Abstract
Several studies strongly suggest that DC differentiated in vitro in the presence of type I IFN acquire more potent immune stimulatory properties, compared with DC differentiated in vitro with IL-4. However, little is known about the molecular mechanisms underlying this phenomenon. To address this question, we compared the Ag-processing machinery (APM) profile in human DC grown in the presence of IFN-α (IFNDC) or IL-4 (IL-4DC). Using a panel of APM component-specific mAb in Western blot experiments, we found that IFNDC preferentially express inducible proteasome subunits (LMP2, LMP7, and MECL1) both at immature and mature stages. In contrast, immature IL-4DC co-express both constitutive (β1, β2, and β5) and inducible subunits, as shown by Western blotting analysis. In addition, immature IFNDC express higher levels of TAP1, TAP2, calnexin, calreticulin, tapasin, and HLA class I molecules than IL-4DC. The different proteasome profiles of IFNDC and IL-4DC were associated with a greater ability of IFNDC to present an immunodominant epitope that requires LMP7 expression for its processing. In general, these data show the impact of cytokines on APM component expression and hence the Ag-processing ability of DC.
Keywords: Antibodies, Antigen processing, Cytokines, Human DC
Introduction
DC play a key role as APC in the initiation of specific immune responses [1, 2]. In recent years, attention has been focused on the possibility that tissue microenvironment could markedly influence the phenotype and functional characteristics of DC, thus shaping the immunological effector mechanisms from the first steps of the immune response. For this reason, understanding the effect on DC maturation exerted by cytokines, known to be released in the peripheral tissue, is a crucial issue in immunology. Changes associated with DC maturation include up-regulation of MHC and co-stimulatory molecule expression, and a progressive change in Ag-processing machinery (APM) component expression [3–5]. The proteasome is a central element of APM; it may be expressed in two forms, constitutive and inducible (also called immunoproteasome), endowed with different protein cleavage specificities [6, 7]. The constitutive proteasome is a four-ring structure; the outer rings contain seven non-catalytic α-type subunits, whereas the inner rings contain seven β-type subunits, three of which are catalytic (delta/β1, Z/β2, and X/β5). The immunoproteasome contains alternative forms of the catalytic subunits (LMP2, MECL1, and LMP7), that replace the corresponding constitutive homologs β1, β2, and β5 [8–10].
Differences in the protein cleavage patterns between the constitutive proteasome and the immunoproteasome have been demonstrated, and the spectrum of antigenic peptides produced by a cell may eventually vary under different physiological and pathological stimuli [11]. Most importantly, it has been found that a number of antigenic peptides, mainly derived from self-proteins, are not processed efficiently by the immunoproteasome [7, 12]; they include tumor antigen epitopes that are utilized as targets of T-cell-based immunotherapy. On the other hand, the replacement of constitutive proteasome by the immunoproteasome in sites of infection [13] suggests that, during the peak phase of viral and bacterial elimination, the CTL response mainly targets immunoproteasome-dependent T-cell epitopes [14, 15]. This notion has important implications for the design of immunotherapeutic strategies and vaccines not only in malignant diseases but also in other pathological conditions, such as viral infections and autoimmunity.
Monocyte-derived mature DC are traditionally generated by in vitro exposure to GM-CSF and IL-4, followed by an additional stimulus, such as CD40L or LPS, which may induce terminal DC maturation. Because IL-4 may not represent the earliest physiological signal in DC generation, the effects of type I IFN on DC differentiation from human monocytes have been recently studied [16]. In particular, data obtained in different models demonstrated that DC generated in the presence of type I IFN (IFNDC) are as potent stimulators of naive CD4+ T cells as DC grown in the presence of IL-4 (IL-4DC), but much more efficient APC in inducing CTL responses [17]. It is noteworthy that the majority of these results were obtained by culturing DC in the presence of FBS.
Little is known, however, about the effect of type I IFN on APM component expression in DC. Taking advantage of a panel of mAb specific for the three constitutive catalytic subunits β1, β2, and β5 and the three inducible homologs LMP2, MECL1, and LMP7, we compared the expression profile of the various components of proteasome in immature and mature DC cultured in human serum conditioned media and differentiated in the presence of GM-CSF/IFN-α and of GM-CSF/IL-4. We here show that DC differentiation in the presence of type I IFN is associated with preferential expression of immunoproteasome subunits at both immature and mature stages. On the contrary, differentiation in the presence of IL-4 entails co-expression of constitutive proteasome and immunoproteasome subunits. IFNDC and IL-4DC also differ in the expression of the other APM components, such as TAP1, TAP2, and tapasin, which are all expressed at higher levels in immature IFNDC and reach similar levels in IL-4DC only following in vitro stimulation with LPS. This different proteasome profile is paralleled by a greater ability of IFNDC to present an immunodominant epitope that requires LMP7 expression for its processing.
Results
Distinct profile of DC generated in the presence of IFN-α and IL-4
We first compared the effect of GM-CSF/IFN-α and GM-CSF/IL-4 on the phenotypic profile of purified CD14+ monocytes cultured in the presence of human serum. Results of a representative experiment out of 12 consecutive experiments are shown in Fig. 1; statistical differences between mean values obtained in the different culture conditions were calculated according to the Mann–Whitney test. As far as the immunophenotypic profile is concerned, following a 3-day culture at 37°C, IFNDC and IL-4DC displayed a comparable expression of CD83 (6±6 versus 8.4±10%; p=0.95). On the contrary, some differences were found in the expression of CD14 and CD86. CD86 had a significantly lower expression on IFNDC than on IL-4DC (66±18.3 versus 85±10.6%; p<0.05), whereas the reverse was true for CD14 (65±19 versus 36±29%; p=0.05). Both IFNDC and IL-4DC expressed HLA class I and class II antigens, but the expression levels were significantly higher on IFNDC for HLA class I molecules (MFI 691±21.7 versus 568±46; p<0.05); HLA class II antigen expression was similar on IFNDC and IL-4DC (MFI 446±52.2 versus 416±6; p=0.89). DC-SIGN, a typical DC marker, was expressed in immature IL-4DC (59.5±5.6%), but not in IFNDC (0.9±0.3%), as previously reported [18] (not shown).
Figure 1.
