Abstract
CD26/dipeptidyl peptidase IV (DPPIV) is a surface antigen with multiple functions, including a role in T-cell activation and the development of certain human cancers. We previously demonstrated that CD26/DPPIV enhanced sensitivity of Jurkat cells to doxorubicin. We now show that expression of CD26/DPPIV enhanced sensitivity of CD26 Jurkat transfectants to G2–M arrest mediated by the antineoplastic agent etoposide. The increased sensitivity to etoposide-induced G2–M arrest was associated with disruption of cell cycle-related events, including hyperphosphorylation of p34cdc2 kinase, change in cdc25C expression and phosphorylation, and alteration in cyclin B1 expression. CD26/DPPIV-associated enhancement of doxorubicin and etoposide-induced G2–M arrest was also observed in serum-free media, suggesting an effect of CD26 on cell-derived processes rather than serum-derived factors. Importantly, our work elucidated a potential mechanism for the enhanced susceptibility of CD26-expressing Jurkat cells to the topoisomerase II inhibitors by demonstrating that CD26/DPPIV surface expression was associated with increased topoisomerase II α levels and enhanced enzyme activity. Besides being the first to show a functional association between the multifaceted molecule CD26 and the key cellular protein topoisomerase II α, our studies provide additional evidence of a potential role for CD26 in the treatment of selected malignancies.
Keywords: CD26/DPPIV, cell cycle, G2–M, topoisomerase II α, Jurkat
CD26 is a 110 kDa surface glycoprotein with diverse functional properties and is expressed on a variety of tissues, including selected lymphoid cells (Morimoto and Schlossman, 1998). Its extracellular domain encodes a membrane-associated dipeptidyl peptidase IV (DPPIV) activity, able to cleave amino-terminal dipeptides from polypeptides with either L-proline or L-alanine at the penultimate position, including chemokines involved in leukocyte function (Oravecz et al, 1997; Proost et al, 1998). CD26 is also involved in T-cell activation, owing to its association with CD45, mannose 6-phosphate/insulin-like growth factor II receptor and adenosine deaminase (ADA) (Morimoto et al, 1989; Dang et al, 1990a,1990b,1990c,1990d,1991; Torimoto et al, 1991; Kameoka et al, 1993; Morrison et al, 1993; Ikushima et al, 2000). Additionally, its DPPIV enzyme activity has a key role in various aspects of T-cell activation, as demonstrated by studies using DPPIV inhibitors, soluble CD26/DPPIV molecules or CD26 genetic mutants (Flentke et al, 1991; Tanaka et al, 1993,1994; Steinbrecher et al, 2001). Besides its involvement in normal T-cell function, CD26 may also have a role in the development of certain tumors (Tanaka et al, 1995; Stecca et al, 1997; Bauvois et al, 1999; Dang and Morimoto, 2002). For example, it is expressed on the surface of such aggressive T-cell malignancies as T-cell lymphoblastic lymphomas/acute lymphoblastic leukaemias and T cell CD30+ anaplastic large cell lymphomas, but not on the more indolent T-cell diseases like mycosis fungoides (Carbone et al, 1995; Jones et al, 2001).
Based on our hypothesis that CD26 expression influences tumour cell biology and potentially its sensitivity to cytotoxic treatments, as suggested by previous work showing that CD26 expression is associated with changes in tumour cell line behaviour in vitro and that the clinical behaviour of selected tumours may be correlated with differences in CD26 expression (Morrison et al, 1993; Carbone et al, 1995), we initiated studies using stable CD26 Jurkat transfectants to investigate the effect of CD26 on tumour sensitivity to selected antineoplastic agents. We recently demonstrated that Jurkat cells expressing CD26/DPPIV displayed enhanced sensitivity to cell cycle arrest induced by doxorubicin (Aytac et al, 2001). Owing to the potential implications of our findings with regards to future treatment of selected cancers, we investigated the effect of CD26/DPPIV on tumour sensitivity to etoposide, another key antineoplastic agent. In this paper, we extend our previous work by showing that expression of CD26/DPPIV resulted in enhanced sensitivity to etoposide-induced G2–M arrest, associated with such changes of key regulators of this checkpoint as hyperphosphorylation of p34cdc2, change in expression and phosphorylation of cdc25C and alteration in cyclin B1 expression. Furthermore, we demonstrate that the effect of CD26/DPPIV on drug sensitivity was independent of serum, as similar results were obtained in serum-free media. Finally and perhaps most importantly, we establish that expression of CD26/DPPIV resulted in enhanced levels of topoisomerase II α that correlated with increased enzyme activity, suggesting a potential mechanism for the observed increase in tumour sensitivity to the topoisomerase II inhibitors doxorubicin and etoposide.
MATERIALS AND METHODS
Cells and reagents
Human T-cell leukaemia Jurkat stable transfectants have been described (Tanaka et al, 1993; Aytac et al, 2001). The Jurkat cell lines include: (1) wild-type CD26-transfected Jurkat cell lines (wtCD26); (2) Jurkat cell lines transfected with mutant CD26 containing an alanine at the putative catalytic serine residue at position 630, resulting in a mutant CD26-positive/DPPIV-negative Jurkat transfectant (S630A); and (3) nontransfected parental Jurkat cells (parental). Jurkat transfectants were maintained in culture media, which consisted of RPMI 1640 supplemented with 10% FCS, penicillin (100 U ml−1), streptomycin (100 μg ml−1), and G418 (0.25 mg ml−1) (Gibco BRL, NY, USA). Nontransfected parental Jurkat cells were maintained in the same culture media without G418. For certain experiments, AIM V serum-free media (Gibco, NY, USA) were used rather than culture media containing RPMI 1640 and 10% FCS. In experiments involving AIM V serum-free media, cells were washed extensively with sterile PBS, and then preincubated for 24 h at 37°C with AIM V-containing culture media to prevent contamination with serum. Following preincubation, cells were washed with sterile PBS, and then further incubate in AIM V-containing culture media with or without chemotherapeutic agents at the indicated doses. Anti-p34cdc2, anti-cdc25C and anti-cyclin B1 were from Santa Cruz (CA, USA), antitopo-isomerase II α (Ki-S1, recognising a carboxyterminal α-isozyme specific epitope) was from Boehringer Mannheim, antiactin was from Sigma, and the anti-CD26 antibody 1F7 was a murine antibody that has been described previously (Morimoto et al, 1989). Tetrazolium salt MTT (3,(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) (Sigma, MO, USA) was dissolved at a concentration of 5 mg ml−1 in sterile PBS at room temperature, with the solution being filter sterilized and stored at 4°C in the dark. Extraction buffer was prepared as follows: 20% w v−1 of SDS was dissolved at 37°C in a solution of 50% each of N,N-dimethyl formamide (DMF) (Sigma, MO, USA) and distilled water; pH was adjusted to 4.7 by the addition of 1 M HCl. Etoposide and doxorubicin were purchased from Calbiochem and was dissolved in sterile PBS. Hybond N+ membrane, [α-32P]dCTP and the Ready-To-Go DNA labelling beads were obtained from Amersham Biosciences. Human topoisomerase IIalpha gene probe YEpWob6 was kindly provided by Dr Leroy F Liu (Robert Wood Johnson Medical School, NJ, USA).
