Abstract
Objective
The purpose of this study was to assess the value of delayed-enhanced magnetic resonance imaging (DE-MRI) to guide ablation of ventricular arrhythmias in patients with non-ischemic cardiomyopathy (NIC).
Background
In patients with NIC, ventricular arrhythmias often are associated with scar tissue. DE-MRI can be used to precisely define scar tissue.
Methods
DE-MRI was performed in 29 consecutive patients (mean age 50±15 years) with NIC (mean ejection fraction 37±9%) referred for catheter ablation of ventricular tachycardia (VT) or premature ventricular complexes (PVCs). Scar was extracted from DE-MRIs and was then integrated into the electroanatomic map. Mapping data were correlated with respect to the localization of scar tissue.
Results
Scar was identified by DE-MRI in 14/29 patients. Nine of these patients had VT and five had PVCs. In 5 of the patients there was predominantly endocardial scar, and mapping and ablation of arrhythmias was effectively performed from the endocardium in all 5 patients. In 2 patients scar was either intramural or epicardial with extension to the endocardium. In both patients with partial endocardial scar extension, the ablation was effective in eliminating some but not all arrhythmias. In 2 patients most of the scar tissue was confined to the epicardium; mapping identified and eliminated an epicardial origin in both patients. No effect on arrhythmias could be achieved in the other 5 patients with predominantly intramural scar.
Conclusions
DE-MRI in patients without prior infarctions can help to identify the arrhythmogenic substrate; furthermore it helps to plan an appropriate mapping and ablation strategy.
Keywords: non-ischemic cardiomyopathy, mapping, ablation, ventricular tachycardia, magnetic reronance imaging
In patients with ischemic and non-ischemic cardiomyopathy ventricular arrhythmias are often associated with the presence of scar tissue(1-3). Whereas scar tissue in post-infarction patients is commonly located in the subendocardium(4), scar in patients with non-ischemic cardiomyopathy is often found in the mid-myocardium(4) or the epicardium(5). Delayed-enhanced magnetic resonance imaging (DE-MRI) precisely defines the extent and location of scar tissue and therefore might be a useful guide to mapping and ablation of ventricular arrhythmias in patients with non-ischemic cardiomyopathy.
The purpose of this study was to determine whether DE-MRI is a useful guide to mapping and ablation of ventricular arrhythmias in patients with non-ischemic cardiomyopathy.
Methods
Patient Characteristics (Table 1 and 2)
Table 1.
Patient Characteristics
| Characteristics | Scar By DE-MRI | No Scar By DE-MRI | P |
|---|---|---|---|
| Patient number | 14 | 15 | |
| Age (years) | 52±13 | 48±17 | 0.5 |
| Gender (M/F) | 9/5 | 7/8 | 0.5 |
| Ejection fraction | 0.38±0.09 | 0.36±0.09 | 0.5 |
| MRI: LV volume enddiastolic (ml) | 194±56 | 184±72 | 0.7 |
| MRI: LV mass (cm3) | 172±53 | 130±46 | 0.07 |
| Spontaneous or inducible VT | 9/14 | 0/15 | <0.001 |
| Frequent PVCs | 7/14 | 15/15 | <0.001 |
| SOO of PVCs (RV / LV) | 0/4 | 7/7 | 0.1 |
| Effective ablation sites of VTs (RV / LV) per patient | 3/4 | 0/0 | 1 |
Abbreviations: DE-MRI= delayed enhanced magnetic resonance imaging; VT= ventricular tachycardia; PVC= premature ventricular complex; ACEI= angiotensin converting enzyme inhibitor; SOO= site of origin; RV= right ventricle; LV= left ventricle.
Table 2.
