Conspectus
Ligand-sensitized, luminescent lanthanide(III) complexes are of considerable importance because their unique photophysical properties (microsecond to millisecond lifetimes, characteristic and narrow emission bands, and large Stokes shifts) make them well suited as labels in fluorescence-based bioassays. The long-lived emission of lanthanide(III) cations can be temporally resolved from scattered light and background fluorescence to vastly enhance measurement sensitivity. One challenge in this field is the design of sensitizing ligands that provide highly emissive complexes with sufficient stability and aqueous solubility for practical applications.
In this Account, we give an overview of some of the general properties of the trivalent lanthanides and follow with a summary of advances made in our laboratory in the development of highly luminescent Tb(III) and Eu(III) complexes for applications in biotechnology. A focus of our research has been the optimization of these compounds as potential commercial agents for use in Homogeneous Time-Resolved Fluorescence (HTRF) technology. Our approach involves developing high-stability octadentate Tb(III) and Eu(III) complexes that rely on all-oxygen donor atoms and using multi-chromophore chelates to increase molar absorptivity; earlier examples utilized a single pendant chromophore (that is, a single “antenna”).
Ligands based on 2-hydroxyisophthalamide (IAM) provide exceptionally emissive Tb(III) complexes with quantum yield values up to ∼60% that are stable at the nanomolar concentrations required for commercial assays. Through synthetic modification of the IAM chromophore and time-dependent density functional theory (TD-DFT) calculations, we have developed a method to predict absorption and emission properties of these chromophores as a tool to guide ligand design. Additionally, we have investigated chiral IAM ligands that yield Tb(III) complexes possessing both high quantum yield values and strong circularly polarized luminescence (CPL) activity.
To efficiently sensitize Eu(III) emission, we have used the 1-hydroxypyridin-2-one (1,2-HOPO) chelate to create remarkable ligands that combine excellent photophysical properties and exceptional aqueous stabilities. A more complete understanding of this chromophore has been achieved by combining low-temperature phosphorescence measurements with the same TD-DFT approach used with the IAM system. Eu(III) complexes with strong CPL activity have also been obtained with chiral 1,2-HOPO ligands. We have also undertaken the kinetic analysis of radiative and non-radiative decay pathways for a series of Eu(III) complexes; the importance of the metal ion symmetry on the ensuing photophysical properties is clear. Lastly, we describe a Tb(III)-IAM compound—now carried through to commercial availability—that offers improved performance in the common HTRF platform and has the potential to vastly improve sensitivity.
Keywords: Antenna, Coordination complex, HTRF assay, Lanthanide, Luminescence
Introduction
The ‘rare earths’ consist of elements Z=21 (Sc), Z=39 (Y), and Z=57-71 (La-Lu).1 Despite this classification, most of these elements are actually relatively abundant: yttrium, for example, is one of the 30 most abundant elements and even the ‘rarest’ of these elements, Thulium (Z=69) and Lutetium (Z=71), are some 100 times more abundant in the Earth’s crust than Gold.2
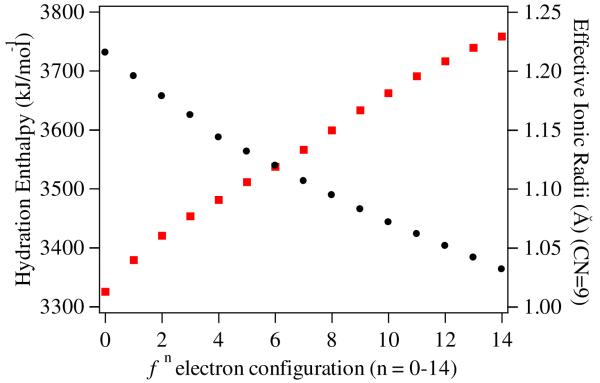
The lanthanides (together with the actinides) are unique in that they have very similar chemical properties, such as hydration enthalpies, common oxidation state, and ionic radius. Nonetheless, variations are observed. For example, from Z = 57-71, the hydration enthalpies of the trivalent cations increase almost linearly3 while the atomic radii decrease regularly across the series in the well known lanthanide contraction (Figure 1).4
Figure 1.
Observed increase in hydration enthalpy (left axis, red squares) and decrease in ionic radii (right axis, black circles) for the trivalent Ln(III) series.
In aqueous solution, the lanthanides exist almost exclusively in their trivalent state, Ln(III), due to the balance between hydration enthalpy of the trivalent cations and the sum of the first three ionization energies. The majority of the electronic wave function for the 4f orbitals lies beneath the occupied 5s and 5p orbitals. Hence, the 4f valence electrons interact minimally with their environment, and bonding in Ln(III) complexes is mainly electrostatic and non-directional. This same property imparts great flexibility on Ln(III) complexes, in terms of coordination number and geometry, since the requirement of maximizing orbital overlap is relaxed.
The Ln(III) ions are ‘hard’ Lewis acids, and display a preference for hard, negatively charged donor atoms such as oxygen (e.g. OH-, H2O) or fluoride (F-) compared to softer bases such as sulfur (e.g. S-) or nitrogen (e.g. NH3), when they act as monodentate ligands. Coordination numbers range from CN = 3 to 12 in the solid state, and are largely determined by the ionic radius of the cation and the charge and steric requirements of the ligand(s), with 8 and 9 being the most common in solution.3, 5 In aqueous solutions, Ln(III) cations readily hydrolyze above ca. pH 6 to form hydroxospecies;6
with reported5 logβ1 values ranging from -8.5 for La(OH)2+ to -7.6 for Lu(OH)2+. Complex formation with organic ligands competes with hydrolysis, and the stability of Ln(III) complexes are typically measured using potentiometric or spectrophotometric methods, with results evaluated in terms of the equilibrium constant for the complexation reaction;
For example, the logβ110 value (i.e. ML complex formation) for La(III) with ethylenediamine-tetraacetic acid (EDTA) is 15.46 (25°C, 0.1 M supporting electrolyte),7 which increases steadily across the Ln(III) series (logβ110 = 19.80 with Lu(III)), due to the increased Lewis acidity. Alternatively, the stability of metal complexes with differing ligand and complex acidities can be evaluated via conditional stability constants, or pM’s, defined as the negative log of the free metal concentration, pM7.4 = -log10[M]free, under conditions of 10 μM total ligand, 1 μM total metal, and pH 7.4.
