Abstract
In human pregnancies mothers and their embryo/fetuses are invariably genetically different. Thus, attenuation of the adaptive maternal immune response, which is programmed to reject “foreign” entities, is required for pregnancy to be initiated and maintained. Unexpectedly, given the propensity of the immune system to dispose of non-self entities, at least 50% of expected human pregnancies reliably go forward. This indicates that to a large extent, effective systems of tolerance have evolved. Although overlapping and redundant mechanisms of tolerance have been identified, production of HLA-G by trophoblast cells derived from the external trophectoderm layer of the blastocyst appears to be of major importance. At this point in time, no pregnancies in which all of the proteins derived from the HLA-G gene are absent have as yet been reported. Many studies have shown that both membrane-bound and soluble isoforms of the proteins derived from this HLA class Ib gene are produced by placental trophoblast cells, with consequences that include but are not restricted to immune suppression at the maternal-fetal interface. Here we report new studies that are leading to a better understanding of the HLA-G proteins, their unique structures, unusual modes of regulation, diverse functions and potential for use in diagnostic and therapeutic procedures related to suboptimal fertility in women.
Keywords: expression patterns, functions, HLA-G, human, LILRB, placenta, regulation
Introduction
In women, unlike certain experimental animal models, pregnancies are essentially never syngeneic. Thus, adjustments of the normal activities of human maternal and fetal immune systems are required in order for pregnancy to be established and maintained in the face of genetic dissimilarities. More than half a century of experimentation has revealed that several varieties of soluble immunosuppressive molecules are produced by the pregnant uterus and the fetal placenta, and several types of leukocytes with immune suppressive profiles inhabit these organs. Furthermore, in the placenta, trophoblast cells, a unique lineage without counterpart in adult tissues that are in immediate apposition to maternal immune cells, finely regulate their expression of immunogenic molecules known to stimulate graft rejection, chief among which are the human leukocyte antigens (HLA). Neither class Ia HLA-A, -B nor class II antigens are expressed. Instead, trophoblast cells display HLA class Ib antigens, HLA-E, -F, and –G.
HLA-G is the best studied of these three genes. Its expression in trophoblast is dependent upon which among several subpopulations is examined; its regulation is related to diverse conditions such as oxygen levels, growth factor levels and available substrate for migration; its function(s) appear to be diverse. Of great functional import, products of the HLA-G gene have been shown to exhibit immunomodulatory properties that could lead to localized tolerance at the maternal-fetal interface. Below we first provide background on the pregnancy-associated setting for high production of HLA-G. Subsequent paragraphs present brief explanations of HLA-G structure, patterns of expression in placental cells, molecular regulation and specific activities of the HLA-G isoforms Particular attention is paid to the soluble isoforms of HLA-G that are readily identified in mother’s blood throughout pregnancy. Although we focus on recent studies, the interested reader may acquire more extensive background and information from current reviews [1–6].
Maternal and fetal tissues in intimate juxtaposition
At the time of implantation of the blastocyst, the uterine endometrium has been hormonally altered and prepared for pregnancy. This process is termed decidualization; it involves stunning changes in the cellular content, synthesized molecules and vascularization of this uterine lining. The blastocyst invades the uterine epithelia and the consequent breach is healed by epithelial cells. Thus, an intimate physical relationship develops between maternal fetal tissues, with the embryo/fetus snugly encased within the mother’s decidua.
Trophoblast cells derived from the outermost trophectoderm layer of the blastocyst form the external cell layers of the placenta. Some of the cells in the trophoblastic villi remain in readiness as precursor villous cytotrophoblastic (CTB) cells, and some of these merge to form syncytiotrophoblast (sTB), a cell layer which provides a barrier between embryonic/fetal cells in the villous placenta and maternal blood carrying bone marrow-derived immune cells. The sTB is also a major site of production of steroid and protein hormones critical to pregnancy and, in addition, is responsible for bidirectional transport of nutrients and wastes between the fetus and mother’s blood. A third general subpopulation termed extravillous CTB invades the decidua where leukocytes are waiting. These migrating extravillous cells have many functions that include production of hormones, chemoattractants and cytokines and many unique qualities such as the ability to invade maternal spiral arteries and replace the endothelial cells. This latter property permits maternal blood to flow over the placenta from approximately week 10 of gestation through parturition.