Scar Characteristics of Patients with Left or Right Ventricular Scar as Detected by DE-MRI
| Patient # | Underlying heart disease | RV normal volume / scar volume (cm3) | LV normal volume / scar volume (cm3) | LV scar % of total LV | LV scar distribution Trm/End/Intr/Epi (%) |
|---|---|---|---|---|---|
| 1 | sarcoidosis | 79.9 / 111.5 | 272 / 71.6 | 21 | 71 / 8 / 7 / 14 |
| 2 | DCM | 54.9 / 0 | 234 / 11.21 | 5 | 0 / 13 / 80 / 7 |
| 3 | sarcoidosis | 45.3 / 1.9 | 145.7 / 14.4 | 10 | 0 / 17 / 76 / 6 |
| 4 | DCM | 34.1 / 0 | 133.5 / 25.2 | 19 | 0 / 0 / 85 / 15 |
| 5 | sarcoidosis | 41.4 / 0 | 148.3 / 21.6 | 15 | 0 / 0 / 11 / 89 |
| 6 | DCM | 56.6 / 0 | 274.2 / 5.0 | 1.8 | 0 / 100 / 0 / 0 |
| 7 | DCM | 53.5 / 0 | 180.1 / 21.9 | 12 | 0 / 100 / 0 / 0 |
| 8 | DCM | 43.3 / 0 | 194.4 / 1.1 | 0.5 | 0 / 100 / 0 / 0 |
| 9 | DCM | 31.4 / 0 | 137.4 / 8.7 | 6 | 24 / 0 / 76 / 0 |
| 10 | DCM | 68.5 / 29.7 | 165.5 / 32.2 | 19.5 | 0 / 24 / 26 / 50 |
| 11 | DCM | 58.3 / 0 | 159 / 6.5 | 4 | 0 / 0 / 0 / 100 |
| 12 | DCM | 25.6 / 0 | 100 / 0.2 | .2 | 0 / 0 / 100 / 0 |
| 13 | DCM | 30.7 / 0 | 127.8 / 21.5 | 17 | 88 / 12 / 0 / 0 |
| 14 | DCM | 28 /0 | 109 / 11 | 10 | 0 / 0 / 100 / 0 |
Abbreviation as above: DCM= dilated crardiomyopathy; RV= right ventricular, LV = left ventricular; Trm= transmural; End= endocardial; Intr= intramural; Epi= epicardial
The subjects of this study were 29 consecutive patients (mean age 50±15 years, 13 women) with non-ischemic cardiomyopathy (mean ejection fraction 39±9%) referred for radiofrequency catheter ablation of symptomatic premature ventricular complexes (PVCs, n=20), or ventricular tachycardia (VT, n=9). None of the patients had contraindications for MRI. In patients with frequent PVCs, the PVC burden was 29±18% of the total QRS count. Treatment with a mean of 1.5±0.9 antiarrhythmic medications (including amiodarone in 6 patients) had been unsuccessful. All patients underwent DE-MRI before the catheter ablation procedure.
Cardiac Magnetic Resonance Imaging
The DE-MRI studies were performed on a 1.5-Tesla magnetic resonance imaging scanner (Signa Excite CV/i, General Electric, Milwaukee, Wis.) with a 4- or 8-element phased array coil placed over the chest of patients in the supine position. Images were acquired with ECG gating during breath-holds. Dynamic short- and long-axis images of the heart were acquired using a segmented, k-space, steady-state, free-precession (SSFP) pulse sequence (repetition time 4.2 ms, echo time 1.8 ms, 1.4×1.4 mm in-plane spatial resolution, 8 mm slice thickness). Fifteen minutes after administration of 0.20 mmol/kg of intravenous gadolinium DTPA (Magnevist, Berlex Pharmaceuticals, Wayne, NJ), 2-D delayed enhancement imaging was performed using an inversion-recovery sequence (6) (repetition time 6.7 ms, echo time 3.2 ms, in-plane spatial resolution 1.4×2.2 mm, slice thickness 8mm) in the short-axis and long-axis of the left ventricle at matching cine-image slice locations. The inversion time (250-350 ms) was optimized to null the normal myocardium. The DE-MRIs were reviewed for the presence or absence of delayed enhancement by 2 observers blinded to the results of mapping and ablation. Discrepancies were resolved by consensus.
Electrophysiologic Study And Mapping
The study protocol was approved by the Human Research Committee. After informed consent was obtained, a 6 Fr quadripolar electrode catheter was introduced into the right femoral vein and positioned at the right ventricular apex. Programmed right ventricular stimulation was performed in all patients with 1-4 extrastimuli to induce VT. Sustained VT was defined as VT lasting >30 seconds or requiring termination secondary to hemodynamic compromise of the patient. In 9/28 patients, sustained monomorphic VT could be induced. In these 9 patients a total of 49 distinct, sustained, monomorphic VTs could be induced (mean of 7±7 VTs per patient). The VTs had a mean cycle length of 366±78 ms. Thirteen had a right bundle branch block morphology and 18 had a left bundle branch block morphology.
Electroanatomical mapping (CARTO, Biosense Webster, Inc) was performed with either a 4-mm-tip ablation catheter (Navistar) or a 3.5-mm-tip, open-irrigation ablation catheter (Thermocool). Multielectrode catheters were positioned in the high right atrium, the His position and the right ventricular apex. Electrograms were filtered at 50-500 Hz. The intracardiac electrograms and leads V1, I, II and III were displayed on an oscilloscope and recorded at a speed of 100 mm/sec. The recordings were stored on optical disc (EP Med, West Berlin, NJ). Depending on the morphology of the targeted ventricular arrhythmias mapping was performed in the right ventricle (for left bundle branch block ventricular arrhythmias) or the left ventricle (for right bundle branch block arrhythmias). A voltage map was generated during sinus rhythm, followed by activation mapping and / or entrainment mapping of the targeted PVCs or VT in the right or left ventricle, as appropriate.
