Abstract
Gait measures have been shown to predict cognitive decline and dementia in older adults. Investigation of the neurobiology associated with locomotor function is needed to elucidate this relationship with cognitive abilities. This study aimed to examine magnetic resonance imaging (MRI; hippocampal volume)- and proton magnetic resonance spectroscopy (MRS; N-acetylaspartate to creatine (NAA/Cr) ratios)-derived hippocampal correlates of quantitative gait function (swing time (seconds), stride length (cm), and stride length variability (standard deviation)) in a subset of 48 nondemented older adults (24 males; mean age=81 years) drawn from the Einstein Aging Study, a community-based sample of individuals over the age of 70 residing in the Bronx, New York. Linear regression analyses controlling for age were used to examine hippocampal volume and neurochemistry as predictors of gait function. We found that stride length was associated with hippocampal volume (β=0.36, p=0.03; overall model R2=0.33, p=0.01), but not hippocampal neurochemistry (β=0.09, p=0.48). Stride length variability was more strongly associated with hippocampal NAA/Cr (β=−0.38, p=0.01; overall model R2=0.14, p=0.04) than hippocampal volume (β=−0.33, p=0.08). Gait swing time was not significantly related to any neuroimaging measure. These relationships remained significant after accounting for memory and clinical gait impairments. These findings suggest that nondemented older adults exhibit increased stride length variability that is associated with lower levels of hippocampal neuronal metabolism, but not hippocampal volume. Conversely, decreased stride length is associated with smaller hippocampal volumes, but not hippocampal neurochemistry. Distinct neurobiological hippocampal substrates may support decreased stride length and increased stride length variability in older adults.
Keywords: Motor processing, Memory, Hippocampus, Locomotion, Neuroimaging, Aging
1. Introduction
The number of adults over the age of 65 is projected to represent a rising proportion of the general population in the coming years (Wan et al., 2005). As the population ages, it is increasingly important to characterize the biological, cognitive, and motor manifestations of the aging process. The extant literature demonstrates that advancing age is associated with declining brain function in both motor and cognitive domains (Anstey and Low, 2004; Christensen, 2001; Fratiglioni et al., 1999) and that gait dysfunction (Camicioli et al., 1998; Verghese et al., 2002; Verghese et al., 2007) and poor performance on cognitive tests (DeCarli et al., 2004; Grober et al., 2000) are associated with increased risk of cognitive decline and dementia. While the neuroimaging correlates of cognitive dysfunction have been extensively examined using measures of hippocampal volume (Jack et al., 1999; Killiany et al., 2000) and neuronal metabolism (Chao et al., 2005; Modrego et al., 2005; Zimmerman et al., 2008), brain based correlates of locomotor abilities require additional investigation.
The objective of the present study was to examine the cross-sectional relationship between MR-derived measures of hippocampal structure and function and quantitative indices of locomotion in nondemented older adults. Previously, (Verghese et al., 2007) we performed a factor analysis on eight different quantitative gait variables in a community-based sample. We found three gait factors (pace, rhythm, and variability) that were predictors of risk of cognitive decline and dementia in that sample. For the current study, we selected the quantitative gait measures that loaded most strongly on each of the three previously determined gait factors (stride length, swing time, and stride length variability, respectively) as our primary gait indices of interest. Stride length is the distance between the heel points of two successive footfalls of the same foot. Swing time, a marker of gait stability, is the amount of time when the foot is in the air from toe off to heel strike of the same foot. Stride length variability is the standard deviation of the variability in length between strides.
Structural magnetic resonance imaging (MRI) is an important neuroimaging tool that promotes the characterization of brain structures that may be involved in pathological processes and provides an important context in which to consider both neurochemical and neurofunctional findings. MRI was used in the current study to obtain measures of hippocampal volume. Magnetic resonance spectroscopy (MRS) is a neuroimaging technique that permits investigation of neuronal cellular chemical activity through examination of neurotransmitters and amino acids. MRS provides a measure of cerebral metabolic change which may reflect pathological insults to brain integrity. Although several different chemical compounds (e.g., choline, myo-inositol) have been examined in older individuals, decreased hippocampal N-acetylaspartate (NAA) and its ratio to creatine (Cr) has been a particularly robust age-associated finding (Angelie et al., 2001; Driscoll et al., 2003; Schuff et al., 1999). NAA is primarily located in neurons and is thought to be a marker of neuronal viability (Pettegrew et al., 2000), while Cr reflects biochemical energy reserves of glia and neurons and is presumed to be stable in the context of pathologic processes (Narayana, 2005). MRS was used in the current study to obtain measures of hippocampal NAA/Cr.
Previous studies examining neuroimaging correlates of gait dysfunction have focused on subcortical white matter lesions and ventriculomegaly (e.g., see (Rosano et al., 2007b; Rosano et al., 2005)) and gray matter volumes (Rosano et al., 2007a). In addition to these brain substrates, several lines of evidence provide support for the role of the hippocampus in locomotion: 1) Animal models of hippocampal lesions reveal behavioral abnormalities that include impairments in learning and memory as well as abnormalities in gait, balance, and motor coordination (e.g., see Ferguson et al., 2000; Paylor et al., 2001); 2) Numerous animal models demonstrate a link between hippocampal neurophysiology and both voluntary and wayfinding locomotor function (e.g., see (Bland, 2004; Oddie et al., 1996)); 3) In our community-based sample, we previously reported that disruptions in gait function in nondemented older adults are an early predictor of the development of memory decline and dementia (Verghese et al., 2007). We have also shown that smaller hippocampal volume and lower hippocampal metabolism are related to memory difficulties in nondemented older adults (Zimmerman et al., 2008). Thus, our hypothesis that locomotor function in older adults is associated with neuroimaging measures of hippocampal volume and hippocampal NAA/Cr is supported by collective consideration of findings from animal studies as well as our own work with locomotion, neuroimaging markers, and memory function in older adults. Investigation of the underlying hippocampal neurobiology associated with gait dysfunction may enhance understanding of the preclinical onset of cognitive decline in elderly adults.