MTT assay
Cell growth assay was performed as described previously (Aytac et al, 2001). Cells were incubated in microplates in the presence of culture media alone, culture media and doxorubicin or etoposide at the indicated concentrations for a total volume of 100 μl (50 000 cells well−1). Following 36–48 h of incubation at 37°C, 25 μl of MTT was added to the wells at a final concentration of 1 mg ml−1. The microplates were then incubated for 2 h at 37°C, followed by the addition of 100 μl of extraction buffer. Following overnight incubation at 37°C, OD measurements at 570 nm were performed, with the s.e. of the mean of the triplicate wells being less than 15%. Cytotoxicity index was calculated as follows:
 |
Cell cycle analysis
Cells were incubated in culture media alone, culture media and doxorubicin or etoposide at indicated concentrations at 37°C for 24 h. Cells were then collected, washed twice with PBS and resuspended in PBS containing 10 μg ml−1 propidium iodide, 0.5% Tween-20 and 0.1% RNase at room temperature for 30 min. Samples were then analysed (FACScan, Becton Dickinson, CA, USA) for DNA content. Cell debris and fixation artefacts were gated out and G0–G1, S, and G2–M populations were quantified using the CellQuest and ModFit LT programs.
Sodium dodecyl – polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
After incubation at 37°C in culture media and doxorubicin or etoposide at the concentrations indicated for 24 h, cells were harvested from wells, washed with PBS and lysed in lysis buffer, consisting of 1% Brij 97, 5 mM EDTA, 0.02 M HEPES, pH 7.3, 0.15 M NaCl, 1 mM PMSF, 0.5 mM NaF, 10 μg ml−1 aprotinin and 0.2 mM sodium orthovanadate. After incubating on ice for 15 min, nuclei were removed by centrifugation and supernatants were collected. 2×sample buffer consisting of 20% glycerol, 4.6% SDS, 0.125 M Tris, pH 6.8 and 0.1% bromophenol blue was added to the appropriate aliquots of supernatants. Following boiling, protein samples were submitted to SDS–PAGE analysis on an 8% gel under standard conditions using a mini-Protean II system (Bio-Rad, CA, USA). For immunoblotting, the proteins were transferred onto nitrocellulose (Immobilon-P, Millipore, MA, USA). Following overnight blocking at 4°C in a blocking solution consisting of 0.1% Tween-20 and 5% bovine serum albumin (BSA) in TBS, membranes were blotted with the appropriate primary antibodies diluted in blocking solution for 1 h at room temperature. Membranes were then washed with blocking solution, and appropriate secondary antibodies diluted in blocking solution were then applied for 1 h at room temperature. Secondary antibodies were goat anti-mouse or goat anti-rabbit HRP conjugates (Dako, CA, USA). Membranes were then washed with blocking solution and proteins were subsequently detected by chemiluminescence (Amersham Pharmacia Biotech, NJ, USA).
Detection of DPPIV enzyme activity
Dipeptidyl peptidase IV (DPPIV) enzyme activity was detected by using an Enzyme Overlay Membrane system (EOM, Enzyme Systems Products, Dublin, CA, USA) to which the substrate Ala-Pro-AFC (7-amino-4-trifluoromethyl Coumarin) has been coupled, as described previously (Aytac et al, 2001). Following incubation at 37°C with media alone or media with etoposide for 24 h, cell lysates were prepared, and sample buffer consisting of 20% glycerol, 4.6% SDS, 0.125 M Tris, pH 6.8, 0.1% bromophenol blue and 2% 2-mercaptoethanol was added at room temperature. Samples were then submitted to SDS–PAGE analysis on an 8% gel under standard conditions. The EOM was moistened in 0.5 M Tris HCl, pH 7.8, placed over the surface of the gel, and incubated at 37°C for 40 min in a humidified atmosphere. The membrane was then removed from the gel and placed atop a long-wavelength UV lamp box to monitor enzymatic reaction, which involves the removal of the dipeptide Ala-Pro from the fluorogenic AFC and results in the appearance of fluorescent bands on the membrane.
Immunofluorescence
All procedures were carried out at 4°C, and flow cytometry analyses were performed (FACScan, Becton Dickinson, CA, USA) as described previously (Dang et al, 1990d). Cells were stained with anti-CD26 antibody and washed two times with PBS and then with goat anti-mouse IgG FITC (Coulter). Cells were then washed two times with PBS prior to flow cytometry analyses. Negative controls were stained with second antibody alone.
Preparation of nuclear extracts for detection of topoisomerase II α
For detection of topoisomerase II α by immunoblotting, isolation of nuclear fractions from Jurkat cells were prepared as follows. In brief, 10×106 cells were harvested and allowed to swell for 15 min on ice in cytoplasmic extraction buffer (10 mM HEPES, 10 mM KCL, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, 2 μg ml−1 leupeptin, 2 μg ml−1 aprotinin, and 0.5 mg ml−1 benzamidine). Then NP-40 (final concentration 0.3%) was added into that cell suspension and vortexed for 10 s. After 1 min of centrifugation at 16 000 g, the supernatant was removed. The pellet was then incubated with nuclear extraction buffer (20 mM HEPES, 400 mM KCL, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 2 μg ml−1 leupeptin, 2 μg ml−1 aprotinin, and 0.5 mg ml−1 benzamidine) for 30 min on ice with intermittent vortexing. The suspension was centrifuged at 16 000 g for 5 min, and supernatant was saved as the nuclear extract. SDS–PAGE and immunoblotting were then performed on the nuclear extracts. Each lane was equally loaded with 15 μg of protein.