Spectroscopic Properties
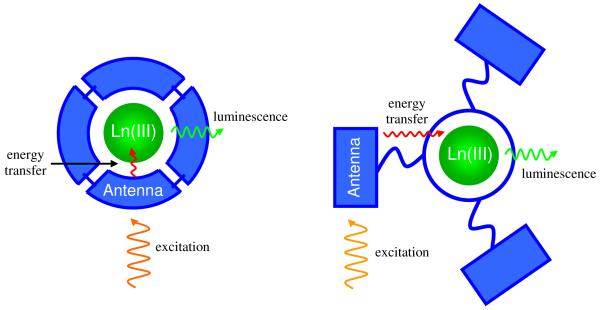
One significant aspect of the lanthanides is their unusual spectroscopic properties. Trivalent cations display absorption and emission bands that correspond to Laporte-forbidden f-f transitions. Because the 4f orbitals are relatively insensitive to the ligand field, these optical bands are line-like and characteristic of each metal. As a consequence of the parity rule (and sometimes due to the change in spin multiplicity), these absorptions have very low molar absorptivities (ε < 1 M-1cm-1), making the direct excitation of the metals very inefficient unless high power laser excitation is utilized. This problem is overcome by using a strongly absorbing chromophore to sensitize Ln(III) emission in a process known as the antenna effect (Figure 2); initial excitation occurs via the chromophore, which transfers energy to the Ln(III), populating the metal centered excited state, which then emits. Given the short lifetimes of the excited singlet states for organic chromophores, energy transfer is usually discussed as proceeding from the excited triplet state, although singlet and charge transfer (CT) states may also play a role.8
Figure 2.
The antenna effect for Ln(III) sensitization, illustrated using the chromophoric chelate (right) and pendant chromophore (left) ligand designs (see text).
The ground and excited states of the Ln(III) ions are shown in Figure 3. While the metal ions on the periphery (Ce-Pm, Ho-Yb) have relatively small energy gaps between adjacent levels, the central metals (Sm-Dy) display larger gaps, particularly for Gd(III). Hence, for the former, non-radiative deactivation process such as coupling to solvent vibrational modes are more efficient, and these ions are only weakly emissive. For the central group, the luminescent electronic transitions also involve a change in spin multiplicity. As a result, their luminescence is particularly long lived (μs-ms). For the purposes of this account we will focus on only Eu(III) and Tb(III), since only these two metals typically have the sizeable quantum yields (> 10 %) and long-lived luminescence lifetimes (0.1 - 1 ms) desirable for commercial applications in biotechnology.
Figure 3.
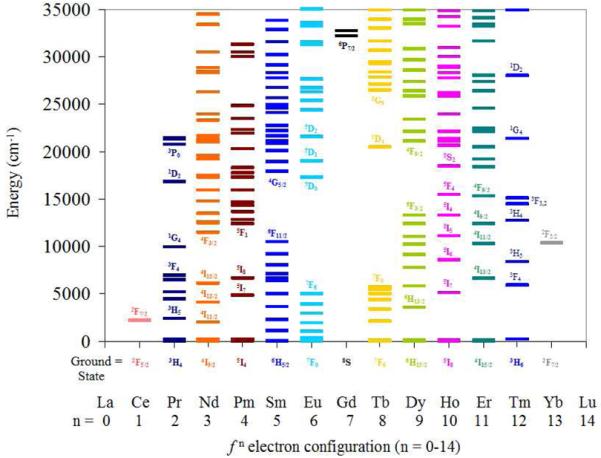
A summary of electronic excited state energy levels for the Ln(III) series.
Lanthanide Complexes in Fluoroimmunoassays
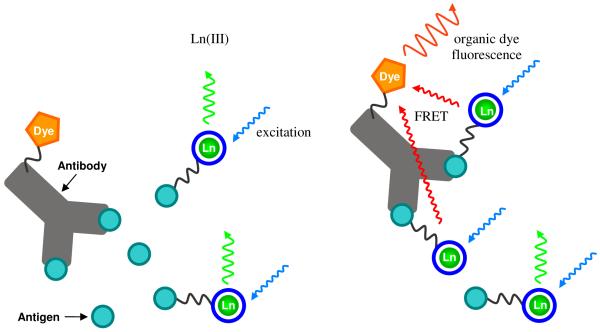
Both Eu(III) and Tb(III) are used in the field of biotechnology, where the ability to assay for analytes at small concentrations and in a complicated matrix presents a challenging problem. Lanthanide luminescence is amenable to these applications since it can be detected at nano- to picomolar concentrations by utilizing spectral and temporal discrimination. One of the first assay techniques developed was the Dissociation Enhanced Lanthanide Fluoro Immuno Assay (DELFIA®). This assay, however, is not ideal because it is heterogeneous and the signal cannot be traced until the end of the assay.9 Hence, the preparation of efficient lanthanide sensitizers that form stable complexes in dilute aqueous solution is an active area, since they can be used in homogenous formats.
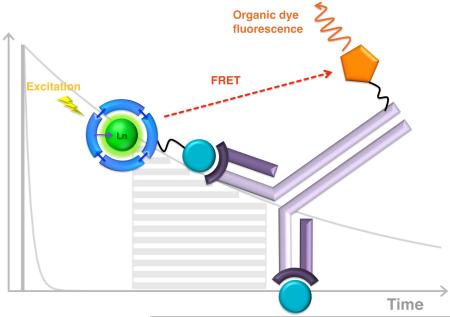
After more than 15 years of development, homogenous time resolved fluorescence (HTRF) assays have become widespread. Of these, a recent survey ranked CisBio’s HTRF technology, which relies on fluorescence resonance energy transfer (FRET), as the most frequently used generic assay,10 and the general format of this assay is shown in Figure 4. When only the Ln(III)-labeled antigen is present, it binds the antibody and efficient FRET between the lanthanide and acceptor takes place, resulting in enhanced emission of the organic dye. When the target antigen is present, the labeled antigen is displaced and the ratio of organic dye to Ln(III) emission is altered. With the long-lived luminescence of a lanthanide, the sensitivity of the assay can be vastly improved by using time gated excitation and detection techniques. This minimizes false signals due to direct excitation of the organic dye molecule and increases sensitivity by removing background autofluorescence from other components present in biological media.
Figure 4.
A cartoon illustration of the general principle for Homogenous Time-Resolved Fluoroimmunoassay (HTRF) format in the presence (left) and absence (right) of the target antigen.
