Abstract
The purpose of the study was to identify a comprehensive prognostic system of pretreatment clinical parameters in 425 patients (pts) with metastatic renal-cell carcinoma treated with different subcutaneous (s.c.) recombinant cytokine-based home therapies in consecutive trials. Treatment consisted of (A) s.c. interferon-α2a (INF-α), s.c. interleukin-2 (IL-2) (n=102 pts), (B) s.c. IFN-α2a, s.c. IL-2, and i.v. 5-fluorouracil (5-FU) (n=235 pts) or (C) s.c. IFN-α2a, s.c. IL-2, and i.v. 5-FU combined with p.o. 13-cis-retinoic acid (13cRA) (n=88 pts). Kaplan–Meier survival analysis, log-rank statistics, and Cox regression analysis were employed to identify risk factors and to create a multiple risk factor model. The following pretreatment risk factors were identified by univariate analysis: (1) three and more metastatic sites, (2) presence of liver, lymph node or bone metastases, (3) neutrophil count ⩾6500 cells μl−1, (4) serum lactate dehydrogenase level (LDH) ⩾220 U l−1, and (5) serum C-reactive protein level (CRP) ⩾11 mg l−1. Cox regression analysis with forward stepwise variable selection identified neutrophil count as the major prognostic factor (hazard ratio=1.9, P<0.001), while serum levels of LDH and CRP, time between diagnosis of tumour and onset of metastatic disease, number of metastatic sites, and bone metastases were significant but somewhat less important prognostic variables within the multiple risk factor model (hazard ratio ⩽1.5). Patients were assigned to one of the three risk groups according to cumulative risk defined as the sum of simplified risk s.c.ores for six pretreatment variables. Low-, intermediate-, and high-risk patients achieved a median overall survival of 32+ months (95% CI 24, 43; 5-year survival of 27%), 18+ months (95% CI 15, 20; 5-year survival of 11%), and 8+ months (95% CI 6, 10; 5-year survival of 5%), respectively. These prognostic categories are helpful both in individual patient care and in the assessment of patients entering prospective clinical trials.
Keywords: renal-cell carcinoma, immunotherapy, survival, prognosis, risk, stratification
Patients (pts) with untreated metastatic renal-cell carcinoma have an overall median survival of no more than 12 months and a 5-year survival of less than 10%. While renal-cell carcinoma responds poorly to single-agent chemotherapy or hormonal therapy, immunotherapies with subcutaneous (s.c.) recombinant interleukin-2 (IL-2) alone or in combination with s.c. recombinant interferon-α (INF-α) yielded significant therapeutic efficacy in renal-cell carcinoma (Atzpodien et al, 1990,2001,2002; Sleijfer et al, 1992).
In previous studies, a variety of prognostic staging factors, notably, performance status, recent weight loss, disease-free interval, pretreatment erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), neutrophils, haemoglobin, extrapulmonary and bone metastases, and a number of metastatic sites were identified as important indicators for survival in metastatic renal-cell carcinoma patients (Elson et al, 1988; Palmer et al, 1992; Lopez-Hänninen et al, 1996; Gelb, 1997; Culine et al, 1998; Hoffmann et al, 1999; Motzer et al, 1999,2002).
Here, we develop a comprehensive new prognostic system for metastatic renal carcinoma patients, by retrospective analysis. All patients were treated with outpatient immunotherapy comprising s.c. IL-2 and s.c. INF-α.
PATIENTS AND METHODS
Patients
Between November 1988 and February 1998, 425 patients with progressive metastatic renal-cell carcinoma were entered on consecutive clinical trials and received either IFN-α2a, IL-2 (therapy A, n=102 pts), IFN-α2a, IL-2, and 5-FU (therapy B, n=235 pts) or IFN-α2a, IL-2, and 5-FU combined with 13cRA (therapy C, n=88 pts). Median follow-up of these patients was 20+ months (range 0–157+ months). Pretreatment included radical tumour nephrectomy (n=412), chemotherapy (n=5), immunotherapy (n=47), chemoimmunotherapy (n=8), and hormone therapy (n=32).
