Abstract
In a previous study, we demonstrated DNA damage, expressed as micronuclei, in binucleate dermal fibroblasts obtained from human skin 2-9 weeks after fractionated radiotherapy. Here we assessed micronuclei in X-irradiated skin fibroblasts from 9-14-week-old female Lewis rats as a function of time after a single dose of radiation to determine the lifetime of such damage in the skin. After irradiation with 5, 10, 15 and 18 Gy, formation of micronuclei at 1 day or 2 months postirradiation increased up to about 10 Gy, with evidence for a plateau at higher doses. The time course of micronuclei present in the skin fibroblasts demonstrated a plateau region (approximately 20 days after 18 Gy and about 2 months after 10 Gy) before the number of micronuclei started to decline. Residual micronuclei were observed for more than 1 year after irradiation. Monomicronucleated cells predominated in fibroblasts from nonirradiated skin, whereas in fibroblasts from irradiated skin, multimicronucleated cells predominated and persisted (together with monomicronucleated cells) in the residual levels of damage at late times. The results suggest that DNA damage in dermal fibroblasts can be assayed by the micronucleus assay in samples from irradiated skin up to 1 month after irradiation for doses up to at least 10 Gy. Further studies are needed to define the dose-response relationship in detail.
INTRODUCTION
In a recent paper (1) we reported that dermal fibroblasts obtained from human skin during surgery for soft tissue sarcoma at 2-9 weeks after the end of a course of fractionated radiotherapy (50 Gy in 25 fractions) demonstrated substantial levels of DNA damage when examined at their first division in culture after removal from the patient. Estimates of the dose received by the skin samples demonstrated that the levels of micronuclei observed were dependent on dose. Latent damage in human skin after irradiation is consistent with results of studies of wound healing after irradiation (2-6) and with reduction of the tolerance to late radiation damage after a second course of radiation treatment to previously irradiated skin (7, 8). However, it is unlikely that DNA damage alone is responsible for these latter effects considering the current evidence that chronic inflammatory responses and generated reactive oxygen species may play a significant role in radiation-induced late damage (9). Nevertheless, tolerance to reirradiation increases with time after irradiation (7, 8). The purpose of the present study was to assess the dynamics of micronuclei in rat skin fibroblasts at various times after exposure to various doses of X radiation comparable to those received by the patients.
MATERIALS AND METHODS
Animals
Female Lewis rats 9-14 weeks of age were used for all the experiments. The animals were maintained in the animal facility at the Princess Margaret Hospital/Ontario Cancer Institute and allowed access to food and water ad libitum. All the experiments were conducted according to protocols approved under the regulations of the Canadian Council for Animal Care.
Irradiation
The skin irradiations were carried out with a double-headed 100 kVp X-ray unit as described previously (10). Each animal was anesthetized with halothane and placed in a Lucite box containing two opposed circular lead collimators to localize the irradiation field (2.5 cm diameter) to an area on the rat's buttock. Both buttocks were irradiated sequentially using this technique. Skin from the scapular region was used as an out-of-field control. In addition, nonirradiated animals were also used as controls. At various times from 1 day to >1 year after the irradiation (0-18 Gy), the animals were killed and biopsies of skin from the irradiated or control regions were taken for analysis of DNA damage using a cytokinesis-blocked micronucleus assay as described below.