Detection of topoisomerase II α activity
Topoisomerase II α catalytic activity was analysed through the use of the topoisomerase II decatenation assay kit according to the manufacturer's instructions (TopoGEN, Inc, OH, USA). Briefly, following incubation of Jurkat cells at 37°C for 24 h in culture media, 10×106 cells were harvested and nuclear extracts were obtained. Appropriate dilutions of nuclear extracts of Jurkat CD26 transfectants or parental Jurkat cells were then incubated with 0.2 μg of kinetoplast DNA (kDNA) in a 1×Topo II assay buffer (50 mM Tris-HCl, pH 8, 120 mM KCl, 10 mM MgCl2, 0.5 mM ATP, 0.5 mM dithiothreitol, and 30 μg ml−1 BSA) for 60 min at 37°C. The reactions were terminated by the addition of 5×stop buffer/gel loading dye (final concentration: 1% Sarkosyl, 0.0005% bromophenol blue and 5% glycerol). DNA products were resolved on 1% agarose gel containing ethidium bromide (0.5 μg ml−1) at 50 V for 60 min. After electrophoresis, the gel was destained in water for 30 min, and fluorescence was photographed by UV imager.
Northern blotting studies
Total RNA was isolated from Jurkat cells by using Trizol Reagent (Life Technologies, NY, USA) according to the manufacturer's instructions. Then, RNA samples (20 μg lane−1) were run on 1% agarose–formaldehyde gel and transferred to a Hybond N+ membrane for the Northern blot analysis by standard procedures. For detection of topoisomerase II α mRNA, YEpWob6 cDNA probe was labelled with [α-32P]dCTP using the Ready-To-Go DNA labelling beads (Amersham Biosciences, NJ, USA) and the blots were hybridised with the labelled probe in ULTRAhyb buffer (Ambion, TX, USA) for 24 h at 42°C. The blots were washed in 2×SSC and 0.1% SDS at 42°C and then washed in 0.1×SSC and 0.1% SDS at 42°C. The blots were exposed to a Phosphor Imager screen (Molecular Dynamics Storm MD 840, NJ, USA) for 24–48 h.
Statistics
Student's t-test was used to determine whether the difference between control (S630A and parental Jurkat cells) and wtCD26 cells was significant (P<0.05 being significant).
RESULTS
Effect of CD26/DPPIV expression on etoposide-mediated growth inhibition and cell cycle arrest at the G2–M checkpoint of Jurkat cells
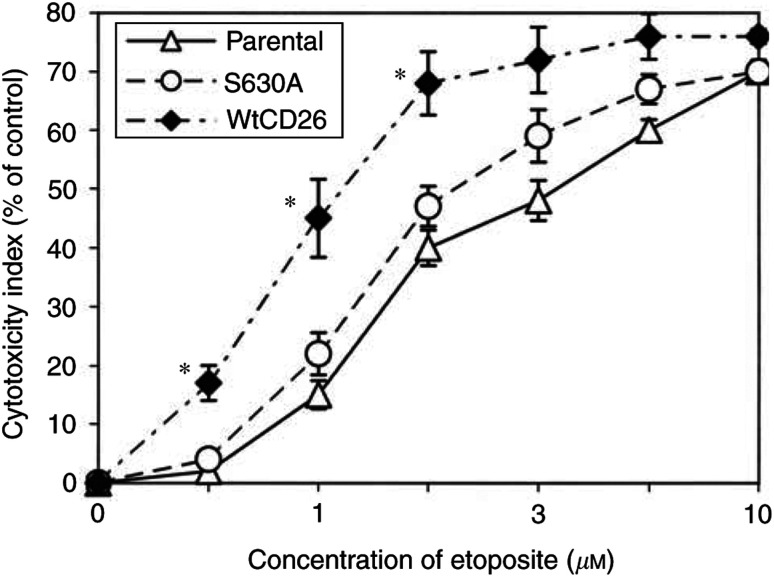
In view of our recent findings regarding the effect of CD26/DPPIV on sensitivity to the topoisomerase II inhibitor doxorubicin, we examined its effect on etoposide, another topoisomerase II inhibitor with antineoplastic activity, using stable CD26 transfectants of the Jurkat T-leukaemia cell line. Figure 1 shows that wtCD26 transfectants (wtCD26) displayed significantly increased sensitivity to etoposide compared to parental cells, as monitored by MTT uptake assays. Vector-only Jurkat transfectants also exhibited the same degree of drug sensitivity as parental cells (data not shown). Significantly, CD26 transfectants mutated at the DPPIV catalytic site (S630A) were also less sensitive to etoposide than wtCD26 transfectants. These data suggested that the presence of CD26, particularly its intrinsic DPPIV enzymatic activity, resulted in enhanced sensitivity to DNA damage mediated by the antineoplastic agent etoposide.
Figure 1.
 |
Cell cycle analysis by PI staining demonstrated that the enhancement in etoposide sensitivity seen with wtCD26 transfectants as compared to parental Jurkat cells was because of an increase in the percentage of cells arresting at G2–M (Table 1 ). Once again, DPPIV enzymatic activity was required to maximise drug sensitivity to etoposide as the G2–M block in the S630A CD26 mutant was significantly less than that seen with wtCD26 transfectants.
Table 1. Enhanced etoposide-induced G2–M arrest associated with CD26/DPPIV.
| %G0–G1 | %S | %G2–M | |
|---|---|---|---|
| Media alone | |||
| Parental | 49 | 38 | 13 |
| S630A | 47 | 42 | 11 |
| wtCD26 | 46 | 40 | 14 |
| Etoposide (0.10 μM) | |||
| Parental | 41 | 38 | 21 |
| S630A | 34 | 37 | 29 |
| wtCD26 | 25 | 19 | 56 |
| Etoposide (0.25 μM) | |||
| Parental | 27 | 32 | 41 |
| S630A | 22 | 30 | 48 |
| wtCD26 | 12 | 19 | 69 |
CD26 Jurkat transfectants and parental cells were incubated at 37°C in media containing etoposide for 24 h. Cells were then harvested and cell cycle analyses were performed with PI staining. Data are representative of three separate experiments.