Since all treatment regimens were designed to be administrated at home, selection of patients with good or excellent performance status was required. Criteria for entry into the study were: histologically confirmed metastatic renal-cell carcinoma; an expected survival duration of more than 3 months; Karnofsky performance status >80%; age between 18 and 80 years; white blood cell count >3500 μl−1; platelet count >100 000 μl−1; haematocrit >30%; serum bilirubin and creatinin <1.25 of the upper normal limit. Exclusion criteria included evidence of congestive heart failure, severe coronary artery disease, cardiac arrhythmias, symptomatic CNS disease or seizure disorders, human immunodeficiency virus infections or positivity for hepatitis B surface antigen or chronic hepatitis, or concomitant corticosteroid therapy. In all patients treated, no chemotherapy, immunomodulatory treatment, or steroid therapy had been performed during the previous 4 weeks. Pregnant and lactating woman were excluded.
The clinical studies were approved by the institutional review board of the Medizinische Hochschule Hannover; written informed consent was obtained from all patients prior to entry into the study.
Treatment design
Patients were treated in an outpatient setting. All patients received outpatient s.c. IFN-α2a and s.c. IL-2. Treatment A consisted of IFN-α2a and IL-2, only. s.c. IFN-α2a was administered at 5×106 IU m−2, day 1, weeks 1+4, and days 1, 3, 5, weeks 2, 3, 5, 6; s.c. IL-2 was administered at 10×106 IU m−2, twice daily days 3–5 weeks 1+4, and at 5×106 IU m−2, days 1, 3, 5, weeks 2, 3, 5, 6; weeks 7 and 8 were therapy free. Treatment B consisted of IFN-α2a, IL-2, and 5-FU. s.c. IFN-α2a was administered at 5×106 IU m−2, day 1, weeks 1+4, and days 1, 3, 5, weeks 2+3, and at 10×106 IU m−2, days 1, 3, 5, weeks 5–8. s.c. IL-2 was administered at 10×106 IU m−2, twice daily days 3–5 weeks 1+4, and at 5×106 IU m−2, days 1, 3, 5, weeks 2+3; i.v. bolus 5-FU was administered at 1000 mg m−2, day 1 weeks 5–8. Treatment C consisted of IFN-α2a, IL-2, 5-FU, and 13-cRA; patients received 20 mg p.o. 13cRA twice daily, in addition to the above dosages of IFN-α2a, IL-2, and 5-FU.
Eight week treatment cycles were repeated for up to three courses unless progression of disease occurred. Re-evaluation of patients' tumour status was performed between treatment cycles.
Concomitant medication was given as needed to control adverse effects of chemoimmuno-therapy.
Statistical analysis
Survival was measured from start of therapy to date of death or to the last date known to be alive. Treatment efficacy was assessed on an intention-to-treat basis. Survival curves were estimated by the Kaplan–Meier method. Univariate risk factor analysis was performed by the log-rank test and multivariate analysis by Cox regression. Continuous pretreatment clinical measurements (e.g. neutrophil count) were analysed as dichotomous variables according to approximately ‘optimal’ cutpoints, determined as follows. The value best discriminating between good and poor survival (i.e., which had the most significant P-value on a log-rank test) was found by testing all possible cutpoints within the central 80% of the distribution of values. All such cutpoints were then rounded to clinically relevant (i.e. convenient) values. The P-values for the clinically relevant cutpoints were corrected for multiple testing (Miller and Siegmund, 1982; Altman et al, 1994). Cox regression analysis with forward stepwise variable selection was employed to build a model with multiple risk factors. To allow for possible joint effects, all predictors were included as candidate variables, whether or not significant in univariate analysis.