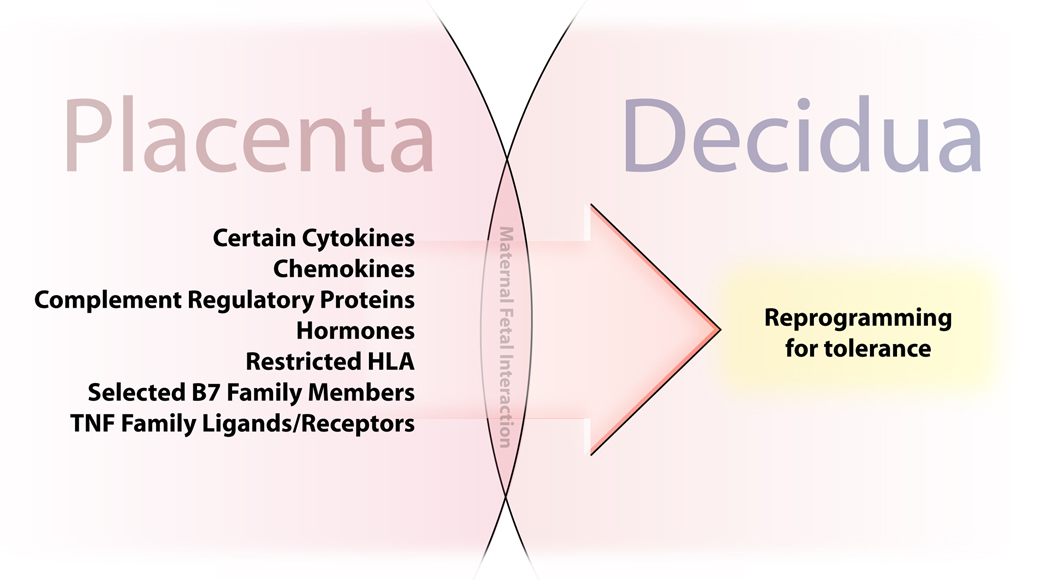
The functional result of sequestering embryonic cells beneath multiple layers of trophoblast is that trophoblast cells assume charge of achieving an immunologically compatible relationship between the mother and the fetus. Thus, as investigators study immune events in pregnancy, the trophoblast is under the microscope. It is clear that the embryo/fetus survives because of a set of cooperative interactions between the fetus and the mother [1–6], many of which are driven by the placenta, as illustrated in Figure 1.
Figure 1. Products of the fetal placenta drive tolerance in the pregnant uterus.
The fetal placenta is directly apposed and in contact with the decidua throughout pregnancy. Placental cells exhibit cell surface molecules and synthesize soluble substances that program a tolerant phenotype in maternal immune cells in the adjacent decidua.
Cooperative interactions between mothers and their embryo/fetuses provide tolerance at the maternal-fetal interface
Early aspects of tolerance generation at the maternal-fetal interface rely mainly on maternal factors such as ovary-derived progesterone, an immunosuppressive hormone that affects receptive cells in much the same way as cortisteroids. Thereafter, maternal factors include inflammatory cytokines from a wave of leukocytes homing to the site of implantation and, subsequently, dramatic alterations in the cellular composition of the decidualizing endometrium. Following implantation, cells of the pregnant uterus, which in the nonpregnant state is inhabited by a variety of leukocytes responsible for innate and adaptive immunity, are re-arranged to establish a site of exclusively innate immunity. Resident bone marrow-derived cells include uterine natural killer cells (uNK), which exhibit an unusual spectrum of markers (CD56bright/CD16-) and comprise ∼20% to 40% of the leukocytes during the first two trimesters (until approximately week 24), then become undetectable [7]. By contrast, macrophages are a stable population throughout gestation that constitutes 10% to 20% of the leukocytes. Other cells present in comparatively small numbers are Treg cells (CD4+/CD25+) [8], dendritic cells and lymphocytes. Many of these are in immunosuppressive modes as illustrated by production of certain cytokines that facilitate tolerance in the pregnant uterus.
In complementary actions, placental trophoblast cells assume major responsibilities for instituting pregnancy immune privilege. These versatile cells produce chemokines such as CXCL12/stromal cell factor-1 [9] that attract cells of the innate immune system thereby playing a major role in the uterine switch from adaptive to innate immunity. Further, trophoblast cells produce other general soluble immunosuppressants such as progesterone and prostaglandins, as well as specific suppressor molecules, which include transforming growth factor-β (TGF-β1) and interleukin-10 (IL-10). Many of these same molecules are synthesized within the villous placenta, some by stromal cells and others by trophoblast.
Novel immunotolerizing molecules are synthesized, displayed and secreted by placental trophoblast cells. These include HLA class Ib proteins, at least one of which, HLA-G, selectively targets to uterine leukocytes bearing receptors that interfere with leukocyte activation. These are the leukocyte immunoglobulin-like receptors LILRB1 (also known as ILT-2) and LILRB2 (also known as ILT-4) [10–12]. In general, lymphocytes exhibit LILRB1 whereas mononuclear phagocytes and their relatives, dendritic cells, express both LILRB1 and LILRB2. The overall impact of ligand/inhibitory receptor interactions is interference with activating signals, thus driving the targeted cells into immune suppressive pathways.
Molecular and protein structures of HLA-G
Understanding molecular and biochemical features of the HLA-G gene and its products can improve our ability to determine opportunities for HLA-G to impact pregnancy. Much is known of HLA-G as it was the first trophoblast HLA class Ib antigen to be identified and continues to be an entity of considerable interest [reviewed in Refs. 1–6]. HLA class I genes reside at the telomeric end of human chromosome 6p21. Most of these genes are gene fragments or pseudogenes. Only six appear to be expressed: HLA class Ia genes (HLA-A, -B and –C) and HLA class Ib genes (HLA-E, -F and –G). Of particular interest to the situation of semiallogeneic pregnancy, the former are highly polymorphic, with many alleles, and the latter have few variants. Moreover, the class Ia antigens are ubiquitously expressed whereas class Ib antigens may be tissue/organ-specific and/or conditional.
