Abstract
Purpose
Recent research indicates that intraocular pressure (IOP) does not decrease significantly during the nocturnal period, although aqueous humor flow decreases by 50% or more at night. This study was undertaken to investigate whether changes in outflow facility, episcleral venous pressure, or uveoscleral flow at night could account for the nocturnal IOP.
Methods
Sixty-eight eyes of 34 healthy subjects (age, 18–44 years; mean, 29) were studied. Aqueous humor flow rate, IOP, and outflow facility were measured with pneumatonometry, anterior chamber fluorophotometry, and Schiotz tonography respectively, in each eye during the mid-diurnal (2–4 PM) and mid-nocturnal (2–4 AM) periods. Nocturnal IOP, flow rate, and outflow facility were compared to the same variables during the diurnal period. Mathematical models based on the modified Goldmann equation were used to assess the conditions under which these results could be reconciled.
Results
Supine IOP decreased slightly from 18.9 ± 2.7 mm Hg in the mid-diurnal period to 17.8 ± 2.5 mm Hg in the mid-nocturnal period (mean ± SD, P = 0.001). Aqueous flow rate decreased from 2.26 ± 0.73 to 1.12 ± 0.75 µL/min (mean ± SD, P < 0.001). There was a nonsignificant trend toward a nocturnal decrease of outflow facility (diurnal, 0.27 ± 0.11 µL/min/mm Hg; nocturnal, 0.25 ± 0.08 µL/min/mm Hg; mean ± SD, P = 0.13).
Conclusions
Outflow facility measured by tonography does not decrease enough during the nocturnal period to compensate for the decreased aqueous humor flow rate. Modeling results indicate that the experimental results could be reconciled only if nocturnal changes in episcleral venous pressure and/or uveoscleral flow occurred.
Intraocular pressure (IOP) when measured in the habitual positions (sitting while awake and supine during sleep) is highest during sleep for most patients.1–5 When combined with the normal drop in systemic blood pressure during sleep,6,7 this elevated IOP may compromise optic nerve head perfusion in susceptible individuals. Nocturnal IOP elevation may play a more important role in glaucomatous progression in some cases than previously has been appreciated. However, the reason that IOP is elevated at night is unclear, because aqueous production decreases significantly during the same period.8–10 Even when position changes are eliminated, IOP does not drop enough at night to account for the decrease in aqueous humor production.1–5
The modified Goldmann equation relates the variables that determine steady state IOP, including conventional (pressure dependent) and uveoscleral (essentially pressure independent) aqueous outflow rates, aqueous outflow facility, and episcleral venous pressure (EVP). Our goal in this study was to investigate the circadian variation of aqueous outflow facility and its role in determining nocturnal IOP. We also investigated the conditions under which experimentally measured circadian changes in IOP, aqueous flow rate, and outflow facility could be reconciled.
METHODS
Study Subjects
Subjects were recruited from volunteers who were students or employees of Mayo Clinic or local area residents. Subjects included men and women in good general health, between the ages of 18 and 45 years, with regular sleep schedules. Patients with chronic disease (e.g., hypertension) were included as long as the condition was stable and did not require treatment other than medications. Patients using systemic steroids or β-adrenergic blockers were excluded, as were subjects older than 45 years or with any history of ocular disease including intraocular surgery or trauma, narrow angles, glaucoma, diabetic eye disease, uveitis, high myopia (>6 D), or high hyperopia (>4 D). Each subject gave informed consent to participate after explanation of the nature and possible consequences of the study. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at Mayo Clinic.
Sample Size
The sample size was estimated to detect a 20% difference between diurnal and nocturnal outflow facilities. Assuming a diurnal outflow facility of 0.24 ± 0.08 µL/min/mm Hg (mean ± SD), similar to that of other healthy subjects,10–13 we estimated that 35 subjects would have to have a 90% chance of detection of a 20% difference between diurnal and nocturnal outflow facility (paired t-test, α = 0.05, β = 0.10).
Measurements
In this study, IOP, aqueous flow rate, and outflow facility were measured or calculated. Comparisons between the diurnal and nocturnal periods were performed to assess circadian changes.
IOP was measured with a pneumatonometer (model 30 Classic; Medtronic Ophthalmics, Jacksonville, FL) in participants in the sitting and supine positions after anesthesia with topical proparacaine 0.5% Subjects were kept sitting or supine for at least 5 minutes before measurement in those positions. The mean of three consecutive measurements was recorded as the IOP.
Aqueous flow rate through the anterior chamber was calculated from the rate of disappearance of fluorescein from the combined cornea and anterior chamber. In brief,
| (1) |
where ΔMf is the mass of fluorescein lost from the combined cornea and anterior chamber during the interval Δt, is the average concentration of fluorescein in the anterior chamber during the same interval (estimated from the initial and final concentrations assuming an exponential decay), and 0.25 µL/min is the assumed flow rate equivalent to the diffusional loss of fluorescein from the eye. Diffusional loss of fluorescein has been shown to be a small fraction (<10%) of the total fluorescein loss, with the bulk flow accounting for the remainder.14–17 The mass of fluorescein in the anterior chamber and cornea was calculated by multiplying the mean concentration in each compartment by its volume. Fluorescein concentrations were measured by using a scanning ocular fluorophotometer.18–20 Anterior chamber volume was measured photogrammetrically,21 and cornea volume was assumed to be 70 µL in all subjects.22,23
Aqueous outflow facility was measured by an electronic Schiotz tonometer (Berkeley Bio-Engineering, Inc., San Leandro, CA) with a 4-minute tracing and was calculated from the pressure decay curves and standard tables.24 Facility was measured at the end of the mid-diurnal and mid-nocturnal periods. Topical proparacaine 0.5% was used for anesthesia before tonography.