Isolation of Fibroblasts and Micronucleus Assay
Animals were injected intraperitoneally with a supralethal dose of Nembutal (100 mg/kg). Fur on dorsal skin and buttocks was shaved and further removed by applying hair remover lotion (Nair) for a short time. Skin was then wiped with alcohol, and biopsies (about 1 × 1 cm) were taken from two different irradiated areas or control areas and kept in ice-cold α-MEM with 2000 mg/ml glucose, 100 mg/ml streptomycin and 100 mg/ml penicillin. Fibroblast isolation from skin biopsies and the cytokinesis-blocked micronucleus (MN) assay were performed as described previously (11, 12) with minor modifications. Briefly, each biopsy was minced with sterile scissors and treated with enzyme cocktail of 0.06 mg/ml collagenase I and 0.5 mg/ml DNase I (Sigma, St. Louis, MO) in α-MEM at 37°C for 1.5 h (mixing every 30 min). The digest was then strained into a 50-ml tube containing 5 ml of ice-cold α-MEM supplemented with 20% fetal bovine serum (CanSera, Etobicoke, Canada) (termed fibroblast isolation medium) on ice through a 70-μm nylon strainer (BD Falcon, Bedford, MA). Remaining tissue was further digested in 1 mg/ml trypsin (Sigma) at 37°C for 30 min (mixing every 10 min). Digestion was stopped by adding an equal amount of fibroblast isolation medium plus 200 μl of DNase stock solution (20 mg/ml), and the cells were strained into the same tube. After centrifugation, the cell pellet was resuspended in fibroblast isolation medium and plated into chamber slides (Nunc, Rochester, NY) for the micronucleus assay. The chamber slides were washed and fresh medium containing 3.2 μg/ml cytochalasin B (Sigma) was added after 24 h, and 72 h later the slides were washed with PBS, incubated in hypotonic KCl (5.56 mg/liter) for 10 min, and fixed in cold methanol for 1.5 min. Just prior to scoring of micronuclei, the slides were stained with Acridine Orange (BD, Sparks, MD) for 2 min, washed with PBS and mounted in PBS. Micronuclei, defined as rounded bodies, no more than one-third the size of the nucleus, having staining color and intensity identical to the staining of nuclei, and completely detached from nuclei (13), were scored in up to 1000 binucleate cells. The data for the two skin samples from the same animal were pooled. We counted micronuclei in 1000 binucleate cells for most of the samples, but in some cases there was a low yield of cells on an individual slide. The minimum number of binucleate cells counted was 200. An analysis demonstrated that the accumulated number of micronuclei/1000 binucleate cells generally stabilized when micronuclei in more than about 200 binucleate cells were counted. The data points for which low numbers of binucleate cells were counted generally did not differ greatly from those for which higher binucleate cells were counted. The small number of binucleate cells apparently containing more than six micronuclei were not scored because it is very difficult to confidently interpret them as micronuclei in a single binucleate cell. The total MN score per 1000 binucleate cells as well as the percentage of cells containing micronuclei were calculated. In addition, the percentages of binucleate cells with one MN and with multiple micronuclei were recorded as well as the percentage of binucleate cells per total number of cells on the slide.
Statistical Analysis
Values are presented as means ± SEM. However, since the data may not be normally distributed, we opted, for point-to-point comparisons of two independent samples, to use the more conservative non-parametric Mann-Whitney test (Prizm 4 for Windows software, version 4.03). P values were from two-sided tests. For analysis of dose-response trends, two-way ANOVA was used. For analysis of the time course of micronuclei after irradiation, a linear regression model was built with the total number of micronuclei as the outcome (dependent variable) and time and dose as explanatory variables. In the model we allowed for random variation (mixed model) due to the possible correlations between the measurements taken within the same animal. Since we were interested whether the total number of micronuclei varies with time in a different way depending on the dose of radiation, we included an interaction term between dose and time in the model.
RESULTS
Dose Response
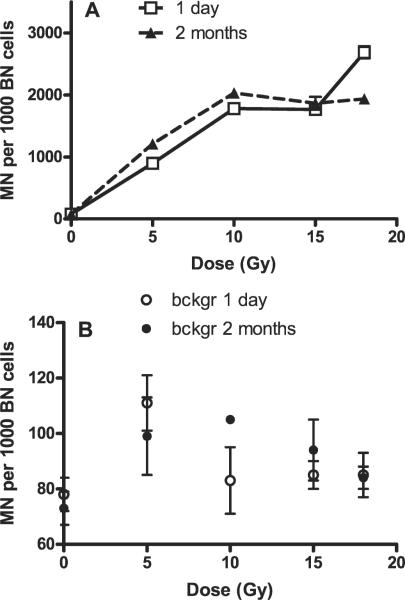
Initially we examined the dose-response relationship for MN formation in rat skin using doses of 0, 5, 10, 15 and 18 Gy, similar to doses we had studied previously for MN formation in rat lung fibroblasts (12). These doses were also chosen to cover a range approximately equivalent to the fractionated treatment received by human skin in our previous study (1). The animals were irradiated at 9 weeks of age, and we assayed the skin 1 day or 2 months after irradiation. These times were chosen based on our previous studies in lung (1 day) and in human skin (1), where it represents the approximate time at which most of the skin specimens were analyzed relative to the end of the course of fractionated radiation treatment. The results of these studies are shown in Fig. 1A, where the total micronuclei per 1000 binucleate cells is plotted as a function of radiation dose. Background levels in out-of-field skin were low but were significantly higher than the background levels in nonirradiated rats (97 ± 4, n = 64 compared to 75 ± 4, n = 12, P = 0.0084), as shown in Fig. 1B. These findings are at odds with our previous observations indicating no out-of-field effect in shielded skin areas in Sprague-Dawley rats (14), but they are consistent with (although much smaller than) the out-of-field effect we observed for MN formation in rat lung (11, 12, 15, 16). The results in Fig. 1B suggest that this small out-of-field effect may decrease with dose, but the trend was not statistically significant (by ANOVA test).