CD26 expression and DPPIV enzyme activity on Jurkat transfectants
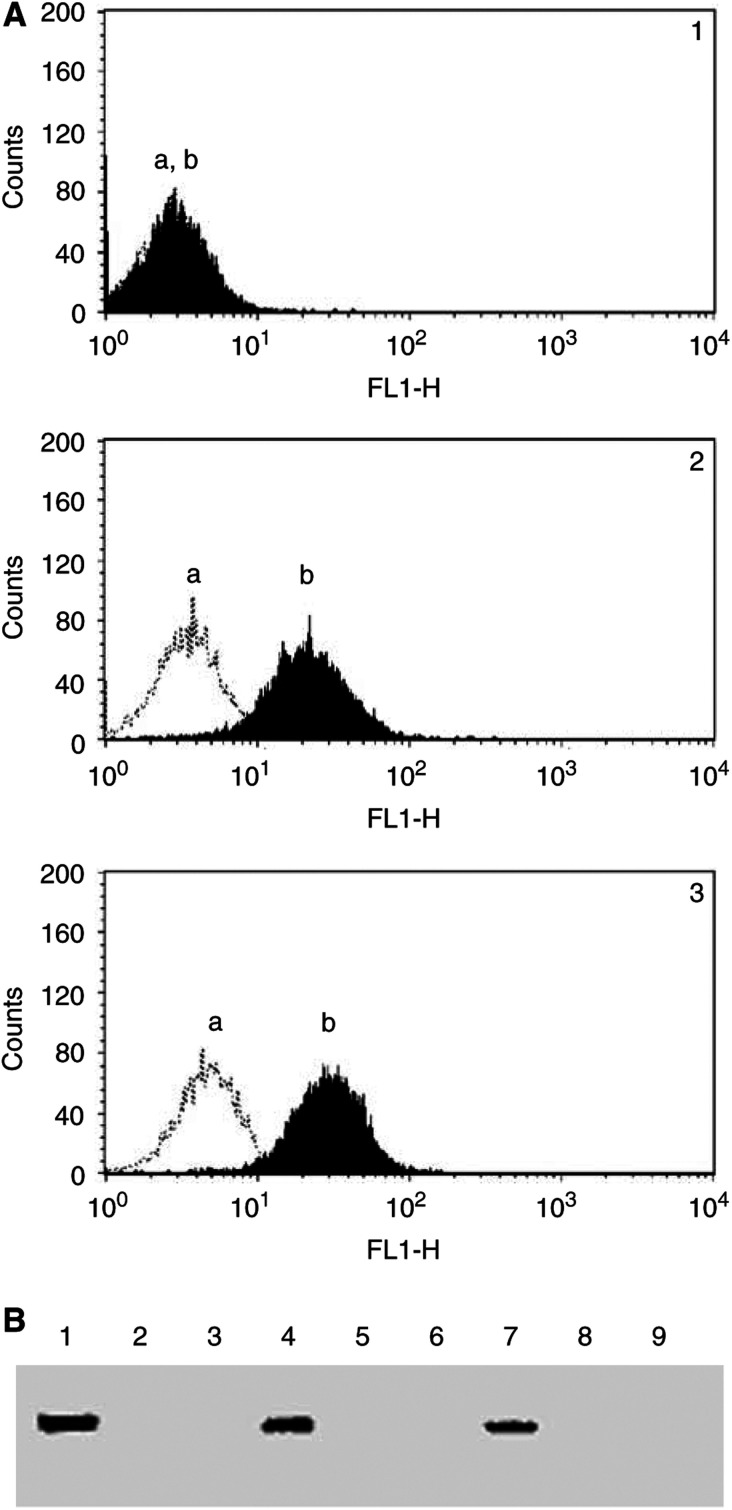
Data from Figure 2A showed that CD26 is expressed on the surface of wtCD26 and S630A Jurkat transfectants, and is not expressed on parental Jurkat cells. Figure 2B showed that only wtCD26 Jurkat transfectants expressed DPPIV enzyme activity, which still remains after treatment with etoposide. On the other hand, S630A transfectants as well as parental Jurkat cells did not exhibit DPPIV enzyme activity. These data therefore indicated that the observed differences in etoposide sensitivity in these various CD26 Jurkat transfectants were associated with differences in DPPIV enzymatic activity.
Figure 2.
CD26 expression and DPPIV enzyme activity on Jurkat transfectants. (A) Jurkat cells were evaluated for CD26 expression by flow cytometry as described in Materials and Methods. panel 1: Parental Jurkat; panel 2: S630A transfectants; panel 3: wtCD26 transfectants; a: negative control; b: anti-CD26 antibody. (B) Jurkat cells were incubated for 24 h at 37°C with media alone or etoposide at 0.10 μM or 0.50 μM. Cells were then harvested, and DPPIV enzyme activity assays were performed as described in Materials and Methods. Lanes 1–3: media alone; lanes 4–6: etoposide at 0.10 μM; lanes 7–9: etoposide at 0.50 μM. Lanes 1, 4, 7: wtCD26; lanes 2, 5, 8: S630A; lanes 3, 6, 9: parental Jurkat cells.
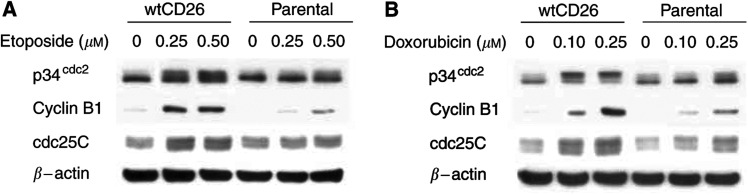
Effect of CD26/DPPIV expression on p34cdc2, cyclin B1 and cdc25C following etoposide treatment of Jurkat cells
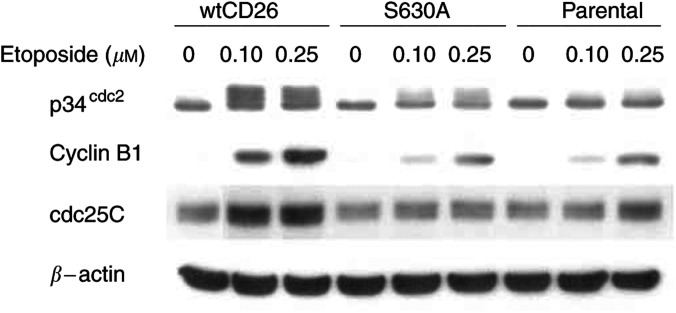
To elucidate the mechanisms involved in CD26/DPPIV-associated enhancement in etoposide-induced G2–M arrest, we evaluated the status of key regulators of this checkpoint (Figure 3). p34cdc2 undergoes hyperphosphorylation at the inhibitory residues Thr14/Tyr15 following etoposide treatment, leading to decreased kinase activity associated with G2–M arrest (King et al, 1994; Poon et al, 1997; Aytac et al, 2001). As assessed by Western blot analysis, etoposide-treated wtCD26 Jurkat transfectants had higher levels of hyperphosphorylated p34cdc2 as compared to parental Jurkat cells and S630A transfectants, which only exhibited a slight enhancement in the level of phosphorylated p34cdc2 at the higher doses of etoposide.