For PVCs, the site with the earliest local activation time resulting in elimination of the PVC during radiofrequency ablation was defined as critical. For hemodynamically tolerated VT, a site was defined as critical in the presence of concealed entrainment resulting in VT termination during radiofrequency energy delivery or in the presence of termination of a VT by mechanical contact with the mapping catheter. For non-tolerated VTs, an isthmus was defined as a site where a match of the QRS morphology between the pace-map and the VT could be identified.
Low voltage was defined as a bipolar voltage amplitude of ≤1.5mV(7). Areas with bipolar voltage ≤1.5 and ≤1.0mV were measured. Activation time was measured with electronic calipers from the onset of the 1st rapid component of the ventricular electrogram to the beginning of the QRS complex. If a VT was hemodynamically tolerated, entrainment mapping also was performed. If PVCs were infrequent or if a targeted VT was not hemodynamically tolerated, pace-mapping was used in order to identify the site of origin. In case of left ventricular mapping, a bolus of 5000 units of heparin was used initially and heparin was then administered to obtain an activated clotting time of 300 sec. For right sided procedures, an initial bolus of 3000 units of heparin was given followed by 1000 units of heparin every hour. In case of an epicardial procedure, heparin was only administered after the pericardial puncture was performed.
A 2 Fr electrode catheter (Pathfinder, CARDIMA, Inc.) was introduced into the coronary venous system in 3 patients in whom an epicardial origin of a ventricular arrhythmia was suspected. In 1 patient, epicardial mapping and ablation was performed using percutaneous access(8) after an ineffective endocardial mapping and ablation.
Registration of DE-MRI and Electroanatomic Mapping Data
Prior to the procedure, scar tissue was identified on the DE-MRI (figure 1). On each short axis DE-MRI image, with reference to long-axis DE-MRI images and short and long axis SSFP dynamic images, the following contours were manually traced: the endocardial and epicardial contours of both the left and right ventricles, and each area of abnormal signal in the left or right ventricular myocardium. Then, using customized software in the MATLAB environment (MathWorks, Natick, MA), 3-D endocardial and epicardial surfaces were generated for both the left and right ventricles. In addition, for each area of abnormal signal in the left and right ventricular myocardium, a 3-D surface representation of the distribution of this abnormal signal was produced (figure 2a and 2b).
Figure 1. Stack of short axis DE-MRI pictures showing delayed enhancement in the epicardial left ventricular lateral wall.
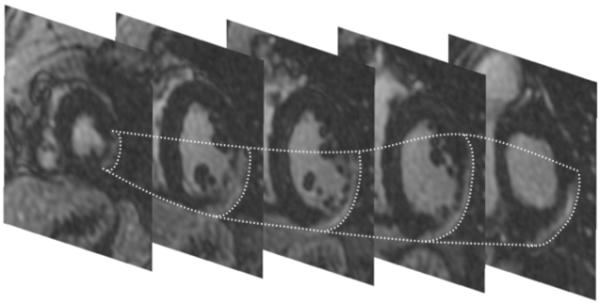
Although the free wall is thinned, the endocardium is relatively preserved. The epicardial contours of the scar are traced in dotted lines. An endocardial radiofrequency catheter ablation procedure for VT was unsuccessful. Epicardial ablation successfully eliminated all 3 inducible VTs.
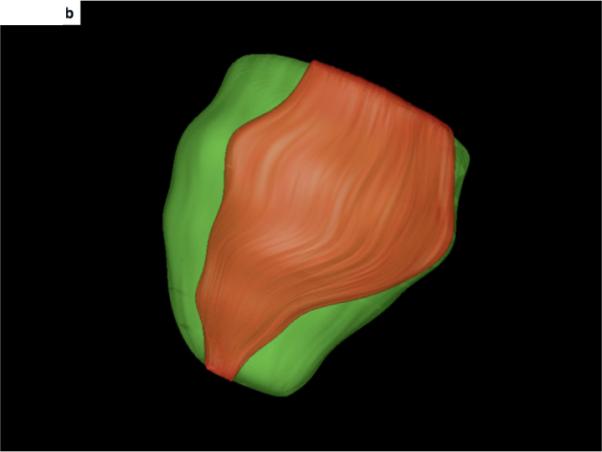
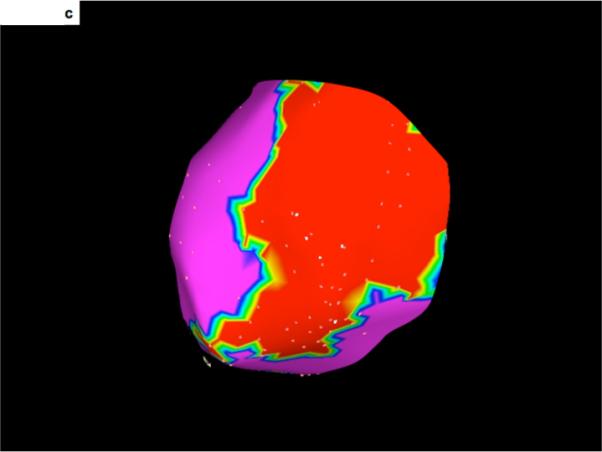
Figure 2. 3-D display and integration of scar within the electroanatomic map.