2. Results
Sample Characteristics
Sample demographics, quantitative gait performance, and hippocampal measurements are presented in Table 1. Sex and ethnicity characteristics were consistent with those of both the larger Einstein Aging Study (EAS) cohort and the U.S. Census Bureau data for Bronx county. Global cognitive status and memory performance were similar between the MRI subsample and the larger EAS cohort. There were no education, sex, or ethnicity differences on the MR-derived or gait variables of interest, with the exception of men exhibiting a longer stride length than women (t=2.20, p=0.03). The effect of gender was considered in all subsequent analyses examining stride length. MRI-derived hippocampal volumetric and midsagittal area measurements were available for 39 and 36 individuals due to scan acquisition limitations. There were no differences in age, education, sex, global cognitive status, memory performance, or hippocampal NAA/Cr between those participants who had hippocampal volumetric data and those that did not.
Table 1.
Sample demographics, verbal memory performance, and hippocampal measurements.
| |
Total Sample (N=48) |
Normal Memory (n=34) |
Mild Memory Impairment (n=14) |
|---|---|---|---|
| Age, years, mean (SD) | 81.18 (5.47) | 80.37 (5.77) | 83.14 (4.2) |
| Sex, N women, (%) | 23 (47.9) | 19 (55.9) | 4 (28.6) |
| Ethnicity, N Caucasian, (%) | 35 (72.9) | 25 (73.5) | 10 (71.4) |
| Education, years, mean (SD) | 13.08 (3.11) | 13.2 (3.50) | 12.7 (1.90) |
| Handedness, N Right, (%)* | 47 (97.8) | 33 (97.1) | 14 (100) |
| BIMC, total errors, median (range) | 2.00 (0−13) | 2.00 (0−6) | 3.5 (0−13) |
| CDR, score, median (range) | 0 (0−1) | 0 (0−0.5) | 0.5 (0−1) |
| Swing time, mean (seconds) | 0.43 (0.05) | 0.43 (0.04) | 0.44 (0.06) |
| Stride length, mean (cm) | 111.39 (19.45) | 112.57 (20.50) | 108.55 (16.97) |
| Stride length variability (SD) | 4.06 (1.79) | 3.59 (1.56) | 5.20 (1.87) |
| Velocity (cm/second) | 93.91 (21.91) | 96.29 (22.90) | 88.13 (18.79) |
| Fall History in Past Year, # yes, (%)* | 6 (12.5) | 2 (5.9) | 4 (28.6) |
| Abnormal Clinical Gait, # yes, (%)* | 7 (14.6) | 5 (14.7) | 2 (14.3) |
| Hippocampal volume, mean (SD)* | 4.27 (0.07) | 4.40 (0.07) | 3.92 (0.01) |
| Hippocampal NAA/Cr, mean (SD) | 1.30 (0.23) | 1.36 (0.82) | 1.17 (0.93) |
Handedness data available for N=45; Falls history data available for N=45; Clinical gait evaluation data available for N=44; Hippocampal volumetric data available for N=39 (Normal Memory: n=29; Mild Memory Impairment: n=10).
Note: MRI volumetric data are given in cubic centimeters; SD=standard deviation; BIMC=Blessed Information Memory Concentration test, total error score; CDR=Clinical Dementia Rating scale; NAA/Cr=N-acetylaspartate/creatine ratio
Clinical Gait Characteristics
Data for self-reported history of neurological disorders associated with gait impairment were available for 47 individuals. Of these, none reported a history of Parkinson's Disease and three reported a history of stroke. Data for the presence of a fall history within the year prior to the gait evaluation were available for 45 individuals. Of these, six reported that they had fallen in the past year. Data from a clinical gait evaluation performed by a neurologist were available for 44 individuals. Of these, seven were determined to have an abnormal clinical gait with possible neurological etiology. Gait subtypes included unsteady (n=5), neuropathic (n=1), and ataxic (n=1). Six out of seven individuals exhibited mild gait disturbances (abnormality detectable on examination, but walked unassisted) and one individual exhibited moderate gait disturbance (abnormality detectable on examination, used walking aid). We have previously reported high reliability for our clinical gait classification (Verghese et al., 2006).
Simple Correlations
Pearson correlation coefficients revealed that increasing age was associated with smaller hippocampal volumes (r=−0.35, p=0.03) and shorter stride length (r=−0.52, p<0.01). The strength of the relationship between age and hippocampal volume was similar after controlling for midsagittal area (r=−0.30, p=0.08). Age was used as a covariate in all subsequent analyses. Individuals with larger hippocampal volumes had longer stride lengths after controlling for midsagittal area and gender (r=0.45, p=0.01). No other primary gait measures of interest were correlated with hippocampal volume. Individuals with lower hippocampal NAA/Cr values had greater variability in stride length (r=−0.36, p=0.02). No other primary gait measures of interest were correlated with hippocampal NAA/Cr. Midsagittal area was not correlated with hippocampal volume (r=0.13, p=0.45), hippocampal NAA/Cr (r=0.17, p=0.31), stride length (r=0.14, p=0.39), stride length variability (r=0.01, p=0.97), or swing (r=0.01, p=0.97). Gait velocity, a commonly used locomotor metric, was associated with stride length (r=0.89, p<0.01) and hippocampal volume (r=0.50, p<0.01).