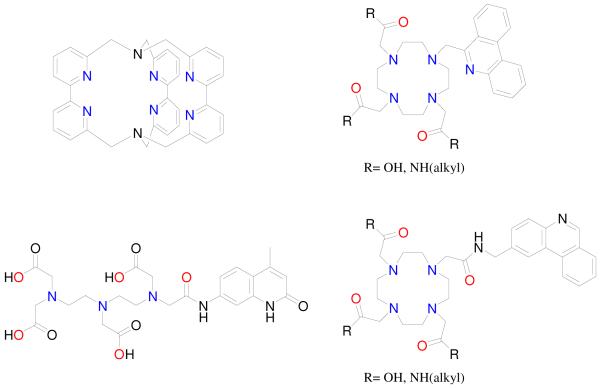
When developing a lanthanide-based label for a homogeneous assay, either of two general strategies can be employed in ligand design (Figure 2). The first approach, which we term the chromophoric chelate, utilizes one or more aromatic groups which absorb incident light and also contain coordinating atoms. As such, these chromophores are directly bound to the Ln(III) cation. Early examples are the tris-bipyridyl cryptands developed by Lehn and coworkers11 (Figure 5), which have been commercially developed by CisBio. However, these complexes often require an augmenting agent (e.g. F-) to optimize their luminescence.12
Figure 5.
Selected examples of compounds based on the cryptate (top left), macrocyclic (e.g. DO3A, top and bottom right) or linear aminocarboxylate (e.g. DTPA, bottom left) platforms utilized for Ln(III) sensitization.
In the design which we term the pendant chromophore, one or more chromophores are linked to a multidentate ligand such as diethylenetriaminepentaacetic acid (DTPA)13 or 1,4,8,11-tetraazacyclotetradecane-1,4,8-triacetic acid (DO3A),14 which bind the Ln(III) ion and saturate the metal’s coordination sphere (Figure 5). The chromophore is covalently attached to the binding unit but not necessarily bound to the lanthanide. Using this approach, a number of chromophores have been attached to various alkyl amine or macrocyclic platforms, which form stable and kinetically inert complexes, facilitating their use in biological applications. Both strategies allow for incorporation of multiple chromophores, which increases the overall absorbance of the complex, however, in the latter, when the chromophore is not directly attached to the metal cation, ligand-to-Ln(III) energy transfer necessarily proceeds via a through-space Förster-type mechanism, while for the former approach, both Förster15 and Dexter16 mechanisms are possible.
To yield highly luminescent Ln(III) complexes, an effective antenna ligand must be optimized in several areas. The ligand must form stable complexes over a wide pH range and resist aqueous hydrolysis at sub-nanomolar concentrations. Furthermore, the ligand should saturate the Ln(III) coordination sphere to prevent water from coordinating to the metal ion, since bound solvent effectively quenches luminescence. For Eu(III) and Tb(III), the inner sphere hydration number, q, can be quantified using the observed luminescence lifetimes, τ, in H2O and D2O respectively, using the general relationship, . Empirical values of A proposed by Horrocks17 are 1.05 ms-1 for Eu(III), and 2.1 ms-1 for Tb(III), with B = 0. The B term was introduced by Beeby and Parker et al. to account for second sphere water molecules, and constants of A = 1.2, B = 0.25 for Eu(III), and A = 5.0 ms-1, B = 0.06 for Tb(III) were proposed,18 which were later further refined for Eu(III) by Horrocks et. al.19 to be A = 1.11, B = 0.31. Due to solvent induced quenching, a multidentate ligand topology is preferred, which will also confer stability at nano- to picomolar concentrations.
In terms of the desired photophysical properties, the chromophore should possess a high molar absorptivity, ε, and the excitation wavelength, λex, should be above ca. 350 nm to facilitate the use of inexpensive excitation sources and avoid excitation of other chromophores in biological media (e.g. tryptophan, NADH, etc). Since sensitization typically occurs via the excited triplet state, the antenna should possess a high intersystem crossing efficiency (ηisc) and the ligand triplet state should be at least ca. 1,850 cm-1 higher in energy than the Ln(III) emitting state to minimize thermal repopulation of the triplet.20 For Eu(III), the energetic requirements of the antenna chromophore are less straightforward, since the metal can accept energy via the 5D2, 5D1 and 5D0 electronic states (Figure 3). Also, while the quenching effects of thermal back transfer can be observed when the ligand triplet and 5D0 levels of Eu(III) are too close in energy, low lying ligand-to-metal charge-transfer (LMCT) states can be readily accessed when higher excitation energies (e.g. ≥ 30,000 cm-1) are used, due to the increased ease of photo-reduction to form Eu(II) at shorter wavelengths.21 In addition to governing the sensitization efficiency (ηsens) of the complex, the ligand can also exert control over the coordination environment, and hence symmetry of the Ln(III) ion, which may affect the overall quantum yield (Φtot) by influencing the intrinsic quantum yield of the metal center (ΦLn).
Sensitization of Tb(III) with the IAM Chromophore
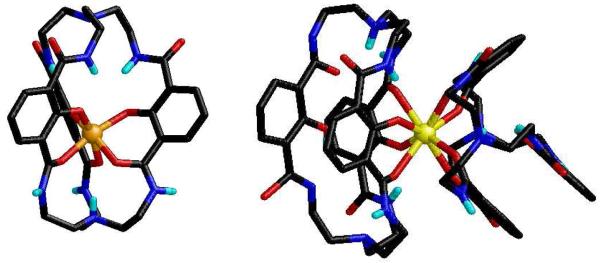
Our interest in ligand-sensitized Ln(III) luminescence originated with the 2-hydroxyisophthalamide (IAM) binding unit (Figure 6), developed to investigate the salicylate binding mode in Fe(III) complexes.22 The X-ray structure of the Fe(III) complex of the Bis-Tren-IAM ligand shows each 2-hydroxyisophthalamide arm is bidentate, binding through the deprotonated phenolate oxygen and an adjacent carbonyl oxygen. The remaining amide group is oriented so the amide proton forms an intermolecular H-bond with the phenolate oxygen (Figure 6), which stabilizes the structure by predisposing the ligand toward metal binding. In addition, we found that Bis-Tren-IAM forms highly luminescent complexes when mixed with solutions of Ln(III) salts,23 which sparked our ongoing interest in this area.
Figure 6.
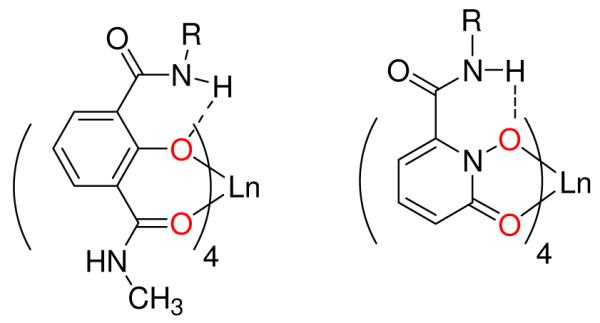
Generalized structures of the 2-hydroxyisophthalamide or ‘IAM’ (left) and 1-hydroxy-pyridin-2-one or ‘1,2-HOPO’ (right) chelates featuring exclusively oxygen donor sets (red), where ‘R’ is typically an alkyl amine backbone.