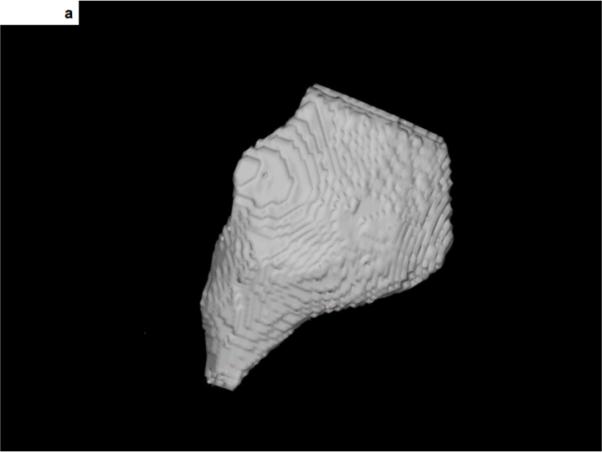
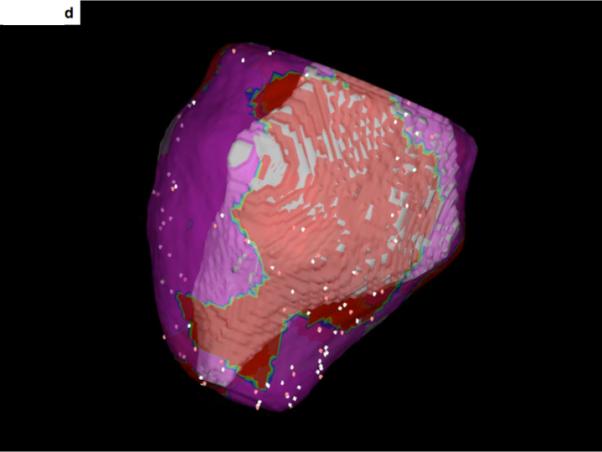
A- The 3-dimensional display of scar tissue (grey) that was extracted from the DE-MRI shown in Figure 1. The inferolateral aspect of the epicardial scar is shown.
B- The 3-dimensional display of scar (orange) and the surrounding epicardium (green) of the left ventricle. The heart is oriented in the same manner as in Figure 2A, showing the inferolateral aspect of the scar extending from the left ventricular base to the apex.
C- An epicardial voltage map. Low voltage areas (<1.5mV) are displayed in red). The scar shown in Figure 2A and 2B corresponds with the low voltage area shown here.
D- Merging of the voltage map with the 3-dimensional reconstruction of the scar seen on DE-MRI. The region of low voltage corresponds to the location of the DE-MRI scar.
This was performed by first transforming the 3-D surface map data into volumetric data corresponding to each surface, and importing this 3-D volumetric DICOM data into the CARTO MERGE software (Biosense Webster, Diamond Bar, California) using its standard volumetric data import functionality. Within the CARTO MERGE software, the 3-D surfaces were recreated by segmentation of the imported volumetric data. 3-D registration of the MRI surface data (Figure 2b) and the electroanatomic mapping data (Figure 2c) was initially accomplished using 3 fixed reference points (aorta, left ventricular apex and mitral annulus) of both MRI data and electroanatomic mapping data, and then refined using surface registration. Mitral annular points sites were selected on the electroanatomic map where both atrial and ventricular signals were present. Using this approach, a 3-D registration of both image modalities could be achieved with an accuracy of <5 mm as assessed by surface registration statistics. The electroanatomic map was then fused to the endocardial surface within the CARTO MERGE software (figure 2d).
Radiofrequency Ablation
Radiofrequency energy was delivered at sites of earliest endocardial activation during PVCs, at sites with concealed entrainment during VT, or at sites of origin as determined by pace mapping. For non-irrigated catheters, the target temperature was set to 60C°; for irrigated tip catheters the initial power setting was 30 Watts; applications of radiofrequency energy were titrated to achieve an impedance drop of 10 Ohms. Ablation was performed during ventricular ectopy or VT whenever possible and energy applications were continued for ≥30 seconds if adequate heating at the electrode-tissue interface was achieved. If the PVCs or VT ceased within 30 seconds, the energy application was continued for 60 seconds and was followed by a 2nd 60-second application. If the PVCs or VT did not cease in ≤30 seconds, the radiofrequency energy application was discontinued and additional mapping was performed. In the pericardial space, only non-irrigated tip catheters were used with a target temperature of 60C°. In the coronary venous system, irrigated tip catheters were used for ablation with an initial power setting of 15 watts. After ablation, programmed right ventricular stimulation was repeated. Successful catheter ablation was defined as cessation of the targeted PVCs or the non-inducibility of VT.