FIG. 1.
(Panel A) Micronuclei (MN) per 1000 binucleate (BN) cells in freshly isolated in situ skin fibroblasts of Lewis rats 1 day and 2 months postirradiation plotted as a function of dose received and (panel B) corresponding background (out-of-field) values compared to 0-Gy values. Values are plotted as means ± SE. Two to four animals (two biopsies per animal) were used.
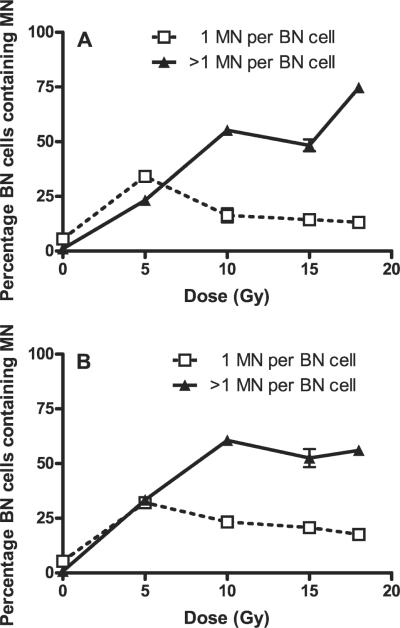
The results for the irradiated skin show similar dose responses for the two assay times (1 day and 2 month after irradiation) with an initial rise up to doses of 10 Gy and then evidence for the development of a plateau. Relative to the results for day 1, there was a small increase in total micronuclei 2 months after 5 Gy (P = 0.029) and 10 Gy (P > 0.05), whereas at 18 Gy there was a relative decrease in total micronuclei at 2 months (P = 0.029). Very similar overall results are obtained if the percentage of multimicronucleated binucleate cells are plotted as a function of radiation dose (Fig. 2).
FIG. 2.
Percentage of binucleate (BN) cells containing micronuclei (MN) (1 micronucleus or >1 micronucleus per cell) in freshly isolated in situ skin fibroblasts of Lewis rats 1 day (panel A) and 2 months (panel B) postirradiation plotted as a function of dose received. Values are plotted as means ± SE. Two to four animals (two biopsies per animal) were used.
Time Course
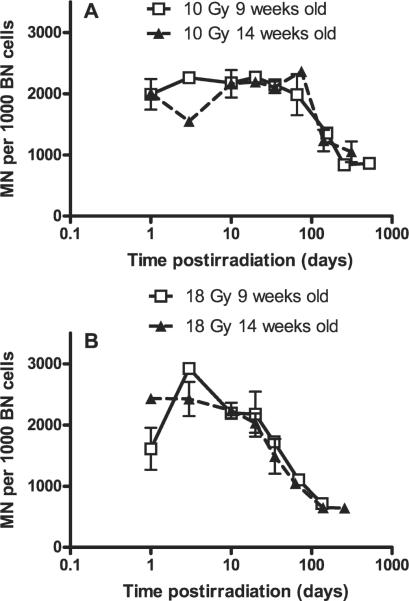
Because the primary focus of our study was to examine the time course of detection of the DNA damage, we next measured the number of micronuclei at multiple times (up to more than 1 year) after single doses of either 10 Gy or 18 Gy. We chose these doses because they are approximately equivalent to the fractionated doses used clinically in our previous study (1) and because they produced high levels of damage, allowing for the maximum possibility to track damage decay over time. The experiments were performed twice with two different groups of rats irradiated at the age of 9 weeks or 14 weeks. The results plotted as micronuclei/1000 binucleate cells as a function of log time are shown separately for the two experiments in Fig. 3 for the animals treated with 10 and 18 Gy, respectively. After 10 Gy (Fig. 3A), there is generally good agreement between the two experiments, and it is seen that the number of micronuclei per 1000 binucleate cells remained essentially unchanged up to 60-70 days postirradiation and then began to decrease by about day 100 but remained well above background at more than a year after irradiation. In this context, it is of interest that background micronuclei were generally found to be independent of the age of the animal, which is in accordance with a previous study using activated lymphocytes (17). For the animals treated with 18 Gy (Fig. 3B), the overall data show a similar pattern to that for 10 Gy, but the time at which the decline in micronuclei per 1000 binucleate cells starts is earlier at 20-30 days after irradiation.
FIG. 3.
Micronuclei (MN) per 1000 binucleate (BN) cells in freshly isolated in situ skin fibroblasts of Lewis rats at various times after 10 Gy (panel A) and 18 Gy (panel B) irradiation. Values are plotted as means ± SE. Two to four animals (two biopsies per animal) were used.