RESULTS
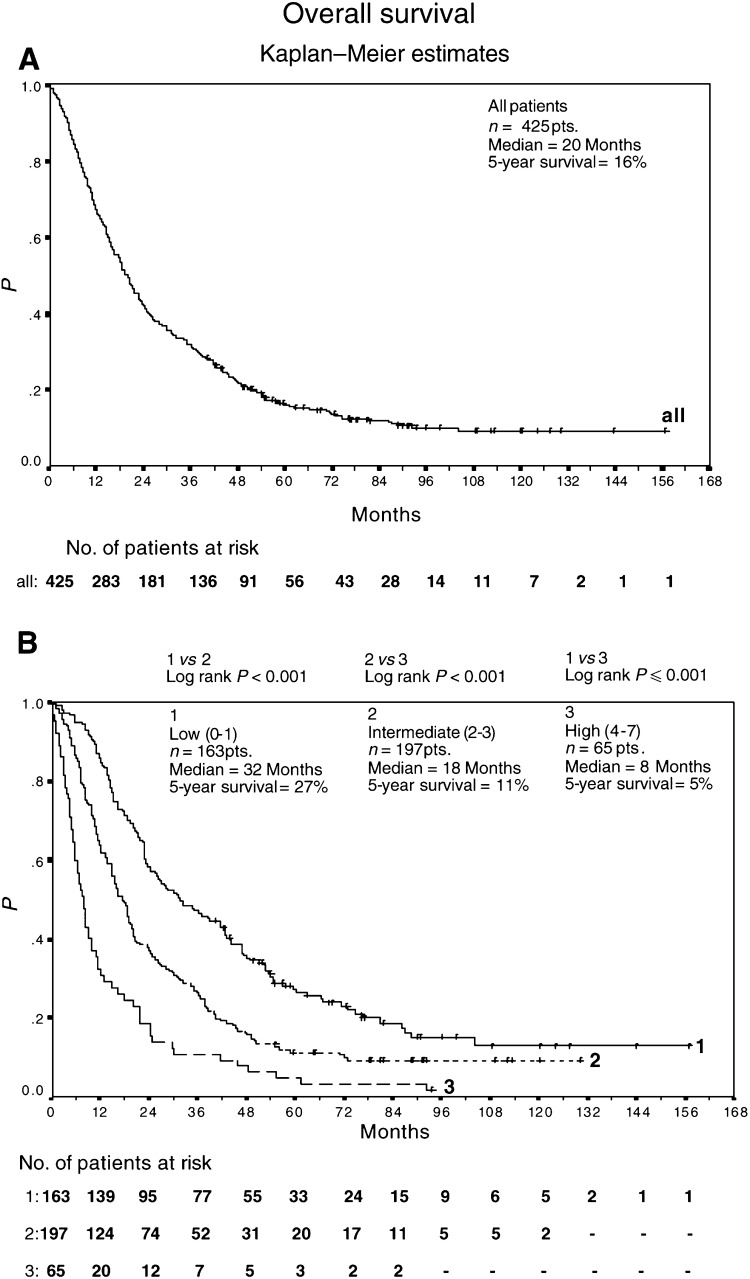
Median survival time was 20+ months (95% CI, 18, 22; 5-year survival of 16%) (Figure 3A); 54 of 425 patients remain alive.
Figure 3.
Overall survival of 425 advanced renal-cell carcinoma patients treated with outpatient s.c. IL-2/INF-α2a therapy (A). Overall survival of 163 low-risk patients, 197 intermediate-risk patients, and 65 high-risk patients treated with outpatient subcutaneous interleukin-2/interferon-α2a therapy (B). Survival was calculated from the start of therapy using Kaplan–Meier method.
Univariate risk factor analysis
As shown in Table 1 , we identified the following pretreatment staging factors as univariate predictors of poor overall survival: (1) three and more metastatic sites, (2) presence of liver, lymph node or bone metastases, (3) neutrophil counts ⩾6500 cells μl−1, (4) serum LDH level ⩾220 U l−1, and (5) serum C-reactive protein level (CRP) ⩾11 mg l−1.
Table 1. Pretreatment clinical factors and their prognostic significance in univariate analysis.
| Risk factorsa | Categories compared | Number of patients | Median survival (months) | P-value (log-rank test) | P-value (correctedb) |
|---|---|---|---|---|---|
| Sex | Female vs male | 114 vs 311 | 20 vs 19 | 0.5 | |
| Age (years) | < 50 vs ⩾50 | 80 vs 345 | 13 vs 21 | 0.06 | 0.5 |
| Pretreatment with IL-2 | Absent vs present | 370 vs 55 | 20 vs 17 | 0.6 | |
| Time from diagnosis of tumour to metastatic disease (years) | < 3 vs ⩾3 | 304 vs 121 | 17 vs 25 | 0.01 | 0.2 |
| Number of metastatic sites | < 3 vs ⩾3 | 350 vs 75 | 21 vs 14 | <0.001 | 0.003 |
| Lung metastases | Absent vs present | 113 vs 312 | 15 vs 21 | 0.2 | |
| Liver metastases | Absent vs present | 362 vs 63 | 20 vs 15 | 0.03 | |
| Lymph node metastases | Absent vs present | 302 vs 123 | 21 vs 15 | 0.02 | |
| Brain metastases | Absent vs present | 400 vs 25 | 20 vs 20 | 0.2 | |
| Bone metastases | Absent vs present | 337 vs 88 | 22 vs 12 | <0.001 | |
| Other metastases | Absent vs present | 284 vs 141 | 21 vs 18 | 0.2 | |
| ESR (mm h−1)c | < 60 vs ⩾60 | 343 vs 82 | 22 vs 11 | 0.002 | 0.05 |
| Hemoglobin (g dl−1)c | < 11 vs ⩾11 | 48 vs 377 | 10 vs 21 | 0.06 | 0.53 |
| Neutrophil counts (cells μl−1)c | < 6500 vs ⩾6500 | 362 vs 63 | 21 vs 8 | <0.001 | <0.001 |
| LDH (U l−1)c | < 220 vs ⩾220 | 330 vs 95 | 22 vs 12 | <0.001 | 0.01 |
| CRP (mg l−1)c | < 11 vs ⩾11 | 222 vs 203 | 24 vs 16 | <0.001 | <0.001 |
Laboratory normal ranges were as follows: ESR: male: 3–8 mm h−1, female: 6–11 mm h−1; haemoglobin: male: 13.5–17.5 g dl−1, female: 12–16 g dl−1; neutrophils: 1500–7500 cells μl−1; LDH: 80–240 U l−1; CRP: <5 mg l−1.