Figure 3.
Effect of CD26/DPPIV expression on p34cdc2/cyclin B1 complex and cdc25C following etoposide treatment. Jurkat cells were incubated for 24 h at 37°C with media containing etoposide at the indicated doses. Cells were then harvested, and immunoblotting studies were performed with appropriate antibodies as described in Materials and Methods.
Meanwhile, as demonstrated previously (Peng et al, 1997; Aytac et al, 2001), lysates from asynchronously growing Jurkat cells contained two major electrophoretic forms of cdc25C differing in serine-216 phosphorylation. Our studies consistently showed that wtCD26 transfectants displayed increased overall expression of cdc25C and enhancement in cdc25C serine-216 phosphorylation as compared to S630A transfectants and parental cells, when these cells were treated with etoposide. The increase in cdc25C serine-216 phosphorylation was therefore concordant with enhanced p34cdc2 inhibitory phosphorylation in etoposide-treated wtCD26 Jurkat transfectants as compared to parental cells or S630A transfectants.
Our studies also showed that the levels of cyclin B1, which associates with p34cdc2 as part of the key complex regulating cell cycle progression at the G2–M checkpoint, were higher in wtCD26 Jurkat transfectants as compared to those in parental cells or S630A transfectants following treatment with etoposide. As we previously noted (Aytac et al, 2001), we found in many experiments that cyclin B1 levels were slightly higher in untreated wtCD26 transfectants as compared to untreated parental cells or S630A transfectants. Nevertheless, treatment with etoposide consistently resulted in a significant rise in cyclin B1 level in wtCD26 transfectants, an increase greater than that seen in parental Jurkat cells or S630A transfectants. The relative level of cyclin B1 expression therefore correlated with the relative sensitivity of CD26 Jurkat transfectants to etoposide, similar to the case seen with p34cdc2 phosphorylation status and cdc25C serine-216 phosphorylation.
Effect of CD26 expression on drug sensitivity was independent of serum
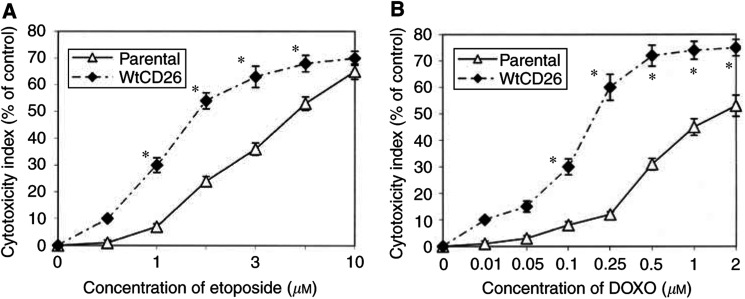
CD26 is able to cleave amino-terminal dipeptides from biological factors with either L-proline or L-alanine at the penultimate position through its DPPIV activity to alter their physiologic functions (Oravecz et al, 1997; Proost et al, 1998). To evaluate the possible role of serum-derived factors in CD26-associated enhancement in drug sensitivity, we incubated wtCD26 transfectants or parental Jurkat cells in AIM V serum-free media, and then performed similar studies as described above following doxorubicin or etoposide treatment. We demonstrated that the presence of CD26 still resulted in enhanced sensitivity to drug-induced G2–M arrest in serum-free media, as assessed by MTT uptake studies (Figure 4) and cell cycle analyses (Table 2 ). Similarly, wtCD26 Jurkat transfectants exhibited greater p34cdc2 phosphorylation, overall expression and serine-216 phosphorylation of cdc25C, and cyclin B1 level when treated with etoposide or doxorubicin as compared to parental Jurkat cells (Figure 5). Hence, these findings suggested that the enhanced drug sensitivity associated with CD26/DPPIV expression in CD26 Jurkat transfectants is because of its effect on cell-derived processes rather than because of its interaction with serum-derived factors.
Figure 4.
 |
Table 2. Enhanced sensitivity to etoposide and doxorubicin in serum-free media.
| %G0–G1 | %S | %G2–M | |
|---|---|---|---|
| Media alone | |||
| Parental | 54 | 36 | 10 |
| wtCD26 | 55 | 34 | 11 |
| Etoposide (0.25 μM) | |||
| Parental | 51 | 30 | 19 |
| wtCD26 | 34 | 23 | 43 |
| Etoposide (0.50 μM) | |||
| Parental | 36 | 28 | 36 |
| wtCD26 | 18 | 18 | 64 |
| Doxorubicin (0.10 μM) | |||
| Parental | 43 | 30 | 27 |
| WtCD26 | 31 | 20 | 49 |
| Doxorubicin (0.25 μM) | |||
| Parental | 36 | 23 | 41 |
| wtCD26 | 14 | 12 | 74 |
Following pretreatment with AIM V serum-free media at 37°C for 24 h, wtCD26 Jurkat transfectants and parental cells were incubated at 37°C in serum-free media with etoposide or doxorubicin for 24 h. Cells were then harvested and cell cycle analyses were performed with PI staining. Data are representative of three separate experiments.
Figure 5.
Effect of CD26 expression on G2/M checkpoint regulators following drug treatment in serum-free media. Following pretreatment with AIM V serum-free media at 37°C for 24 h, wtCD26 and parental Jurkat cells were incubated at 37°C in serum-free media containing etoposide (A) or doxorubicin (B) for 24 h at 37°C. Cells were then harvested and immunoblotting studies were conducted with appropriate antibodies as described in Materials and Methods.