Consistent with a need for tolerance, human placental trophoblast cells do not express HLA-A or –B (nor do they express the HLA class II antigens). HLA-C is present but it is as yet poorly understood. In contrast, all of the known HLA class 1b antigens are expressed by trophoblast cells [13]. Although HLA-G has 5 alleles most of these do not alter the amino acid sequence; those that do are not predicted to change secondary structures of the heavy chains. One important deletion has been identified, which is a single base pair (bp) deletion at nucleotide 1597, which causes a frameshift at amino acid 130. The deletion of a cytosine (C) residue at codon 130 results in a null allele (called G*0105N), which cannot encode functional HLA-G1 or -G5 protein isoforms. This novel mutation, called 1597ΔC, occurs in the homozygous form in healthy individuals, indicating that the HLA-G1 and -G5 isoforms are not essential for fetal survival [14]. In these individuals, other isoforms may be functionally adequate but less effective than HLA-G1 and –G5. The null allele is associated with increased risk for recurrent miscarriage. In the untranslated region, a 14-bp insertion/deletion polymorphism is present in exon 8 that may have a different impact on function. For additional associations and discussion of the impact of single base pair polymorphisms, alleles and isoforms see references [15–20] and recent reviews [1–6].
Functionality of HLA-G is influenced not only by the low numbers of alleles but also by generation of seven alternatively spliced transcripts from the single mRNA. From this transcript four membrane-bound (-G1, -G2, -G3, -G4) and three soluble (-G5, -G6, -G7) soforms may be produced. The full length isoform, HLA-G1, and the –G5 isoforms are structurally similar except that due to a stop sequence in intron 4, -G5 has neither transmembrane nor intracellular amino acids. The same is true of –G2 and –G6 but in this case, the α2 region is deleted due to a stop codon in intron 2. HLA-G1 and –G5 are able to bind light chain,β2-microglobulin (β2m) where as –G2 and –G6 are not and instead appear to form HLA class II-like homodimers. HLA-G3, -G4 and -G7 mRNAs are scarce in placentas and the functions of their putative protein products remain unknown.
Free heavy chains
HLA class I molecules are often found as free heavy chains. As described in greater detail below, recent studies in our laboratory have shown that this is the case for HLA-G5 synthesized in placental villous cytotrophoblast cells [21]. The inability of these cells to form heterodimeric light chain:heavy chain heterodimers is due to low levels of β2m mRNA and a consequent inability to produce β2m protein. As expected from many reports indicating that the mAb W6/32, which prefers β2m in the binding site together with HLA class I heavy chain, identifies migrating extravillous CTB cells, Apps et al. [22] have reported that extravillous CTB cells exhibit cell surface, homodimeric β2m-associated HLA-G, although of which isoform is not certain.
Where is HLA-G found?
Despite the fact that HLA-G was first reported in 1987 and that since that time, many antibodies have been generated to detect the products of this gene, expression patterns remain a subject of considerable discussion. Early work indicated that HLA class I antigen expression was restricted to the extravillous CTB cell population, with proteins being particularly prominent in the cells in the trophoblastic shell at the distal end of trophoblastic columns. Although some investigators remain firmly attached to the concept that only extravillous CTB cells produce HLA-G [23], others are not so sure as evidence mounts for synthesis in other trophoblast subpopulations.
Early on, detection was mainly achieved using the monoclonal antibody (mAb) W6/32, which identifies all varieties of HLA class I heavy chains, particularly when they are associated with the common light chain, β2m. The problem here is that neither free heavy chains nor isoforms such as HLA-G2 and –G6 that cannot associate with β2m would be detected. Studies in our laboratory indicate, as mentioned above, that one of the soluble isoforms, HLA-G5, is synthesized in placental villous cytotrophoblast cells as free heavy chains [21]. Importantly, HLA-G5 as free heavy chain interacts differently with HLA-G receptors than heavy chain:light chain heterodimers [24]. HLA-G5 has also been reported in villous CTB cells and secretions of placental explants by several experienced teams of investigators [21,25,26].
In sum, it appears that HLA-G1, β2m-associated HLA-G5 and HLA-G2/G6 are present only at the leading edge of trophoblast columns [27] and in the chorion membrane CTB cells immediately adjacent to the decidua (Platt JS and Hunt JS, unpublished). HLA-G5 is ubiquitous in trophoblast subpopulations and may be produced as either free heavy chain (from placental CTB cells) or associated with β2m (by extravillous CTB cells). The question has been raised as to whether HLA-G found in maternal sera [28] is cleaved HLA-G1 or HLA-G5 but several investigators have, independently, identified production of HLA-G5 from the villous placenta. Further, in another context, it is HLA-G5, not cleaved HLA-G1, that predominates [29].
The soluble isoform, HLA-G6, deserves mention. The mAb we generated that recognizes HLA-G2 and -G6 [26-2H11] shows that one or another or both of these two isoforms are prominent in/on extravillous CTB cells distal to the placental villi and infiltrating the decidua as well as the chorion membrane CTB cells immediately adjacent to term decidua [27; Platt JS and Hunt JS, unpublished]. Possibly, HLA-G2/G6 comprises an adequate functional substitute in women carrying the HLA-G1/G5 null allele mentioned above.
Regulation of HLA-G isoforms in trophoblast cells
Production of HLA-G may be a consequence of either genetic or environmental factors [14–20]. For examples of genetic factors, Hviiid has identified differences related to HLA-G alleles and the Ober group has described differential regulation related to specific polymorphisms in the promoter region.