Remarkably, the IAM chromophore can sensitize all four of the visible emitting Ln(III) cations, Tb(III), Eu(III), Sm(III) and Dy(III) (Figure 8). The Tb(III) complex in particular displays a quantum yield in aqueous solution of Φtot = 61 %, which remains one of the highest values reported. The X-ray structure of the Eu(III) complex reveals that Bis-Tren-IAM acts as a tetradentate ligand, binding in a 2:1 stoichiometry, with only two of the three 2-hydroxyisophthalamide units chelated (Figure 7).23 To better suit the Ln(III) coordination requirements, an octadentate ligand, H(2,2)-IAM (Figure 9), was prepared, and its Tb(III) complex retains the favorable photophysical properties (ε = 26,800 M-1 cm-1, Φtot = 59 %, τ = 2.6 ms) seen with Bis-Tren-IAM.
Figure 8.
Observed emission from Ln(III) cations sensitized by an IAM ligand irradiated with a standard laboratory UV-lamp (λex = 365 nm). From left to right: ligand only, Tb(III), Eu(III), Dy(III) and Sm(III) complexes.
Figure 7.
X-ray structures of [Fe(Bis-Tren-IAM)] (left) and [Eu(Bis-Tren-IAM)2] (right) (selected H atoms omitted).
Figure 9.
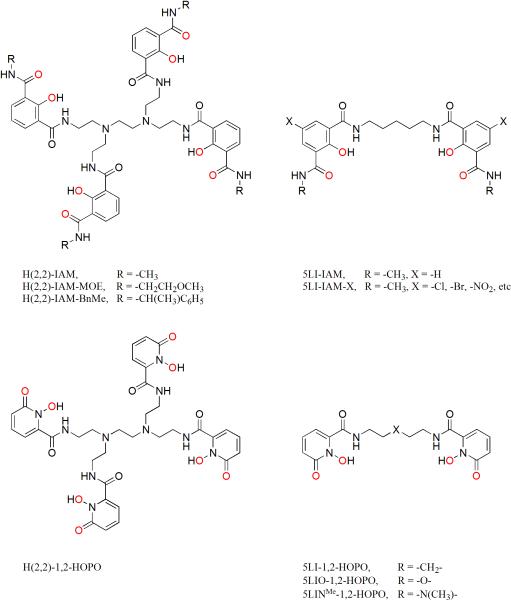
Chemical structures of ligands based on the 2-hydroxyisophthalamide (IAM) and 1-hydroxypyridin-2-one (1,2-HOPO) chelates.
To quantify the stability of this new class of luminescent species, it was necessary to improve their aqueous solubility, which was achieved by replacing the methyl amide with a 2-methoxyethyl (MOE) amide group (Figure 9). For the [Ln(H(2,2)-IAM-MOE)] complexes, logβ110 values of 14.7 (1) and 14.4 (1) were measured for Tb(III) and Eu(III), with corresponding pM values of 14.7 and 14.3.24 Spectrophotometric titration data was used to assign the protonation sequence of the ligand, revealing a central amine in the backbone to be the most basic site, with a pKa value of ca. 9.5. The high basicity of the amine is retained in the Ln(III) complexes, which have pKa values of 9.4 and 9.2 with Tb(III) and Eu(III) respectively. The Ln(III) affinities of the fully deprotonated versus mono-protonated ligand suggest that the latter benefits from a stabilizing hydrogen bond involving the tertiary backbone amines, predisposing the ligand for metal binding.24 Another important result was that the photophysical properties of the more soluble [Tb(H(2,2)-IAM-MOE)] complex remain essentially unchanged (ε ∼ 27,000 M-1 cm-1, Φtot = 56 %, τ = 2.6 ms). The Gd(III) complexes of both H(2,2)-IAM and H(2,2)-IAM-MOE were prepared to measure the zero phonon energy of the lowest energy ligand triplet state (T0-0), resulting in values of ca. 23,350 cm-1 and 23,170 cm-1 respectively. The lowest excited singlet and triplet states for the IAM ligands are quite close in energy (ΔEavg(S1 → T0-0) = 850 cm-1), suggesting a relatively high rate of intersystem crossing due to the similarity of the excited state geometries, which may contribute to the high quantum yields observed. Furthermore, the mean T0-0 energy is only ca. 2,750 cm-1 higher in energy than the Tb(III) 5D4 emitting state, which is within the optimal region for efficient ligand to Tb(III) energy transfer.20
Altering the IAM chromophore via modification of the secondary amide group on the ‘open’ face of the ligand does not alter the photophysical properties of the Tb(III) complexes. However, in the same report,24 we showed that altering the length of the central amine backbone can have a dramatic impact on the photophysical behavior of the metal complexes by altering the solvent accessibility. Estimations of q for the Tb(III) complexes using the equations derived by Beeby and Parker et al.18 showed that for the [Tb(H(2,2)-IAM-MOE)] complex, there are no bound water molecules; in contrast, complexes formed with analogous ligands that have slightly longer propyl- and butyl-linkages between the two central amines have a single water molecule bound to the metal, leading to decreased quantum yields.
Modifying the IAM amide linkage can also introduce chirality into the ligand, yielding Ln(III) complexes with circularly polarized luminescence (CPL) activity. Employing both enantiomers of α-methylbenzylamine, the chiral ligands H(2,2)-IAM-R(+)-α-BnMe and H(2,2)-IAM-S(-)-α-BnMe were produced (Figure 9).25 Their Ln(III) complexes retain the brightness of the parent achiral forms and also display CPL activity, reported as the luminescence dissymmetry factor, glum, which measures the difference in left and right circularly polarized emission (IL or IR) compared to the total emission;
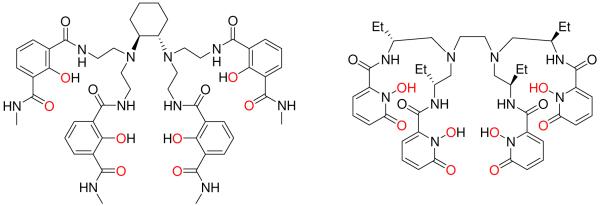
These [Ln(H(2,2)-IAM-BnMe)] complexes display very high |glum| values of ca. 0.3 with Eu(III), and we also reported the first CPL active Sm(III) complexes formed from chiral ligands. One drawback of these complexes is their low solubility, preventing studies in water. An alternative approach where the chiral center was incorporated into the ligand backbone (CyH(2,2)-IAM, Figure 10) displays both improved aqueous solubility and an increased |glum| value of 0.078 compared to [Tb(H(2,2)-IAM-BnMe)] (|glum| = 0.048).26
Figure 10.