CD26/DPPIV expression is associated with enhanced topoisomerase II α level and activity
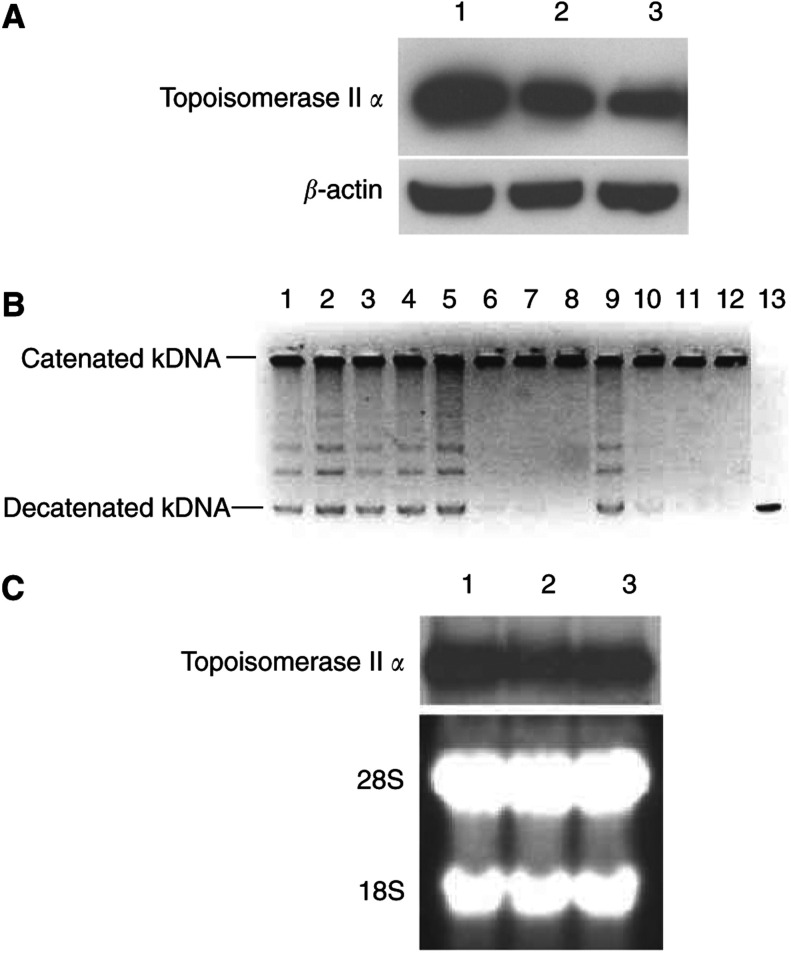
As antineoplastic agents with key role in the treatment of haematological malignancies, doxorubicin and etoposide both target topoisomerase II α (Burden and Osheroff, 1998). To further explore the potential mechanism of CD26/DPPIV-associated enhancement in drug sensitivity, we evaluated topoisomerase II α expression in CD26 Jurkat transfectants. We found that wtCD26 Jurkat transfectants expressed higher level of topoisomerase II α than parental Jurkat cells or S630A transfectants (Figure 6A), showing that the increased drug sensitivity in Jurkat cells expressing CD26/DPPIV was associated with enhanced topoisomerase II α level. Furthermore, we demonstrated that the enhanced topoisomerase II α protein level correlated with increased topoisomerase II α enzyme activity in wtCD26 Jurkat cells as compared to S630A transfectants or parental Jurkat cells. While decatenated DNA was observed with high concentrations of nuclear extracts from wtCD26, S630A and parental Jurkat cells, it was detected only in wtCD26 nuclear extracts at lower concentrations (Figure 6B). On the other hand, Northern blotting studies consistently detected similar levels of topoisomerase II α mRNA in wtCD26, S630A and parental Jurkat cells (Figure 6C).
Figure 6.
Topoisomerase II α expression, catalytic activity and mRNA level associated with CD26/DPPIV. (A) Jurkat cells were incubated in normal culture media, and nuclear extracts were collected for immunoblotting studies to evaluate topoisomerase II α protein levels as described in Materials and Methods. Each lane was equally loaded with 15 μg of protein. Lane 1: wtCD26; lane 2: S630A cells; lane 3: parental Jurkat. Data are representative of three separate experiments. (B) Topoisomerase II decatenation assay was performed as described in Materials and Methods, with nuclear extracts from wtCD26 transfectants, S630A transfectants or parental Jurkat cells being assayed at the indicated concentrations. Lanes 1–4: wtCD26 Jurkat transfectants; lanes 5–8: S630A Jurkat transfectants; lanes 9–12: parental Jurkat cells. Lanes 1, 5, 9: 0.85 μg of nuclear extracts; lanes 2, 6, 10: 0.75 μg of nuclear extracts; lanes 3, 7, 11: 0.65 μg of nuclear extracts; lanes 4, 8, 12: 0.50 μg of nuclear extracts; lane 13: decatenated kDNA marker. Data are representative of three separate experiments. (C) Total cellular RNA (20 μg) was isolated from Jurkat cells and subjected to Northern blot analysis with a 32P-radiolabelled topoisomerase II α cDNA probe as described in Materials and Methods. Lane 1: wtCD26 Jurkat; lane 2: S630A; lane 3: parental Jurkat. The lower panel represents ethidium bromide staining of RNA gel prior to transfer. Data are representative of three separate experiments.
DISCUSSION
In this paper, we extended our earlier work by showing that the presence of DPPIV enzymatic activity of CD26-enhanced sensitivity of the human T-leukaemia line Jurkat to etoposide-induced DNA damage. Similar to our previous findings with the antineoplastic agent doxorubicin (Aytac et al, 2001), we demonstrated that etoposide exhibited greater effect on wtCD26 transfectants than parental Jurkat cells or CD26-positive/DPPIV-negative Jurkat transfectants through its effect on major regulators of the G2–M checkpoint, including p34cdc2, cdc25C and cyclin B1. We also showed that CD26-associated enhancement in sensitivity to doxorubicin and etoposide was independent of serum-derived factors, since similar effects were seen when cells were incubated in the presence of serum-free media. Significantly, our studies elucidated a potential mechanism involved in CD26/DPPIV-associated susceptibility to doxorubicin and etoposide-induced G2/M arrest by revealing a correlation between topoisomerase II α activity and protein level and CD26-associated sensitivity to the topoisomerase II inhibitors. However, we detected similar levels of topoisomerase II α mRNA in wtCD26, S630A and parental Jurkat cells. Results of our Northern blotting studies therefore suggested that the observed enhancement in topoisomerase II α level seen in Jurkat transfectants expressing CD26/DPPIV was not because of an overall increase in the steady-state level of topoisomerase II α mRNA. Topoisomeras II α levels are regulated through a number of mechanisms by various factors, including transcriptional rate, post-transcriptional alterations and post-translational modifications (Isaacs et al, 1998). Our laboratory is now actively conducting work to elucidate the particular mechanism(s) involved in CD26/DPPIV-associated enhancement in topoisomerase II α protein level and enzyme activity.