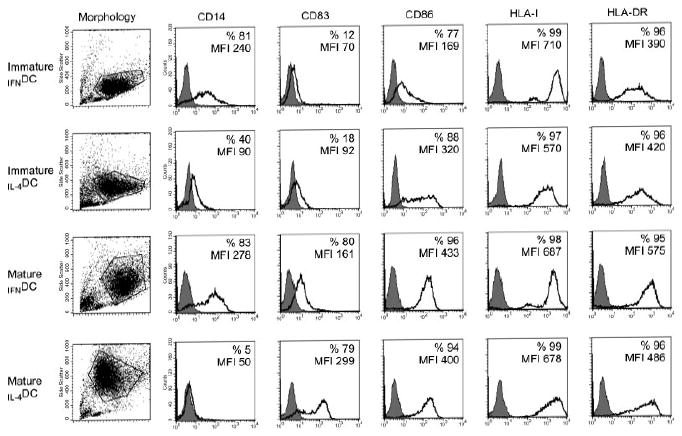
Differential antigenic profile of immature and mature IFNDC and IL-4DC. The morphological pattern of each DC population studied is shown in the first left panel by analyzing side scatter/forward scatter profile, and the area delimited in the dot plot panel excludes monocytes, debris, and dead cells. For each DC population (indicated on the left), the markers analyzed are depicted at the top of each column; the percentage of positive cells and the geometric MFI values are reported in each panel. Shaded histograms represent staining with an isotype-matched control mAb. One representative experiment out of 12 independent experiments is shown.
After 48 h of incubation at 37°C in the presence of LPS, both IFNDC and IL-4DC showed signs of maturation. CD83 expression was up-regulated on IFNDC and to a greater extent on IL-4DC (67.2±12.8 versus 81±16.6%, respectively). In addition, IFNDC and IL-4DC expressed comparable levels of CD86 (98.6±2.2 versus 97.6±3.1%) and of HLA class I antigens (MFI 681±31.4 versus 672±5.6). However, IFNDC expressed higher levels of HLA-DR antigens (MFI 562±13.5 versus 495±12.2; p<0.05 according to the Mann–Whitney test) and of CD14. The latter was strongly down-regulated on IL-4DC (4.4±12.6 versus 67±28% in IFNDC). As far as CD14 is concerned, its rather high expression might argue for a poor differentiation from the monocyte stage. However, it is noteworthy that the MFI values of CD14+ cells in IFNDC and IL-4DC, both at immature (geometric MFI 203±40 versus 67±27, respectively) and mature (geometric MFI 210±68 versus 54±3, respectively) stages, were significantly lower than the MFI values of monocytes (510±6). We could speculate that a pronounced CD14 expression is a feature of immature and mature DC grown in the presence of human serum. Indeed, as previously reported [19], when DC were grown in human serum, CD1a was not detectable (or barely detectable) on both IFNDC and IL-4DC (data not shown). Lastly, DC-SIGN (not shown) was still expressed in mature IL-4DC (39.5±5.6%), but not in mature IFNDC (0.4±0.2%).
To evaluate whether differences in DC phenotype induced by cytokines correlated with T-cell-stimulatory capacity, DC precultured with cytokines for 3 days were tested for their ability to stimulate monocyte-depleted allogeneic PBL at different stimulator/responder ratios. Proliferative responses were compared with those obtained with LPS-treated DC and CD14+-untreated monocytes. A comparable stimulatory capacity of immature IFNDC and IL-4DC was observed. As expected, LPS-treated DC and untreated CD14+ monocytes induced the highest and the lowest level of proliferation, respectively (Fig. 2).
Figure 2.
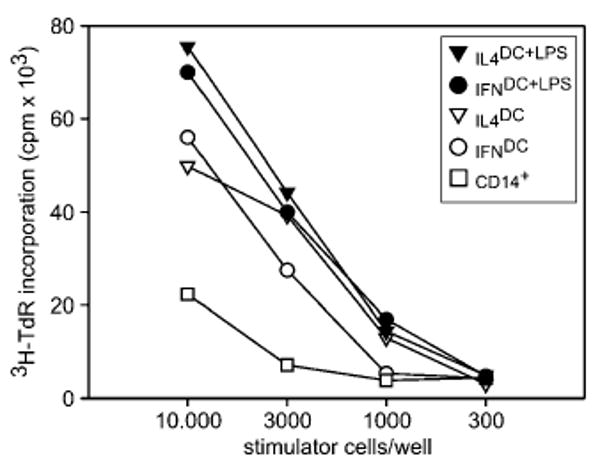
MLR with IFNDC and IL-4DC. Different numbers of allogeneic immature DC, LPS-treated DC, or CD14+ monocytes, obtained as described in the Materials and methods, were added to monocyte-depleted PBMC. Proliferative responses of 6-day cultures were measured by 3H-TdR incorporation. One representative experiment out of three independent experiments is shown.
Because immature DC are very effective in capturing and processing Ag, the ability of IFNDC and IL-4DC to take-up apoptotic tumor cells was also compared. To this end, IFNDC and IL-4DC, labeled with the fluorescent dye PKH-67 (green), were mixed with apoptotic melanoma cells labeled with the fluorescent dye PKH-26 (red), and incubated for 20 h at 37°C to allow phagocytosis. After incubation, the uptake of apoptotic cells by DC was evaluated by FACS analysis as the percentage of double-stained cells. As shown in Fig. 3A and C, in control cultures maintained on ice, the phagocytic activity of both immature IFNDC and IL-4DC was negligible. On the other hand, when apoptotic bodies were mixed with DC at 37°C, IFNDC and IL-4DC showed strong and comparable phagocytic activity (Fig. 3B and D). LPS treatment of IFNDC and IL-4DC did not abolish, but strongly reduced their phagocytic activity (64±8 versus 19±9 in immature and mature IFNDC, respectively; 69±7 versus 11±8 in immature and mature IL-4DC, respectively), indicating that both types of immature DC were fully susceptible to undergo terminal differentiation, that paralleled the increase in the expression of HLA and other accessory molecules as mentioned at the beginning of this section.
Figure 3.
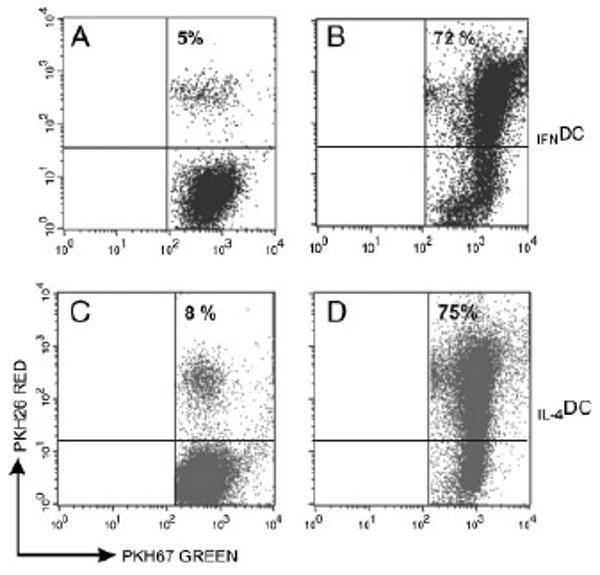
Phagocytic activity of immature IFNDC and IL-4DC. Phagocytosis was evaluated as the number of double-positive DC by flow cytometry. (A) and (C) depict the percentage of positive IFNDC and IL-4DC in control cultures maintained at 4°C, whereas (B) and (D) show the percentage of positive IFNDC and IL-4DC incubated with apoptotic PDO-267-MEL cells at 37°C. One representative experiment out of three is shown.