Experimental Protocol
The subjects were given a complete dilated eye examination, and an updated medical history was recorded if one had not been recorded within the past 12 months. One week before the study session, the subjects were asked to maintain a regular sleep cycle of 8 hours asleep and 16 hours awake. They were equipped with a wrist actigraph to monitor physical activity (Actiwatch AW-L; Mini Mitter, Bend, OR) and were also asked to maintain a log with sleep and awake times. Subjects’ sleep patterns were used to adjust the actual time of the measurements during the study, so that the nocturnal period for each subject was equivalent to 11 PM to 7 AM. Contact lens wearers were asked to stop wearing their contact lenses 1 week before the study.
On the day of the study, the subjects were asked to maintain a regular schedule with normal activities. The subjects instilled 3 to 5 drops of topical 2% fluorescein in each eye between 1 and 2 AM on the study day. The number of drops was varied by age, to produce the optimal anterior chamber fluorescein concentrations. Subjects 25 years and less received 5 drops; 26 to 35 years received 4 drops; and over 35 years received 3 drops. Subjects carefully cleaned excess fluorescein from their lashes and lid margins before returning to sleep.
Subjects reported to the Mayo Clinic General Clinical Research Center (GCRC) at 1 PM on the day of the study. They were permitted to leave the GCRC for brief periods but were asked to remain in the hospital. Food and water were given ad libitum, and the subjects could continue with normal activities while at the GCRC, except that they were asked to refrain from exercise.
At the beginning of the mid-diurnal period (2 PM), fluorescein concentration was measured in the anterior segment of each eye, followed by IOP measurement of each eye. These measurements were repeated at the end of the mid-diurnal period (4 PM) and were followed by 4-minute Schiotz tonography. Immediately after tonography, the subjects instilled another 2 to 5 drops of topical 2% fluorescein in each eye, depending on the measured anterior chamber fluorescein concentration. After cleaning the excess fluorescein from the lashes and lid margins, the subjects lay supine for 20 to 30 minutes with eyes closed, to ensure an even distribution of the fluorescein. They were asked to go to sleep at their regular times based on their sleep patterns from the previous week.
The subjects were awakened at the beginning of the mid-nocturnal period (2 AM) for a third set of fluorophotometry and IOP measurements. At the end of the mid-nocturnal period (4 AM), anterior segment fluorescence and IOP were measured a fourth and final time, followed by a second 4-minute Schiotz tonography. During the nocturnal period, IOP and facility measurements were performed under reduced light conditions (~10 lux white light). A previous report indicates that moderate light exposure at night does not affect the nocturnal IOP pattern.25 Fluorophotometry was performed in a darkened room, but the anterior segment was briefly illuminated with the scanning laser. Total time for all measurements in the mid-nocturnal period was 20 to 25 minutes.
Statistical Analysis
Mean IOP, aqueous flow rates and, outflow facility from the mid-diurnal period were compared with values from the mid-nocturnal period. All eyes were used to determine the means, and diurnal and nocturnal data were compared by using generalized estimating equation models to account for potential correlation between the two eyes of each subject. Differences were considered significant at P < 0.05. The diurnal versus nocturnal analysis was repeated for two subgroups: age ranges, 18 to 29 and 30 to 45 years. As a result of our recruitment methods, the sample population was skewed toward the younger end of the eligibility spectrum. This subgroup analysis was undertaken to ensure that our results were not adversely affected by this bias. The results from the subgroups were compared with each other and the group as a whole.
EPV and Mathematical Models
Mid-diurnal and mid-nocturnal EVP was calculated by using the modified Goldmann equation
| (2) |
where Q is the aqueous humor flow rate, c is the outflow facility, U is the pressure-independent uveoscleral outflow rate, Pi is the IOP, and Pe is the EVP. This calculation was modified as:
| (3) |
where u is the fraction of flow through a pressure independent uveoscleral pathway. Since the correct value of u is not known, we calculated Pe for uveoscleral flow fractions ranging from 0 to 1.0.
The modified Goldmann equation was also used to create three mathematical models to reconcile circadian changes in IOP and aqueous humor flow rate. In the first model, we calculated circadian changes in outflow facility, assuming that EVP and the uveoscleral flow fraction remained constant. From equation 3, outflow facility can be calculated as:
| (4) |
Based on equation 4, the fractional change in outflow facility from day to night necessary to produce the measured changes in IOP and aqueous humor flow is given by
| (5) |
where Pe is calculated from equation 3 and c, Q, and Pi are the diurnal facility, aqueous humor flow rate, and IOP, respectively, and c′, Q′, and are the nocturnal facility, aqueous humor flow rate, and IOP, respectively. Experimentally measured IOP and aqueous humor flow rate data were used in this model.
In the second model, we examined the circadian changes in EVP necessary to reconcile diurnal-to-nocturnal changes in IOP and aqueous humor flow rate. Based on equation 3, the fractional change in EVP from day to night is given by
| (6) |
where c, Q, Pi, and Pe are the diurnal facility, aqueous humor flow, IOP, and EVP respectively, and c′, Q′, , and are the nocturnal facility, aqueous humor flow, IOP, and EVP, respectively. The uveoscleral flow fraction was assumed to remain constant throughout the circadian cycle. Experimentally measured IOP, aqueous humor flow rate, and outflow facility data were used in this model.
In the third model, we examined whether circadian changes in uveoscleral flow fraction could reconcile diurnal-to-nocturnal changes in IOP and aqueous humor flow rate, assuming that EVP remained constant. From equation 3, the uveoscleral flow fraction can be calculated as:
| (7) |
The diurnal-to-nocturnal change in uveoscleral flow fraction is given by
| (8) |
where u, c, Q, and Pi, are the diurnal uveoscleral flow fraction, outflow facility, aqueous flow rate, and IOP, respectively, and u′, c′, Q′, and are the nocturnal uveoscleral flow fraction, outflow facility, aqueous flow rate, and IOP, respectively, and Pe is calculated from equation 3 Experimentally measured IOP, aqueous humor flow rate, and outflow facility data were used in this model.