As for environmental conditions, HLA-G mRNA is modestly upregulated by interferons (IFNs) despite lacking many elements of the IFN response system. Furthermore, low oxygen induces all HLA-G transcripts in term CTB cells [30], but changes in protein levels have not been identified. Epidermal growth factor (EGF), a well known trophoblast growth and syncytialization factor, as well as collagen IV, a substrate on which vCTB cells from term placentas may be cultured and may migrate, have dramatic effects, enhancing protein expression of HLA-G5 [1,21]. Neither upregulates expression of HLA-G6. In recent studies, Diaz-Lagares et al. have shown that in certain cell lines and blastocysts, nitric oxide diminishes HLA-G in cells while increasing shedding into culture media [31]. Much work remains to be done before these differentiation pathways are outlined but some intriguing information is now available on allele-specific miRNA targets in HLA-G, and our laboratory is currently focusing on trophoblast miRNA involvement in regulation, a concept recently discussed by Veit and Chies [32].
Receptors for HLA-G
New information on leukocyte inhibitory receptors that block activating signals has opened the door to understanding how HLA-G might influence leukocytes in the systemic circulation and in the decidua. The maternal adaptive immune response does recognize HLA-G with many mothers producing anti-HLA-G unrelated to disparity with paternal HLA-G allele [33]. However, this does not place restrictions on fertility or successful pregnancy; these mothers have multiple, normal pregnancies as is also the case for mothers synthesizing anti-paternal HLA-A, -B and -D.
The major HLA-G receptors on leukocytes are the LILRB. LILRB are expressed by T and B lymphocytes as well as by NK cells and mononuclear phagocytes, and act by interfering with activating signals. Although LILRB1 appears to be the main binding protein for lymphocytes, LILRB2 is probably the main receptor for HLA-G on monocytes/macrophages and dendritic cells although LILRB1 may also be present. Early studies in our laboratory have shown that decidual macrophages exhibit readily detectable LILRB1 and LILRB2 [34]. At this point in time, little is known of HLA-G signal transduction pathways via LILRB, but all of the evidence points to interference with activating LILR, Toll-like receptors (TLR4) and IFNs. One clear end result is inhibition of activation markers such as CD8 (but not CD4) on peripheral blood lymphocytes [27] and diminished production of inflammatory cytokines by monocytes and macrophages. This latter effect has been demonstrated by many investigators and reported in several recent reviews [1–6].
McCormick et al. [35] have recently reported that when transfected into a human cell line with trophoblast characteristics, SGHPL-4, soluble HLA-G inhibits motility if the cells have been stimulated with hepatocyte growth factor or EGF. Although intriguing, HLA-G receptor expression on this line is not clear and there is not, at present, evidence for expression of the inhibitory LILRB on trophoblast in situ.
Evidence for immunomodulation
Tolerance induction by the proteins derived from the HLA-G gene is the best reported of all effects of HLA-G on immune cells. Effects include 1) impact on NK cell killing, migration and cell viability as well as proliferation and IFNγ production, 2) regulation of cytokine production in blood mononuclear cells and cytotoxic T lymphocytes (CTL), 3) suppression of CTL killing and viability, 4) inhibition of proliferation and induction of a suppressive phenotype in TH cells, and 5) alteration of dendritic cell stimulatory capacity as well as maturation of this lineage [1–6]. Recently, Guillard et al. [36] have shown an unusual function: HLA-G has an activating effect on NK cells via stimulation of NFκB that is independent of LILRB1.
Leukocytes exposed to the eukaryotic recombinant proteins we generated in HEK293 cells several years ago exhibit properties associated with tolerance induction. Both rHLA-G5 and –G6 reduced CD8 mRNA and protein in IFNγ-activated blood mononuclear cells without affecting CD3 or causing cell death [27]. However, others have reported cell death in lymphocytes. For example, tumor cell HLA-G was reported to kill PHA-activated T cells through induction of the Fas/FasL pathway [37]. In a more recent study reported by Zhong et al. [38], generation of alloreactive T cells was inhibited by soluble HLA-G/IgG dimers via reducing proliferation of both CD4+ and CD8+ lymphocytes.
Returning to mononuclear phagocytes, Figure 2 shows the results of MTT assays conducted on CD14+ blood monocytes. No reduction in proliferation was observed. In CD14+cells, we have found that HLA-G5 and –G6 diminish production of IFNγ-stimulated inflammatory cytokines while leaving anti-inflammatory cytokines at baseline levels (D. Wheaton and J.S. Hunt, unpublished). Suppression is not dramatic, decreases of ∼15% to ∼20% of controls untreated with HLA-G are characteristic in our assays. An example of a Luminex cytokine assay identifying concentrations of inflammatory and anti-inflammatory cytokines in the supernatant culture media of one harvest of monocytes (either resting or IFNγ-activated) is shown in Figure 3. Similarly, Gros et al. [39] have recently reported that NK cell cytotoxicity is mildly inhibited by soluble HLA-G via actions on dendritic cells. Whether altered B7-1 expression on dendritic cells might be involved has been addressed in studies by Smith et al. [40], whose experiments were conducted using HLA-G tetramers in a non-HLA-G-expressing model, the rat. Some studies on HLA-G signaling pathways are emerging: in dendritic cells, differentiation induced by HLA-G and LILRB2 reportedly use the interleukin-6/STAT-3 pathway [41], and NK cytotoxicity may be reduced by blocking the MEK/ERK activating signal pathway [42].
Figure 2. Recombinant HLA-G produced in HEK293 cells does not kill CD14+ blood monocytes.