Corrected for testing multiple cutpoints on continuous factors (Miller and Siegmund, 1982).
Cutoff does not reflect normal range.
Sex, age, time from diagnosis of tumour to metastatic disease, the presence of lung, brain or other metastases, ESR, haemoglobin level, and IL-2-pretreatment were also tested, but rendered not significant by univariate analysis after correction (where necessary) of P-values by using the formula of Miller and Siegmund (1982) (see also Altman et al, 1994).
Multivariate analysis of risk factors and overall survival
To build a multiple risk factor model, we used multivariate Cox regression containing all predictors as candidate variables, since factors that are not univariately significant may nevertheless become significant when included together in the model. Six factors were found to be significant in a multivariate fashion. Neutrophil count was identified as the major prognostic factor (hazard ratio=1.9, P<0.001), while serum level of LDH (hazard ratio 1.3; P=0.02) and CRP (hazard ratio 1.4; P=0.001), time between diagnosis of tumour and metastatic disease (hazard ratio 0.7; P=0.001), number of metastatic sites (hazard ratio 1.4; P=0.01), and bone metastases (hazard ratio 1.5; P=0.001) were significant but less important prognostic variables within the multiple risk factor model (Table 2). However, caution should be exercised regarding the significance of these P-values since methodology seems to be unavailable to correct for the selection of ‘optimal’ cutpoints within a multivariate modelling framework.
Table 2. Multivariate risk factor model for overall survival in metastatic renal carcinoma.
| Risk factor | Categories compared | Hazard ratioa | 95% CIa | P-valuea | Weight (contribution to cumulative risk score) |
|---|---|---|---|---|---|
| Neutrophil counts (cells μl−1) | <6500 vs ⩾6500 | 1.9 | 1.5–2.6 | <0.001 | 0 vs 2 |
| LDH (U l−1) | <220 vs ⩾220 | 1.3 | 1.0–1.7 | 0.02 | 0 vs 1 |
| CRP (mg l−1)a | <11 vs ⩾11 | 1.4 | 1.1–1.7 | 0.001 | 0 vs 1 |
| Time from diagnosis of tumour to metastatic disease (years) | <3 vs ⩾3 | 0.7 | 0.5–0.9 | 0.001 | 1 vs 0 |
| Number of metastatic sites | <3 vs ⩾3 | 1.4 | 1.1–1.9 | 0.01 | 0 vs 1 |
| Bone metastases | Absent vs present | 1.5 | 1.2–2.0 | 0.001 | 0 vs 1 |
In multivariate Cox regression model.