Using these models, we examined the diurnal-to-nocturnal changes in outflow facility, EVP, and uveoscleral flow fraction necessary to produce the measured circadian changes in IOP and aqueous humor flow rate.
RESULTS
Experimental Results
Thirty-five subjects were enrolled in the study, but the data from one subject were eliminated because equipment failure prevented recording of the nocturnal data. Measurements for 68 eyes from 34 subjects were analyzed. The ages of the subjects ranged from 18 to 44 years (28.9 ± 8.0, mean ± SD). The ages of subjects in the two subgroups ranged from 18 to 28 years (23.1 ± 2.6) and 30 to 44 years (37.1 ± 5.2). The characteristics of each group are shown in Table 1. Although there was a trend toward higher myopia, thinner central cornea thickness, and lower clinic IOP in the younger group (18–29 years) compared with the older group (30–45 years), there were no statistically significant differences in any of the baseline characteristics between the groups (P > 0.05). None of the subjects were using systemic antihypertensive medications. The most common medication was oral contraceptive pills, used by six subjects. One subject was using salbutamol inhalers as required for asthma, and one subject was using rizatriptan benzoate for migraine headache as required, but neither subject used these medications during the study measurements.
TABLE 1.
Study Population Characteristics
| Group | Age (y) |
Refraction (D) |
CCT (µm) |
Clinic IOP (mm Hg) |
|---|---|---|---|---|
| All Ages (n = 68 eyes) | 28.9 ± 8.0 | − 1.64 ± 1.57 | 548 ± 28 | 13.2 ± 2.6 |
| 18–29 y (n = 40 eyes) | 23.1 ± 2.6 | − 1.93 ± 1.44 | 541 ± 30 | 12.8 ± 30 |
| 30–45 y (n = 28 eyes) | 37.1 ± 5.2 | − 1.24 ± 1.68 | 558 ± 21 | 13.6 ± 1.8 |
Data are expressed as the mean ± SD.
IOP measured in subjects in the sitting position declined slightly from a mid-diurnal pressure of 138 ± 2.3 mm Hg to a mid-nocturnal pressure of 12.9 ± 2.4 mm Hg (mean ± SD, P = 0.02). Diurnal-to-nocturnal changes were similar in the two subgroups, although the change was not statistically significant in the younger group (Table 2).
TABLE 2.
Experimental Results for Diurnal and Nocturnal IOP, Aqueous Flow Rate, and Outflow Facility
| Sitting IOP (mm Hg) |
Supine IOP (mm Hg) |
Aqueous Flow Rate (µL/min) |
Outflow Facility (µL/min/mm Hg) |
|
|---|---|---|---|---|
| All Ages | ||||
| Day | 13.8 ± 2.3 | 18.9 ± 2.7 | 2.26 ± 0.73 | 0.27 ± 0.11 |
| Night | 12.9 ± 2.4 | 17.8 ± 2.5 | 1.12 ± 0.75 | 0.25 ± 0.08 |
| P | 0.02 | 0.001 | <0.001 | 0.13 |
| 18–29 y | ||||
| Day | 13.8 ± 1.9 | 18.7 ± 2.4 | 2.21 ± 0.70 | 0.27 ± 0.12 |
| Night | 131 ± 2.5 | 17.6 ± 2.4 | 1.08 ± 0.75 | 0.25 ± 0.09 |
| P | 0.18 | 0.03 | <0.001 | 0.42 |
| 30–45 y | ||||
| Day | 139 ± 2.8 | 19.2 ± 2.9 | 2.33 ± 0.76 | 0.28 ± 0.09 |
| Night | 12.7 ± 2.4 | 18.0 ± 2.7 | 1.18 ± 0.77 | 0.25 ± 0.08 |
| P | 0.05 | 0.006 | <0.001 | 0.10 |
Data are expressed as the mean ± SD.
Intraocular pressure measured in the supine position also declined slightly from 18.9 ± 2.7 mm Hg in the mid-diurnal period to 17.8 ± 2.5 mm Hg in the mid-nocturnal period (mean ± SD, P = 0.001). Changes were similar and significant in both subgroups (Table 2).
The aqueous humor flow rate decreased by approximately 50% between the mid-diurnal and the mid-nocturnal periods. During the mid-diurnal period, the mean flow rate was 2.26 ± 0.73 µL/min, whereas during the mid-nocturnal period, it was 1.12 ± 0.75 µL/min (mean ± SD, P < 0.001). Both subgroups had similar results, with significant differences between diurnal and nocturnal measurements (Table 2).