CD14+ blood monocytes incubated for 48 hr with 100 nM/ml of recombinant HLA-G5 or HLA-G6 (or no HLA-G) generated in HEK293 cells showed little change. Although some statistical significance suggesting increased cell numbers, the changes were not dramatic.
Figure 3. A schematic drawing illustrating the effects of recombinant HLA-G5 on resting and IFNγ-activated blood monocytes.
After 48 hr of incubation with HLA-G5 CD14+ cells that were resting (no IFNγ) demonstrated diminished synthesis of TNFα and IL-1 β in comparison with untreated cells but levels of IL-6 and the anti-inflammatory cytokine, IL-10, were unchanged. By contrast, when the cells were activated with 100 U/ml of IFNγ and simultaneously treated with recombinant HLA-G5 there were a modest increases in levels of TNFα and IL-1 β but a dramatic increase in the anti-inflammatory cytokine, IL-10. The data shown represent values obtained in one of three similar assays.
These results of mild rather than profound suppression as the consequence of leukocyte encounter with HLA-G support a new view of HLA-G at the maternal-fetal interface. It seems reasonable to postulate that this modest degree of immunomodulation indicates that the pregnant uterus must be ever ready to switch to an activation profile so as to combat infection. Importantly, the major effects of HLA-G5 and –G5 will be local with a concentration gradient likely. CTL may be less effective in recognizing HLA class I-presented foreign peptides, and macrophages, which are steady inhabitants of the pregnant uterus, will be selectively driven to produce specific, beneficial and appropriate cytokine sets.
Immunomodulation may be only one of many functions of HLA-G. Although recent reports of other actions are as yet unverified in more than one laboratory the family of HLA-G proteins may also influence angiogenesis in the decidua and vascularity in the placenta [43,44]. Further, there is considerable evidence mounting to suggest that HLA-G may be involved in other conditions of transplantation. For example, a recent report from Le Maux et al [45] indicates that in combination with Treg, soluble HLA-G molecules in circulating blood may improve stem cell transplantation.
Clinical implications
Multiple reports indicate that levels of HLA-G may predicate reproductive success [1–6]. As a consequence, fertility physicians are anxious to identify commercially available ELISA or other assays that will accurately report levels of HLA-G in the blood of patients with suboptimal fertility. The best known and most frequently employed at present is an ELISA from EXBIO Praha, a.s. (Czech Republic), which identifies β2m-associated HLA-G1 (cleaved from cell surfaces) and the soluble isoform, HLA-G5, when it is β2m-associated by using anti-β2m as a developing agent. We know from recent studies that free heavy chains of HLA-G5 are abundant in maternal blood but also have evidence that β2m-associated cleaved HLA-G1 or soluble β2m-associated G5 is likely to be present (D. Wheaton and J. S. Hunt, unpublished). Free HLA-G5 heavy chains as well as HLA-G2 and –G6, none of which bind β2m, would not be identified by the MEM-G/9 mAb used for “capture” in the ExBio ELISA. Our work discussed above would suggest that ELISA intended for diagnosis would most profitably focus on the soluble isoform, HLA-G5, and employ reagents that would identify both β2m-associated and non-associated heavy chains of this protein. Concurrent exploration of carriers of the 14bp deletion in the 3’untranslated region of the HLA-G gene might not be profitable in preeclampsia [46] but single base-pair mutations [47] as well as the G*0106 null allele [48] could be of interest in other adverse conditions of pregnancy, including diseases of infants and children [49].
The functional effects of systemic HLA-G circulating in mothers throughout pregnancy remain to be defined as all assays indicate concentration-dependent effects of these proteins, and levels are undoubtedly highest proximal to the placenta.
Perspectives
As HLA-G and the other two HLA class Ib genes and their proteins become better understood, it may be reasonably postulated that new aspects of immunity and tolerance will be revealed by studying these genes and their proteins within the natural condition of pregnancy. Many of these studies must be conducted in women although two primate models have been developed that may permit closing the cause and effect loop between lack of HLA-G and deficient reproductive capacity. The monkey and the baboon, while not perfect mirrors of human conditions, can certainly be used to conduct experiments that may reveal unsuspected functions [44,50].
In the interim, scientists can work toward learning whether diagnostic tests for aberrant levels of HLA-G can be improved with appropriate, well characterized reagents in accord with comments by Vercammen et al. [51] as well as Shaikly et al. [52] and whether treatment with recombinant proteins may improve fertility in some subsets of women. We expect the field of HLA-G biology, which supports its own highly successful bi-annual international meeting, to continue successfully drawing together workers in the field for idea exchange and thoughtful discussion.