Hippocampal Volume and Gait Performance
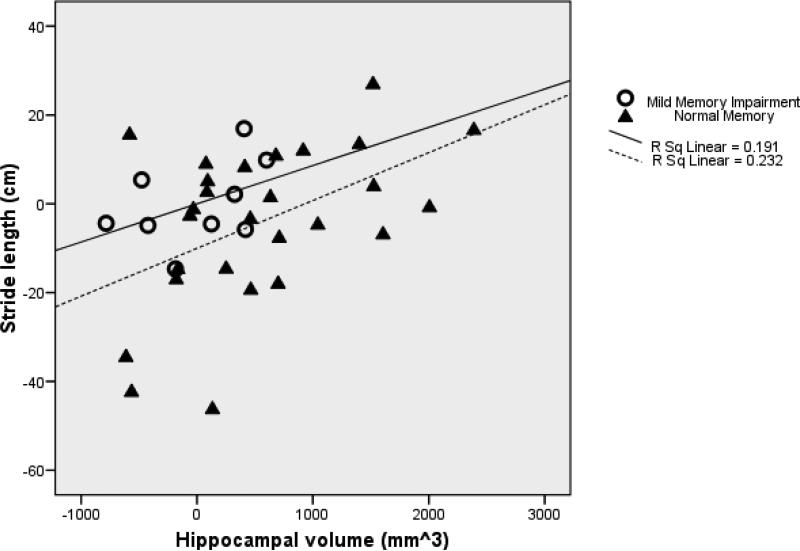
The model of the prediction of stride length by hippocampal volume controlling for age and midsagittal area (F(3,32)=5.17, p<0.01, R2=0.33, n=36) indicated that shorter stride length was associated with smaller hippocampal volume (β=0.36, t=2.35, df=32, p=0.03; Figure 1) and older age (β=−0.33, t=−2.15, df=32, p=0.04). Inclusion of gender and weight in the model did not meaningfully change the results. Inclusion of gait velocity in the model altered the results such that hippocampal volume was no longer a significant predictor (β =0.01, t=0.13, df=36, p=0.90). The model for the prediction of swing time and stride length variability by hippocampal volume was not significant.
Figure 1.
Partial regression plot of the relationship between hippocampal volume and stride length controlling for age and midsagittal area in older adults
Hippocampal NAA/Cr and Gait Performance
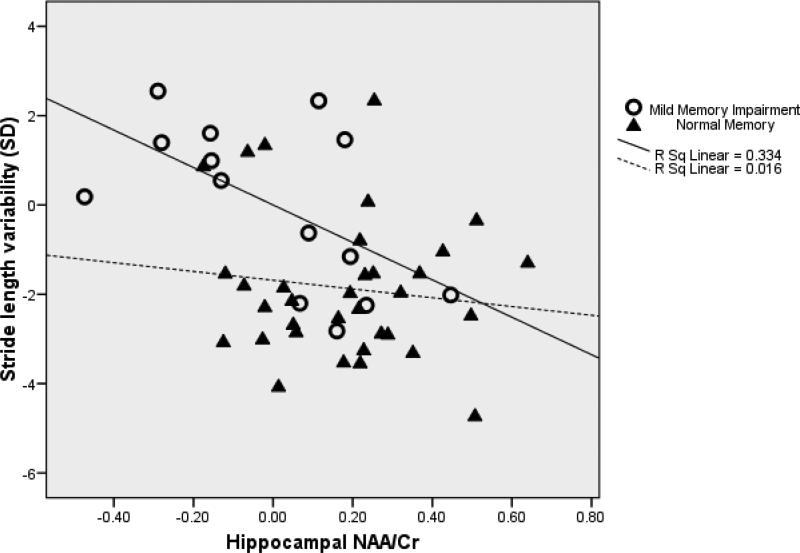
The model of the prediction of stride length variability by hippocampal NAA/Cr controlling for age (F(2,45)=3.57, p=0.04, R2=0.14, n=48) indicated that greater variability in stride length was associated with lower levels of hippocampal NAA/Cr (β=−0.38, t=−2.67, df=45, p=0.01; Figure 2). Inclusion of weight and gait velocity in the model did not meaningfully change the results. The model for the prediction of swing time and stride length by hippocampal NAA/Cr was not significant.
Figure 2.
Partial regression plot of the relationship between hippocampal NAA/Cr and stride length variability controlling for age in older adults
Hippocampal Volume, Hippocampal NAA/Cr, and Gait Performance
The model of the prediction of stride length by hippocampal volume and NAA/Cr controlling for age and midsagittal area (F(4,31)=4.14, p=0.01, R2=0.35, n=36) revealed significant effects for both hippocampal volume (β=0.42, t=2.56, df=31, p=0.02) and age (β=−0.33, t=−2.15, df=31, p=0.04). Inclusion of gender and weight in the model did not meaningfully change the results. Inclusion of gait velocity in the model altered the results such that hippocampal volume was no longer a significant predictor (β =0.05, t=0.05, df=36, p=0.61). The model for the prediction of swing time and stride length variability by hippocampal volume and NAA/Cr was not significant. Table 2 depicts a summary of all three regression models for the prediction of stride length, while Table 3 depicts a summary of all three regression models for the prediction of stride length variability.
Table 2.