The graphs suggest that the number of micronuclei per 1000 binucleate cells is stable until at least 20 days, although there is some variability at 1 and 3 days. A model of the data up to 20 days (two separate experiments for each dose pooled together) supports this hypothesis. The estimated slope for 10 Gy was 8 micronuclei per 1000 binucleate cells per day (95% confidence interval -19; 35) and for 18 Gy was -10.5 micronuclei per 1000 binucleate cells per day (95% confidence interval -35; 14). Both confidence intervals contain the value 0 and extend well around this value, suggesting that the number of micronuclei per 1000 binucleate cells remains stable for both doses within this time frame. Furthermore, the model found no significant difference between levels of micronuclei per 1000 binucleate cells in the plateau region for 10 and 18 Gy. To model the apparent sigmoid shape of these curves over the whole range of time, we applied a transformation of the number of micronuclei (log((MN - 390)/(3500 - MN))). The model indicated that the number of micronuclei per 1000 binucleate cells decayed faster in the sigmoid region of the curve after 18 Gy than after 10 Gy (P < 0.0001). The number of residual micronuclei per 1000 binucleate cells at approximately 1 year was not significantly different between the 10-Gy and 18-Gy groups.
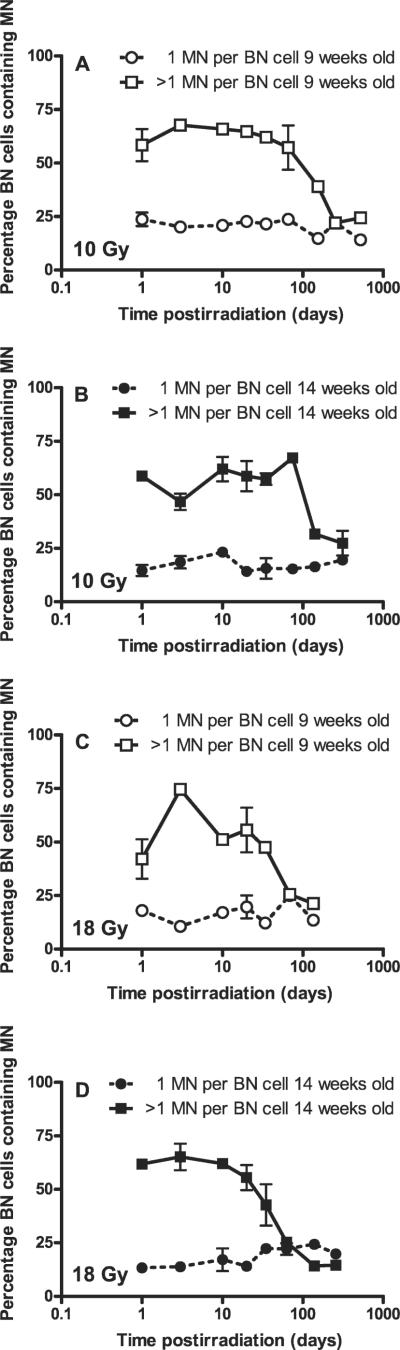
The percentages of binucleate cells containing micronuclei are plotted as a function of log time after irradiation in Fig. 4. It is of interest that the reduction in the number of micronuclei at the late times after irradiation is due to analogous changes in the percentage of multimicronucleated binucleate cells rather than the percentage of monomicronucleated binucleate cells.
FIG. 4.
Percentage of binucleate (BN) cells containing micronuclei (MN) (1 micronucleus or >1 micronucleus per cell) in freshly isolated in situ skin fibroblasts of Lewis rats at various times: after 10 Gy, 9-week-old animals (panel A), 10 Gy, 14-week-old animals (panel B), or at various times after 18 Gy, 9-week-old animals (panel C) and 18 Gy, 14-week-old animals (panel D). Values are plotted as means ± SE. Two to four animals (two biopsies per animal) were used.
DISCUSSION
Dose Response
The results presented here for rat skin are generally consistent with our results reported previously for human skin (1). The results confirm that DNA damage in dermal fibroblasts can be assayed by measuring micronuclei in samples obtained from irradiated skin and, as seen in Fig. 1, there is a dose-response relationship, which appears to extend up to doses of about 10 Gy (single dose). Above this dose there is evidence for the development of a plateau in the number of micronuclei per 1000 binucleate cells, despite the fact that the percentage of binucleate cells expressing micronuclei remains below 100% (Fig. 2). This finding may suggest that some fibroblasts successfully rejoin all their DNA breaks despite high levels of initial damage. Alternatively, it may indicate that some forms of DNA damage are not reflected in micronucleus formation and that there is an upper limit to the amount of damage that can be detected by this assay.