Essential for cellular proliferation as a key regulator of mitosis, DNA topoisomerase II enzyme catalyses many types of interconversions between different DNA topological isomers (Wang, 1996). Eukaryotes have two isoforms of topoisomerase II, α and β, which are coded by two distinct genes (Drake et al, 1989). A key factor in drug resistance is the intracellular level of topoisomerase II α, as several studies demonstrated that increased enzyme level is correlated with increased sensitivity, and drug resistance is often associated with decreased topoisomerase II α level (Davies et al, 1988; Fry et al, 1991; Larsen and Skladanowski, 1998). Consistent with these previous findings, our work showed that expression of CD26/DPPIV in Jurkat transfectants was associated with higher level of topoisomerase II α. While these findings suggested that a mechanism for the enhanced sensitivity to doxorubicin and etoposide was the increased intracellular level of their target enzyme, our data did not necessarily exclude the potential involvement of other additional factor(s) (Beck et al, 1993; Larsen and Skladanowski, 1998). Furthermore, a functional association between CD26 and topoisomerase II α may also prove to be an important and generalised aspect of CD26 biology. In fact, this interaction may play a role in CD26 function in T-cell physiology, particularly in view of well-established findings from multiple groups demonstrating CD26 involvement in T-cell activation and proliferation.
CD26/DPPIV cleaves selected cytokines at the NH2 terminus to alter their biological functions, resulting in lower chemotactic potency, impaired signalling effects and altered receptor specificity (Oravecz et al, 1997; Proost et al, 1998). Our studies with serum-free media showed that the presence of CD26/DPPIV was still associated with enhanced sensitivity to doxorubicin and etoposide, by influencing the key components of the G2–M checkpoint following drug treatment. These findings therefore suggested that CD26/DPPIV affects certain intrinsic cell-derived factors, independent of serum, to interfere with selected intracellular pathways, leading to enhanced drug sensitivity.
Previous studies have demonstrated that CD26 is expressed on the surface of selected aggressive T-cell malignancies (Carbone et al, 1995; Jones et al, 2001). Meanwhile, topoisomerase II α is highly expressed on aggressive B-cell non-Hodgkin's lymphomas; on the other hand, its expression on T-cell malignancies appears to be generally low, although this conclusion is based on studies with limited samples (Holden et al, 1995; Turley et al, 1997; Korkolopoulou et al, 2001). Our current data with the human T-cell leukaemia cell line Jurkat suggest that the expression of CD26 may be potentially linked to high topoisomerase II α level in selected T-cell malignancies, which are generally aggressive and often difficult to treat at the present time. These findings therefore suggest a correlation between CD26 and topoisomerase II α expression in haematological malignancies as well as perhaps selected solid tumours, which may also have potential clinical implications in view of the use of topoisomerase II inhibitors as major components of the treatment of these diseases. Along with our recent study demonstrating in vitro and in vivo antitumour effect of anti-CD26 antibody (Ho et al, 2001), our current work may therefore provide additional insights into the potential treatment strategies for selected cancers based on CD26 biology.
Acknowledgments
NHD is supported by the MD Anderson Physician-Scientist Program, the Gillson Longenbaugh Foundation and the V Foundation. KS is supported by a grant from the Eli Lilly Japan International Fellowship. KO is the recipient of a grant from the Japan Society for the Promotion of Science.
References
- Aytac U, Claret F-X, Ho L, Sato K, Ohnuma K, Mills GB, Cabanillas F, Morimoto C, Dang NH (2001) Expression of CD26 and its associated dipeptidyl peptidase IV enzyme activity enhances sensitivity to doxorubicin-induced cell cycle arrest at the G2/M checkpoint. Cancer Res 61: 7204–7210 [PubMed] [Google Scholar]
- Bauvois B, De Meester I, Dumont J, Rouillard D, Zhao HX, Bosmans E (1999) Constitutive expression of CD26/dipeptidylpeptidase IV on peripheral blood B lymphocytes of patients with B chronic lymphocytic leukaemia. Br J Cancer 79: 1042–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck WT, Danks MK, Wolverton JS, Kim R, Chen M (1993) Drug resistance associated with altered DNA topoisomerase II. Adv Enzyme Regul 33: 113–127 [DOI] [PubMed] [Google Scholar]
- Burden DA, Osheroff N (1998) Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta 1400: 139–154 [DOI] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Zagonel V, Aldinucci D, Gattei V, Degan M, Improta S, Sorio R, Monfardini S, Pinto A (1995) The expression of CD26 and CD40 ligand is mutually exclusive in human T-cell non-Hodgkin's lymphomas/leukemias. Blood 86: 4617–4626 [PubMed] [Google Scholar]
- Dang NH, Hafler DA, Schlossman SF, Breitmeyer JB (1990a) FcR-mediated crosslinking of Ta1 (CDw26) induces human T lymphocyte activation. Cell Immunol 125: 42–57 [DOI] [PubMed] [Google Scholar]
- Dang NH, Morimoto C (2002) CD26: an expanding role in immune regulation and cancer. Histol Histopathol 17: 1213–1226 [DOI] [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Deusch K, Schlossman SF, Morimoto C (1990b) Co-mitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol 144: 4092–4100 [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Schlossman SF, Morimoto C (1990c) Human CD4 helper T cell activation: functional involvement of two distinct collagen receptors, 1F7 and VLA integrin family. J Exp Med 172: 649–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Shimamura K, Tanaka T, Daley JF, Schlossman SF, Morimoto C (1991) 1F7 (CD26): a marker of thymic maturation involved in the differential regulation of the CD3 and CD2 pathways of human thymocyte activation. J Immunol 147: 2825–2832 [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Sugita K, Daley JF, Schow P, Prado C, Schlossman SF, Morimoto C (1990d) Cell surface modulation of CD26 by anti-1F7 monoclonal antibody: analysis of surface expression and human T cell activation. J Immunol 145: 3963–3971 [PubMed] [Google Scholar]