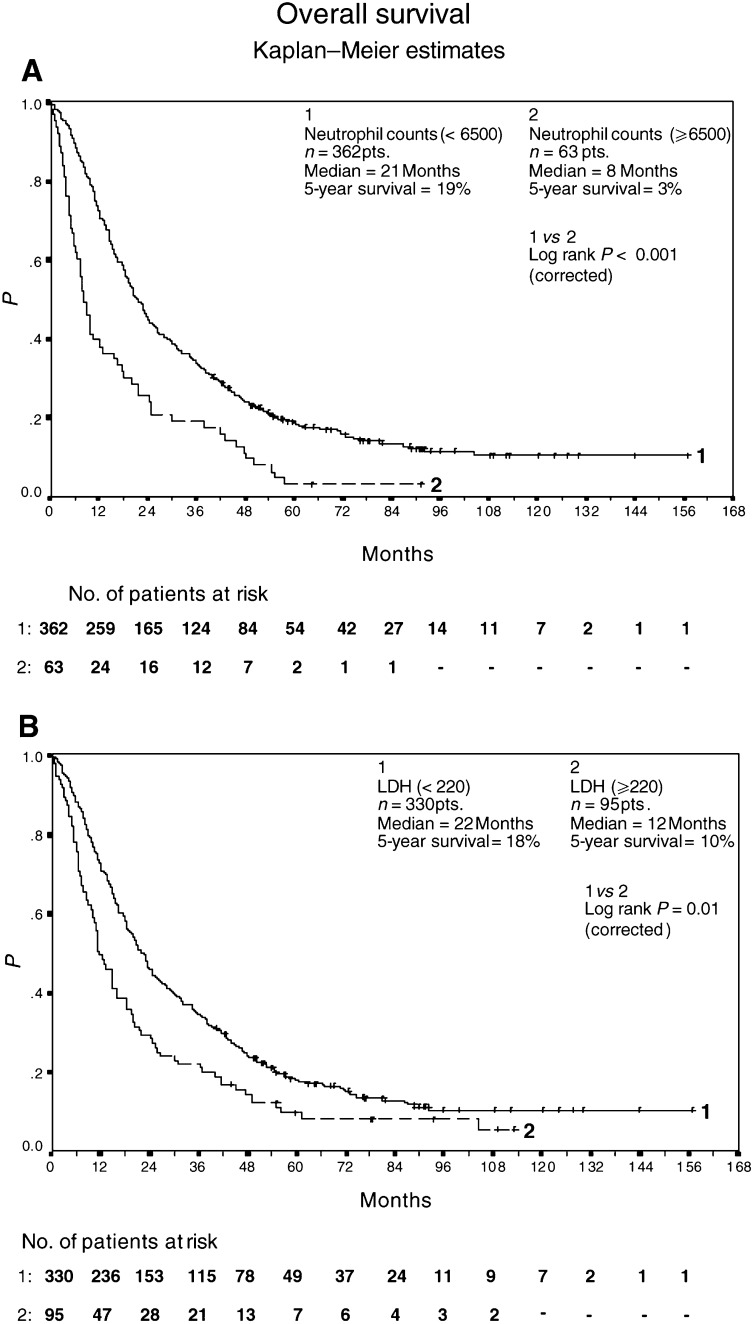
The largest and most significant association with an unfavourable outcome was observed in patients with elevated neutrophil count (⩾6500 cells μl−1) (Figure 1A). A total of 63 patients with elevated levels of neutrophils count achieved a median overall survival of 8+ months (95% CI 6, 12; 5-year survival of 3%), while 362 patients with less than 6500 cells μl−1 yielded a median overall survival of 21+ months (95% CI 19, 24; 5-year survival of 19%). Similarly, 95 patients with elevated LDH levels yielded a median overall survival of 12+ months (95% CI 10, 16; 5-year survival of 10%), in contrast to 330 patients with LDH levels less than 220 U l−1, and a median overall survival of 22+ months (95% CI 19, 25; 5-year survival of 18%).
Figure 1.
Overall survival of 362 patients with neutrophil counts <6500 cells μl−1 and 63 patients with neutrophil counts ⩾6500 cells μl−1 (A). Overall survival of 330 patients with LDH levels <220 U l−1 and 95 patients with LDH levels ⩾220 U l−1 (B). All patients were treated with outpatient s.c. IL-2/IL-α2a therapy. Survival was calculated from the start of therapy using Kaplan–Meier method.