Differential proteasome subunit expression in IFNDC and IL-4DC
Subsequently, we investigated the expression of proteasome subunits in IFNDC and IL-4DC, utilizing a panel of subunit-specific mAb. When the mAb were preliminarily tested in Western blotting with control T2 and Colo38 melanoma cells, T2 cells expressed the constitutive subunits β1, β2, and β5 (Fig. 4A). As expected, LMP2 and LMP7 were not detectable, because T2 cells lack the encoding genes (Fig. 4A). On the other hand, Colo38 cells showed the concomitant expression of constitutive and inducible subunits (Fig. 4A). Treatment with IFN-γ or IFN-α was associated with a marked conversion of the proteasome profile toward immunoproteasome expression (Fig. 4A).
Figure 4.
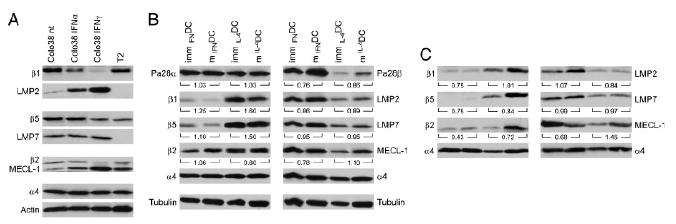
Differential proteasome subunit expression in IFNDC and IL-4DC. β1-specific mAb SY-5, β5-specific mAb SJJ-3, β2-specific mAb NB1, LMP2-specific mAb SY-1, MECL1-specific mAb TO-6, LMP7-specific mAb HB2, and rabbit polyclonal antisera to PA28α or PA28β were tested in Western blotting with lysates of T2 cells and untreated or Type I/Type II IFN-treated Colo38 melanoma cells (A), with lysates of immature and mature IFNDC and IL-4DC (B), and with components immunoprecipitated by the α2-specific mAb from immature and mature IFNDC and IL-4DC (C). Actin and tubulin were used as loading controls in (A) and (B), respectively. Antibodies against the non-catalytic α4 chain were used in (B) and (C) as a control for total proteasome contents. The numbers under each DC pair indicate the ratio of the intensity of the bands between immature and mature DC, calculated as detailed in the Materials and methods.
Western blot analysis showed a different constitutive and inducible proteasome subunit profile in DC, differentiated in the presence of IFN-α or IL-4 (Fig. 4B). Immature IFNDC preferentially expressed the immunoproteasome subunits (Fig. 4B). In contrast, immature IL-4DC co-expressed both constitutive and inducible proteasome subunits (Fig. 4B). Following LPS-induced maturation, both IFNDC and IL-4DC did not substantially change their proteasome profile (Fig. 4B), thus indicating that IL-4DC were substantially unable to skew their proteasome profile in favor of the synthesis and assembly of inducible proteasome subunits. The loading control with the constitutively expressed α4 chain (Fig. 4B) indicated no apparent change in proteasome contents in either culture conditions. In addition, no difference was detected in PA28α expression by mature and immature IFNDC and IL-4DC (Fig. 4B). On the contrary, the PA28β subunit showed a much higher expression in immature IFNDC than IL-4DC. In addition, LPS-induced maturation was associated with a slight increase in its expression in DC grown in either conditions (Fig. 4B).
To address the potential interference of free subunits not incorporated into proteasome structures, DC lysates were first immunoprecipitated with a mAb specific for the non-catalytic subunit α2, which recognizes all proteasome complexes. As shown in a representative experiment (Fig. 4C), a clear profile of inducible subunit expression was obtained. Again, internal controls with the non-catalytic α4 subunit showed no difference in proteasome content in the various experimental conditions (Fig. 4C). Altogether, these results are compatible with the possibility that IFNDC preferentially express inducible proteasome subunits at both immature and mature stages, whereas immature and mature IL-4DC co-express both proteasome forms.
Differential TAP1, TAP2, calnexin, calreticulin, and tapasin expression in IFNDC and IL-4DC
We then tested whether the differences in proteasome subunit profile observed in IFNDC and IL-4DC were associated with differential expression of other APM components. In all the experiments, T2 and Colo38 cells were used as a negative and a positive control, respectively. As expected, T2 cells were not stained by TAP1- and TAP2-specific mAb, whereas Colo38 cells did show strong expression of both molecules following IFN-γ stimulation (Fig. 5A). As far as immature DC is concerned, marked differences emerged for all these components, in particular for TAP1, TAP2, and tapasin, which consistently showed a higher expression in immature IFNDC than in immature IL-4DC (Fig. 5B). The geometric MFI values were 229 and 116 for TAP1, 317 and 162 for TAP2, and 240 and 157 for tapasin, respectively. After 48 h of stimulation with LPS at 37°C, the expression of these components was comparable in IFNDC and IL-4DC (Fig. 5B). The geometric MFI values were 190 and 209 for TAP1, 220 and 238 for TAP2, and 219 and 247 for tapasin, respectively. On the whole, our results indicate that immature IFNDC, compared with IL-4DC, express a substantially different profile of most APM components. Following terminal DC maturation, most of the differences between DC grown in the presence of the two cytokine cocktails were no longer evident.
Figure 5.
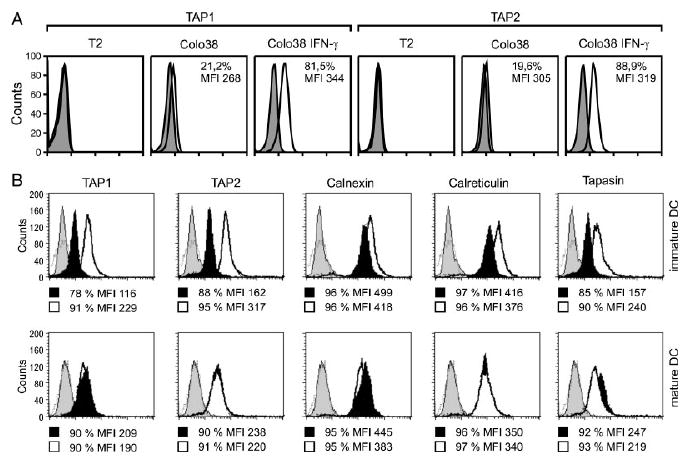
Differential APM component expression in immature and mature IFNDC and IL-4DC. (A) Permeabilized control T2 and Colo38 cells were incubated with TAP1- and TAP2-specific mAb and analyzed by flow cytometry as a control. The percentage of positive cells and MFI are reported in each panel. (B) Permeabilized immature and mature IFNDC (bold line histogram) and IL-4DC (black filled histogram) were incubated with TAP1-specific mAb NOB1, TAP2-specific mAb NOB2, calreticulin-specific mAb TO-11, calnexin-specific mAb TO-5, and tapasin-specific mAb TO-3 and subsequently stained with FITC-conjugated goat anti-mouse IgG antibodies. Stained cells were analyzed by flow cytometry. Cells incubated only with FITC-labeled goat anti-mouse IgG antibodies (gray filled and broken line histogram) were used as a negative control. The percentage of positive cells and MFI are reported under each panel.