- Davies SM, Robson CN, Davies SL, Hickson ID (1988) Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem 263: 17724–17729 [PubMed] [Google Scholar]
- Drake FH, Hofmann GA, Bartus HF, Mattern MR, Crooke ST, Mirabelli CK (1989) Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry 28: 8154–8160 [DOI] [PubMed] [Google Scholar]
- Flentke GR, Munoz E, Huber BT, Plaut AG, Kettner CA, Bachovchin WW (1991) Inhibition of dipeptidyl aminopeptidase IV (DP-IV) by Xaa-boro-Pro dipeptides and use of these inhibitors to examine the role of DP-IV in T cell function. Proc Nat Acad Sci USA 88: 1556–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Chresta CM, Davies SM, Walker MC, Harris AL, Hartley JA, Masters JR, Hickson ID (1991) Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res 51: 6592–6595 [PubMed] [Google Scholar]
- Ho L, Aytac U, Stephens LC, Ohnuma K, Mills GB, McKee KS, Neumann C, LaPushin R, Cabanillas F, Abbruzzese JL, Morimoto C, Dang NH (2001) In vitro and in vivo antitumor effect of the anti-CD26 monoclonal antibody 1F7 on human CD30+ anaplastic large cell T cell lymphoma Karpas 299. Clin Cancer Res 7: 2031–2040 [PubMed] [Google Scholar]
- Holden JA, Perkins SL, Snow GW, Kjeldsberg CR (1995) Immunohistochemical staining for DNA topoisomerase II in non-Hodgkin's lymphomas. Am J Clin Pathol 104: 54–59 [DOI] [PubMed] [Google Scholar]
- Ikushima H, Munakata Y, Ishii T, Iwata S, Terashima M, Tanaka H, Schlossman SF, Morimoto C (2000) Internalization of CD26 by mannose 6-phosphate/insulin-like growth factor II receptor contributes to T cell activation. Proc Nat Acad Sci USA 97: 8439–8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs RJ, Davies SL, Sandri MI, Redwood C, Wells NJ, Hickson ID (1998) Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta 1400: 121–137 [DOI] [PubMed] [Google Scholar]
- Jones D, Dang NH, Duvic M, Washington LT, Huh YO (2001) Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol 115: 885–892 [DOI] [PubMed] [Google Scholar]
- Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C (1993) Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 261: 466–469 [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW (1994) Mitosis in transition. Cell 79: 563–571 [DOI] [PubMed] [Google Scholar]
- Korkolopoulou P, Angelopoulou M, Siakantari M, Mitropoulos F, Vassilakopoulos T, Zorzos H, Rassidakis G, Androulaki A, Patsouris E, Kittas C, Davaris P, Pangalis GA (2001) Evaluation of DNA topoisomerase II α expression provides independent prognostic information in non-Hodgkin's lymphomas. Histopathology 38: 45–53 [DOI] [PubMed] [Google Scholar]
- Larsen AK, Skladanowski A (1998) Cellular resistance to topoisomerase-targeted. [DOI] [PubMed]
- Morimoto C, Schlossman SF (1998) The structure and function of CD26 in the T-cell immune response. Immunol Rev 161: 55–70 [DOI] [PubMed] [Google Scholar]
- Morimoto C, Torimoto Y, Levinson G, Rudd CE, Schrieber M, Dang NH, Letvin NL, Schlossman SF (1989) 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol 143: 3430–3439 [PubMed] [Google Scholar]
- Morrison ME, Vijayasaradhi S, Engelstein D, Albino AP, Houghton AN (1993) A marker for neoplastic progression of human melanocytes is a cell surface ectopeptidase. J Exp Med 177: 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oravecz T, Pall M, Roderiquez G, Gorrell MD, Ditto M, Nguyen NY, Boykins R, Unsworth E, Norcross MA (1997) Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal t cells expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med 186: 1865–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C-Y, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3- protein binding by phosphorylation of cdc25C on serine-216. Science 277: 1501–1505 [DOI] [PubMed] [Google Scholar]
- Poon RYC, Chau MS, Yamashita K, Hunter T (1997) The role of cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res 57: 5168–5178 [PubMed] [Google Scholar]
- Proost P, De Meester I, Schols D, Struyf S, Lambeir A-M, Wuyts A, Opdenakker G, De Clercq E, Scharpe S, Van Damme J (1998) Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV: conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. J Biol Chem 273: 7222–7227 [DOI] [PubMed] [Google Scholar]
- Stecca BA, Nardo B, Chieco P, Mazziotti A, Bolondi L, Cavallari A (1997) Aberrant dipeptidyl peptidase IV (DPPIV/CD26) expression in human hepatocellular carcinoma. J Hepatol 27: 337–345 [DOI] [PubMed] [Google Scholar]
- Steinbrecher A, Reinhold D, Quigley L, Gado A, Tresser N, Izikson L, Born I, Faust J, Neubert K, Martin R, Ansorge S, Brocke S (2001) Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta1 secretion in vivo. J Immunol 166: 2041–2048 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Duke-Cohan JS, Kameoka J, Yaron A, Lee I, Schlossman SF, Morimoto C (1994) Enhancement of antigen-induced T-cell proliferation by soluble CD26/dipeptidyl peptidase IV. Proc Nat Acad Sci USA 91: 3082–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kameoka J, Yaron A, Schlossman SF, Morimoto C (1993) The costimulatory activity of the CD26 antigen requires dipeptidyl peptidase IV enzymatic activity. Proc Nat Acad Sci USA 90: 4586–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Umeki K, Yamamoto I, Sakamoto F, Noguchi S, Ohtaki S (1995) CD26 (dipeptidyl peptidase IV/DPPIV) as a novel molecular marker for differentiated thyroid carcinoma. Int J Cancer 64: 326–331 [DOI] [PubMed] [Google Scholar]
- Torimoto Y, Dang NH, Vivier E, Tanaka T, Schlossman SF, Morimoto C (1991) Coassociation of CD26 (dipeptidyl peptidase IV) with CD45 on the surface of human T lymphocytes. J Immunol 147: 2514–2517 [PubMed] [Google Scholar]
- Turley H, Comley M, Houlbrook S, Nozaki N, Kikuchi A, Hickson ID, Gatter K, Harris AL (1997) The distribution and expression of the two isoforms of DNA topoisomerase II in normal and neoplastic human tissues. Br J Cancer 75: 1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC (1996) DNA topoisomerases. Annu Rev Biochem 65: 635–692 [DOI] [PubMed] [Google Scholar]