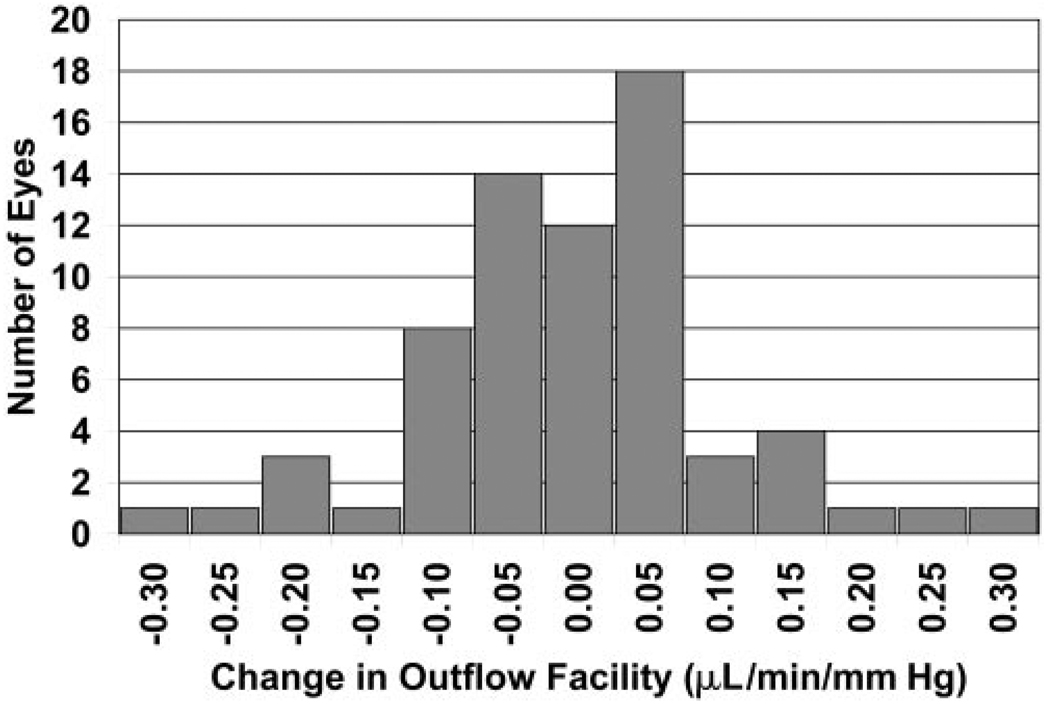
The mean outflow facility of all 68 eyes was 0.27 ± 0.11 µL/min/mm Hg during the day and 0.25 ± 0.08 µL/min/mm Hg (mean ± SD) during the night (Table 2). The diurnal-to-nocturnal changes in outflow facility were approximately Gaussian in distribution (Fig. 1), but the mean difference was not significantly different from 0 (P = 0.13). The two subgroups had similar results for diurnal and nocturnal outflow facilities (Table 2).
FIGURE 1.
Histogram of the experimentally measured diurnal-to-nocturnal outflow facility change in individual eyes, showing an approximately Gaussian distribution (mean ± SD = −0.02 ± 0.10 µL/min/mm Hg, n = 68, P = 0.13).
For each of the measured parameters, there were statistically significant correlations between right and left eyes, except for aqueous flow rate measured at the mid-nocturnal period (mid-diurnal: IOP sitting r = 0.74 P < 0.001, IOP supine r = 0.77 P < 0.001, flow r = 0.75 P < 0.001, facility r = 0.81 P < 0.001; mid-nocturnal: IOP sitting r = 0.70 P < 0.001, IOP supine r = 0.70 P < 0.001, flow r = −0.04 P = 0.82, facility at 4 AM r = 0.52, P = 0.002). These results highlight the need for the generalized estimating equation models used in the statistical analysis.
Results of Mathematical Models
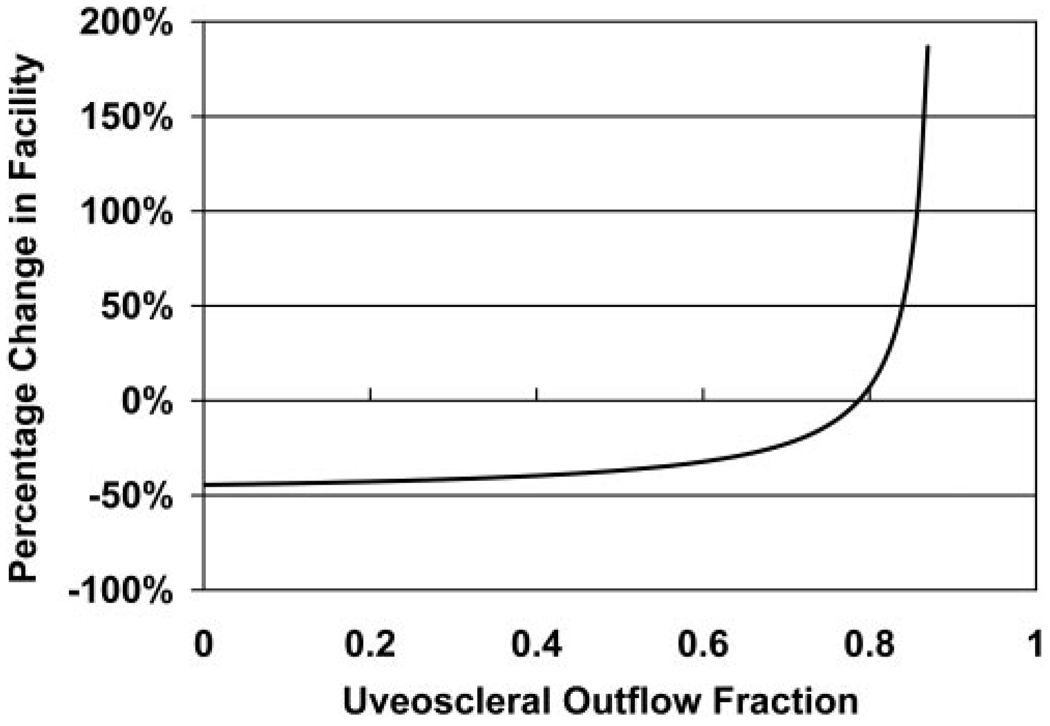
The mathematical model result for the nocturnal change in outflow facility necessary to reconcile the measured IOP and aqueous flow results (equation 4 and equation 5) is shown in Figure 2. For a nocturnal outflow facility change of 10% or less, similar to the small but nonsignificant change measured experimentally, the uveoscleral flow fraction ranged from 76% to 81%. Within this range, our model suggests that the circadian variations in IOP could be reconciled with the circadian variations in aqueous humor flow with little or no change in outflow facility. However, for uveoscleral flow fractions of 50% or less, the nocturnal decrease in outflow facility needed to be 37% to 44% to be compatible with the experimentally measured IOP and aqueous flow rate.