Regression models for the effect of age, hippocampal volume, and hippocampal NAA/Cr on stride length in older adults.
| |
Predictors |
R2 |
Standardized Beta |
p-value |
|---|---|---|---|---|
| Model 1 | Age | 0.33 | −0.33 | 0.04* |
| Midsagittal Area | 0.03 | 0.83 | ||
| |
Hippocampal Volume |
|
0.36 |
0.03* |
| Model 2 | Age | 0.28 | −0.49 | 0.00** |
| |
Hippocampal NAA/Cr |
|
0.09 |
0.48 |
| Model 3 | Age | 0.35 | −0.33 | 0.04* |
| Midsagittal Area | 0.05 | 0.73 | ||
| Hippocampal Volume | 0.42 | 0.02* | ||
| Hippocampal NAA/Cr | −0.16 | 0.32 |
Significant at the p<0.05 level.
Significant at the p<0.01 level.
Note: NAA/Cr=N-acetylaspartate/creatine ratio.
Table 3.
Regression models for the effect of age, hippocampal volume, and hippocampal NAA/Cr on stride length variability in older adults.
| |
Predictors |
R2 |
Standardized Beta |
p-value |
|---|---|---|---|---|
| Model 1 | Age | 0.10 | −0.09 | 0.62 |
| Midsagittal Area | 0.03 | 0.86 | ||
| |
Hippocampal Volume |
|
−0.33 |
0.08 |
| Model 2 | Age | 0.14 | −0.11 | 0.46 |
| |
Hippocampal NAA/Cr |
|
−0.38 |
0.01* |
| Model 3 | Age | 0.19 | −0.09 | 0.60 |
| Midsagittal Area | 0.07 | 0.67 | ||
| Hippocampal Volume | −0.20 | 0.29 | ||
| Hippocampal NAA/Cr | −0.34 | 0.06 |
Significant at the p<0.01 level.
Note: NAA/Cr=N-acetylaspartate/creatine ratio.
To account for anthropomorphic features that may impact gait, we repeated primary analyses using normalized gait measures (gait divided by height) as dependent variables. The overall pattern and strength of the findings remained unchanged (data not shown).
Memory Impairment
Pearson correlation analyses indicated that lower levels of hippocampal NAA/Cr were associated with shorter stride length (r=0.56, p=0.04) and increased stride length variability (r=−0.56, p=0.04) among older adults with mild memory impairments, but not among older adults with normal memory. Smaller hippocampal volume was associated with shorter stride length (r=0.51, p=0.01) among older adults with normal memory, but not among older adults with mild memory impairments. This pattern of results was similar when analyses were repeated using nonparametric statistics.
Clinical Gait Abnormalities
As described previously, seven individuals were diagnosed with neurological clinical gait abnormalities on qualitative examination by the study neurologist. Linear regressions were repeated with the removal of these individuals and the models restricted to subjects with clinically normal gaits remained significant for the prediction of stride length by hippocampal volume (F(3, 22)=6.74, p<0.01, R2=0.48), the prediction of stride length by hippocampal volume and NAA/Cr (F(4, 21)=4.88, p=0.01, R2=0.48), and the prediction of stride length variability by hippocampal NAA/Cr (F(2, 34)=3.49, p=0.04, R2=0.17).
3. Discussion
The goal of this study was to examine hippocampal structural and functional correlates of quantitative locomotor performance in nondemented older adults. Our findings indicate that in older adults, shorter stride length is associated with smaller hippocampal volume, but not neuronal metabolism. Conversely, increased variability in stride length is associated with poorer hippocampal metabolism, but not volume. When hippocampal volume and neuronal metabolism are considered together, only hippocampal volume remained a predictor of stride length in older adults. Further examination of these relationships in the context of memory performance reveals that smaller hippocampal volume is related to shorter stride length in individuals with normal memory abilities, while lower levels of hippocampal neuronal metabolism are related to both shorter stride length and increased stride length variability in individuals with mild memory impairments. This overall pattern of findings suggests a series of specific brain-behavior relationships in older adults that may vary as a function of hippocampal integrity, locomotor activity, and memory performance.
Several studies have examined neuroimaging correlates of locomotor performance in elderly individuals. Subcortical white matter changes, such as white matter hyperintensities, consistently have been shown to predict gait disturbance (e.g., see (Baloh et al., 2003; Rosano et al., 2007b; Rosano et al., 2005; Whitman et al., 2001; Wolfson et al., 2005)), balance dysfunction (Baloh et al., 1995; Starr et al., 2003; Tell et al., 1998), and disequilibrium (Kerber et al., 1998) in older adults, even after accounting for cerebrovascular disease (Briley et al., 1997). Generalized ventricular enlargement and sulcal widening have also been linked to poor balance and motor performance in nondemented older adults (Rosano et al., 2005; Tell et al., 1998). Recent studies have utilized region of interest analysis approaches to examine brain volume changes in individuals with locomotor dysfunction. A large population-based study (Guo et al., 2001) reported temporal lobe atrophy in elderly women with motor impairments using computed tomography (CT) scans. A recent study by Rosano and colleagues (Rosano et al., 2007a) reported associations between smaller gray matter volumes in left cerebellum and left prefrontal cortex and slower gait performance that persisted after accounting for the presence of white matter hyperintensities. However, Rosano and colleagues also examined associations between gait measures and the gray matter of the parahippocampal gyrus (Rosano et al., 2007a) and hippocampus (Rosano et al., 2008) in a large community-based study and did not find significant relationships. Considered together, the findings from these important studies provide support for a wide variety of brain abnormalities in older adults with lower extremity motor dysfunction. None of these studies, however, specifically examined hippocampal total volume or neuronal metabolism in relation to quantitative gait measures. Our findings complement and extend previous research through identification of neuroimaging-derived biological substrates that are associated with distinct locomotor functions in older adults.
