. The majority were male (58%) and African American (75%). The median gestational age at birth was 27.0 weeks [25.9, 28.5] and post-conceptional age at enrollment was 28.3 weeks [27.3, 30.9]. The median age at enrollment was 4 days [3, 20]. One of the neonates had positive cultures from blood, urine, and cerebrospinal fluid which grew Coagulase-negative Staphylococcus. Two other neonates had urinary tract infections (Klebsiella and Enterococcus).
Table 1.
Demographics
| Duke study | Heresi et al7 | |
|---|---|---|
| Gender | ||
| Male (%) | 7 (58) | 14 (64) |
| Female (%) | 5 (42) | 8 (36) |
| Race | ||
| White (%) | 2 (16) | 14 (64) |
| African American (%) | 9 (75) | 8 (36) |
| Native American (%) | 1 (8) | 0 (0) |
| Gestational age (weeks) | ||
| Mean +/− SD | 27.1 +/− 1.8 | 26.2 +/− 2.2 |
| Range | 23 – 29 | 24 – 34 |
| Postconceptional age (weeks) | ||
| Mean +/− SD | 29.4 +/− 3.6 | 31.5 +/− 3.0 |
| Range | 24 – 36 | 26 – 39 |
| Age (days) | ||
| Mean +/− SD | 16.2 +/− 24.6 | 37.2 +/− 15.9 |
| Range | 2 – 82 | 7 – 56 |
| Weight (g) | ||
| Mean +/− SD | 996 +/− 330 | 1374.5 +/− 389.4 |
| Range | 540–1622 | 580 – 2201 |
SD - standard deviation
Pharmacokinetic profile of micafungin
The calculated population PK indices are presented in Table 2. The parameters cohorted by weight at enrollment are presented in Table 3, and the micafungin concentration time curve is shown in Figure 1. There was no statistical difference in the AUC0–24, CL, Cmax, Vdss, or Vdβ between neonates weighing < 1000 g and ≥ 1000 g.
Table 2.
Population pharmacokinetic parameters of micafungin*
| Parameter (population mean) | Estimate value | Relative Standard Error (%) |
|---|---|---|
| CL (L/hr)* | 0.0365 | 10.8 |
| V (L)* | 0.507 | 8.52 |
| Q (L/hr) | 0.0316 | 22.8 |
| Vss (L)* | 1.6 | 36.9 |
| Inter-individual variability (CV%) | 48.8 | 30.5 |
| Residual sum of squares (CV%) | 29.2 | 28.4 |
V – Volume; Q - intercompartmental clearance; CV - coefficient of variation
assuming the same η for CL, V and Vss
Relative standard error = (100*SE/estimate)
Table 3.
Pharmacokinetic profile of micafungin (mean, SD)
| Cohort n=12 | <1000 grams (n=7) |
≥1000 grams (n=5) |
P value | |
|---|---|---|---|---|
| AUC (ug· h/mL) | 437.5(99.4) | 412.7(121.4) | 472.2(49.6) | 0.33 |
| CL (mL/min/kg) | 0.575(0.196) | 0.622(0.250) | 0.510(0.054) | 0.36 |
| Cmax (ug/mL) | 38.4(8.8) | 38.6(11.6) | 38.2(3.3) | 0.95 |
| Vdss (L) | 1.5(0.5) | 1.2(0.5) | 1.8(0.3) | 0.06 |
| Vdss (L/kg) | 1.515(0.516) | 1.637(0.657) | 1.344(0.141) | 0.35 |
| Vdβ (L/kg) | 0.613(0.282) | 0.636(0.370) | 0.581(0.106) | 0.75 |
Vdβ – volume of distribution in the elimination phase
Figure 1.