Data Analysis
Scar volume (cm3) and endocardial as well as epicardial scar area (cm2) was determined after endocardial and epicardial contours were manually traced and areas with delayed enhancement were identified and traced. Location of scar was classified as predominantly endocardial (figure 3), predominantly epicardial (figure 1) or transmural if endo and epicardial areas were involved (figure 3). The intramural scar component was quantified As 100% if there was no extension to epi- or endocardial surface (figure 4) and <100% in case of extension to endo/epicardium (figure 4). The distribution of areas with delayed enhancement was classified as monofocal (figure 1) or multifocal (figure 3,4). For right ventricular scar, scar was defined as present or absent. If the septum revealed scar on the right ventricular endocardium, this was considered to be right ventricular scar.
Figure 3. A short-axis view of the mid-portion of the right and left ventricles.
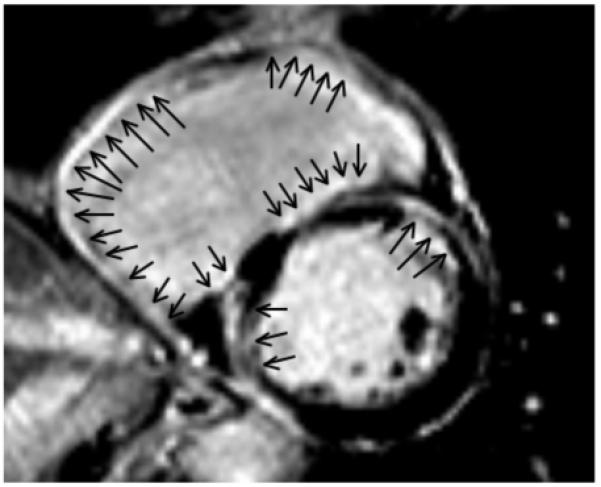
The scar involves predominantly the right ventricular endocardium. The right ventricle shows enodcardial delayed enhancement (arrow) that is transmural in the right ventricular free wall and mostly endocardial at the right ventricular septum. Endocardial scar also is present in the left ventricle, with some transmural components. All 4 inducible VTs were ablated at the right ventricular endocardium in this patient.
Figure 4. A short-axis view of the mid-portion of the left ventricle.
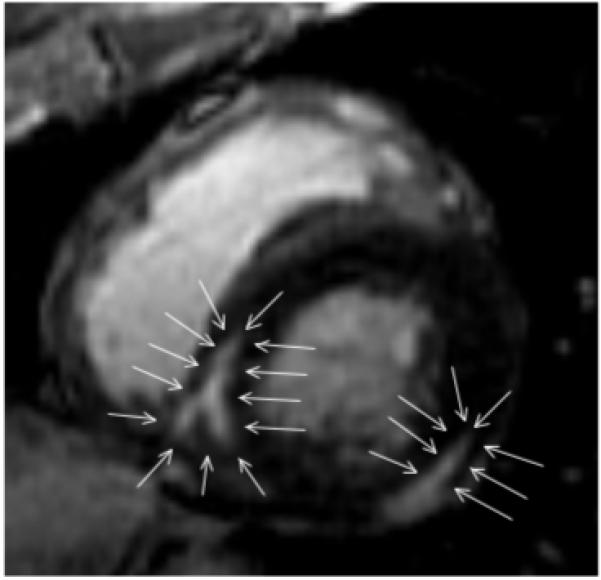
There is delayed enhancement that is predominantly mid-myocardial at the inferior free wall. The scar is intramural (arrows) and extends to the epicardium, with sparing of the endocardium. The scar located at the postero-septal wall is completely intramural (arrows). Endocardial radiofrequency catheter ablation was unsuccessful in this patient. Limited epicardial mapping from within the coronary sinus did not show early activation during the induced VTs, and mapping was not performed within the pericardial space.
Follow-Up
All patients were seen in follow-up 3-6 months after the ablation procedure. Patients with ICDs were seen every 6 months in a device clinic. The mean duration of follow-up was 19±11 months. Post ablation antiarrhythmic medication was continued in 4 patients in whom the ablation was considered to be only partially effective (n=2) or ineffective (n=2). In the remaining patients, antiarrhythmic medications were not continued.
Statistical Analysis
Variables are expressed as the mean ± 1 standard deviation. Variables were compared by Student’s t test, the Fisher exact test, or by Chi-square analysis, as appropriate. If a cell size was < 5, the Fischer exact test was used. Changes in the PVC burden and changes in ejection fraction were assessed by paired t-tests. Pearson correlation coefficient (R) was calculated to assess linear relationship between two continuous variables. A p value of <0.05 was considered statistically significant.