FIGURE 2.
The diurnal-to-nocturnal change in outflow facility necessary to reconcile the experimentally measured IOP and aqueous flow rate results, assuming constant uveoscleral outflow fractions ranging from 0 to 0.85.
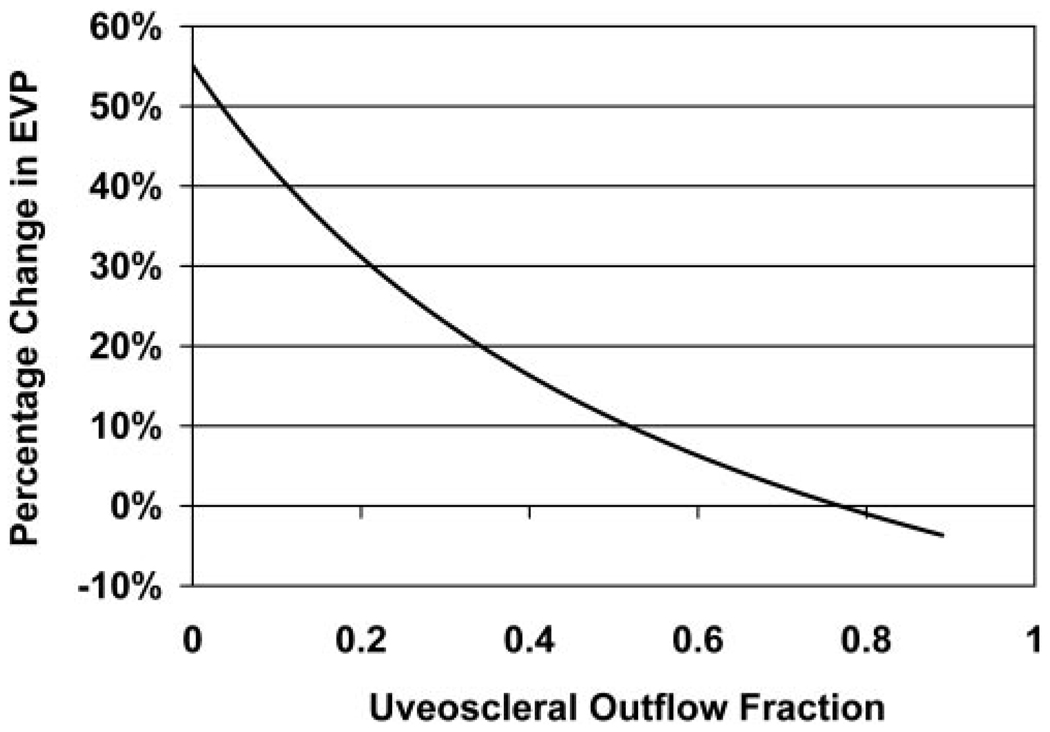
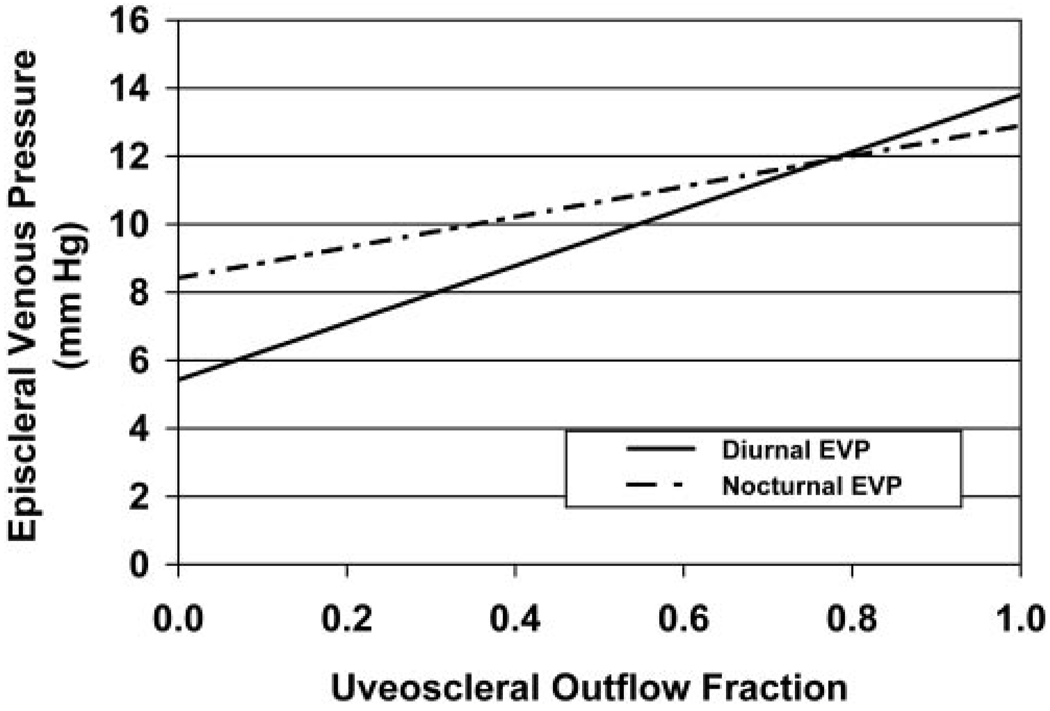
Our model for nocturnal change in EVP necessary to reconcile the measured IOP and aqueous flow rate changes (equation 6) is shown in Figure 3. Relatively small changes in nocturnal EVP produced the observed IOP pattern. For uveoscleral flow fractions of 10% to 75%, EVP changes of 1% to 42% produced the experimentally measured circadian IOP pattern. Within this range of uveoscleral flow fraction, the EVP calculated using equation 3 varied from 6.3 to 11.7 mm Hg during the day and 8.9 to 11.8 mm Hg during the night (Fig. 4).