Differential functional activity of IFNDC and IL-4DC
The differences in proteasome subunit expression profile observed in IFNDC and IL-4DC prompted us to compare the in vitro ability of these cells to present antigens via the MHC class I pathway by using two approaches. In a first set of experiments, we evaluated the ability of IFNDC and IL-4DC, obtained from HLA-A2+ donors and incubated with the HIV p17-Gag protein, to induce the expansion of autologous epitope-specific CD8+ T lymphocytes. The p17-Gag protein was chosen because the presentation of its immunodominant A2-restricted Gag77–89 epitope (SL9) strictly depends on the proteolytic activity of the LMP7 subunit [20]. To determine the percentage of antigen-specific T lymphocytes in cultures primed and expanded with the different DC preparations, recovered cells were analyzed by four-color immunofluorescence with CD8-specific and CD3-specific mAb in association with specific and irrelevant multimers. As shown in Fig. 6A, IFNDC incubated with the whole HIV p17-Gag protein showed a greater ability to expand SL9-specific CD8+ T lymphocytes than IL-4DC. Interestingly, the opposite was true when IFNDC and IL-4DC loaded with the synthetic SL9 peptide were used as stimulators (Fig. 6A). On the other hand, no difference was detected when IFNDC and IL-4DC, loaded with the highly immunogenic MelanA/A2 modified peptide, were used in control cultures to generate autologous MelanA/A2-specific CD8+ T lymphocytes (Fig. 6B). These results argue against the possibility that the capability of IFNDC incubated with the whole p17-Gag protein to initiate and expand a primary response would be the result of intrinsic superior immunostimulatory properties.
Figure 6.
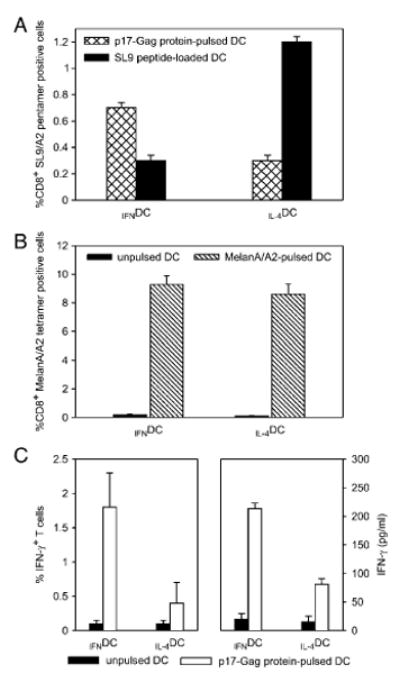
Differential stimulatory activity of IFNDC and IL-4DC pulsed with HIV p17-Gag protein. (A) Cytofluorimetric analysis of SL9 peptide-specific CD8+ T lymphocytes in cultures generated by in vitro stimulation with p17-Gag protein-pulsed (hatched columns) or SL9 peptide-loaded (closed columns) IFNDC and IL-4DC. Mean values ± 1 SD of three consecutive experiments are shown. (B) Cytofluorimetric analysis of MelanA/A2 peptide-specific CD8+ T lymphocytes in cultures generated by in vitro stimulation with IFNDC and IL-4DC unpulsed and pulsed with the MelanA26–35 modified peptide. Results show mean values ± 1 SD of three consecutive experiments. Closed columns depict values in control cultures incubated with unpulsed DC. (C) Intracellular IFN-γ staining (left box) and IFN-γ production in culture supernatants (right box) of a SL9 peptide-specific CD8+ T-cell line stimulated with p17-Gag protein-pulsed IFNDC or IL-4DC. Incubation of CD8+ T cells with either DC type was carried out for 5 h in the case of intracellular staining, and for 48 h in the case of IFN production. Results show mean values ± 1 SD of three consecutive experiments. Closed columns depict values in control cultures incubated with unpulsed DC. Both intracellular IFN-γ staining and IFN-γ release in culture supernatants after stimulation with IFNDC were significantly higher than in the corresponding co-cultures containing IL-4DC (p<0.01 according to the Mann–Whitney test).
To further confirm the greater ability of IFNDC to present the SL9 epitope, we generated a p17-specific CD8+ T-cell line to assess the presentation of the processed epitope in a short-term readout such as IFN-γ release. To this end, an SL9-specific CD8+ T-cell line was incubated for 5 h with IFNDC and IL-4DC, pulsed with the p17-Gag protein, and IFN-γ production was measured by both intracellular staining and ELISA. As shown in Fig. 6C, the percentage of SL9-specific CD8+ T cells showing intracellular IFN-γ staining after stimulation with IFNDC was significantly higher than that found in the corresponding co-cultures containing IL-4DC (Fig. 6C, left panel, p<0.01 according to the Mann–Whitney test). In addition, significantly higher levels of IFN-γ in the culture supernatant of specific CD8+ T cells incubated with IFNDC were also found (Fig. 6C, right panel; p<0.01). On the whole, these data suggest that the pattern of proteasome composition induced by type I IFN may play a role in modulating the processing ability of DC and this can have a substantial impact on T-cell activation.
Discussion
In this study, we compared the phenotypic profile of human DC differentiated from circulating monocytes in the presence of GM-CSF/IFN-α or GM-CSF/IL-4, as well as their patterns of expression of proteasome subunits and other APM components. In addition, we addressed the possible functional significance of the differential APM component expression in these two cell types. The most relevant finding emerging from our study is the observation that IFNDC preferentially express inducible proteasome subunits (LMP2, LMP7, and MECL1) at both immature and mature stages, whereas immature IL-4DC co-express both constitutive and inducible proteasome subunits. As far as the other APM components are concerned, we also showed that immature DC differentiated in the presence of IFN-α express higher levels of TAP1, TAP2, and tapasin, compared with IL-4DC. On the other hand, the difference in the expression of these APM components was no longer evident when DC were terminally matured in the presence of LPS, and only the different proteasome profile differentiated the two DC types. These findings, along with the higher HLA class I antigen expression, suggest a substantially more advanced differentiation profile of immature IFNDC, compared with immature IL-4DC. However, this profile was not associated with a clear evidence of maturation, as immature IFNDC showed intact phagocytic properties, a function that is considered a reliable marker of an immature status [21], and underwent terminal differentiation after LPS treatment. In agreement with findings by Macagno et al. [3], however, we found a substantial inability of IL-4DC to skew their proteasome profile toward the synthesis and assembly of inducible proteasome subunits. Indeed, following LPS activation, as also shown by others [5], the expected switch toward an immunoproteasome phenotype was not observed. Nevertheless, we cannot exclude the presence of hybrid, “asymmetric” proteasomes that could account for the apparently puzzling finding that DC maturation is not always associated with a decrease in constitutive and an increase in inducible subunit expression [22, 23]. Unfortunately, immunoprecipitation approaches with α subunit-specific antibodies do not help in discriminating between mature proteasomes and precursor or hybrid forms.