Interestingly, comparison of the current results with the dose-response data obtained in human skin fibroblasts (from STS patients) gathered approximately 2 months (49-65 days) postirradiation [data from ref. (1)] shows that the number of micronuclei per 1000 binucleate cells was lower in human cells than in rat fibroblasts (Fig. 5). This may be due to a variety of causes, including higher radiosensitivity of rats relative to humans, difficulties with accurate dosimetry at the skin surface in the human studies, or an incorrect conversion of fractionated to equivalent single dose for the human skin studies. The patients received fractionated radiation, and the dose plotted in Fig. 5 was calculated as an equivalent single dose for early radiation effects based on the α/β formula (α/β = 10), which may not be applicable for DNA damage measured by scoring of micronuclei.
FIG. 5.
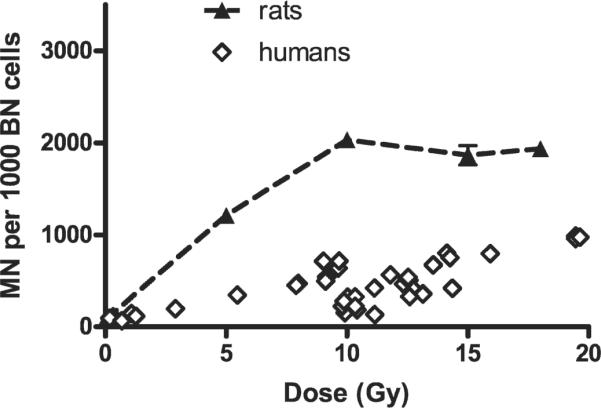
Micronuclei (MN) per 1000 binucleate (BN) cells in freshly isolated in situ skin fibroblasts of Lewis rats (9 weeks old) and humans plotted as a function of (equivalent) single dose. Rat fibroblasts were isolated 2 months after single-dose irradiation; human fibroblasts were isolated 49-65 days after fractionated irradiation (equivalent single dose was calculated using the linear-quadratic formula and assuming α/β = 10). Values for rat fibroblasts are plotted as means ± SE. Values for humans are for individual skin samples.
Background and Out-of-Field Effects
Background MN levels in out-of-field skin were higher than the background levels in nonirradiated rats (Fig. 1B; P = 0.0084 by Mann-Whitney), suggesting a possible out-of-field effect. Background levels for the time-course series are presented separately in Table 1. There is scatter in these data, but in general the numbers of micronuclei are similar to those seen in Fig. 1B, and they show no evidence for change with time. While resembling the out-of-field effect found in rat lung, the increase in micronuclei was only about 30% higher than the 0-Gy background, and the ranges of MN values overlapped (34-166 in out-of-field compared to 59-101 in nonirradiated). For comparison, the increase in micronuclei in shielded lung was 6-14 times higher than the 0-Gy background (11, 12, 15). Taking into account that no out-of-field effect was found in rat skin in previous studies using another irradiation device (14), it is possible that some radiation scatter rather than an out-of-field effect may explain the results. However, there is no clear dose-dependent trend for these background values (see Fig. 1B), which would be expected if the observations were due to radiation scatter.
TABLE 1.
Background Values for Total Micronuclei and Percentage of Binucleate Cells with 1 or More Micronuclei for the Various Groups after Irradiation
| 10 Gy, 9 weeks old |
10 Gy, 14 weeks old |
18 Gy, 9 weeks old |
18 Gy, 14 weeks old |
||||
|---|---|---|---|---|---|---|---|
| Days postirradiation | Mean ± SE | Days postirradiatioi | Mean ± SE | Days postirradiation | Mean ± SE | Days postirradiation | Mean ± SE |
| Total micronuclei per 1000 binucleate cells | |||||||
| 1 | 62 ± 19 | 1 | 89 | 1 | 94 ± 6 | 1 | 95 ± 9 |
| 3 | 105 ± 11 | 3 | 69 | 3 | 123 ± 7 | 3 | 96 ± 8 |
| 10 | 95 ± 9 | 50 | 101 ± 66 | 10 | 108 ± 1 | 10 | 92 ± 3 |
| 20 | 113 ± 2 | 20 | 97 ± 33 | 20 | 131 ± 8 | 20 | 60 ± 2 |
| 35 | 75 ± 37 | 35 | 182 | 34 | 109 | 35 | 24 ± 3 |
| 66 | 113 ± 15 | 75 | 138 ± 50 | 69 | 175 ± 17 | 63 | 39 |
| 155 | 114 ± 50 | 141 | 136 ± 36 | 135 | 195 | 139 | 73 ± 5 |