Hippocampal involvement in locomotor activity is supported by animal models of hippocampal lesions that produce impairments in learning and memory as well as impairments in motor coordination, balance, and spontaneous perseverative turning (Ferguson et al., 2000; Mickley et al., 1989; Paylor et al., 2001; Sams-Dodd et al., 1997). A substantial animal literature using electroencephalogram (EEG) also describes hippocampal rhythmical synchronous activity (RSA), or theta oscillation, that is associated with voluntary motor movements in the mouse that include walking, running, jumping, and swimming (Bland, 2004; Oddie et al., 1996; Vanderwolf, 1969). Reported findings of relationships between hippocampal cellular firing rates and walking speed of the guinea pig suggest that neuronal excitation may be a contributor to changes in theta rhythms (Rivas et al., 1996). Finally, a study of patients with right medial temporal lobectomy (Philbeck et al., 2004) found that these individuals demonstrated poor path integration and linear displacement during a walking challenge, providing further evidence for the role of the hippocampus in locomotor activity. Although speculative, we propose that these findings suggest that the hippocampus may play a role in the timing or rhythmicity of locomotion that may be compromised in the older adult.
Hippocampal long-term potentiation (LTP), or synaptic transmission, is believed to support learning and memory cognitive processes (Bliss and Collingridge, 1993; Morris and Frey, 1997). Animal studies that use walking to stimulate acetylcholine release report associated modulation in LTP in the CA1 subregion of the hippocampus (Leung et al., 2003) and increased hippocampal regional cerebral blood flow in both young (Nakajima et al., 2003) and aged rats (Uchida et al., 2006). Hippocampal LTP has also been proposed to support the firing of place cells (Muller and Stead, 1996), which are associated with the active exploration of the environment by the rat (Foster et al., 1989).
The dissociation of our findings by hippocampal biology, gait function, and memory performance warrants further discussion. In our study, hippocampal volume was associated with stride length in both the overall sample of nondemented older adults as well as the follow-up analyses of individuals with normal cognition. Several studies have reported hippocampal volumetric decline that is commonly observed in healthy older adults across the lifespan (e.g., see (Pruessner et al., 2001; Raz et al., 2005; Raz et al., 2004; Zimmerman et al., 2006)). Our findings also indicated that lower levels of hippocampal NAA/Cr, a metabolite thought to be an indicator of neuronal integrity, were associated with shorter stride length and increased stride length variability among individuals with mild memory impairments. Decreased NAA/Cr in the hippocampus may be a predictor of both incident and prevalent dementia (Chao et al., 2005; Dixon et al., 2002; Modrego et al., 2005; Spencer et al., 2003). Memory impairment is also a well-known risk factor for development of dementia in older adults (e.g., see (DeCarli et al., 2004; Grober et al., 2008; Grober et al., 2000; Kawas et al., 2003)). Although speculative, this overall pattern of findings suggests that that the volume of the hippocampus may play a role in the motor processes involved in stride length in healthy elderly adults with normal memory functions, whereas abnormal hippocampal neuronal metabolism may underlie preclinical disease in older adults with mild memory impairment and increased stride length variability, both of which have been shown to predict dementia. Thus, the hippocampal NAA/Cr measurement in nondemented older adults may provide a more sensitive index of neuronal damage compared to hippocampal volume.
There are several limitations to the current study. Our MR protocol was designed to evaluate volumetry and neurochemistry of hippocampus; we cannot determine the specificity of our findings as we do not have similar data from other brain regions. Locomotor abilities are likely supported by a network of brain regions in older adults. Hippocampal volume data were only available for a subset of participants and thus the sample size varied across analysis models. Additionally, our sample was drawn from a larger study of community-dwelling adults over the age of 70 and we were not able to examine relationships between hippocampal integrity and locomotor activity across the entire adult lifespan or in individuals with clinical diagnoses, such as dementia. An important consideration for future investigations is the possibility that the observed associations may vary as a function of age and cognitive status. Findings regarding memory performance should be interpreted with caution given the relatively small number of mild memory impaired individuals in our sample. It is also possible that the spatial resolution of the spectroscopic method may have resulted in acquisition of the NAA signal from other surrounding structures. Finally, our cross-sectional design and correlational analyses prevent the formulation of causational conclusions. Additional investigation is necessary to examine the longitudinal sequence and relationships of locomotor, cognitive, neurochemical, and anatomic changes that emerge across the spectrum from normal aging to advanced dementia.
4. Experimental Procedure
Participants
We previously reported an association between memory performance and hippocampal volume and neurochemistry in this sample of participants (Zimmerman et al., 2008). Between February 2004 and August 2006, a subset of 48 older adults was drawn from the EAS, a community-based sample of individuals over the age of 70 living in the Bronx, New York. The subset was systematically selected to obtain a range of memory performance on the Free and Cued Selective Reminding Test – Immediate Recall (FCSRT-IR; discussed further below) in older adults who did not meet diagnostic criteria for dementia based on the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (American, 1994). In the absence of contraindications to MRI, all individuals with mild memory impairment (free recall FCSRT-IR ≤24, n=14) and a random sample of individuals with normal memory (free recall FCSRT-IR>24, n=34) were invited to participate in the neuroimaging studies. FCSRT-IR free recall impairment cut-scores are described in previous studies (Grober and Kawas, 1997; Grober et al., 2000). Of the 48 participants in the current study, eight reported a subjective memory complaint as determined by a positive response to the item, “Do you feel that you have more problems with memory than most?” from the 15-item Geriatric Depression Scale (Yesavage et al., 1982). Of these participants with memory complaints, six exhibited memory within normal limits and two exhibited mild memory impairment.