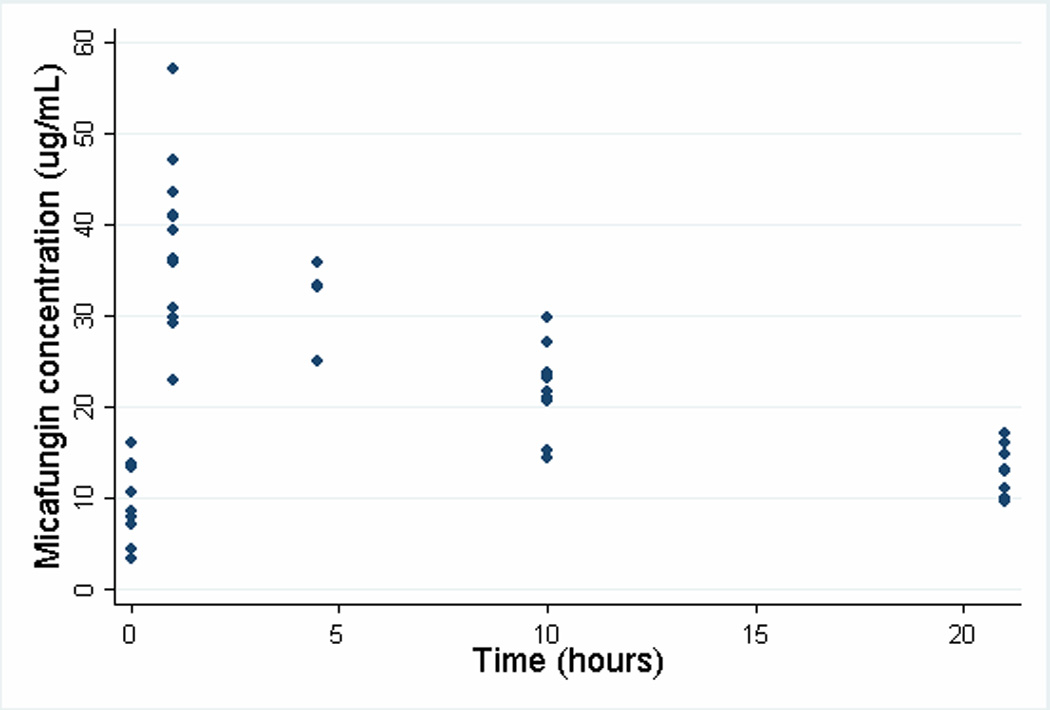
Plasma micafungin concentration-time profile
Safety and tolerability
All 12 neonates experienced at least 1 AE. None of the AEs were felt to be related to micafungin administration. One 540 g neonate died on day of life 11 from necrotizing enterocolitis, 3 days after completing the course of micafungin. The most commonly reported AEs were hyponatremia (42%), hypochloremia (33%), hypokalemia (25%), and monocytosis (25%). Hematology and serum chemistry measurements prior to and after the final dosing of micafungin are shown in Table 4. Serum potassium was higher at the end of therapy, 4.6 vs. 4.0 mmol/L (P=0.03). Other serum chemistries, including total bilirubin, remained statistically unchanged from baseline to the end of the therapy. No evidence of renal toxicity or significant changes in hematology parameters was noted.
Table 4.