Chemical structure of CyH(2,2)-IAM (left) and EtH(2,2)-1,2-HOPO (right) ligands for aqueous CPL measurements.
We were also interested in determining how the photophysical properties of the antenna can be further optimized. For example, it remains challenging to easily and reliably predict the effects of antenna modification on the ligand and hence ensuing photophysical properties. A series of IAM ligands substituted para to the phenolate group (IAM-X) were developed to determine the relationship between chromophore modification and the resulting photophysical properties of the complexes.27 This was achieved using a simplified tetradentate 5LI-IAM (Figure 9) ligand scaffold, which forms ML2 complexes with Tb(III) that retain most of the favorable photophysical properties (ε = 25,100 M-1 cm-1, Φtot = 36 %, τ = 2.5 msec) compared to the octadentate derivatives.
A wide selection of X groups covering a broad range of electron withdrawing and electron donating abilities was chosen. With the exception of the nitro-substituted ligand (5LI-IAM-NO2) (vide infra), the ligand absorption, fluorescence and phosphorescence energies were all found to increase linearly with the resonance component of the substituent Hammett parameter (R).27 The overall quantum yield of the Tb(III) emission, , was also found to increase with the triplet energy of the ligand, an effect which we attribute in part to a decrease in non-radiative deactivation caused by thermal repopulation of the ligand excited triplet state. The luminescence behavior observed for the Tb(III) complexes agrees well with results reported by Latva et al.,20 which show that quantum yield values display marked decreases when the ligand triplet energy falls below ca. 22,000 cm-1.
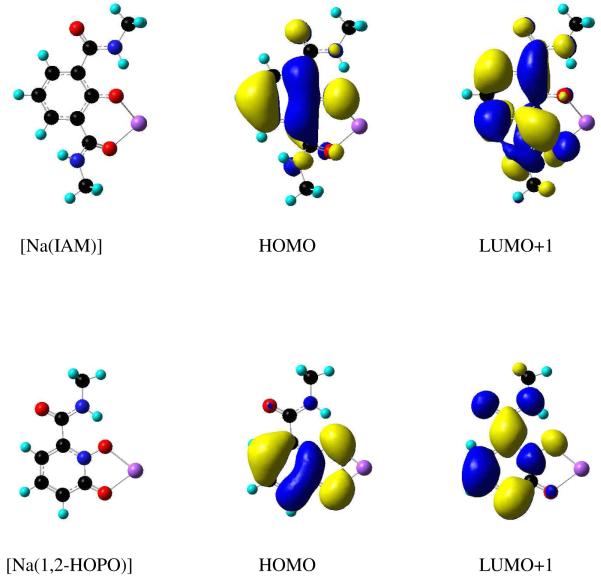
To complement these findings and to develop a more general way to screen antenna ligands, Time-Dependent Density Functional Theory (TD-DFT) calculations were performed27 on simplified bidentate IAM chelates, using Na+ in place of Tb(III). The singlet and triplet values obtained from these calculations agree (within ca. 5 %) with the experimentally determined absorption maxima and triplet energies. An examination of the orbitals involved in the transitions (Figure 12) also confirmed that they can be described as essentially π-π*, except in the case of the IAM-NO2. The excited state transitions for this chromophore showed significant intra-ligand charge transfer (ILCT) character, which explains why this ligand fails to adhere to the trends seen for the remainder of the IAM-X ligand series. Together the experimental and computational results serve as complementary techniques useful to quickly and accurately assess the photophysical properties of potential antenna chromophores prior to ligand synthesis.
Figure 12.
Results from TD-DFT (B3LYP/6-311G++ (d,p)) for simplified model ‘[Na(IAM)]’ (top) and ‘[Na(1,2-HOPO)]’ (bottom) chromophores, revealing the π→π* character of the low lying excited singlet and triplet states.
Sensitization of Eu(III) with the ‘1,2-HOPO’ Chromophore
Given the success with the IAM chromophore as a sensitizer for Tb(III), an improved chromophore for Eu(III) was sought. A systematic screening of ligands developed in our laboratory was undertaken, and a ligand utilizing a 6-amide derivative of 1-hydroxy-pyridin-2-one (1,2-HOPO) (Figure 6) was found to sensitize Eu(III) with high efficiency.28 Previous examples of sensitized Tb(III) and Eu(III) emission using 1,2-HOPO exist, but the chromophore lacked the 6-amide group.29,30 Since our initial communication, we have progressed in the development and characterization of complexes that feature the 1,2-HOPO chelate group.
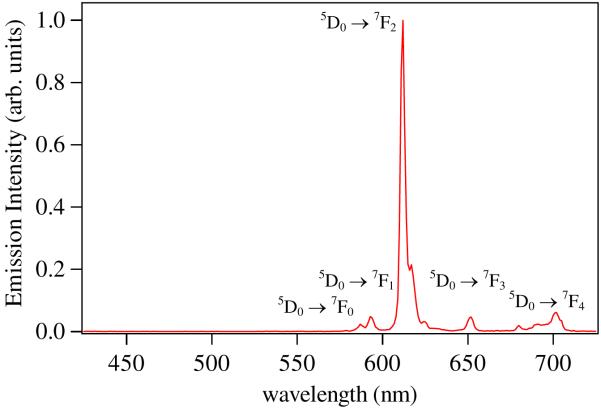
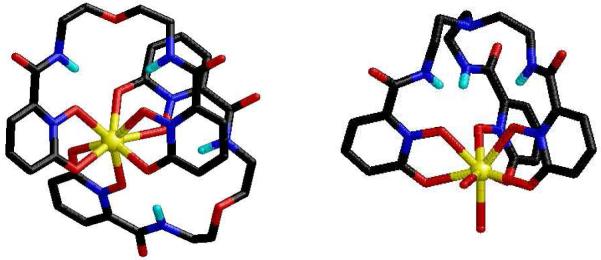
The tetradentate ligand, 5LI-1,2-HOPO (Figure 9), is remarkable since its EuL2 complex combines both exceptional photophysical properties (ε = 18,800 M-1 cm-1, Φtot = 20.7 %, τH2O = 737 μsec, q = 0.1) with excellent aqueous stability (logβ120 = 22.6 (1), pM = 18.4).31 As an example, the metal centered emission spectrum observed for [Eu(5LI-1,2-HOPO)2]- in aqueous solution is shown in Figure 13. As a rationale for the impressive aqueous stability of this complex, we note the use of an all-oxygen donor atom set and that the bidentate 1,2-HOPO chelating units form a 5-membered ring with Eu(III). This binding mode of the 1,2-HOPO chelate has been confirmed in X-ray structures with Eu(III), Sm(III) and Gd(III), (Figure 14),31,32 and is in contrast to the less favorable 6-membered ring formed in IAM complexes. From the 77K phosphorescence spectrum of [Gd(5LIO-1,2-HOPO)2]-, the ligand T0-0 energy was measured at 21,260 cm-1, explaining the observations that 6-amide 1,2-HOPO derivatives do not efficiently sensitize luminescence from Tb(III) at room temperature. The ligand triplet state is too close in energy to the 5D4 accepting state of Tb(III) (ΔE(T0-0 → 5D4) = 760 cm-1), but is appropriately positioned to sensitize the 5D1 state of Eu(III), located at 19,030 cm-1 (ΔE(T0-0 → 5D1) = 2230 cm-1). Using the same TD-DFT technique developed for the IAM ligands, we have performed calculations on a simplified Na+ adduct of a bidentate 1,2-HOPO chromophore (Figure 12).31 The close agreement (within ca. 5 %) observed between the experimental T0-0 energy and the calculated value of 21,365 cm-1 is further evidence to support the generality of this approach in making a rapid assessment of the potential of a given antenna system prior to synthetic efforts.