Results
Findings on DE-MRI (table 2)
In 14/29 cardiac MRIs there was evidence of delayed enhancement in the MRIs. Delayed enhancement was either present in a single focus (n=6) or multifocal (n=8). It affected the left ventricle in all 14 patients; right ventricular involvement was present in 3/14 patients. The distribution was predominantly endocardial (n=7 with intramural extension in 2/7), midmyocardial (n=5) or epicardial (n=2). If an electroanatomic map was performed in the chamber or surface displaying DE, there was always low voltage present matching with the area of DE (figure 2). Endocardial size of the scar as assessed by DE-MRI (26±34cm2) correlated well with the endocardial scar size as defined by voltage mapping (<1.0mV: 33±42cm2;R=0.96; p<0.0001 and <1.5mV: 45±14cm2, R=0.94; p<0.0001). Image registration of the 3 D MRI volume of the chamber in which mapping was performed resulted in a positional error of 4.8±3.6 mm with surface registration. Eighty-seven percent of low voltage points projected on scar tissue.
Relationship between DE-MRI and Results of Catheter Mapping (table 2-4)
Table 4.
Scar Involvement Relative to Mapped Chamber and Identification of Target Sites
| Patient | Mapped Chamber | % scar involving endocardium of mapped chamber | % of ablated arrhythmias | % scar involving intramural or epicardial part of mapped chamber | Limited epicardial mapping identifying SOO (% of mapped arrhythmias) |
|---|---|---|---|---|---|
| 1 | RV | 100 | 100 | 0 | NA |
| 2 | LV | 13 | 33 | 87 | NA |
| 3 | LV | 17 | 0 | 82 | 0 |
| 4 | LV | 0 | 0 | 100 | 0 |
| 5 | LV endocardium LV epicardium |
Endocardial: 0 Epicardial: 100 |
0 100 |
0 100 |
NA |
| 6 | LV endocardium | 100 | 100 | 0 | NA |
| 7 | LV endocardium | 100 | 100 | 0 | NA |
| 8 | LV endocardium | 100 | 100 | 0 | NA |
| 9 | LV endocardium | 23 | 0 | 76% | 0 |
| 10 | RV / LV endocardium | 100 / 24% | 79% / 71% | 0 / 76% | 0 |
| 11 | LEMO | NA | 100 | 100% | 100% |
| 12 | LV | 0 | 0 | 100% | NA |
| 13 | LV endocardium | 100 | 100 | 0 | NA |
| 14 | LV endocardium / LEMO | 0 | 0 | 100% | 0 |
Abbreviations: as above
All of the patients with inducible VT or a history of sustained VT had evidence of delayed enhancement. In each patient in whom delayed enhancement was present and in whom a critical component of an arrhythmia could be identified, the critical component of the arrhythmia was confined to the scar tissue. Isolated potentials could be identified in all of the patients displaying delayed enhancement on the MRI unless the scar was intramural in location. After integration of the scar into the electroanatomic map, activation / entrainment and or pace-mapping confirmed that the critical area for ventricular arrhythmias was confined to endocardial scar tissue in 7/ 14 patients (50%) with delayed enhancement. Epicardial mapping and ablation identified critical components of VT confined to epicardial scar in 2/14 patients (14%) with delayed enhancement. In 5/14 patients (36%) with delayed enhancement, scar was predominantly located intramurally, and an ablation procedure was ineffective.
In the 7 patients where ventricular arrhythmias could be identified in an area of endocardial scar, all arrhythmias (VT in 4 patients and PVCs in 3 patients) were confined to the endocardium in 5/7 patients. In the remaining 2 patients, although there was an endocardial extension of the scar, the majority of the scar (80%) was intramural in one patient and was intramural or extended to the epicardium where mapping was not performed in another patient. Critical areas for arrhythmias (both patients with VT) could be identified at the endocardium for 20/33 VTs in both patients. In the 2 patients where the scar was limited to the epicardium, one had a prior failed ablation. In this patient a transcutaneous puncture of the pericardial space followed by epicardial mapping delineated the epicardial extent of the scar and allowed localization of critical components for all 3 inducible VTs. In the other patient, a limited epicardial mapping procedure via the coronary venous system identified the critical area of the VT. A complete epicardial mapping procedure was not required in this patient. In 5 /14 remaining patients with delayed enhancement, the scar was predominantly intramural with minimal extension of the scar towards either endocardium or epicardium. Mapping within the endocardium failed to identify suitable target sites or areas with isolated potentials in these patients.
Acute Response to Catheter Ablation (table 3 and 4)
Table 3.