| 255 | 114 ± 13 | 313 | 85 | 257 | 122 ± 6 | ||
| 523 | 162 ± 46 | ||||||
| Percentage of binucleate cells with 1 micronuclei | |||||||
| 1 | 4.6 ± 1.1 | 1 | 1.7 | 1 | 6.3 ± 0.2 | 1 | 6.4 ± 0.6 |
| 3 | 7.3 ± 0.6 | 3 | 6.6 ± 0.3 | 3 | 8.9 ± 1.6 | 3 | 5.8 |
| 10 | 5.8 ± 0.9 | 10 | 10.1 ± 6.6 | 10 | 7.3 ± 0.4 | 10 | 8.0 ± 0.7 |
| 20 | 7.4 ± 0.6 | 20 | 6.6 ± 2.6 | 20 | 8.9 | 20 | 5.5 ± 0.2 |
| 35 | 6.7 ± 2.8 | 35 | 18.2 | 34 | 4.6 | 35 | 1.8 ± 0.8 |
| 66 | 6.1 ± 0.9 | 75 | 12.8 ± 5.9 | 69 | 13. ± 1.5 | 63 | 0.6 ± 0.6 |
| 155 | 7.2 ± 0.9 | 141 | 7.1 ± 0.2 | 135 | 8.8 | 139 | 4.0 ± 0.6 |
| 255 | 8.1 ± 1.4 | 313 | 4.9 | 257 | 7.6 ± 1.8 | ||
| 523 | 11.7 ± 2.7 | ||||||
| Percentage of binucleate cells with >1 micronuclei | |||||||
| 1 | 0.7 ± 0.1 | 1 | 3.5 | 1 | 1.2 ± 0.1 | 1 | 1.1 ± 0.3 |
| 3 | 1.2 ± 0.1 | 3 | 0.9 ± 0.5 | 3 | 1.3 ± 0.7 | 3 | 1.8 ± 0.2 |
| 10 | 1.5 | 10 | 0.0 | 10 | 1.1 ± 0.1 | 10 | 0.4 ± 0.2 |
| 20 | 1.5 | 20 | 1.2 ± 0.3 | 20 | 1.9 ± 0.3 | 20 | 0.1 ± 0.1 |
| 35 | 0.3 ± 0.1 | 35 | 0.0 | 34 | 3.1 | 35 | 0.2 ± 0.1 |
| 66 | 2.0 | 75 | 0.4 ± 0.2 | 69 | 1.6 ± 0.2 | 63 | 0.6 ± 0.3 |
| 155 | 1.6 ± 0.9 | 141 | 1.5 ± 0.1 | 135 | 53 | 139 | 1.6 |
| 255 | 1.5 | 313 | 1.6 | 257 | 2.1 ± 0.2 | ||
| 523 | 2.0 ± 0.8 | ||||||
Time Course
The detailed analysis of the time course (after 10 and 18 Gy) over which DNA damage can be observed in dermal fibroblasts (Figs. 3 and 4) shows that until at least 20 days the number of micronuclei per 1000 binucleate cells is stable and starts to decay afterward depending on dose. The limited data at 5 Gy also suggest no reduction in micronuclei over the first 2 months. A possible interpretation of these results is that the fibroblasts sustain DNA damage, which is manifested primarily at division (apoptosis is generally found to be rare in fibroblasts). Thus the delay in the decrease in micronuclei could reflect the slow turnover of fibroblasts in the skin and the effects of the radiation on the cell cycle and proliferation rate. The faster decrease in micronuclei after 18 Gy than after 10 Gy may further reflect an increased demand for fibroblast proliferation as a result of the higher level of damage caused by the higher dose. Alternatively or additionally, radiation can induce maturation in fibroblasts toward non-proliferative fibrocytes (18, 19), which could reduce the number of (severely) damaged cells able to undergo proliferation and thus be scored as expressing micronuclei in the assay.
To examine the proliferative potential of the fibroblasts, we analyzed the percentage of binucleate cells in the cultures (data not shown). In general this percentage declined with dose and time after irradiation but with different kinetics for the two doses (it decreased slowly with time after 10 Gy but, after 18 Gy, decreased sharply on day 1 after irradiation and remained low). These observations are consistent with maturation of fibroblasts into fibrocytes after irradiation as a possible explanation of the observed differences in the rate of decay of micronuclei with time after the two different doses. However, no clear correlation was observed between the kinetics of the changes in micronuclei with time and the percentage of binucleate cells. Furthermore, the induced proliferation in vitro cannot be assumed to reflect the proliferation occurring in vivo. Thus, although the induced proliferative capacity of skin fibroblasts in vitro generally decreased with the decrease in micronuclei, and to a greater extent when the decrease in micronuclei was faster, it is unknown whether this effect actually occurs in irradiated skin.