Figure 13.
Representative emission spectrum of a Eu(III)-1,2-HOPO complex in aqueous solution, pH 7.4.
Figure 14.
X-ray structures of [Eu(5LIO-1,2-HOPO)2]- (left) and [Eu(Tren-1,2-HOPO)(H2O)2] (right) (selected H atoms omitted).
Utilizing the same ligand backbone employed for the IAM chromophore, an octadentate ligand, H(2,2)-1,2-HOPO (Figure 9) was prepared, which was expected to form a more thermodynamically stable complex than the tetradentate analogs.33 The low aqueous solubility (> 0.1 mM) of [Eu(H(2,2)-1,2-HOPO)]- below pH 7 prevented stability measurements by potentiometry, so stability was assessed by competition batch titration versus DTPA, at pH 6.1, 7.4 and 8.5, from which logβ110 = 21.8(5), logβ111 = 31.2 (3) and logβ112 = 38.1 (2) values were extracted. These can be compared directly with those for the corresponding [Tb(H(2,2)-IAM-MOE)]- complex of logβ110 = 14.7 (1), logβ111 = 24.1 (1) and logβ112 = 30.3 (1), showing that the 1,2-HOPO based chelate is ca. 7 orders of magnitude more stable than the IAM derivative.
The increased stability toward dilution achieved using an octadentate ligand rather than a tetradentate scaffold was also highlighted by comparing the properties of the Eu(III) complexes of H(2,2)-1,2-HOPO, and a structurally similar tetradentate ligand, 5LINMe-1,2-HOPO (Figure 9). For a fixed tolerance of 1 % free Eu(III) at pH 7.4, the resistance of the octadentate [Eu(H(2,2)-1,2-HOPO)]- complex toward aqueous hydrolysis is improved by ca. 8 orders of magnitude compared with the tetradentate [Eu(5LINMe-1,2-HOPO)2]- complex.33
The photophysical properties of [Eu(H(2,2)-1,2-HOPO)]- were measured in aqueous solution and while the absorption properties (ε = 18,200 M-1 cm-1) and emission spectra are similar to those observed for the Eu(III) complexes with 5LI- and 5LIO-1,2-HOPO, the overall quantum yield is significantly reduced, to Φtot = 3.6 %. Solvent induced quenching was suspected due to the reduced luminescence lifetime in aqueous solution (τH2O= 480 μsec), and the presence of an inner sphere water molecule was confirmed by measurements in D2O (τD2O= 1220 μsec, q = 1.1). Hence, while bis-tetradentate complexes receive adequate protection from non-radiative deactivation, the H(2,2)-1,2-HOPO complex has a single bound water molecule, strongly enhancing the non-radiative decay rate and explaining the poor luminescence performance. The addition of ethyl substituents on the arms of the H(2,2)-backbone displace second sphere water molecules has the benefit of imbuing chirality in the ligand and hence CPL activity (Figure 10). However, the Eu(III) complex still retains one inner sphere water molecule, and the improved quantum yield was limited to Φtot = 7.7 %.26
To further investigate the photophysical properties of these Eu(III) complexes, we examined the metal centered radiative (kr) and non-radiative (knr) decay rate constants using the approach reported by Verhoeven34 and Beeby.35 As expected, the rate of non-radiative decay, knr, is significantly higher for the [Eu(H(2,2)-1,2-HOPO)(H2O)]- complex, when compared to [Eu(5LI-1,2-HOPO)2]- for example, due to the bound inner sphere water molecule. However, analysis of the kinetic parameters also revealed the radiative decay rate constant, kr, is lower for [Eu(H(2,2)-1,2-HOPO)(H2O)]-, presumably due to the different symmetries of the coordination environments experienced by Eu(III) (e.g. CN = 8 vs. CN = 9). For complexes of Eu(III), it has been shown that the steady state emission spectra can be used to probe the solution structure, and our analysis of these 1,2-HOPO complexes has shown that, while the eight coordinate Eu(III) cation in bis-tetradentate ML2 complexes such as [Eu(5LI-1,2-HOPO)2]- likely have average D2d site symmetry in solution,31 the nine coordinate [Eu(H(2,2)-1,2-HOPO)(H2O)]- complex has local C2 symmetry,33 highlighting the importance of symmetry on the photophysical properties of these luminescent Eu(III) complexes.
From the kinetic analysis, the efficiency of the sensitization process, ηsens, can be quantified by comparing the observed total quantum yield, Φtotal, with the calculated intrinsic quantum yield value using the relationship, . This is related to both the rate of intersystem crossing, kisc, and the kinetics of the electronic energy transfer step, kEET. A comparison of the data for the Eu(III) complexes with 5LI-1,2-HOPO and H(2,2)-1,2-HOPO has shown that while the efficiency of these processes approaches 50 % for the former, it is as low as ca. 20 % for the octadentate complex with Eu(III). Recent indications are that the T0-0 energy of [Gd(H(2,2)-1,2-HOPO)(H2O)]- is ca. 875 cm-1 lower than that of [Gd(5LI-1,2-HOPO)2]-, which could alter either kisc or kEET, and decrease the efficiency of sensitization.