Einstein Aging Study design and methods are described elsewhere (Lipton et al., 2003). Briefly, participants were recruited through systematic sampling from Medicare or voter registration lists for Bronx County. Participants were excluded if they were non-English speakers or if they reported severe sensory loss (i.e., poor visual acuity) or medical conditions that would interfere with neuropsychological evaluation. General cognitive status was assessed using the Blessed Information-Memory-Concentration test (BIMC) (Blessed et al., 1968). Participant diagnosis was established at a case conference attended by a study neurologist and neuropsychologist.
Quantitative Gait Evaluation
Quantitative gait studies were performed on a computerized walkway mat (180 × 35.5 × 0.25 inches) with embedded pressure sensors (GAITRite, CIR systems, USA) (Verghese et al., 2004). Subjects were asked to walk on the mat at their ‘normal pace’ for two trials in a well-lit hallway wearing comfortable footwear. Start and stop points were marked by white lines on the floor placed three feet from the mat edge for initial acceleration and terminal deceleration. Computer software automatically computes gait parameters as the mean of two trials based on footfalls recorded on the walkway. Excellent reliability (Bilney et al., 2003; Verghese et al., 2004) of the GAITRite system has been reported in our and other centers, and the system is widely used in both clinical and research settings. In our previous work (Verghese et al., 2007), we showed that three gait factors (pace, rhythm, and variability) were related to future risk of cognitive decline and dementia. In order to minimize the number of statistical comparisons, we selected the quantitative gait measure that loaded most strongly on each factor (stride length, swing time, and stride length variability, respectively) for examination in the current study. Swing time, a marker of gait stability, is the duration in seconds from when the foot is in the air to the time taken from toe off to heel strike of the same foot. Stride length is the distance in centimeters between heel points of two consecutive footfalls of the same foot. Stride length variability is the standard deviation of the variability in length between strides.
Magnetic Resonance Imaging
MR imaging was performed on a Varian-Magnex 4T imaging system at the Gruss Magnetic Resonance Research Center at the Albert Einstein College of Medicine, Bronx, NY. All neuroimaging was performed within an average of 3.94 ± 2.94 months from the time of the gait assessment. T1-weighted inversion recovery gradient echo images were acquired with 1.5mm slice thickness, field of view (FOV)=240, and 160 × 160 resolution, with pixel size of 1.5×1.5×1.5mm3. One-hundred-twenty slices were taken parallel to the planum temporale with 180mm coronal-axial coverage. Images were reformatted orthogonal to the planum temporale and manual volumetric measurements of the hippocampus were performed using previously described methodology (Jack et al., 1992; Watson et al., 1992). Briefly, the limit of the posterior hippocampal tail was identified from the oblique coronal image where the crus of the fornix was visualized. Anteriorly, the pes hippocampus was disarticulated from the amygdala by identification of the alveus, or by extending the horizontal line defined by the uncal recess of the temporal horn. Laterally and superiorly, the temporal horn cerebrospinal fluid demarcated the hippocampus. Medially, the uncal and ambient cistern were used. Inferiorly, the subiculum was separated from the parahippocampal gray matter with a straight line. Whole brain volume was not available in this dataset; therefore, intracranial midsagittal area was acquired to account for individual differences in head size (Ferguson et al., 2005). Volumetrics were obtained by a single trained rater with high intrarater reliability (ICC=0.99). Hippocampal volume was the primary variable of interest obtained from this image acquisition.
Magnetic Resonance Spectroscopy
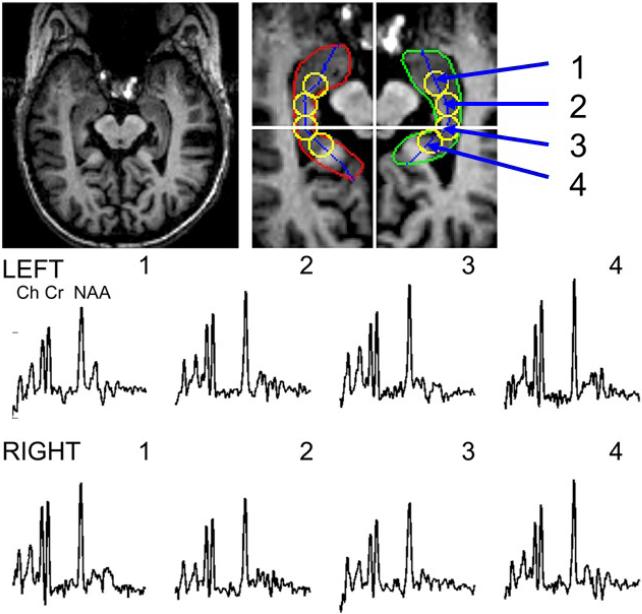
All data were acquired at 4T using a Varian INOVA console and quadrature head coil using a modified LASER sequence (10mm thickness, 80×100mm in-plane FOV selection) in combination with two dimensions of phase encoding (24×24, FOV=192×192mm, 19.2 minutes) angulating the plane along the temporal pole (Hetherington et al., 2002). The magnetic field homogeneity was adjusted for each subject (Hetherington et al., 1996). To provide reproducible selection criteria, hippocampal voxels were reconstructed using a voxel shifting method (Hetherington et al., 2004; Twieg et al., 1989). Centers of reconstructed spectroscopic voxels were defined from structural images using anatomically defined criteria. Hippocampal boundaries were manually delineated on anatomical images and a mid-line between the medial and lateral boundaries was calculated. Four loci, one positioned at the level of the aqueduct along the midline, two anterior loci, and one posterior locus were selected by translating along the midline in 9mm increments (see Figure 3). These coordinates were used in voxel shifting reconstruction to provide spectroscopic voxels centered over these loci. Spectral data were obtained using a convolution difference of 250Hz followed by 3Hz of Gaussian broadening and Fourier transformation in the spectral domain. Data were fit using Gaussian line shapes and the NAA/Cr ratio was determined by using the ratios of the resonance areas. The NAA/Cr ratio was the primary variable of interest obtained from this image acquisition.