There were some differences in time-course dynamics of micronuclei between the 9-week-old and 14-week-old animals. Surprisingly, at 18 Gy, 9-week-old animals showed a transient increase in micronuclei per 1000 binucleate cells on day 3 compared to day 1; in addition, micronuclei on day 1 were lower than in 14-week-old animals. This effect was repeated with a separate group of animals, which suggests that it might represent unusual experimental variation associated with younger animals. This might be due to increased turnover in the dermal fibroblasts of younger animals combined with a postirradiation effect associated with a large radiation-induced inflammatory response. It is noteworthy that the data for 18 Gy in Fig. 1 (for which 9-week-old animals were used) show the increase on day 1 whereas time-course series show it on day 3. This suggests that early transient increases in micronuclei per 1000 binucleate cells indeed occur at 18 Gy but that the exact time may fluctuate in a range of few days. The modeling of the data also does not show the trend of the data at early times as being significantly different for the two doses used. We have also observed such early fluctuations in micronuclei in lung fibroblasts after irradiation (16). Moreover, in cultured confluent dermal fibroblasts, an increase in micronuclei at 14-21 days after 10 Gy has been reported (20). We have not ruled out the possibility that some fraction of the micronuclei observed are being generated postirradiation as a result of increased levels of reactive oxygen (and/or nitrogen) species induced by an inflammatory response, as we have observed in rat lung (12, 15, 16). However, preliminary results using antioxidants that are effective in reducing micronuclei in lung fibroblasts (15) show minimal evidence that micronuclei in mouse skin fibroblasts can be reduced by such treatment given postirradiation (Jelveh and Hill, unpublished observations, 2008).
Mono- and Multimicronucleated Binucleate Cells
The differential analysis of the percentage of monomicronucleated and multimicronucleated binucleate cells demonstrates predominance of monomicronucleated cells in background and nonirradiated samples (Figs. 2 and 4). In induced micronuclei, however, multimicronucleated cells predominated. It is noteworthy that at the doses used in our studies most of the response dynamics is due to changes in the frequency of multimicronucleated cells. In our study the dynamics of multimicronucleated cells followed the dynamics of the total percentage of multimicronucleated cells. At late times, multimicronucleated cells persisted in the residual levels of damage, together with monomicronucleated cells (Fig. 4). Normally, it is assumed that monomicronucleated cells are more likely to survive damage and can carry genomic abnormalities to further generations of cells (21). On the other hand, multimicronucleated cells may persist for 3-4 years in human peripheral blood lymphocytes, despite their faster decline during the 2 years after irradiation (22).
Residual Damage
As noted in the Introduction, our findings of residual DNA damage are consistent with the known effects of prior irradiation on both wound healing and tolerance to subsequent radiation treatment. Previous studies have suggested that the persistence of reduced tolerance of skin to a second radiation treatment is dependent on whether early or late end points are being assessed. In mice, prior radiation treatment reduced tolerance to early effects in skin of a second treatment for about 6 months after the initial irradiation (23). For late effects in skin, reduced tolerance lasted longer and depended on whether the initial treatment was close to tolerance (7, 8). Our results may thus be consistent with the general belief that early responses in skin primarily reflect damage to the epidermal layer whereas late responses such as dermal atrophy, telangiectasia, necrosis and fibrosis reflect damage to the various elements of the dermis (together with the damage of vascular network) and can occur months to over a year after irradiation (9). Further studies are required to determine if the dynamics of micronuclei in the dermal fibroblasts reflects late tolerance to reirradiation.
Conclusions
The persistence of DNA damage in the irradiated skin demonstrated in this study indicates the long-term nature of radiation damage in non-proliferating cell populations. It suggests the possibility that it would be practical to use the MN assay for biodosimetry even if samples are not obtained for up to a month after irradiation. Our results suggest a dose limit in the range of 10 Gy; however, more detailed analysis of dose-response relationships is required. The unusual results at 1 and 3 days in the 9-week-old rats (Figs. 3 and 4) after a dose of 18 Gy may suggest that younger animals are more sensitive to MN formation at early times. However, further studies are required (and are currently under way in our laboratory) to examine effects of age, gender and strain of animals, all of which may affect the results obtained.
ACKNOWLEDGMENTS
This work was funded by an IHRT grant from the CIHR (CRT43818) and by a grant from the NIAID/NIH (U19 AI-067733).