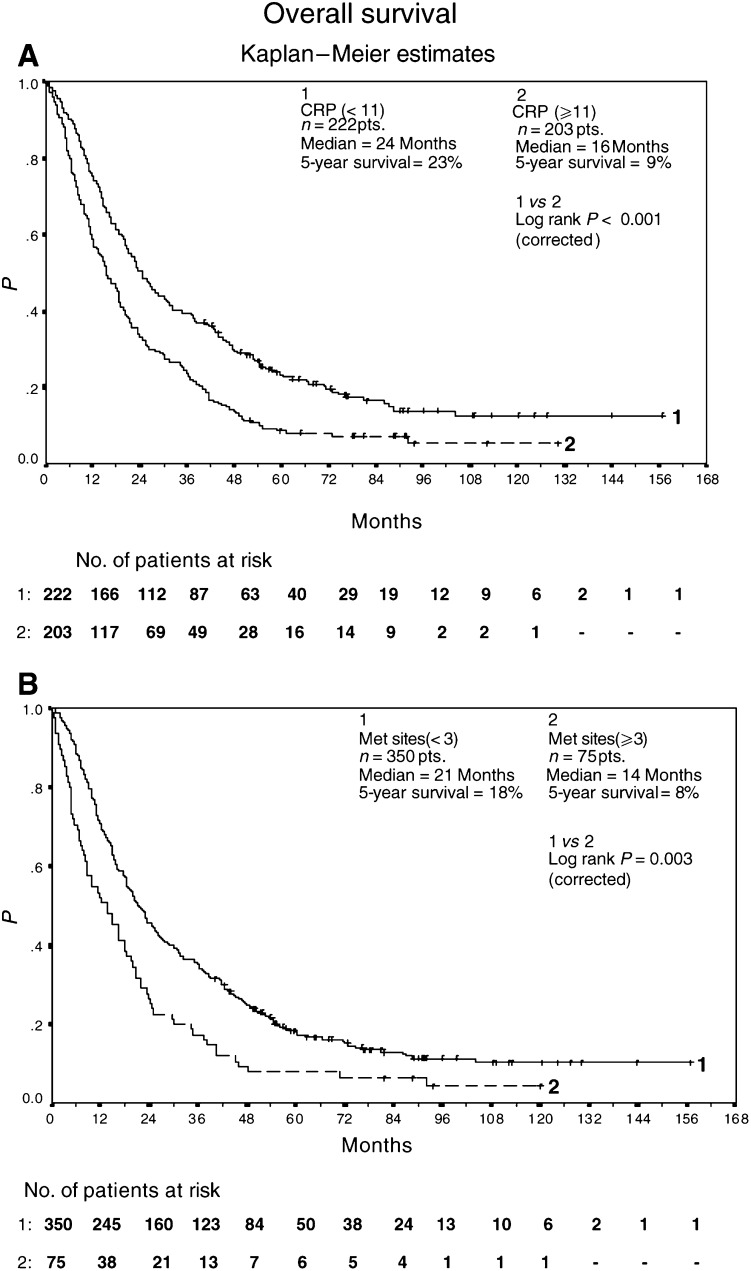
It was calculated that 203 patients with elevated serum levels of CRP achieved a median overall survival of 16+ months (95% CI 12, 19; 5-year survival of 9%), while 222 patients with CRP levels less than 11 mg l−1 yielded a median overall survival of 24+ months (95% CI 21, 30; 5-year survival of 23%) (Figure 2A). In addition, 75 patients with three and more metastatic sites had a median overall survival of 14+ months (95% CI 8, 18; 5-year survival of 8%), compared to 350 patients with one or two metastatic sites and a median overall survival of 21+ months (95% CI 19, 25; 5-year survival of 18%) (Figure 2B).
Figure 2.
Overall survival of 222 patients with CRP levels <11 mg l−1 and 203 patients with CRP levels ⩾11 mg l−1 (A). Overall survival of 350 patients with <3 metastatic sites and 75 patients with ⩾3 metastatic sites (B). All patients were treated with outpatient s.c. IL-2/INF-α2a therapy. Survival was calculated from the start of therapy using Kaplan–Meier method.
Prognostic system
Based on the rounded regression coefficients (log hazard ratios in the final Cox model) of variables, we defined the weights of prognostic features as follows: neutrophil count was assigned weight 2, the remaining variables (serum level of CRP and LDH, time between diagnosis of tumour and metastatic disease, number of metastatic sites, bone metastases) were given weight 1. A prognostic score consisting of the sum of the weights of these six variables was used to assign patients to low (0⩾score⩽1), intermediate (2⩾score⩽3), and high risk (4⩾score⩽7) groups, respectively (Table 3).
Table 3. Definition of risk groups from cumulative risk score.
| Risk group | Cumulative risk score | Contribution of individual prognostic variables |
|---|---|---|
| Low risk (n=163) | 0, 1 | Absence |
| One minor prognostic variablea | ||
| Intermediate (n=197) | 2, 3 | Two minor prognostic variables |
| Three minor prognostic variables | ||
| One minor plus major prognostic variableb | ||
| High risk (n=65) | 4, 5, 6, 7 | Four or five minor prognostic variables |
| ⩾Three minor plus major prognostic variable |
i.e. with weight 1.
i.e. with weight 2.
Median overall survival of low (n=163), intermediate (n=197), and high-risk (n=65) patients was 32+ months (95% CI 24, 43; 5-year survival of 27%), 18+ months (95% CI 15, 20; 5-year survival of 11%), and 8+ months (95% CI 6, 10; 5-year survival of 5%), respectively (Figure 3B).