The different proteasome composition of IFNDC and IL-4DC entailed functional consequences too, and was associated with a differential ability to present an immunodominant peptide, the generation of which from the native protein is strictly dependent on the presence of the inducible LMP7 subunit [20]. Nonetheless, we could not attribute the greater ability of IFNDC to process p17 protein solely to the expression of inducible proteasome subunits. Indeed, this might also be due, at least in part, to a differential binding of regulatory components in DC grown under different conditions. In fact, although apparently equal amounts of PA28α were observed in all DC preparations (Fig. 4B), the expression of PA28β, which is a key limiting component of the regulatory complex [24], was much higher in immature and mature IFNDC. However, we can rule out trivial explanations for the greater presenting capacity of IFNDC, such as co-stimulation through Toll receptors due to endotoxin contamination of p17-Gag preparations that was below the detection limits of a Limulus assay.
Surprisingly, when DC were directly pulsed with the SL9 peptide, IL-4DC showed a greater ability to stimulate SL9-specific T cells than IFNDC. This puzzling observation was reproducible in different experiments, and comparable results were obtained with both immature and LPS-matured DC. This apparent discrepancy could not be attributed to different immunostimulatory properties of IFNDC and IL-4DC, because: (i) both DC types showed comparable stimulatory ability in mixed lymphocyte reaction (MLR) readout (Fig. 2); (ii) incubation with a modified MelanA/A2 peptide did not reveal any significant difference in the ability of DC grown under different conditions to expand specific CD8+ T lymphocytes (Fig. 6B); and (iii) a significant difference in accessory molecule expression was not observed in our phenotypic analysis (Fig. 1). Thus, we have no plausible explanation for the observation that IFNDC incubated with the p17-Gag protein give rise to a higher epitope presentation than IL-4DC, whereas the reverse is found when a processing-independent synthetic peptide of this protein is used. We can only advance that the SL9 peptide is preferentially generated by IFNDC in limiting amounts when the cells are fed with the whole protein, whereas a molar excess of exogenous peptide could bring about massive saturation of the HLA molecules, thus overwhelming the intrinsic presenting capability of the cells. In any case, these observations also suggest that in vitro incubation of DC with peptides may not entirely reflect what is happening under physiological conditions, and may invite one to exercise caution when drawing conclusions from studies performed with DC pulsed in vitro with exogenous peptides.
The data reported here provide important insights into the role that different cytokine combinations could exert on the APM profile during the differentiation process of human monocyte-derived DC. Based on these findings and in view of the different proteolytic activity of constitutive and inducible proteasome subunits, two major Ag-processing scenarios could be envisaged, depending on the cytokines involved in DC differentiation. On one hand, the concomitant expression of constitutive and inducible proteasome subunits, that seems to characterize DC generated in the presence of IL-4, would reasonably help in enhancing the diversity of Ag processing. On the other hand, the rapid skewing toward immunoproteasome assembly observed when DC undergo differentiation in the presence of IFN-α, could be an efficient mechanism to generate more restricted sets of epitopes, thus polarizing the immune response already at an early stage. Because type I IFN is one of the earliest factors produced in vivo after a microbial challenge, we might speculate that the early induction of immunoproteasome in DC could serve to focus the immune response toward T-cell epitopes exclusively or preferentially generated in inflamed tissues. On the other hand, we cannot exclude that the activity demonstrated here by type I IFN is inscribed in a more complex and still undefined network of cytokine activities. For example, it is known that type I IFN up-regulates IL-15 production from DC [25] and other cells of innate immune response [26], and IL-15 in turn is able to increase DC activation by enhancing APM component expression [27]. Thus, acting in concert, several cytokines involved in the early steps of immune response could cooperate in modulating its outcome.
Materials and methods
Antibodies
The β1-specific mAb SY-5, the β5-specific mAb SJJ-3, the β2-specific mAb NB1, the LMP2-specific mAb SY-1, the LMP7-specific mAb HB2, the MECL1-specific mAb TO-6, the calnexin-specific mAb TO-5, the calreticulin-specific mAb TO-11, and the tapasin-specific mAb TO-3 were developed and characterized as described [28, 29]. The TAP1-specific mAb NOB1, and the TAP2-specific mAb NOB2 were developed and characterized utilizing the strategy described elsewhere [28]. α2-specific purified mAb (clone MCP21), α4-specific purified mAb (clone MCP34), rabbit polyclonal antiserum specific for PA28α subunit, and rabbit polyclonal antiserum specific for PA28β subunit were purchased from Biomol International, L.P. (Plymouth Meeting, PA, USA). Actin-specific Ab (IgG fraction of goat antiserum) and tubulin-specific mAb (clone TU-02) were both purchased from Santa Cruz Biotechnology (Santa Cruz, CA USA). CD14-specific PE-labeled mAb, HLA-DR-specific FITC-labeled mAb, CD3-specific FITC-conjugated mAb (clone SK7), and CD8-specific PerCP-labeled mAb (clone SK1) were purchased from Becton Dickinson (San Diego, CA, USA), HLA-A, B, C-specific FITC-conjugated mAb and CD1a-specific FITC-conjugated mAb were purchased from Dako (Glostrup, Denmark), CD86-specific FITC-conjugated mAb was purchased from Calbiochem (San Diego, CA USA), and CD83-specific PE-labeled mAb was purchased from Immunotech (Marseilles, France).
Purified mouse IgG2b isotype control and purified mouse IgG1 isotype control were purchased from Southern Biotech (Birmingham, AL, USA). PE-labeled rabbit anti-mouse IgG antibodies were purchased from Dako. HRP-linked donkey anti-rabbit IgG antibodies were purchased from Amersham Bioscience (Little Chalfont, Buckinghamshire, UK). Mouse TrueBlot™ ULTRA HRP anti-mouse IgG and an FITC-conjugated anti-human DC-SIGN mAb (clone eB-h209) were both purchased from eBioscience (San Diego, CA, USA).
Cytokines
rGM-CSF, rIFN-γ, rIL-4, and rIL-7 were purchased from PeproTech (London, UK). rIFN-α2b was purchased from Schering-Plough (Milan, Italy). IL-2 was purchased from Chiron Corporation (Emeryville, CA, USA).