Targeted Arrhythmias with Mapping and Ablation Sites
| Patient # | Inducible VT/Sponateneous VT | # inducible VTs / targeted PVCs | # of targeted RV Arrhythmias | Effective RV endocardial Ablation | # of targeted LV arrhythmias | Effective LV endocardial RF | Effective epicardial RF |
|---|---|---|---|---|---|---|---|
| 1 | Yes / Yes | 4 / 0 | 4 | 4 | 0 | NA | NA |
| 2 | Yes / Yes | 12 / 0 | 0 | NA | 12 | 4 | NA |
| 3 | Yes / Yes | 6 / 0 | 0 | NA | 6 | 0 | 0 |
| 4 | Yes / Yes | 1 / 0 | 0 | NA | 1 | 0 | 0 |
| 5 | Yes / Yes | 3 / 0 | 0 | NA | 4 | 0 | 3 |
| 6 | No / No | 0 / 1 | 0 | NA | 1 | 1 | NA |
| 7 | Yes / No | 1 / 1 | 0 | NA | 2 | 2 | NA |
| 8 | No / No | 0 / 3 | 0 | NA | 3 | 3 | NA |
| 9 | No / No | 0 / 7 | 0 | NA | 7 | 0 | 0 |
| 10 | Yes / No | 21/0 | 14 | 11 | 7 | 5 | 0 |
| 11 | No / Yes | 1 / 1 | 0 | NA | 2 | 0 | 2 |
| 12 | No / No | 0 / 1 | 1 | NA | 1 | 0 | NA |
| 13 | Yes / Yes | 1 / 0 | 1 | NA | 1 | 1 | NA |
| 14 | No / No | 0 / 2 | 0 | NA | 2 | 0 | NA |
Abbreviations: abbreviations as above, NA= not applicable; RF= radiofrequency ablation, LEMO= limited epicardial mapping only
In 7/14 patients with delayed enhancement, in whom there was an endocardial extension of the scar, at least one arrhythmia could be ablated from the endocardium. All arrhythmias (VTs in 2 patients and PVCs in 3 patients) could be endocardially eliminated in 5/14 patients with delayed enhancement. In the remaining 2 patients with endocardial scar extensions 20 / 33 inducible VTs could be ablated from the endocardium. In one of these 2 patients, the scar was predominantly intramural in location. In the other patient, part of the scar was intramural and epicardial; a limited epicardial map failed to identify any critical epicardial target sites. In this patient, VTs originated from the right and left ventricle and critical sites could be identified endocardially in both ventricles.
In 2 / 14 patients with delayed enhancement, the scar was predominantly located in the left ventricular epicardium. An epicardial mapping procedure with transcutaneous epicardial access eliminated all 3 VTs in a patient who failed a prior endocardial ablation procedure. A limited epicardial ablation procedure was effective in eliminating the one and only VT in one of the patients when radiofrequency energy was delivered within the greater cardiac vein.
In 5 /14 patients with delayed enhancement where the scar was predominantly located intramurally, radiofrequency ablation failed to eliminate the targeted ventricular arrhythmias in all patients (VT in 2 patients and PVCs in 3 patients).
In the 15 patients without delayed enhancement, 9 patients had monomorphic PVCs, and 7/9 could be effectively ablated (4 in the right ventricle and 3 in the left ventricle). The remaining 6 patients had pleomorphic PVCs, and at least one morphology could be eliminated in all but one patient in whom no appropriate target sites could be identified by endocardial mapping. This resulted in a decrease of the PVC burden from 31±17 to 5.2±9% (p=0.005). VT was not inducible in any of these patients.
Follow-up
Total follow-up time was 19±11 months. All but one patient (n=8) with inducible VT received a cardioverter defibrillator. One patient refused implantation of a defibrillator. Six of the 14 patients (43%) with delayed enhancement had recurrent arrhythmias (3 had VT, 3 had PVCs). Recurrences occurred only in patients in whom not all arrhythmias could be eliminated during the initial ablation procedure. Four patients were on amiodarone prior to the ablation procedure; this was discontinued 6 months post ablation in 1 patient (this was 24 months prior to his last follow-up); this patient had one episode of VT during the follow-up period. In the other patients, amiodarone was continued.
The ejection fraction in patients without delayed enhancement increased from 0.37±0.09 to 0.51±0.17 within 6 months post procedure (p<0.001). In patients with delayed enhancement the ejection fraction did not change post ablation (0.36±0.09 vs 0.44±0.12; p=0.2).
Discussion
Main Findings
This study demonstrates that scar tissue can be identified by DE-MRI in approximately 50% of patients with non-ischemic cardiomyopathy and frequent PVCs or monomorphic VT. The DE-MRI can be integrated into an electroanatomical map and the location of the scar is a reliable guide to catheter ablation. If the scar is endocardial, endocardial mapping and ablation will be necessary, and if the scar is epicardial, this indicates that an epicardial approach is very likely to be needed for mapping and ablation. Of note, catheter ablation of frequent PVCs or VT was unsuccessful in all patients who had a predominantly intramural scar. Therefore, an intramural scar on DE-MRI identifies patients in whom the critical arrhythmogenic substrate might be difficult to reach with the catheter technology used in this study.