FIGURE 3.
The diurnal-to-nocturnal change of EVP necessary to reconcile the experimentally measured IOP and aqueous flow rate results, assuming constant uveoscleral outflow fractions ranging from 0 to 0.90.
FIGURE 4.
Calculated EVPs during the diurnal and nocturnal periods using measured IOP, aqueous flow rate, and outflow facility, assuming constant uveoscleral flow fractions ranging from 0 to 1.
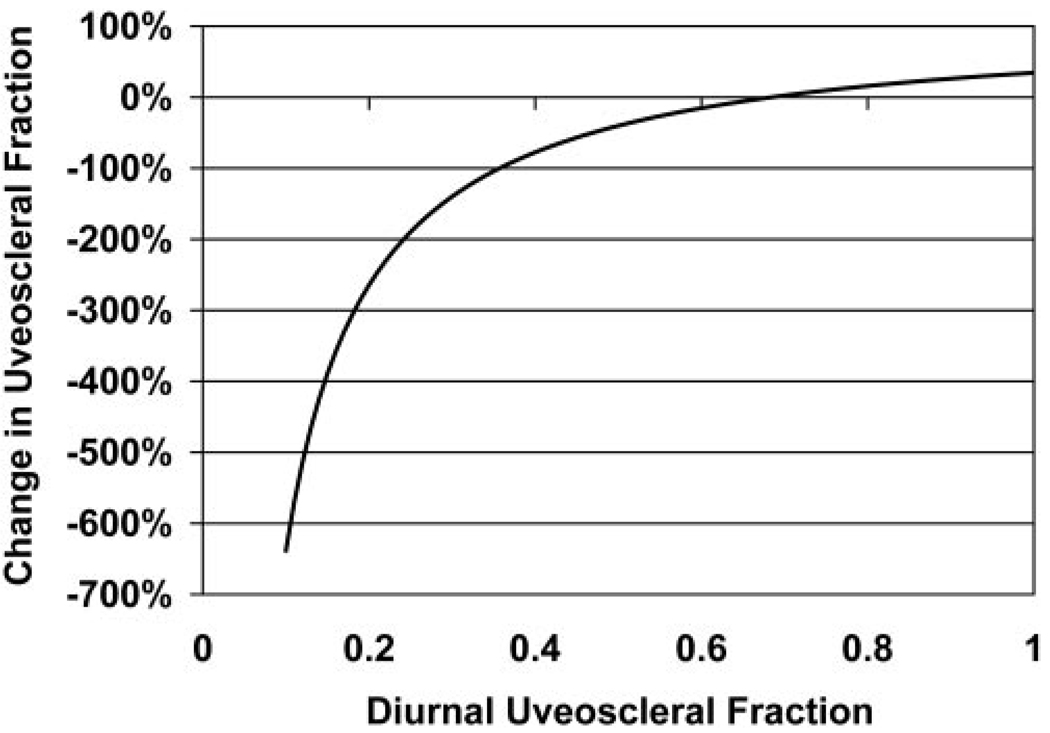
The results of our model of nocturnal changes in uveoscleral flow fraction (equation 7 and equation 8) are shown in Figure 5. To be compatible with the measured IOP, aqueous flow rate and outflow facility changes, the model indicated that diurnal uveoscleral flow fraction (u) had to be at least 36%. Below this level, the nocturnal decrease of the uveoscleral flow fraction had to be greater than 100%. For diurnal uveoscleral flow fractions from 36% to 100%, the nocturnal change ranged from a decrease of 100% to an increase of 35%.
FIGURE 5.
The diurnal-to-nocturnal change in uveoscleral outflow fraction necessary to reconcile the experimentally measured IOP and aqueous flow rate for diurnal uveoscleral outflow fractions ranging from 0.1 to 1.0, assuming constant EVP.
DISCUSSION
Diurnal variation in IOP has been well documented, starting with the work of Drance in the 1960s.26 In subsequent work, investigators examined the 24-hour pattern of IOP variation and found that patients with glaucoma exhibit a decrease in IOP during the nocturnal period.27,28 However, in these studies IOP was measured by Goldmann applanation tonometry with the subjects in the sitting position. Liu et al.1,2,5 demonstrated that IOP measured in the habitual positions (sitting while awake and supine while asleep) was significantly higher during the nocturnal period than the diurnal period. When IOP was measured in the supine position only, patients with glaucoma showed a slight decrease in IOP at night,5 whereas healthy subjects showed a slight increase.1,2 In our study, we found a slight decrease in nocturnal IOP compared with diurnal IOP. Although we examined healthy subjects in our study, the results are consistent with previous reports, since we measured at only four time points and may not have captured the peak diurnal or peak nocturnal pressures. In addition, our subjects were mostly moderate myopes, and Liu et al.3 demonstrated that myopes have a slightly lower nocturnal IOP when measured in the supine position.
The nocturnal elevation of IOP, or even slight decrease in nocturnal IOP, is a paradox considering the 50% or greater decrease in aqueous humor flow at night, which was consistent with previous results.8 This paradox could be explained in two ways. First, as indicated by the Goldmann equation (equation 2), IOP can be influenced by outflow facility, aqueous humor flow rate, pressure-independent uveoscleral flow, and EVP. Nocturnal changes in any of these variables can determine IOP. A second set of possibilities is that nocturnal measurements of aqueous dynamics parameters do not accurately reflect the sleeping state.