Cell lines
The melanoma cell line Colo38 was maintained at 37°C in a 5% CO2 atmosphere in RPMI 1640 medium (Euroclone, Life Sciences Division, Pero, Milan, Italy) supplemented with 10% heat-inactivated FBS (Life Technologies, Gaithersburg, MD, USA), l-arginine (116 mg/mL), l-asparagine (36 mg/mL), l-glutamine (216 mg/mL), streptomycin (0.1 mg/mL), and penicillin (200 U/mL). The T2-cell line, a human T- and B-lymphoblastoid cell hybrid [30], that shows large chromosomal deletions and loss of the genes encoding LMP2 and LMP7 subunits along with a number of other genes, including those encoding TAP1 and TAP2, was maintained in IMDM (Invitrogen, Carlsbad, CA, USA) supplemented with heat-inactivated FBS, l-arginine, l-asparagine, l-glutamine, streptomycin, and penicillin, at the same concentrations as those used for Colo38 cells, at 37°C, 8% CO2 in air. The melanoma-cell line PDO-267-MEL that had been established in our laboratory, was maintained in the same medium as that used for the T2-cell line, supplemented with 10nM hydrocortisone, insulin (5 μg/mL), transferrin (100 μg/mL), 10nM 17 β-estradiol, and 30nM sodium selenite.
Preparation of DC
PBMC were isolated from the peripheral blood of healthy volunteers, as reported elsewhere [31]. Monocytes were purified by negative selection using the MACS monocyte isolation kit (Miltenyi Biotec, Bergish Gladbach, Germany), according to the manufacturer's instructions. The purity of recovered cells was >80%, as determined by cytofluorimetric analysis with CD14-specific mAb (data not shown). Monocytes were plated at a concentration of 6 × 105 cells/mL in RPMI 1640 medium supplemented with 10% human serum (BioWhittaker, Walkersville, MD, USA) in the presence of rGM-CSF (50 ng/mL), and either rIL-4 (1000 U/mL) or rIFN-α2b (1000 U/mL) in 24-well tissue culture plates (Falcon BD Labware, Franklin Lakes, NJ, USA). Cells were harvested on day 3 and analyzed for the expression of CD1a, CD14, CD83, CD86, HLA-DR, and HLA class I antigens by flow cytometry as detailed in the Cytofluorimetric analysis section. To induce DC maturation, LPS from Escherichia coli (Sigma-Aldrich, St. Louis, MO, USA; 1 μg/mL) was added on day 3, and incubation was continued for an additional 48 h at 37°C. DC maturation was monitored by measuring the expression of CD14, CD83, CD86, HLA-DR, and HLA class I antigens as described in the Cytofluorimetric analysis section.
MLR
Monocyte-depleted PBL were seeded into round bottom 96-well plates (Falcon BD Labware) at 105 cells/well in IMDM medium supplemented with 10% human serum, l-arginine, l-asparagine, l-glutamine, streptomycin, and penicillin, as in the Cell lines section. Allogeneic IFNDC and IL-4DC, harvested on day 3 of culture as described in the Preparation of DC section and CD14+-untreated monocytes, were irradiated with 3000 rad, washed five times, diluted in fresh culture medium, and added to each well in triplicate at different stimulator/responder ratios. Following 6 days of incubation at 37°C in an 8% CO2 atmosphere, cell cultures were pulsed with 37 Bq methyl-3[H]thymidine (3H-TdR, Amersham) per well for 18 h and 3H-TdR incorporation was measured by liquid scintillation counting.
Phagocytosis of apoptotic tumor cells by DC
PDO-267-MEL cells were labeled with PKH-26 red dye (Sigma-Aldrich) according to the manufacturer's instructions, plated in Petri dishes (4 × 106), and irradiated for 2 min with an UV-B lamp (Vilber Lourmat, Marne-La-Vallée, France) to induce apoptosis. Cytofluorimetric analysis of apoptotic cells was performed as described elsewhere [32]. After irradiation, PDO-267-MEL cells were kept for 20 h in culture at 37°C to allow apoptosis to occur. The labeled apoptotic cells were harvested, washed, and finally incubated for 20 h at 37°C with immature or LPS-treated DC stained with PKH-67 green dye (Sigma-Aldrich) at a tumor cell:DC ratio of 2:1. Cytofluorimetric analysis of phagocytosis was performed as described elsewhere [22].
Cytofluorimetric analysis
Surface staining was performed by washing DC (2 × 105) with PBS containing 1% BSA and incubating them with an excess of labeled mAb for 30 min in an ice bath. Cells were then washed twice with PBS containing 1% BSA and analyzed on an FACScalibur flow cytometer (Becton Dickinson) using CellQuest Software (Becton Dickinson), as described elsewhere [33]. Intracytoplasmic staining of cells with APM component-specific mAb was performed as described elsewhere [5], with minor modifications. Briefly, cells were washed with PBS containing 1% BSA and fixed with 2% paraformaldehyde for 20 min at room temperature, washed extensively with PBS 1% BSA, re-suspended in 10 mL of PBS 0.5% BSA, transferred into glass flasks and subjected to microwave treatment for 60 s at low power (200 W). Cells were then chilled on ice for 10 min, washed, and placed in tubes at a concentration of 2–5 × 105 cells/tube. Cells were permeabilized for 30 min in PBS buffer containing 1% BSA and 0.1% NP40 (Sigma-Aldrich) and incubated with the primary mAb (0.1–10 μg/mL NP40 buffer) for 1 h at 4°C. After extensive washing with PBS 1% BSA, cells were incubated with secondary antibodies. T2 cells were used as a negative control for TAP1 and TAP2 expression. Colo38 treated with IFN-γ were used as a positive control for TAP1 and TAP2 expression. Controls with an isotype-matched mAb were run in parallel. The geometric MFI values were calculated by subtracting the MFI value of the background staining, determined with an isotype-matched mAb, from the MFI value of the staining with the tested mAb.
Staining of epitope-specific CD8+ T lymphocytes with HLA-A2-peptides complexes
For the identification of epitope-specific CD8+ T lymphocytes, 1–1.5 × 106 cells were washed, re-suspended in PBS with 1% human serum and incubated for 15 min at room temperature with the PE-labeled MelanA/A2 tetramer, produced as previously described [33], and APC-labeled SL9/A2 pentamer (Proimmune, Oxford, UK). FITC-labeled CD3-specific mAb and PerCP-labeled CD8-specific mAb were then added, and after 20 min of incubation at 4°C, cells were washed and analyzed by the FACSCalibur (Becton Dickinson).