Scar and Non-ischemic Cardiomyopathy
In this consecutive series of patients with non-ischemic cardiomyopathy referred for catheter ablation of frequent PVCs or VT, scar was detected by DE-MRI in approximately 50% of patients. These results confirm that scar is present in a large proportion of patients who have ventricular arrhythmias in the absence of coronary artery disease and prior myocardial infarction(1,2,9). However, this is the first study to demonstrate that the scar may be directly associated with a critical component of the targeted PVCs or VT, and that the location of the scar facilitates mapping and catheter ablation.
As in prior studies, the inducibility of monomorphic VT correlated with the scar burden(1). This suggests that there may be a threshold of scar volume above which reentrant VT is likely to occur, as has been reported to be the case in patients with ischemic cardiomyopathy(10). Furthermore none of the patients without scar had inducible VT despite the presence of a compromised ejection fraction. It must be kept in mind, though, that a reversible form of cardiomyopathy secondary to frequent PVCs might have accounted for a reduced ejection fraction in some of the patients without scar on DE-MRI(11).
Image Registration
Pre-procedural scar registration allowed rapid integration of the potential arrhythmogenic substrate into the electroanatomical map of the right or left ventricle. Our study further extends results from a prior study that used PET / CT imaging to target ventricular arrhythmias by localizing scar tissue very precisely using DE-MRI(12). The position of the scar in the wall of the ventricle proved to be predictive of the location of the effective target sites. When the DE-MRI showed delayed enhancement predominantly in the epicardium or intramurally without extension to the endocardium, endocardial mapping and ablation was always unsuccessful. When an intramural scar extended to the epicardium, successful ablation was feasible from within the pericardial space. Therefore, scar location on DE-MRI is a reliable indicator of whether an endocardial or epicardial approach to mapping and ablation will be needed in the almost 50% of patients with non-ischemic cardiomyopathy and frequent PVCs or VT who have a demonstrable scar.
Patients without Scar Tissue
Scar can be identified in a minority of patients with non-ischemic cardiomyopathy(4). In this study of patients with frequent PVCs and impaired left ventricular function in whom PVCs could be eliminated, the left ventricular function either improved or normalized. A reversible form of PVC-induced cardiomyopathy has been described before (11), and might explain improvement of ejection fraction in these patients. The arrhythmogenic substrate could not be identified by MRI in this subset of patients.
Limitations
A limitation of this study is that not all forms of non-ischemic cardiomyopathy were included in this study, and it is possible that the results do not apply to all types of non-ischemic cardiomyopathy.
Another limitation is that epicardial mapping from within the pericardial space was not performed in all patients who failed to respond to endocardial mapping. Therefore, we cannot rule out that some of the ventricular arrhythmias associated with intramural scar may have been successfully ablated using an epicardial approach. Larger studies including especially patients with epicardial and intramural scar are required to confirm our preliminary data. Furthermore, scar was defined by manual tracing and therefore might not be as objective as a semiautomatic method that is frequently used to define scar in post-infarction patients.(13)
Clinical Implications
DE-MRI plays an important role in patients with non-ischemic cardiomyopathy who are candidates for catheter ablation of frequent PVCs or VT. Delayed enhancement indicative of scar is present in approximately 50% of these patients, and if a scar is present, its location can be used to plan the approach to catheter ablation. If the scar is epicardial and does not have an endocardial component, epicardial mapping and ablation may be necessary. On the other hand, if the scar is intramural and does not have an endocardial or epicardial extension, catheter ablation is less likely to be successful, and additional attempts at pharmacologic therapy may be more appropriate than radiofrequency catheter ablation of the frequent PVCs or VT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest related to the data presented in this manuscript.
Disclosure for Hakan Oral:
Co-founder and equity owner of Ablation Frontiers; Research grants from Boston Scientific and St. Jude Medical.
References
- 1.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JA, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–5. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 3.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 5.De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Del Maschio A. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47:1649–54. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 6.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 7.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–96. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 8.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–6. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 9.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Fractionated electrograms in dilated cardiomyopathy: origin and relation to abnormal conduction. J Am Coll Cardiol. 1996;27:1071–8. doi: 10.1016/0735-1097(95)00612-5. [DOI] [PubMed] [Google Scholar]
- 10.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, Kadish AH, Goldberger JJ. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–8. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 11.Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, Jongnarangsin K, Marine JE, Chugh A, Pelosi F, Oral H, Morady F. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–7. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Dickfeld T, Lei P, Dilsizian V, Jeudy J, Dong J, Voudouris A, Peters R, Saba M, Shekhar R, Shorofsky S. Integration of Three-Dimensional Scar Maps for Ventricular Tachycardia Ablation with Positron Emission Tomography-Computed Tomography. J Am Coll Cardiol Img. 2008;1:73–82. doi: 10.1016/j.jcmg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–9. doi: 10.1016/j.jacc.2004.09.020. [DOI] [PubMed] [Google Scholar]