Concerning the first set of possibilities, we examined outflow facility and found that there was a trend toward a small decrease during the nocturnal period, but the change was not statistically significant. It is possible that a real decrease occurs, but the difference between the diurnal and nocturnal measurements was smaller than the detection threshold for the sample size of this study. Nevertheless, the change would be too small to compensate for the change in aqueous flow under virtually all conditions, as indicated by our mathematical models based on the modified Goldmann equation (Fig. 2). We found that the uveoscleral flow fraction had to be 76% to 81% to reconcile the experimentally measured IOP, aqueous flow rate, and outflow facility. These values are substantially higher than any previously reported for experimentally measured or calculated uveoscleral outflow.29,30 Therefore, nocturnal changes in outflow facility alone are not likely to account for the observed 24-hour IOP pattern.
Using our mathematical models, we also examined the possibility that changes in EVP could explain the observed IOP pattern. Measurements of EVP in humans are difficult and have poor reproducibility. Mean EVP in healthy human eyes has been reported between 7 and 14 mm Hg, with values between 8 and 11 mm Hg being most common.31 Our mathematical models indicated that relatively modest increases in EVP from the diurnal to the nocturnal period could account for the observed circadian IOP pattern (Fig. 3). In addition, the calculated EVP for uveoscleral flow fractions between 10% and 75% during both the diurnal and nocturnal periods (Fig. 4) was consistent with reported values. This indicates that the observed 24-hour IOP pattern could plausibly be the result of changes in EVP.
We also investigated the possibility that diurnal-to-nocturnal changes in uveoscleral flow fraction could produce the observed IOP pattern. However, the diurnal uveoscleral flow fraction had to be at least 36%, at which point a 100% drop from the diurnal-to-nocturnal period was necessary to produce the observed IOP pattern (Fig. 5). For a more reasonable 50% decrease in nocturnal flow fraction, a diurnal uveoscleral flow fraction of 47% would be required. This value is at the upper limit of reported values for uveoscleral flow calculated from measurements of IOP, aqueous flow, and EVP. Townsend and Brubaker32 calculated a uveoscleral fraction of 36% in healthy subjects, assuming a fixed EVP of 8 mm Hg. Toris et al.30 calculated a uveoscleral fraction of 54% in younger healthy subjects, and 46% in older healthy subjects, assuming that EVP remained unchanged under treatment with timolol, betaxolol, or acetazolamide. Results from our mathematical model are also significantly greater than reported values for directly measured uveoscleral flow.29 Direct measurement of uveoscleral outflow in living humans has been reported only once in the literature. In a study by Bill and Phillips,33 experiments were performed on patients in whom eyes were to be enucleated for choroidal melanoma. Radiolabeled albumin was perfused into the anterior chamber followed by a washout with inactive fluid. Uveoscleral flow was calculated based on the amount of radioactive material recovered in the ocular and episcleral tissues. This technique showed a uveoscleral flow fraction ranging from 0% to 27%, with a range of 4% to 14% in eyes without medical treatment. This result along with the results of our mathematical model suggest that it is possible, but unlikely, that a change in uveoscleral flow alone produces the observed 24-hour IOP pattern.
It is possible that multiple mechanisms are involved in the circadian IOP change. In two of our models, we assumed that the pressure-independent uveoscleral flow was a constant fraction of the total aqueous flow. If the uveoscleral flow were instead a constant fixed volume per unit of time, the effect would be an increase in uveoscleral fraction during the nocturnal period. If this occurred, an even greater change in EVP and/or outflow facility would be necessary to account for the diurnal-to-nocturnal change in IOP and aqueous flow rate. Conversely, if the uveoscleral flow fraction decreased at night, the necessary change in EVP and/or facility would be less.
A second set of possible explanations for the observed circadian IOP and aqueous flow patterns is that the experimental measurements in the nocturnal period do not accurately reflect the sleeping state. Of the three parameters measured directly in this study, aqueous flow measurements are most likely to reflect accurately both the sleeping and waking states. In our protocol, mid-nocturnal fluorophotometry scans were performed at the beginning and end of a 2-hour interval, during which subjects were sleeping. The calculated aqueous flow rate is an average for this time interval and not a measurement of instantaneous flow rate at the time of the scans themselves. Therefore, the aqueous flow rate calculations reflect the sleeping state and our results are consistent with previous studies of nocturnal aqueous flow.8,15 Furthermore, previous studies have indicated that the decrease in aqueous production at night is related to circadian rhythm and not body position or eyelid closure, with nocturnal decreases in aqueous flow occurring even in awake, sleep-deprived subjects.34
The question of whether nocturnal IOP and outflow facility measurements reflect the sleeping state is more complicated. Both of these are essentially instantaneous measurements performed at night in the waking state with the assumption that the measured values are representative of the sleeping state. Moderate light exposure at night does not appear to affect the nocturnal IOP pattern.25 However, the process of waking does appear to affect IOP, with previous studies reporting significant IOP elevations on waking with a rapid decay over several minutes to a baseline level.35 It is unclear whether this elevation is representative of true sleeping IOP, or a transient change that results from the process of waking. In our study, IOP measurements occurred after aqueous flow measurements and were followed by facility measurements. The consequence of this protocol is that the subjects were awake for approximately 5 to 10 minutes before IOP measurements. This amount of time would be sufficient for a return to baseline in most subjects,35 but the question of whether this is a “sleeping” baseline or an “awake” baseline cannot be answered at this time. Some biological parameters will remain entrained to the circadian rhythm, despite sleep disturbance. This situation holds true for aqueous flow, as well as production of neuroendocrine markers such as thyroid-stimulating hormone and cortisol, which require at least 2 days to reset to a new circadian rhythm.36 In contrast, other biological parameters, such as blood pressure, are influenced more by the sleeping and waking states than is the circadian rhythm.37 Determining whether IOP and outflow facility are entrained to the circadian rhythm or change rapidly from the sleeping state to the waking state will require the development of technology to measure these parameters in sleeping subjects.