Laboratory Evaluations (median values)
| Prior to 1st dose | After 5th dose | P value | |
|---|---|---|---|
| Serum Chemistry (IQR) | |||
| Sodium (mmol/L) | 136 (128, 139) | 134 (127, 138) | 0.72 |
| Potassium (mmol/L) | 4.0 (3.4, 4.4) | 4.6 (4.2, 5.2) | 0.03 |
| Chloride (mmol/L | 101 (93, 108) | 97 (90, 101) | 0.37 |
| Bicarbonate (mmol/L) | 24 (21, 27) | 24 (22, 27) | 0.81 |
| BUN (mg/dL) | 27 (10, 30) | 21 (10, 42) | 0.73 |
| Creatinine (mg/dL) | 1.1 (0.6, 1.2) | 0.9 (0.6, 1.4) | 0.77 |
| Total bilirubin (mg/dL) | 4.9 (4.7, 5.9) | 5.2 (3.4, 6.2) | 0.77 |
| Hematology (IQR) | |||
| White blood cell count(×109/mm3) | 10.0 (5.2, 13.2) | 16.0 (12.3, 28.9) | 0.08 |
| Hematocrit | 34 (32, 40) | 34 (31, 36) | 0.48 |
| Platelet count (×109/mm3) | 174 (115, 281) | 164 (136, 319) | 0.46 |
BUN - blood urea nitrogen
DISCUSSION
This study evaluated, for the first time, the safety and PK of a repeated dose regimen of an elevated dose of micafungin in a cohort of critically ill preterm neonates. Accurate assessment of the PK of micafungin in this population is of critical importance given that candidemia is common and often fatal in the preterm neonate,2 1 and that antifungal activity of the drug is dependent upon attainment of a sufficient systemic exposure. Unfortunately, PK and efficacy data of antifungal agents are sparse in this population.9–13
The PK of micafungin following a single intravenous dose have been previously characterized in neonates.7 The investigators enrolled 18 neonates ≥ 1000 g and administered 1 of 3 doses of micafungin: 0.75, 1.5, and 3.0 mg/kg. Six additional neonates < 1000 g were given 0.75 mg/kg of micafungin. The mean CL of micafungin for the 18 neonates > 1000 g was 0.65 ml/min/kg. Based on the data from 4 patients, there appeared to be a nearly 2-fold greater value (1.32 ml/min/kg) for the neonates < 1000g. This study adds seven < 1000 g preterm neonates. The results from this larger cohort suggest lesser degree of increased clearance in the < 1000 g weight group. In contrast, the CL of micafungin reported for older patients was lower: 0.38 ml/min/kg, 0.28 ml/min/kg, and 0.16 ml/min/kg for children ages 2–8 years, 9–17 years and adults, respectively.5, 14 These apparent developmental differences in micafungin CL remain unexplained on the basis of drug metabolism but may be associated, in part, with differences in drug protein binding between preterm neonates and older patients. Likewise, the apparent greater Vdss of micafungin in neonates from the present (0.62 L/kg) and previous (0.44 L/kg) study as compared to values previously reported from adult studies (0.26 L/kg) may be associated with age-dependent changes in either protein binding or body composition.7
The mean AUC0–24 in this study was 437.5 µg*hr/mL, and the highest observed AUC0–24 was 555.6 µg*hr/mL. In adults, a 150 mg dose of micafungin results in an average AUC of 166.7 µg*hr/mL with values of < 600 µg*hr/mL being associated with good safety profiles.6, 15 AUC data from the current study in preterm neonates suggest that a 15 mg/kg dose of micafungin in preterm neonates provides a similar systemic exposure to a dose of approximately 5 mg/kg dosing in adults.16 High micafungin plasma concentrations such as these may be important in neonates with candidemia secondary to concern for meningo-encephalitis, a common complication of neonatal candidemia.17 Successful treatment of central nervous system infections in neutropenic rabbits with micafungin has been previously documented.18
At present, reports of antifungal efficacy in the treatment of neonatal candidemia are limited to case series or underpowered trials.19–21 Neonatal dosing information is unavailable for anidulafungin and is limited with older antifungal agents such as amphotericin B deoxycholate, lipid preparations of amphotericin B, and fluconazole. Optimal agent and length of therapy for neonatal candidemia is unknown. Exposure data from this study in premature neonates suggest that a dose of 15 mg/kg of micafungin in premature neonates provides similar systemic exposure to approximately 5 mg/kg given to an adult. Further investigation is warranted to evaluate treatment response with similar exposures in this population.
Acknowledgements
Funding was provided by Astellas Pharma US, Inc., Deerfield, IL and in part by grant #5U10HD045962-04, Network of Pediatric Pharmacology Research Units, National Institutes of Health/National Institute for Child Health and Human Development (NIH/NICHD), Bethesda, MD.