Figure 3.
T1-weighted MRI and localized proton MRS with illustrated regions of interest and representative spectra showing the resonances of choline (Ch), creatine (Cr) and N-acetylaspartate (NAA) in bilateral hippocampus of older adults.
Separate left and right measurements were acquired for both hippocampal NAA/Cr and volume. There were no laterality differences in hippocampal NAA/Cr (t(1,47)=0.09, p=0.93) or volume (t(1,38)=−0.17, p=0.85); therefore, all left and right variables were combined (volumes) or averaged (NAA/Cr) to reduce the number of statistical comparisons.
Memory Evaluation
All participants were administered a comprehensive neuropsychological assessment that included the FCSRT-IR (Buschke, 1984; Grober and Buschke, 1987), a verbal memory task that controls attention and strategy use to maximize learning and provide a measurement of memory that is not confounded by deficits in other cognitive abilities. In the free recall condition, a measure of self-organized retrieval, the participant is asked to recall 16 objects that were previously presented. If the participant fails to correctly recall an object, they are provided with a category cue to test cued recall. There are a total of three free and cued recall trials and total scores range from zero to 48.
Statistical Analyses
Age, education, and gender were examined as potential covariates using Pearson product-moment correlation coefficients and independent t-tests. Ethnicity was examined as a potential covariate using ANOVA. Pearson correlation coefficients were utilized to examine relationships among quantitative gait performance (swing time, stride length, stride length variability), hippocampal volume, and hippocampal NAA/Cr.
A series of linear regression analyses were performed to examine MR-derived predictors of each of the three quantitative gait performance variables. The first linear regression examined the effect of hippocampal volume on gait performance with age and midsagittal area as covariates. The second linear regression examined the effect of hippocampal NAA/Cr on gait performance with age as a covariate. The third linear regression analysis examined the effect of both hippocampal neurochemistry and volume on gait performance with age and midsagittal area as covariates.
Acknowledgements
The Authors would like to thank Charlotte Magnotta for assistance with participant recruitment; Danielle Coyle, Betty Forro, Alicia Gomez, and Mary Joan Sebastian for assistance with neuropsychological assessment; Rebecca Gottlieb for quantitative gait assessments; Cynthia Pan for assistance with hippocampus volumetric quantification; and all of the study participants who gave their time in support of this research. The work presented in this paper was supported by National Institute on Aging Grants AG03949 (PI: R.B. Lipton) and AG025119 (PI: J. Verghese).
Abbreviations
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- NAA/Cr
N-acetylaspartate to creatine ratio
- EAS
Einstein Aging Study
- FCSRT-IR
free and reminding test-immediate recall
- BIMC
Blessed memory-concentration test
- LTP
long term
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American PA. Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Angelie E, et al. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2001;22:119–27. [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Low LF. Normal cognitive changes in aging. Aust Fam Physician. 2004;33:783–7. [PubMed] [Google Scholar]
- Baloh RW, et al. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60:835–9. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- Baloh RW, et al. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol. 1995;52:970–4. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- Bilney B, et al. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- Bland BH. The power of theta: providing insights into the role of the hippocampal formation in sensorimotor integration. Hippocampus. 2004;14:537–8. doi: 10.1002/hipo.20027. [DOI] [PubMed] [Google Scholar]
- Blessed G, et al. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Briley DP, et al. Cerebral white matter changes (leukoaraiosis), stroke, and gait disturbance. J Am Geriatr Soc. 1997;45:1434–8. doi: 10.1111/j.1532-5415.1997.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. Journal of Clinical Neuropsychology. 1984;6:433–40. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Camicioli R, et al. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50:1496–8. doi: 10.1212/wnl.50.5.1496. [DOI] [PubMed] [Google Scholar]
- Chao LL, et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64:282–9. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. What cognitive changes can be expected with normal ageing? Aust N Z J Psychiatry. 2001;35:768–75. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- DeCarli C, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–7. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RM, et al. Longitudinal quantitative proton magnetic resonance spectroscopy of the hippocampus in Alzheimer's disease. Brain. 2002;125:2332–41. doi: 10.1093/brain/awf226. [DOI] [PubMed] [Google Scholar]
- Driscoll I, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–51. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, et al. Deficits in memory and motor performance in synaptotagmin IV mutant mice. Proc Natl Acad Sci U S A. 2000;97:5598–603. doi: 10.1073/pnas.100104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KJ, et al. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging. 2005;15:76–8. doi: 10.1177/1051228404270243. [DOI] [PubMed] [Google Scholar]
- Foster TC, et al. Spatial selectivity of rat hippocampal neurons: dependence on preparedness for movement. Science. 1989;244:1580–2. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, et al. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–75. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- Grober E, et al. Neuropsychological strategies for detecting early dementia. J Int Neuropsychol Soc. 2008;14:130–42. doi: 10.1017/S1355617708080156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer's disease. Psychology & Aging. 1997;12:183–8. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- Grober E, et al. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–32. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Guo X, et al. A population-based study on brain atrophy and motor performance in elderly women. J Gerontol A Biol Sci Med Sci. 2001;56:M633–7. doi: 10.1093/gerona/56.10.m633. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, et al. 1H and 31P spectroscopic imaging of epilepsy: spectroscopic and histologic correlations. Epilepsia. 2004;45(Suppl 4):17–23. doi: 10.1111/j.0013-9580.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, et al. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magn Reson Med. 1996;36:21–9. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, et al. 1H and 31P spectroscopy and bioenergetics in the lateralization of seizures in temporal lobe epilepsy. Journal of Magnetic Resonance Imaging. 2002;16:477–483. doi: 10.1002/jmri.10177. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–8. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, et al. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60:1089–93. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Kerber KA, et al. Disequilibrium in older people: a prospective study. Neurology. 1998;51:574–80. doi: 10.1212/wnl.51.2.574. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–9. [PubMed] [Google Scholar]