REFERENCES
- 1.Hill RP, Kaspler P, Griffin AM, O'Sullivan B, Catton C, Alasti H, Abbas A, Heydarian M, Ferguson P, Bell RS. Studies of the in vivo radiosensitivity of human skin fibroblasts. Radiother. Oncol. 2007;84:75–83. doi: 10.1016/j.radonc.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantzer D, Ferguson P, Hill RP, Keating A, Kandel RA, Wunder JS, O'Sullivan B, Sandhu J, Waddell J, Bell RS. Effect of radiation and cell implantation on wound healing in a rat model. J. Surg. Oncol. 2003;83:185–190. doi: 10.1002/jso.10242. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson PC, Boynton EL, Wunder JS, Hill RP, O'Sullivan B, Sandhu JS, Bell RS. Intradermal injection of autologous dermal fibroblasts improves wound healing in irradiated skin. J. Surg. Res. 1999;85:331–338. doi: 10.1006/jsre.1999.5664. [DOI] [PubMed] [Google Scholar]
- 4.Gorodetsky R, McBride WH, Withers HR. Assay of radiation effects in mouse skin as expressed in wound healing. Radiat. Res. 1988;116:135–144. [PubMed] [Google Scholar]
- 5.Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur. J. Surg. Oncol. 2002;28:75–79. doi: 10.1053/ejso.2001.1213. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Zee B. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 7.Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin. Radiat. Oncol. 2000;10:200–209. doi: 10.1053/srao.2000.6593. [DOI] [PubMed] [Google Scholar]
- 8.Stewart FA. Re-treatment after full-course radiotherapy: is it a viable option? Acta Oncol. 1999;38:855–862. doi: 10.1080/028418699432545. [DOI] [PubMed] [Google Scholar]
- 9.Brush J, Lipnick SL, Phillips T, Sitko J, McDonald JT, McBride WH. Molecular mechanisms of late normal tissue injury. Semin. Radiat. Oncol. 2007;17:121–130. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Bristow RG, Hill RP. Comparison between in vitro radiosensitivity and in vivo radioresponse in murine tumor cell lines. II: In vivo radioresponse following fractionated treatment and in vitro/in vivo correlations. Int. J. Radiat. Oncol. Biol. Phys. 1990;18:331–345. doi: 10.1016/0360-3016(90)90098-5. [DOI] [PubMed] [Google Scholar]
- 11.Khan MA, Hill RP, Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int. J. Radiat. Oncol. Biol. Phys. 1998;40:467–476. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- 12.Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother. Oncol. 2003;66:95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 13.Fenech M. The in vitro micronucleus technique. Mutat. Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 14.Hill RP. Radiation effects on the respiratory system. Br. J. Radiol. Suppl. 2005;27:75–81. [Google Scholar]
- 15.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother. Oncol. 2006;79:231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int. J. Radiat. Biol. 2005;81:887–899. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- 17.Joksic G, Petrovic S, Ilic Z. Age-related changes in radiation-induced micronuclei among healthy adults. Braz. J. Med. Biol. Res. 2004;37:1111–1117. doi: 10.1590/s0100-879x2004000800002. [DOI] [PubMed] [Google Scholar]
- 18.Herskind C, Johansen J, Bentzen SM, Overgaard M, Overgaard J, Bamberg M, Rodemann HP. Fibroblast differentiation in subcutaneous fibrosis after postmastectomy radiotherapy. Acta Oncol. 2000;39:383–388. doi: 10.1080/028418600750013159. [DOI] [PubMed] [Google Scholar]
- 19.Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc. Natl. Acad. Sci. USA. 1988;85:5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurent C, Pouget JP, Voisin P. Modulation of DNA damage by pentoxifylline and alpha-tocopherol in skin fibroblasts exposed to gamma rays. Radiat. Res. 2005;164:63–72. doi: 10.1667/rr3383. [DOI] [PubMed] [Google Scholar]
- 21.Jagetia GC, Baliga M. Shrinath. Vincristine increases the genomic instability in irradiated cultured human peripheral blood lymphocytes. Toxicol. Lett. 2002;126:179–186. doi: 10.1016/s0378-4274(01)00455-6. [DOI] [PubMed] [Google Scholar]
- 22.Chang WP, Tsai MS, Hwang JS, Lin YP, Hsieh WA, Huang SY. Follow-up in the micronucleus frequencies and its subsets in human population with chronic low-dose gamma-irradiation exposure. Mutat. Res. 1999;428:99–105. doi: 10.1016/s1383-5742(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 23.Terry NH, Tucker SL, Travis EL. Time course of loss of residual radiation damage in murine skin assessed by retreatment. Int. J. Radiat. Biol. 1989;55:271–283. doi: 10.1080/09553008914550301. [DOI] [PubMed] [Google Scholar]