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 3.Rowen JL. Mucocutaneous candidiasis. Seminars in Perinatology. 2003;27:406–413. doi: 10.1016/s0146-0005(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 4.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 5.Seibel NL, Schwartz C, Arrieta A, et al. Safety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother. 2005;49:3317–3324. doi: 10.1128/AAC.49.8.3317-3324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiemenz J, Cagnoni P, Simpson D, et al. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother. 2005;49:1331–1336. doi: 10.1128/AAC.49.4.1331-1336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heresi GP, Gerstmann DR, Reed MD, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr Infect Dis J. 2006;25:1110–1115. doi: 10.1097/01.inf.0000245103.07614.e1. [DOI] [PubMed] [Google Scholar]
- 8.Hope WW, Mickiene D, Petraitis V, et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis. 2008;197:163–171. doi: 10.1086/524063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baley JE, Meyers C, Kliegman RM, et al. Pharmacokinetics, outcome of treatment, and toxic effects of amphotericin B and 5-fluorocytosine in neonates. Journal of Pediatrics. 1990;116:791–797. doi: 10.1016/s0022-3476(05)82674-5. [DOI] [PubMed] [Google Scholar]
- 10.Starke JR, Mason EO, Jr, Kramer WG, et al. Pharmacokinetics of amphotericin B in infants and children. J Infect Dis. 1987;155:766–774. doi: 10.1093/infdis/155.4.766. [DOI] [PubMed] [Google Scholar]
- 11.Wurthwein G, Groll AH, Hempel G, et al. Population pharmacokinetics of amphotericin B lipid complex in neonates. Antimicrob Agents Chemother. 2005;49:5092–5098. doi: 10.1128/AAC.49.12.5092-5098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nahata MC, Tallian KB, Force RW. Pharmacokinetics of fluconazole in young infants. Eur J Drug Metab Pharmacokinet. 1999;24:155–157. doi: 10.1007/BF03190361. [DOI] [PubMed] [Google Scholar]
- 13.Saxen H, Hoppu K, Pohjavuori M. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clinical Pharmacology & Therapeutics. 1993;54:269–277. doi: 10.1038/clpt.1993.147. [DOI] [PubMed] [Google Scholar]
- 14.Hebert MF, Smith HE, Marbury TC, et al. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol. 2005;45:1145–1152. doi: 10.1177/0091270005279580. [DOI] [PubMed] [Google Scholar]
- 15.Sirohi B, Powles RL, Chopra R, et al. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:47–51. doi: 10.1038/sj.bmt.1705398. [DOI] [PubMed] [Google Scholar]
- 16.Mycamine (package insert) Deerfield, IL: Astellas Pharma; 2005. [Google Scholar]
- 17.Benjamin DK, Jr, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634–640. doi: 10.1542/peds.112.3.634. [DOI] [PubMed] [Google Scholar]
- 18.Petraitis V, Petraitiene R, Groll AH, et al. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 2002;46:1857–1869. doi: 10.1128/AAC.46.6.1857-1869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarcella A, Pasquariello MB, Giugliano B, et al. Liposomal amphotericin B treatment for neonatal fungal infections. Pediatric Infectious Disease Journal. 1998;17:146–148. doi: 10.1097/00006454-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Linder N, Klinger G, Shalit I, et al. Treatment of candidaemia in premature infants: comparison of three amphotericin B preparations. J Antimicrob Chemother. 2003;52:663–667. doi: 10.1093/jac/dkg419. [DOI] [PubMed] [Google Scholar]
- 21.Lopez Sastre JB, Coto Cotallo GD, Fernandez Colomer B. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am J Perinatol. 2003;20:153–163. doi: 10.1055/s-2003-40008. [DOI] [PubMed] [Google Scholar]