Indirect immunoprecipitation and Western blotting
Cells (5 × 106) were re-suspended in iced lysis buffer containing 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 5mM EDTA, 1% NP-40, and protease inhibitors (Sigma-Aldrich). Following removal of insoluble material, the protein content of cell lysates was determined, and samples (50 μg/lane) were electrophoresed on 14% SDS-PAGE. The immunoprecipitation step was performed as previously described [34], with minor modifications. Briefly, an aliquot of cell lysates (500 μg) was incubated overnight at 4°C with purified α2-specific mAb (1 μg). TrueBlot™ Anti-Mouse IgG Beads were then added, and incubation was continued for an additional 1 h at 4°C under continuous rotation. Immunoprecipitates were washed three times with lysis buffer, boiled in loading buffer (Tris 0.5 M, pH 6.8, 10% SDS, 87% glycerol, 0.25% bromophenol blue, 0.6M 2-ME), and separated by SDS-PAGE as above, followed by transfer onto polyvinylidene fluoride membranes (Millipore, Bedford, MA USA). After overnight soaking in PBS containing 2% non-fatty milk and 0.1% Tween 20, membranes were washed and incubated overnight at 4°C in PBS–0.1% Tween–3% BSA with an optimal amount (1–10 μg/mL) of the subunit-specific mAb. After washing, membranes were incubated with an optimal amount of HRP rabbit anti-mouse IgG antibodies or Mouse TrueBlot™ ULTRA HRP anti-mouse IgG and developed by Supersignal West-Pico (Pierce Biotechnology, Rockford, IL, USA), according to the manufacturer's instructions. For quantification of Western blot data, densitometric analysis was performed by Image Quant 5.2; the band intensity was normalized against the relevant α4 control, and the ratio between values obtained in immature and mature DC was calculated.
In vitro expansion of Ag-specific CD8+ T lymphocytes
DC were obtained from HLA-A2+ donors as described in the Preparation of DC section. On the third day of culture, IFNDC and IL-4DC (1 × 106) were incubated for 4 h at 37°C with HIV p17-Gag protein (50 μg/mL) (kindly donated by Professor A. Caruso, Institute of Microbiology, University of Brescia, Brescia, Italy) in serum-free medium. This protein was brought to a >98% purity by reverse-phase fast protein liquid chromatography, and endotoxin contamination did not exceed the detection limits of a Limulus amoebocyte assay (0.1 U/mL) [35]. At the end of incubation, 10% human serum was added and cells were incubated for two additional days at 37°C. In parallel, IFNDC and IL-4DC (1 × 106) were pulsed for 2 h at 37°C with 20 μM SL9 peptide in serum-free medium (HLA-A2-restricted HIV p17-Gag77–85 peptide SLYNTVATL) or for 1 h at room temperature with 5 μM MelanA/A2 peptide in 1% human serum (HLA-A2-restricted MelanA/A226-35 modified peptide ELAGIGILTV) (both peptides purchased from Tecnogen, Caserta, Italy). HIV p17-Gag protein- and SL9 peptide-pulsed DC were irradiated with 3000 rad, washed, and cultured with autologous immunobead-purified CD8+ T cells (Miltenyi Biotec) at a T-cell:DC ratio of 5:1 in 48-well cluster plates (Falcon BD Labware). Cells were cultured at 37°C in an 8% CO2 atmosphere in IMDM, supplemented with 10% human serum, l-arginine (116 mg/mL), l-asparagine (36 mg/mL), l-glutamine (216 mg/mL), IL-2 (10 U/mL), IL-4 (10 ng/mL), IL-7 (10 ng/mL), streptomycin (0.1 mg/mL), and penicillin (200 U/mL). CD8+ T cells were stimulated every week with irradiated pulsed DC for 3–5 weeks. CD8+ T cells cultured under the same experimental conditions with unpulsed DC were used as a negative control.
Assay for LMP7-dependent epitope presentation
An SL9-specific CD8+ T-cell line, to be used as a short-term readout for LMP7-dependent epitope presentation, was generated by stimulating immunobead-purified CD8+ T lymphocytes weekly with autologous SL9-loaded DC, obtained under standard conditions (IL-4+GM-CSF), in the presence of 2–5 U/mL of IL-2 [36]. After three rounds of stimulation, SL9-specific CD8+ T cells were enriched by cytofluorimetric sorting with SL9/A2 pentamer and re-stimulated twice with p17-Gag protein-pulsed IFNDC or IL-4DC. IFN-γ production was tested by intracellular staining after a 5 h-incubation of the SL9-specific CD8+ T-cell line with IFNDC and IL-4DC (stimulator/responder ratio 1:1) in the presence of 1 μM monensin, according to manufacturer's instructions (Cytofix/Cytoperm™, Becton Dickinson), by double fluorescence with FITC-labeled anti-IFN-γ (Immunotech), and anti-CD8 mAb. In addition, supernatants of SL9-specific CD8+ T cells, stimulated with p17-Gag protein-pulsed IFNDC or IL-4DC for 48 h, were tested for IFN-γ content by a commercial ELISA (Endogen-Thermo Fisher, Rockford, IL, USA).
Statistical analysis
Data were analyzed utilizing the StatGraphics software (version 2.6), as previously reported [37]. Unless otherwise indicated, all the results were expressed as mean values ± 1 SD. The non-parametric Mann-Whitney test was used to compare quantitative variables.
Acknowledgments
We thank Dr. S. Minuzzo for her precious support in the immunoprecipitation and Western blotting procedures and P. Gallo for his invaluable help in the artwork preparation. This study was supported by grants from the Italian Association for Research on Cancer (AIRC); the Italian Federation for Research on Cancer (FIRC); MIUR-PRIN; the Ministry of Health; and PHS grants CA67609, CA110249, and CA113861 awarded by the National Cancer Institute, DHHS. In addition, we gratefully acknowledge the financial support of the Istituto Superiore di Oncologia (ISO) and the Fondazione Cariverona (Bando 2004).