Conclusions
Over the past several years, we have made substantial efforts toward further optimizing the IAM chromophore and understanding the stability and photophysical properties of the Tb-IAM complexes. To a similar extent, considerable progress in understanding the Eu-1,2-HOPO system has also been achieved. While the IAM group forms slightly less stable complexes than the 1,2-HOPO chelate, they nonetheless retain sufficient aqueous stability and resistance toward hydrolysis for use in vitro in homogenous assays. Another key feature of Tb-IAM complexes is the relative insensitivity of the luminescence lifetime to the ligand topology. By contrast, Eu(III) 1,2-HOPO-based complexes show significant variation, in terms of the luminescence lifetimes and also overall quantum yields. A current limitation of the octadentate [Eu(H(2,2)-1,2-HOPO)(H2O)]- complex is the residual inner sphere water molecule, which quenches luminescence. For the IAM group, this is less problematic, likely due to the steric protection provided by the methyl amide group on the ‘open’ face of the [Tb(H(2,2)-IAM)]- complex. We are currently exploring methods to increase the steric bulk of 1,2-HOPO based ligands to improve the performance of octadentate Eu(III) complexes. The lower thermodynamic stability of the Tb(III) IAM complexes can be offset by the kinetic stability of macrotricyclic cryptate complexes.
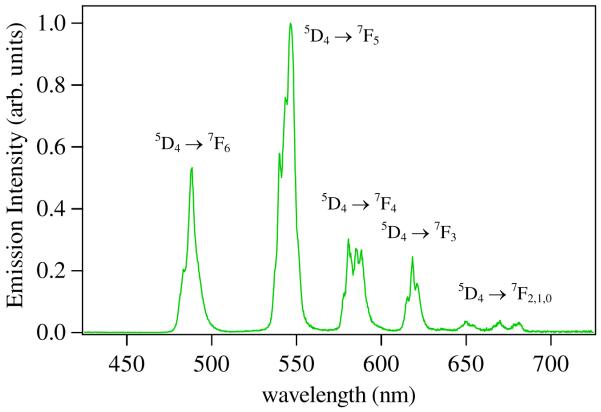
The exceptional performance of Tb-IAM complexes has culminated in the recent commercialization of an IAM based cryptate (Lumi4-Tb) by Lumiphore, Inc and subsequent marketing of this compound by CisBio for use in their HTRF platform. This product is twice as sensitive as the previously utilized Eu(III) complexes, as measured by a comparative IP-One GPCR secondary messenger assay,36 and, more importantly, this comparison was made at a shared common emission wavelength of 620 nm, close to the maximum intensity of the Eu(III) 5D0 → 7F2 transition, but accounting for only ca. 8 % of the overall Tb(III) centered luminescence (Figure 11). Detection and TR-FRET using the Lumi4-Tb cryptate can also be performed at 545 nm, and 488 nm corresponding to the metal centered 5D4 → 7F5 and 5D4 → 7F6 emission bands (which account for ca. 49 % and 21 % respectively of the overall Tb(III) luminescence). Since FRET is dependent upon the overlap of donor emission and acceptor absortion,37 greater efficiency can be expected by using blue shifted organic dyes as HTRF acceptors (e.g. Rhodamine B, , Fluorescein, ), which will further enhance the sensitivity achievable in these assays.
Figure 11.
Representative emission spectrum of a Tb(III)-IAM complex in aqueous solution, pH 7.4.
Acknowledgements
This work was partially supported by the NIH (Grant HL69832) and supported by the Director, Office of Science, Office of Basic Energy Sciences, and the Division of Chemical Sciences, Geosciences, and Biosciences of the U.S. Department of Energy at LBNL under Contract No. DE-AC02-05CH11231. This technology is licensed by the University of California to Lumiphore, Inc., in which some of the authors have a financial interest.
Biographies
Evan G. Moore completed his B.S. and Ph.D. at the University of Queensland under the supervision of Prof. Paul Bernhardt, before commencing postdoctoral work at U.C. Berkeley. He is currently an independent APD research fellow at the University of Melbourne, Australia.
Amanda P. S. Samuel received her B.S. in chemistry from Yale University in 2003 and her Ph.D. from U.C. Berkeley in 2008 under the direction of Prof. Kenneth N. Raymond. She is currently undertaking postdoctoral studies in Prof. Michael R. Wasielewski’s laboratory at Northwestern University.
Kenneth N. Raymond received a B.A. from Reed College in 1964 and his Ph.D. from Northwestern University in 1968 under the direction of Prof.’s Fred Basolo and James Ibers. He joined the faculty of U.C. Berkeley in 1967, and is currently U.C. Berkeley Chancellor’s Professor and Director of the Glenn T. Seaborg Center, Lawrence Berkeley National Laboratory.
References
- 1.Connelly NG, Damhus T, Hartshorn RM, Hutton AT. Nomenclature of Inorganic Chemistry: IUPAC Recommendations. RSC Publishing; Cambridge, UK: 2005. [Google Scholar]
- 2.Cox PA. The Elements, Their Origin, Abundance and Distribution. Oxford University Press; Oxford, UK: 1989. [Google Scholar]
- 3.Bunzli J-CG, Choppin GR. Lanthanide Probes in Life, Chemical and Earth Sciences: Theory and Practice. Elsevier; Amsterdam: 1989. [Google Scholar]
- 4.Goldschmidt VM, Barth T, Lunde G. Geochemical distribution law of the elements. V. Isomorphy and polymorphy of the sesquioxides. The contraction of the “lanthanums” and its consequences. Skrifter Norske Videnskaps-Akademi i Oslo, I. Mat.-Naturv. Klasse. 1925;7:59. [Google Scholar]
- 5.Richardson FS. Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chem. Rev. 1982;82:541–552. [Google Scholar]
- 6.Baes CF, Mesmer RE. The Hydrolysis of Cations. Wiley-Interscience; New York: 1976. [Google Scholar]
- 7.Smith RM, Martell AE. Critical Stability Constants. Plenum Press; New York: 1976. [Google Scholar]
- 8.D’Aleo A, Picot A, Beeby A, Williams JAG, Le Guennic B, Andraud C, Maury O. Efficient Sensitization of Europium, Ytterbium, and Neodymium Functionalized Tris-Dipicolinate Lanthanide Complexes through Tunable Charge-Transfer Excited States. Inorg. Chem. 2008;47:10258–10268. doi: 10.1021/ic8012969. [DOI] [PubMed] [Google Scholar]
- 9.Hemmila I, Dakubu S, Mukkala VM, Siitari H, Lovgren T. Europium as a label in time-resolved immunofluorometric assays. Anal. Biochem. 1984;137:335–343. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- 10.Comley J. TR-FRET Based Assays-Getting Better With Age. Drug Discovery World, Spring. 2006:22–37. [Google Scholar]