Acknowledgements
The author expresses her gratitude for the many outstanding contributions of technical associates, graduate students, post-doctoral fellows and visiting scientists to this work. The studies are currently supported by grants from the National Institutes of Health to JSH (HD24212; HD04940).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt JS. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Bouteiller P, Tabiasco J, Parinaud J. Soluble HLA-G and embryo implantation: frequently asked questions. Gynecol Obstet Invest. 2007;64:134–137. doi: 10.1159/000101736. PMID: 17934308. [DOI] [PubMed] [Google Scholar]
- 3.Pistoia V, Morandi F, Wang X, Ferrone S. Soluble HLA-G: Are they clinically relevant? Semin Cancer Biol. 2007;17:469–479. doi: 10.1016/j.semcancer.2007.07.004. PMID: 17825579. PMCID: PMC2200630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol. 2008;29:125–132. doi: 10.1016/j.it.2007.11.005. PMID: 18249584. [DOI] [PubMed] [Google Scholar]
- 5.Carosella ED, HoWangYin KY, Favier B, LeMaoult J. HLA-G-dependent suppressor cells: Diverse by nature, function, and significance. Hum Reprod. 2008;69:700–707. doi: 10.1016/j.humimm.2008.08.280. PMID: 18817832. [DOI] [PubMed] [Google Scholar]
- 6.Baricordi OR, Stignani M, Melchiorri L, Rizzo R. HLA-G and inflammatory diseases. Inflamm Allergy Drug Targets. 2008;7:67–74. doi: 10.2174/187152808785107615. [DOI] [PubMed] [Google Scholar]
- 7.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 8.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136:373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCS12/stromal cell-derived factor-1. J Immunol. 2005;175:61–68. doi: 10.4049/jimmunol.175.1.61. [DOI] [PubMed] [Google Scholar]
- 10.Allan DS, McMichael AJ, Braud VM. The ILT family of leukocyte receptors. Immunobiol. 2000;202:34–41. doi: 10.1016/S0171-2985(00)80050-9. 65. [DOI] [PubMed] [Google Scholar]
- 11.Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64:215–225. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 12.Katz HR. Inhibition of inflammatory responses by leukocyte Ig-like receptors. Adv. Immunol. 2006;91:251–272. doi: 10.1016/S0065-2776(06)91007-4. [DOI] [PubMed] [Google Scholar]
- 13.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Maquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interactional functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. 2003;172:1376–1384. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 14.Ober C, Aldrich C, Rosinsky B, Robertson A, Walker MA, Willadsen S, Verp MS, Geraghty DE, Hunt JS. HLA-G1 protein expression is not essential for fetal survival. Placenta. 1998;19:127–132. doi: 10.1016/s0143-4004(98)90000-5. [DOI] [PubMed] [Google Scholar]
- 15.Hviid TV, Hylenius S, Rorbye C, Nielsen LG. HLA-G allelic variants are associated with differences in the HLA-G mRNA isoform profile and HLA-G mRNA levels. Immunogenet. 2003;55:63–79. doi: 10.1007/s00251-003-0547-z. [DOI] [PubMed] [Google Scholar]
- 16.Hviid TV, Christiansen OB, Johansen JK, Hvidd UR, Lundegaard C, Moller C, Morling N. Characterization of a new HLA-G allele encoding a nonconservative amino acid substitution in the alpha3 domain (exon 4) and its relevance to certain complications in pregnancy. Immunogenet. 2001;53:48–53. doi: 10.1007/s002510100296. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau P, Le Discorde M, Mouillot G, Marcou C, Carosella ED, Moreau P. The 14 bp deletion-insertion polymorphism in the 3' UT region of the HLA-G gene influences HLA-G mRNA stability. Hum Immunol. 2003;64:1005–1010. doi: 10.1016/j.humimm.2003.08.347. [DOI] [PubMed] [Google Scholar]
- 18.Hviid TV, Hylenius S, Rorbye C, Nielsen LG. HLA-G allelic variants are associated with differences in the HLA-G mRNA isoform profile and HLA-G mRNA levels. Immunogenet. 2003;55:63–79. doi: 10.1007/s00251-003-0547-z. [DOI] [PubMed] [Google Scholar]
- 19.Aldrich C, Verp MS, Walker MA, Ober C. A null mutation in HLA-G is not associated with preeclampsia or intrauterine growth retardation. J Reprod Immunol. 2000;47:41–48. doi: 10.1016/s0165-0378(00)00052-8. [DOI] [PubMed] [Google Scholar]
- 20.Ober C, Aldrich CL, Chervoneva I, Billstrand C, Rahimov F, Gray HL, Hyslop T. Variation in the HLA-G promoter region influences miscarriage rates. Am J Hum Genet. 2003;72:1425–1435. doi: 10.1086/375501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales PJ, Pace JL, Platt JS, Langat DL, Hunt JS. Synthesis of β2-microglobulin-free, disulfide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunol. 2007;122:179–188. doi: 10.1111/j.1365-2567.2007.02623.x. This article is the first to report that cytotrophoblast cells in normal placental villi are capable of producing HLA-G5 heavy chains and that these are β2m–ree due to low transcription and translation of the β2m gene in the cells.