DISCUSSION
The objective of this study was to devise a comprehensive new prognostic system for survival of metastatic renal carcinoma patients.
Using a multivariate risk model derived from the retrospective analysis of 425 patients with metastatic renal-cell carcinoma, we categorised patients into three distinct risk groups based on the following six prognostic factors for poor survival: (1) neutrophil count ⩾6500 cells μl1, (2) serum level of LDH ⩾220 U l−1, (3) serum level of CRP ⩾11 mg l−1, (4) time between diagnosis of tumour and metastatic disease less than 3 years, (5) three and more metastatic sites, and (6) the presence of bone metastases.
These prognostic variables in advanced renal cancer were comparable to clinical features reported previously by others, notably with regard to the number of metastatic sites (Elson et al, 1988), bone metastases (Mani et al, 1995), time between diagnosis of tumour and metastatic disease (Maldazys and deKernion, 1986; Elson et al, 1988; Motzer et al, 2002), and serum level of LDH (Motzer et al, 1999,2002).
In this current and in our previous study (Lopez-Hänninen et al, 1996), for the first time, we could identify pretreatment neutrophil count as a highly statistically significant predictor for overall survival in advanced renal-cell carcinoma. While the biological interpretation of increased neutrophil counts is not evident, Blay et al (1997) demonstrated that IL-6 associated neutrophilia in renal-cell carcinoma could be decreased via the suppression of IL-6 or IL-6-associated paraneoplastic inflammatory syndrome in renal carcinoma. Notably, Paesmans et al (1995),(2000) and Kapp et al (1983) also showed its impact as a prognostic factor for survival in small-cell lung cancer and uterine cervix carcinoma, respectively.
Surprisingly, in this report the presence of brain or CNS metastases (n=25) had no selective impact on survival, which may have been because of concomitant extensive multiorgan disease. Alternatively, the small number of such patients may have reduced the statistical power to detect such an impact in the presence of other important risk factors.
Similar to Motzer et al (2002), who categorised IFN-α-treated advanced renal-cell cancer patients into three different groups with low (18%; overall survival 30 months), intermediate (62%; overall survival 14 months), and high-risk patients (20%; overall survival 5 months), respectively, we established three distinct survival subgroups that is, low-risk patients (38%) with an overall median survival of 32 months, intermediate-risk patients (47%) with an overall median survival of 18 months, and high-risk patients (15%) with an overall median survival of 8 months. Notably, while Motzer et al (2002) also included LDH, in our current model, performance status and serum calcium were not tested, and serum haemoglobin was not identified as a significant statistical predictor for overall survival. In the present prognostic model, risk groups exhibited well-separated survival curves that reflected the prognosis of good/excellent performance status of metastatic renal-cell carcinoma patients receiving outpatient IL-2/IFN-α2a. Overall, this group was highly selected as demonstrated by the relatively large number of patients who had a delay between primary diagnosis and metastatic disease in excess of 3 years.
The identification of prognostic features for overall survival in metastatic renal carcinoma patients has a pivotal role in defining future individualised molecular treatment approaches. The low proportion of patients achieving long-term survival suggest the need for further clinical trials of new therapeutic agents.
While there is a partial consensus between different prognostic models in metastatic renal-cell carcinoma, validation of our proposed model will require testing in a prospectively designed study.