- Leung LS, et al. Cholinergic activity enhances hippocampal long-term potentiation in CA1 during walking in rats. J Neurosci. 2003;23:9297–304. doi: 10.1523/JNEUROSCI.23-28-09297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, et al. Screening for dementia by telephone using the memory impairment screen. Journal of the American Geriatrics Society. 2003;51:1382–90. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- Mickley GA, et al. Spontaneous perseverative turning in rats with radiation-induced hippocampal damage. Behav Neurosci. 1989;103:722–30. doi: 10.1037//0735-7044.103.4.722. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, et al. Conversion from mild cognitive impairment to probable Alzheimer's disease predicted by brain magnetic resonance spectroscopy. Am J Psychiatry. 2005;162:667–75. doi: 10.1176/appi.ajp.162.4.667. [DOI] [PubMed] [Google Scholar]
- Morris RG, Frey U. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Philos Trans R Soc Lond B Biol Sci. 1997;352:1489–503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Stead M. Hippocampal place cells connected by Hebbian synapses can solve spatial problems. Hippocampus. 1996;6:709–19. doi: 10.1002/(SICI)1098-1063(1996)6:6<709::AID-HIPO13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Nakajima K, et al. The effect of walking on regional blood flow and acetylcholine in the hippocampus in conscious rats. Auton Neurosci. 2003;103:83–92. doi: 10.1016/s1566-0702(02)00263-1. [DOI] [PubMed] [Google Scholar]
- Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging. 2005;15:46S–57S. doi: 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddie SD, et al. Intraseptal procaine abolishes hypothalamic stimulation-induced wheel-running and hippocampal theta field activity in rats. J Neurosci. 1996;16:1948–56. doi: 10.1523/JNEUROSCI.16-05-01948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, et al. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol Behav. 2001;73:781–92. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, et al. Molecular insights into neurodevelopmental and neurodegenerative diseases. Brain Res Bull. 2000;53:455–69. doi: 10.1016/s0361-9230(00)00376-2. [DOI] [PubMed] [Google Scholar]
- Philbeck JW, et al. Path integration deficits during linear locomotion after human medial temporal lobectomy. J Cogn Neurosci. 2004;16:510–20. doi: 10.1162/089892904323057254. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, et al. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–8. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Rivas J, et al. Changes in hippocampal cell discharge patterns and theta rhythm spectral properties as a function of walking velocity in the guinea pig. Exp Brain Res. 1996;108:113–8. doi: 10.1007/BF00242908. [DOI] [PubMed] [Google Scholar]
- Rosano C, et al. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007a;62:1048–55. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Rosano C, et al. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007b;29:193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, et al. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–54. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F, et al. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–10. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Schuff N, et al. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–85. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer DC, et al. MRS in relation to hippocampal volume in the oldest old. Neurology. 2003;60:1194–6. doi: 10.1212/01.wnl.0000052822.22994.86. [DOI] [PubMed] [Google Scholar]
- Starr JM, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–8. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell GS, et al. Relationship between balance and abnormalities in cerebral magnetic resonance imaging in older adults. Arch Neurol. 1998;55:73–9. doi: 10.1001/archneur.55.1.73. [DOI] [PubMed] [Google Scholar]
- Twieg DB, et al. Phosphorus-31 magnetic resonance spectroscopy in humans by spectroscopic imaging: localized spectroscopy and metabolite imaging. Magnetic Resonance in Medicine. 1989;12:291–305. doi: 10.1002/mrm.1910120302. [DOI] [PubMed] [Google Scholar]
- Uchida S, et al. Responses of acetylcholine release and regional blood flow in the hippocampus during walking in aged rats. J Physiol Sci. 2006;56:253–7. doi: 10.2170/physiolsci.SC001706. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–18. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Verghese J, et al. Reliability and validity of a telephone-based mobility assessment questionnaire. Age Ageing. 2004;33:628–32. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- Verghese J, et al. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54:255–61. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, et al. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med. 2002;347:1761–8. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- Verghese J, et al. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–35. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, et al. 65+ in the united states. U.S. census bureau current population reports. U.S. Government Printing Office; Washington, D.C.: 2005. pp. 23–209. [Google Scholar]
- Watson C, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–50. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Whitman GT, et al. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–4. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- Wolfson L, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232:23–7. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, et al. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatr Psychiatry. 2006;14:823–33. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, et al. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology. 2008;70:1594–600. doi: 10.1212/01.wnl.0000306314.77311.be. [DOI] [PMC free article] [PubMed] [Google Scholar]