- 22.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–1937. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Gonen-Gross T, Achdout H, Arnon TI, Gazit R, Stern N, Horejsi V, Goldman-Wohl, Yagel S, Mandelboim O. The CD84J/leukocyte inhibitory receptor-2 distinguishes between conformed and β2-microglobulin-free HLA-G molecules. J Immunol. 2005;175:4866–4874. doi: 10.4049/jimmunol.175.8.4866. [DOI] [PubMed] [Google Scholar]
- 25.Le Bouteiller P. Human villous trophoblast and the lack of intron 4-retaining soluble HLA-G secretion: beware of possible methodological biases. Mol Hum Reprod. 2005;11:711–713. doi: 10.1093/molehr/gah211. [DOI] [PubMed] [Google Scholar]
- 26.Hunt JS, Geraghty DE. Commentary. Soluble HLA-G isoforms, HLA-G5 and HLA-G6: technical deficiencies lead to misinterpretations. Mol Hum Reprod. 2005;11:715–717. doi: 10.1093/molehr/gah223. [DOI] [PubMed] [Google Scholar]
- 27.Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, Hunt JS. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol. 2003;171:6215–6224. doi: 10.4049/jimmunol.171.11.6215. [DOI] [PubMed] [Google Scholar]
- 28.Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol. 2000;183:682–688. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 29.Fainardi E, Rizzo R, Melchiorri L, Stignani M, Casdtellazzi M, Caniatti ML, Baldi E, Tola MR, Granieri E, Baricordi OR. Soluble HLA-G molecules are released as HLA-G5 and not as soluble HLA-G1 isoforms in CSF of patients with relapsing-remitting multiple sclerosis. Neuroimmunol. 2007;192:219–225. doi: 10.1016/j.jneuroim.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Hunt JS, Langat D, McIntire RH, Morales P. The role of HLA-G in human pregnancy. Reprod Biol Endocrinol Endocrinol. 4 Suppl 1:S10. doi: 10.1186/1477-7827-4-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Lagares A, Alegre E, Lemaoult J, Carosella ED, Gonzalez A. Nitric oxide produces HLA-G nitration and induces metalloprotease-dependent shedding creating a tolerogenic milieu. Immunol. 2008 doi: 10.1111/j.1365-2567.2008.02911.x. Epub ahead of print. PMID:18764882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veit TD, Chies JA. Tolerance versus immune response — Micro RNAs as important elements in the regulation of the HLA-G gene expression. Transpl Immunol. 2009;20:229–231. doi: 10.1016/j.trim.2008.11.001. PMID: 19038339. In this article the authors discuss the hypothesis that production of HLA-G may be regulated via miRNAs. Such control could impact certain pathologies of pregnancy as well as transplantation and cancer.
- 33.Hunt JS, Pace JL, Morales PJ, Ober C. Immunogenicity of the soluble isoforms of HLA-G. Mol Hum Reprod. 2003;9:729–735. doi: 10.1093/molehr/gag087. [DOI] [PubMed] [Google Scholar]
- 34.Petroff MG, Sedlmayr P, Azzola D, Hunt JS. Decidual macrophages are potentially susceptible to inhibition by class Ia and class Ib HLA molecules. J. Reprod Immunol. 2002;56:3–17. doi: 10.1016/s0165-0378(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 35.McCormick J, Whitley GS, LeBouteiller P, Cartwright JE. Soluble HLA-G regulates motility and invasion of the trophoblast-derived cell line SGHPL-4. Hum Reprod. 2009 doi: 10.1093/humrep/dep026. Epub ahead of print, PMID 19223288. [DOI] [PubMed] [Google Scholar]
- 36.Guillard C, Zidi I, Marcou C, Menier C, Carosella ED, Moreau P. Role of HLA-G in innate immunity through direct activation of NK-κB in natural killer cells. Mol Immunol. 2008;45:419–427. doi: 10.1016/j.molimm.2007.06.160. PMID: 17675239. This article challenges the concept that HLA-G binding to LILRB1 (ILT-2) expressed by NK cells leads to inhibition of NK activation. The evidence includes experiments showing that HLA-G induces the phosphorylation and degradation of IκBα, thus permitting NFκB movement to the nucleus to facilitate transcription.
- 37.Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, Bensussan A, Le Bouteiller P. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164:6100–6104. doi: 10.4049/jimmunol.164.12.6100. [DOI] [PubMed] [Google Scholar]
- 38.Zhong M, Weng X, Liang Z, Lu S, Li J, Chen X, Li Q, Sun W, Song Y, Shen G, Wu X. Dimerization of soluble HLA-G by IgG-Fc fragment augments ILT-2-mediated inhibition of T-cell alloresponse. Transplant. 2009;87:8–15. doi: 10.1097/TP.0b013e31818b6141. PMID 19136885. Here, the authors provide support for the idea that dimers of HLA-G are more active than monomers in inhibiting T cell responses. Studies show that soluble HLA-G dimers reduce both CD4+ and CD8+ T cells and suppress CTL generation.
- 39.Gros F, Cabillic F, Toutirais O, Maux AL, Sebti Y, Amiot L. Soluble HLA-G molecules impair natural killer/dendritic cell crosstalk via inhibition of dendritic cells. Eur J Immunol. 2008;38:742–749. doi: 10.1002/eji.200736918. PMID:18266268. This article is the first to propose that soluble HLA-G exerts its inhibitory effects on NK cells via interaction with dendritic cells. The studies were done using differentiated monocytes, which showed decreases of CD80 as well as HLA-DR expression and IL-12 production following exposure to soluble HLA-G.
- 40.Smith M, Bittner JG, White S, Smith D, Horuzsko A. HLA-G-treated tolerogenic dendritic cells induce tolerogenic potential by increasing expression of B7-1 (CD80) molecules. Transplant Proc. 2008;40:1598–1603. doi: 10.1016/j.transproceed.2008.01.062. PMID 18589158. This article defines the concept that HLA-G plays a critical role in reproduction by regulating B7 family members exhibited by dendritic cells, thus modulating antigen presentation. Rat dendritic cells cultured from bone marrow showed decreased alloproliferative responses and decreased CD86 but not altering MHC class II expression following treatment with soluble HLA-G.