References
- Altman DG, Lausen B, Sauerbrei W (1994) Danger of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Nat Cancer Inst 86(11): 829–835 [DOI] [PubMed] [Google Scholar]
- Atzpodien J, Kirchner H, Illiger HJ, Metzner B, Ukena D, Schott H, Funke PJ, Gramatzki M, von Jürgenson S, Wandert T, Patzelt T, Reitz M. (2001) IL-2 in combination with IFN-α and 5-FU versus tamoxifen in metastatic renal-cell carcinoma: long-term results of a controlled randomised clinical trial. Br J Cancer 85(8): 1130–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, Fornaia P, Loening SA, Roigas J, Müller SC, Westerhausen H, Helbig W, Bodenstein H, Pomer S, Metzner B, Rebmann U, Hofstetter A, Oberneder R, Siebels TI, Wandert T, Patzelt T, Reitz M, DGCIN-Group (2002) 13-CIS-retinoic acid, IFN-alpha2a, IL-2 and chemotherapy in advanced renalcell carcinoma: results of a prospectively randomized trial of The German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). J Clin Oncol (submitted). [DOI] [PubMed]
- Atzpodien J, Korfer A, Franks CR, Knuver-Hopf J, Lopez-Hanninen E, Fischer M, Mohr H, Dallmann I, Hadam M. (1990) Home therapy with recombinant interleukin-2 and interferon-alpha 2b in advanced human malignancies. Lancet; 335: 1509–1512 [DOI] [PubMed] [Google Scholar]
- Blay JY, Rossi JF, Wijdenes J, Menetrier-Caux C, Schemann S, Negrier S, Philip T, Favrot M (1997) Role of interleukin-6 in the paraneoplastic inflammatory syndrome associated with renal-cell carcinoma. Int J Cancer: 72(3): 424–430 [DOI] [PubMed] [Google Scholar]
- Culine S, Bekradda M, Kramar A, Rey A, Escudier B, Droz JP (1998) Prognostic factors for survival in patients with brain metastases from renal-cell carcinoma. Cancer 83(12): 2548–2553 [PubMed] [Google Scholar]
- Elson PJ, Witte RS, Trump DL (1988) Prognostic factors for survival in patients with recurrent or metastatic renal-cell carcinoma. Cancer Res 48(24 Pt 1): 7310–7313 [PubMed] [Google Scholar]
- Gelb AB. (1997) Renal cell carcinoma: current prognostic factors. Union Internationale Contre le Cancer (UIC) and American Joint Committee on Cancer (AJCC). Cancer 80(5): 981–986 [PubMed] [Google Scholar]
- Hoffmann R, Franzke A, Buer J, Sel S, Oevermann K, Duensing A, Probst M, Duensing S, Kirchner H, Ganser A, Atzpodien J (1999) Prognostic impact of invivo soluble cell adhesion molecules in metastatic renal cell carcinoma. Br J Cancer 79(11–12): 1742–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp DS, Fischer D, Gutierrez E, Kohorn EI, Schwartz PE. (1983) Pretreatment prognostic factors in carcinoma of the uterine cervix: a multivariable analysis of the effect of age, stage, histology and blood counts on survival. Int J Radiat Oncol Biol Phys 9(4): 445–455 [DOI] [PubMed] [Google Scholar]
- Lopez-Hänninen E, Kirchner H, Atzpodien J (1996) Interleukin-2 based home therapy of metastatic renal cell carcinoma: risks and benefits in 215 consecutive single institution patients. J Urol 155: 19–25 [PubMed] [Google Scholar]
- Maldazys JD, deKernion JB (1986) Prognostic factors in metastatic renal carcinoma. J Urol 136(2): 376–379 [DOI] [PubMed] [Google Scholar]
- Mani S, Todd MB, Katz K, Poo WJ (1995) Prognostic factors for survival in patients with metastatic renal cancer treated with biological response modifiers. J Urol 154(1): 35–40 [PubMed] [Google Scholar]
- Miller R, Siegmund D (1982) Maximally selected chi-square statistics. Biometrics 38: 1011–1016 [Google Scholar]
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M (2002) Interferon-alfa as a comparative treatment for clinical trials of new therapies aganst advanced renal cell carcinoma. J Clin Oncol 20(1): 289–296 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal-cell carcinoma. J Clin Oncol 17(8): 2530–2540 [DOI] [PubMed] [Google Scholar]
- Paesmans M, Sculier JP, Lecomte J, Thiriaux J, Libert P, Sergysels R, Bureau G, Dabouis G, Van Cutsem O, Mommen P, Ninane V, Klastersky J (2000) Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer 89(3): 523–533 [DOI] [PubMed] [Google Scholar]
- Paesmans M, Sculier JP, Libert P, Bureau G, Dabouis G, Thiriaux J, Michel J, Van Cutsem O, Sergysels R, Mommen P et al (1995) Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analysis including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol 13(5): 1221–1230 [DOI] [PubMed] [Google Scholar]
- Palmer PA, Vinke J, Philip T, Negrier S, Atzpodien J, Kirchner H, Oskam R, Francs CR (1992) Prognostic factors for survival in patients with advanced renal-cell carcinoma treated with recombined inerleukin-2. Ann Oncol 3(6): 475–480 [DOI] [PubMed] [Google Scholar]
- Sleijfer DT, Janssen RA, Buter J, de Vries EG, Willemse PH, Mulder NH (1992) Phase II study of subcutaneous interleukin-2 in unselected patients with advanced renal-cell cancer on an outpatient basis. J Clin Oncol 10(7): 1119–1123 [DOI] [PubMed] [Google Scholar]