Abbreviations
- APM
antigen-processing machinery
- IFNDC
DC grown in the presence of IFN-α
- IL-4DC
DC grown in the presence of IL-4
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Macagno A, Gilliet M, Sallusto F, Lanzavecchia A, Nestle FO, Groettrup M. Dendritic cells up-regulate immunoproteasomes and the proteasome regulator PA28 during maturation. Eur J Immunol. 1999;29:4037–4042. doi: 10.1002/(SICI)1521-4141(199912)29:12<4037::AID-IMMU4037>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Schuler-Thurner B, Schuler G, Huber C, Seliger B. Bipartite regulation of different components of the MHC class I antigen-processing machinery during dendritic cell maturation. Int Immunol. 2001;13:1515–1523. doi: 10.1093/intimm/13.12.1515. [DOI] [PubMed] [Google Scholar]
- 5.Whiteside TL, Stanson J, Shurin MR, Ferrone S. Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J Immunol. 2004;173:1526–1534. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 6.Groettrup M, Soza A, Kuckelkorn U, Kloetzel PM. Peptide antigen production by the proteasome: complexity provides efficiency. Immunol Today. 1996;17:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 7.Morel S, Levy F, Burlet-Schiltz O, Brasseur F, Probst-Kepper M, Peitrequin AL, Monsarrat B, et al. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000;12:107–117. doi: 10.1016/s1074-7613(00)80163-6. [DOI] [PubMed] [Google Scholar]
- 8.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 9.York IA, Goldberg AL, Mo XY, Rock KL. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol Rev. 1999;172:49–66. doi: 10.1111/j.1600-065x.1999.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 10.Kloetzel PM, Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;16:76–81. doi: 10.1016/j.coi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2:179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- 12.Chapiro J, Claverol S, Piette F, Ma W, Stroobant V, Guillaume B, Gairin JE, et al. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J Immunol. 2006;176:1053–1061. doi: 10.4049/jimmunol.176.2.1053. [DOI] [PubMed] [Google Scholar]
- 13.Khan S, van den Broek M, Schwarz K, de Giuli R, Diener PA, Groettrup M. Immunoproteasomes largely replace constitutive proteasomes during an antiviral and antibacterial immune response in the liver. J Immunol. 2001;167:6859–6868. doi: 10.4049/jimmunol.167.12.6859. [DOI] [PubMed] [Google Scholar]
- 14.Gileadi U, Moins-Teisserenc HT, Correa I, Booth BL, Jr, Dunbar PR, Sewell AK, Trowsdale J, et al. Generation of an immunodominant CTL epitope is affected by proteasome subunit composition and stability of the antigenic protein. J Immunol. 1999;163:6045–6052. [PubMed] [Google Scholar]
- 15.Chen W, Norbury CC, Cho Y, Yewdell JW, Bennink JR. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J Exp Med. 2001;193:1319–1326. doi: 10.1084/jem.193.11.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171:3385–3393. doi: 10.4049/jimmunol.171.7.3385. [DOI] [PubMed] [Google Scholar]
- 18.Gagliardi MC, Teloni R, Giannoni F, Pardini M, Sargentini V, Brunori L, Fattorini L, Nisini R. Mycobacterium bovis Bacillus Calmette-Guerin infects DC-SIGN-dendritic cell and causes the inhibition of IL-12 and the enhancement of IL-10 production. J Leukoc Biol. 2005;78:106–113. doi: 10.1189/jlb.0105037. [DOI] [PubMed] [Google Scholar]
- 19.Duperrier K, Eljaafari A, Dezutter-Dambuyant C, Bardin C, Jacquet C, Yoneda K, Schmitt D, et al. Distinct subsets of dendritic cells resembling dermal DCs can be generated in vitro from monocytes, in the presence of different serum supplements. J Immunol Methods. 2000;238:119–131. doi: 10.1016/s0022-1759(00)00147-2. [DOI] [PubMed] [Google Scholar]
- 20.Sewell AK, Booth BL, Jr, Cerundolo V, Phillips RE, Price DA. Differential processing of HLA A2-restricted HIV type 1 cytotoxic T lymphocyte epitopes. Viral Immunol. 2002;15:193–196. doi: 10.1089/088282402317340332. [DOI] [PubMed] [Google Scholar]
- 21.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klare N, Seeger M, Janek K, Jungblut PR, Dahlmann B. Intermediate-type 20 S proteasomes in HeLa cells: “asymmetric” subunit composition, diversity and adaptation. J Mol Biol. 2007;373:1–10. doi: 10.1016/j.jmb.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Drews O, Wildgruber R, Zong C, Sukop U, Nissum M, Weber G, Gomes AV, Ping P. Mammalian proteasome subpopulations with distinct molecular compositions and proteolytic activities. Mol Cell Proteomics. 2007;6:2021–2031. doi: 10.1074/mcp.M700187-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Ossendorp F, Fu N, Camps M, Granucci F, Gobin SJ, van den Elsen PJ, Schuurhuis D, et al. Differential expression regulation of the alpha and beta subunits of the PA28 proteasome activator in mature dendritic cells. J Immunol. 2005;174:7815–7822. doi: 10.4049/jimmunol.174.12.7815. [DOI] [PubMed] [Google Scholar]
- 25.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 26.Yamaji K, Nabeshima S, Murata M, Chong Y, Furusyo N, Ikematsu H, Hayashi J. Interferon-alpha/beta upregulate IL-15 expression in vitro and in vivo: analysis in human hepatocellular carcinoma cell lines and in chronic hepatitis C patients during interferon-alpha/beta treatment. Cancer Immunol Immunother. 2006;55:394–403. doi: 10.1007/s00262-005-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tourkova IL, Shurin GV, Chatta GS, Perez L, Finke J, Whiteside TL, Ferrone S, Shurin MR. Restoration by IL-15 of MHC class I antigen-processing machinery in human dendritic cells inhibited by tumor-derived gangliosides. J Immunol. 2005;175:3045–3052. doi: 10.4049/jimmunol.175.5.3045. [DOI] [PubMed] [Google Scholar]
- 28.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:385–393. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 29.Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, et al. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005;66:185–194. doi: 10.1111/j.1399-0039.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 30.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T–B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 31.Stievano L, Tosello V, Marcato N, Rosato A, Sebelin A, Chieco-Bianchi L, Amadori A. CD8+alpha beta+ T cells that lack surface CD5 antigen expression are a major lymphotactin (XCL1) source in peripheral blood lymphocytes. J Immunol. 2003;171:4528–4538. doi: 10.4049/jimmunol.171.9.4528. [DOI] [PubMed] [Google Scholar]
- 32.Merchant SH, Gonchoroff NJ, Hutchison RE. Apoptotic index by Annexin V flow cytometry: adjunct to morphologic and cytogenetic diagnosis of myelodysplastic syndromes. Cytometry. 2001;46:28–32. doi: 10.1002/1097-0320(20010215)46:1<28::aid-cyto1034>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Mandruzzato S, Rossi E, Bernardi F, Tosello V, Macino B, Basso G, Chiarion-Sileni V, et al. Large and dissimilar repertoire of Melan-A/MART-1-specific CTL in metastatic lesions and blood of a melanoma patient. J Immunol. 2002;169:4017–4024. doi: 10.4049/jimmunol.169.7.4017. [DOI] [PubMed] [Google Scholar]
- 34.Indraccolo S, Minuzzo S, Zamarchi R, Calderazzo F, Piovan E, Amadori A. Alternatively spliced forms of Igalpha and Igbeta prevent B cell receptor expression on the cell surface. Eur J Immunol. 2002;32:1530–1540. doi: 10.1002/1521-4141(200206)32:6<1530::AID-IMMU1530>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.De Francesco MA, Caruso A, Fallacara F, Canaris AD, Dima F, Poiesi C, Licenziati S, et al. HIV p17 enhances lymphocyte proliferation and HIV-1 replication after binding to a human serum factor. AIDS. 1998;12:245–252. doi: 10.1097/00002030-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Kan-Mitchell J, Bisikirska B, Wong-Staal F, Schaubert KL, Bajcz M, Bereta M. The HIV-1 HLA-A2-SLYNTVATL is a help-independent CTL epitope. J Immunol. 2004;172:5249–5261. doi: 10.4049/jimmunol.172.9.5249. [DOI] [PubMed] [Google Scholar]
- 37.Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, Clementi M, Chieco-Bianchi L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]