- 41.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6-STAT3 signaling pathway. Proc Natl Acad Sci USA. 2008;105:8357–8362. doi: 10.1073/pnas.0803341105. PMID: 18550825. This is one of two articles exploring the signal transduction pathways by which HLA-G exhibits its tolerogenic effects. Dendritic cells express LILRB2 (ILT4), which HLA-G recognizes, activating an interleukin-6 signaling pathway and STAT3.
- 42.Yu Y, Wang Y, Feng M. Human leukocyte antigen-G1 inhibits natural killer cytotoxicity through blocking the activating signal transduction pathway and formation of activating immunologic synapse. Hum Immunol. 2008;69:16–23. doi: 10.1016/j.humimm.2007.11.005. PMID: 18295671. This second article explores signal transduction pathways in NK cells. The authors report that HLA-G1 engagement blocks a MEK/ERK activating signaling pathway in activated NK cells. Taken together the two articles suggest that pathway is defined by cell type.
- 43.Le Bouteiller P, Fons P, Herault JP, Bono F, Chabot S, Cartwright JE, Bensdussan A. Soluble HLA-G and control of angiogenesis. J Reprod Immunol. 2007;76:17–22. doi: 10.1016/j.jri.2007.03.007. [DOI] [PubMed] [Google Scholar]; The idea that HLA-G may have multiple functions that include control of angiogenesis in the decidua and placenta is discussed in this thoughtful review.
- 44.Bondarenko GI, Burleigh DW, Durning M, Breburda EE, Grendell RL, Golos TG. Passive immunization against the MHC class I molecule Mamu-AG disrupts rhesus placental development and endometrial responses. J Immunol. 2007;179:8042–8050. doi: 10.4049/jimmunol.179.12.8042. In this paper, evidence for disruption of normal placental and decidual vessels by administration of anti-HLA-G in monkeys is shown. Thus, an HLA-G-like molecule has, in this primate, functions other than induction of tolerance.
- 45.Le Maux A, Noel G, Birebent B, Grosset JM, Vu N, De Guilbert S, Bernard M, Semana G, Amiot L. Soluble human leukocyte antigen-G molecules in peripheral blood haematopoietic stem cell transplantation: a specific role to prevent acute graft-versus-host disease and a link with regulatory T cells. Clin Exp Immunol. 2008;152:50–56. doi: 10.1111/j.1365-2249.2008.03598.x. PMCID:PMC2384077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iverson AC, Nguyen OT, Tommerdal LF, Eide IP, Landsem VM, Acar N, Myhre R, Klungland H, Austgulen R. The HLA-G 14 bp gene polymorphism and decidual HLA-G 14bp gene expression in pre-eclamptic and normal pregnancies. J Reprod Immunol. 2008;7:158–165. doi: 10.1016/j.jri.2008.03.001. PMID 18423887. [DOI] [PubMed] [Google Scholar]
- 47.Yie SM, Li LH, Xiao R, Librach CL. A single base-pair mutation in the 3’untranslated region of HLA-G mRNA is associated with pre-eclampsia. Mol Hum Reprod. 2008;14:649–653. doi: 10.1093/molehr/gan059. PMID: 18952696. [DOI] [PubMed] [Google Scholar]
- 48.Moreau P, Contu L, Alba F, Lai S, Simoes R, Orru S, Carcassi C, Roger M, Rabreau M, Carosella ED. Carosella HLA-G: gene polymorphism in human placentas: possible association of G*0106 allele with preeclampsia and miscarriage. Biol Reprod. 2008;79:459–467. doi: 10.1095/biolreprod.108.068874. PMID: 18509163. [DOI] [PubMed] [Google Scholar]
- 49.Kim JJ, Hong SJ, Hong YM, Kim S, Kang MJ, Kim KJ, Seo EJ, Yoo HW, Cheong HS, Shin HD, Park IS, Lee JK. Genetic variants in the HLA-G region are associated with Kawasaki disease. Hum Immunol. 2008;69:867–871. doi: 10.1016/j.humimm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Langat DK, Morales PJ, Fazleabas AT, Mwenda JM, Hunt JS. Potential regulatory sequences in the untranslated regions of the baboon MHC class Ib gene, Paan-AG, more closely resemble those in the human MHC class Ia genes than those in the class Ib gene, HLA-G. Immunogenet. 2004;56:657–666. doi: 10.1007/s00251-004-0727-5. [DOI] [PubMed] [Google Scholar]
- 51.Vercammen MJ, Verioes A, Van de Velde H, Haentjens P. Accuracy of soluble human leukocyte antigen-G for predicting pregnancy among women undergoing fertility treatment: metanalysis. Hum Reprod Update. 2008;14:209–218. doi: 10.1093/humupd/dmn007. Epub ahead of print. These authors thoughtfully review the evidence for associations between expression of HLA-G by preimplantation embryos and successful treatment for fertility. Their conclusion is that more research is needed
- 52.Shaikly VR, Morrison IE, Taranissi M, Noble CV, Withey AD, Cherry RJ, Blois SM, Fernandez N. Analysis of HLA-G in maternal plasma, follicular fluid and preimplantation embryos reveal an asymmetric pattern of expression. J Immunol. 2008;180:4330–4337. doi: 10.4049/jimmunol.180.6.4330. PMID: 18322247. Evidence presented by this group is consistent with the idea that HLA-G is involved in preimplantation embryo development. The authors affirm the conclusion of Vercammen et al. that more studies are needed to decide on the value of embryo screening and selection.