- 11.Alpha B, Lehn J-M, Mathis G. Energy-transfer luminescence of europium(III) and terbium(III) with macrobicyclic polypyridine ligands. Angew. Chem. 1987;99:259–261. [Google Scholar]
- 12.Cross JP, Dadabhoy A, Sammes PG. The sensitivity of the Lehn cryptand-europium and terbium(III) complexes to anions compared to a coordinatively saturated systems. J. Lumin. 2004;110:113–124. [Google Scholar]
- 13.Ge P, Selvin PR. Carbostyril derivatives as antenna molecules for luminescent lanthanide chelates. Bioconj. Chem. 2004;15:1088–1094. doi: 10.1021/bc049915j. [DOI] [PubMed] [Google Scholar]
- 14.Clarkson IM, Beeby A, Bruce JI, Govenlock LJ, Lowe MP, Mathieu CE, Parker D, Senanayake K. Experimental assessment of the efficacy of sensitized emission in water from a europium ion, following intramolecular excitation by a phenanthridinyl group. New J. Chem. 2000;24:377–386. [Google Scholar]
- 15.Förster T. Excitation transfer and internal conversion. Chem. Phys. Lett. 1971;12:422–424. [Google Scholar]
- 16.Dexter DL. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953;21:836–850. [Google Scholar]
- 17.Horrocks WD, Sudnick DR. Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J. Am. Chem. Soc. 1979;101:334–340. [Google Scholar]
- 18.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, Sousa A. S. d., Williams JAG, Woods M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 2. 1999:493–504. [Google Scholar]
- 19.Supkowski RM, Horrocks W, De W., Jr. On the determination of the number of water molecules, q, coordinated to europium(III) ions in solution from luminescence decay lifetimes. Inorg. Chimica Acta. 2002;340:44–48. [Google Scholar]
- 20.Latva M, Takalo H, Mukkala V-M, Matachescu C, Rodriguez-Ubis JC, Kankare J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997;75:149–169. [Google Scholar]
- 21.Kusaba M, Nakashima N, Kawamura W, Izawa Y, Yamanaka C. Higher yield of photoreduction from europium (3+) to europium (2+) with shorter wavelength irradiation. Chem. Phys. Lett. 1992;197:136–40. [Google Scholar]
- 22.Cohen SM, Petoud S, Raymond KN. A novel salicylate-based macrobicycle with a “split personality”. Inorg. Chem. 1999;28:4522–4529. doi: 10.1021/ic990411k. [DOI] [PubMed] [Google Scholar]
- 23.Petoud S, Cohen SM, Bunzli J-C, Raymond K. Stable lanthanide luminescence agents highly emissive in aqueous solution: multidentate 2-Hydroxyisophthalamide complexes of Sm3+, Eu3+, Tb3+, Dy3+ J. Am. Chem. Soc. 2003;125:13324–13325. doi: 10.1021/ja0379363. [DOI] [PubMed] [Google Scholar]
- 24.Samuel APS, Moore EG, Melchior M, Xu J, Raymond KN. Water-soluble 2-Hydroxyisophthalamides for sensitization of lanthanide luminescence. Inorg. Chem. 2008;47:7535–7544. doi: 10.1021/ic800328g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petoud S, Muller G, Moore EG, Xu J, Sokolnicki J, Riehl JP, Le UN, Cohen SM, Raymond KN. Brilliant Sm, Eu, Tb, and Dy chiral lanthanide complexes with strong circularly polarized luminescence. J. Am. Chem. Soc. 2007;129:77–83. doi: 10.1021/ja064902x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seitz M, Moore EG, Ingram AJ, Muller G, Raymond KN. Enantiopure, octadentate ligands as sensitizers for europium and terbium circularly polarized luminescence in aqueous solution. J. Am. Chem. Soc. 2007;129:15468–15470. doi: 10.1021/ja076005e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuel APS, Xu J, Raymond KN. Predicting Efficient Antenna Ligands for Tb(III) Emission. Inorg. Chem. 2008 doi: 10.1021/ic801904s. accepted, in press. [DOI] [PubMed] [Google Scholar]
- 28.Moore EG, Xu J, Jocher CJ, Werner EJ, Raymond KN. “Cymothoe sangaris”: An extremely stable and highly luminescent 1,2-Hydroxypyridinonate chelate of Eu(III) J. Amer. Chem. Soc. 2006;128:10648–10649. doi: 10.1021/ja062597+. [DOI] [PubMed] [Google Scholar]
- 29.Tedeschi C, Azema J, Gornitzka H, Tisnes P, Picard C. A solid-state study of eight-coordinate lanthanide(III) complexes (Ln = Eu, Gd, Tb, Dy) with 1-hydroxy-2-pyridinone. Dalton Trans. 2003:1738–1745. [Google Scholar]
- 30.Tedeschi C, Picard C, Azema J, Donnadieu B, Tisnes P. First crystal structure of a Tb3+ complex derived from an aromatic hydroxamate ligand: sensitized luminescence properties. New J. Chem. 2000;24:735–737. [Google Scholar]
- 31.Moore EG, Xu J, Jocher CJ, Castro-Rodriguez I, Raymond KN. Highly luminescent lanthanide complexes of 1-Hydroxy-2-pyridinones. Inorg. Chem. 2008;47:3105–3118. doi: 10.1021/ic702144n. [DOI] [PubMed] [Google Scholar]
- 32.Jocher CJ, Moore EG, Xu J, Avedano S, Botta M, Aime S, Raymond KN. 1,2-Hydroxypyridonates as contrast agents for magnetic resonance imaging: TREN-1,2-HOPO. Inorg. Chem. 2007;46:9182–9191. doi: 10.1021/ic700985j. [DOI] [PubMed] [Google Scholar]
- 33.Moore EG, Jocher CJ, Xu J, Werner EJ, Raymond KN. An octadentate luminescent Eu(III) 1,2-HOPO chelate with potent aqueous stability. Inorg. Chem. 2007;46:5468–5470. doi: 10.1021/ic700364t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werts MHV, Jukes RTF, Verhoeven JW. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002;4:1542–1548. [Google Scholar]
- 35.Beeby A, Bushby LM, Maffeo D, Williams JAG. Intramolecular sensitisation of lanthanide(III) luminescence by acetophenone-containing ligands. The critical effect of para-substituents and solvent. J. Chem. Soc., Dalton Trans. 2002:48–54. [Google Scholar]
- 36.Trinquet E, Gregor N, Degorce F, Tardieu J-L, Seguin P. Introduction of a New TR-FRET Terbium Cryptate (Poster 11023); Presented at Society for Biomolecular Sciences, Annual Conference and Exhibition; St. Louis, MO, USA. April 6th-10th, 2008; see also http://www.htrf.com/files/resources/poster_ip-one-tb_sbs_08_ss-coupe.pdf. [Google Scholar]
- 37.Reifernberger JG, Ge P, Selvin PR. Progress in lanthanides as luminescent probes. Rev. Fluorescence. 2005;2:399–431. [Google Scholar]