Based on the data in the present study, the most likely explanation for the observed 24-hour IOP pattern is circadian variations in EVP or a combination of factors. This suggests that EVP may be a critical parameter for the control of nocturnal IOP elevation. However, no current glaucoma medications are known to influence EVP.38 Future therapies may have to target this parameter if optimum control of nocturnal IOP is to be achieved. Further work is necessary to elucidate fully the nature of circadian variation of aqueous humor dynamics. Such work should involve investigating whether the circadian patterns of aqueous dynamics in this study are maintained in older healthy subjects, as well as patients with glaucoma. Subsequent studies should also measure diurnal-to-nocturnal changes in EVP, in addition to IOP, aqueous flow rate, and outflow facility.
Acknowledgments
Supported in part by National Institutes of Health Grant UL1-RR24150, Center for Translational Science Activities, and the Mayo Foundation for Medical Education and Research.
Footnotes
Presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2007.
Disclosure: A.J. Sit, None; C.B. Nau, None; J.W. McLaren, None; D.H. Johnson, None; D. Hodge, None
References
- 1.Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- 2.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- 3.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in young adults with moderate to severe myopia. Invest Ophthalmol Vis Sci. 2002;43:2351–2355. [PubMed] [Google Scholar]
- 4.Liu JH, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44:4439–4442. doi: 10.1167/iovs.03-0349. [DOI] [PubMed] [Google Scholar]
- 5.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 6.Graham SL, Drance SM, Wijsman K, Douglas GR, Mikelberg FS. Ambulatory blood pressure monitoring in glaucoma: the nocturnal dip. Ophthalmology. 1995;102:61–69. doi: 10.1016/s0161-6420(95)31053-6. [DOI] [PubMed] [Google Scholar]
- 7.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol. 1999;43(suppl 1):S10–S16. doi: 10.1016/s0039-6257(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 8.Reiss GR, Lee DA, Topper JE, Brubaker RF. Aqueous humor flow during sleep. Invest Ophthalmol Vis Sci. 1984;25:776–778. [PubMed] [Google Scholar]
- 9.Larsson LI, Rettig ES, Sheridan PT, Brubaker RF. Aqueous humor dynamics in low-tension glaucoma. Am J Ophthalmol. 1993;116:590–593. doi: 10.1016/s0002-9394(14)73201-5. [DOI] [PubMed] [Google Scholar]
- 10.Larsson LI, Rettig ES, Brubaker RF. Aqueous flow in open-angle glaucoma. Arch Ophthalmol. 1995;113:283–286. doi: 10.1001/archopht.1995.01100030037018. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum CS, Podos SM, Lustgarten JS. Comparison of standard and computerized tonography instruments on human eyes. Am J Ophthalmol. 1985;99:403–410. doi: 10.1016/0002-9394(85)90006-6. [DOI] [PubMed] [Google Scholar]
- 12.Feghali JG, Azar DT, Kaufman PL. Comparative aqueous outflow facility measurements by pneumatonography and Schiotz tonography. Invest Ophthalmol Vis Sci. 1986;27:1776–1780. [PubMed] [Google Scholar]
- 13.Takeda Y, Azuma I. Diurnal variations in outflow facility. Ann Ophthalmol. 1978;10:1575–1580. [PubMed] [Google Scholar]
- 14.Brubaker RF. The flow of aqueous humor in the human eye. Trans Am Ophthalmol Soc. 1982;80:391–474. [PMC free article] [PubMed] [Google Scholar]
- 15.Brubaker RF. Flow of aqueous humor in humans: The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1991;32:3145–3166. [PubMed] [Google Scholar]
- 16.McLaren JW, Ziai N, Brubaker RF. A simple three-compartment model of anterior segment kinetics. Exp Eye Res. 1993;56:355–366. doi: 10.1006/exer.1993.1046. [DOI] [PubMed] [Google Scholar]
- 17.McLaren JW, Brubaker RF. Measurement of fluorescein and fluorescein monoglucuronide in the living human eye. Invest Ophthalmol Vis Sci. 1986;27:966–974. [PubMed] [Google Scholar]
- 18.McLaren JW, Brubaker RF. A two-dimensional scanning ocular fluorophotometer. Invest Ophthalmol Vis Sci. 1985;26:144–152. [PubMed] [Google Scholar]
- 19.Brubaker RF, McLaren JW. Uses of fluorophotometry in glaucoma research. Ophthalmology. 1985;92:884–890. doi: 10.1016/s0161-6420(85)33939-8. [DOI] [PubMed] [Google Scholar]
- 20.Maus TL, McLaren JW, Brubaker RF. A comparison of two methods of measuring aqueous flow in humans: fluorophotometry and flare measurement. Curr Eye Res. 1993;12:621–628. doi: 10.3109/02713689309001841. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SB, Coakes RL, Brubaker RF. A simple photogrammetric method of measuring anterior chamber volume. Am J Ophthalmol. 1978;85:469–474. doi: 10.1016/s0002-9394(14)75243-2. [DOI] [PubMed] [Google Scholar]
- 22.Brubaker RF. chap 46. Clinical evaluation of the circulation of aqueous humor. In: Duane TD, editor. Clinical Ophthalmology. Philadelphia: Harper and Row; 1986. [Google Scholar]
- 23.McLaren JW, Herman DC, Brubaker RF, et al. Effect of ibopamine on aqueous humor production in normotensive humans. Invest Ophthalmol Vis Sci. 2003;44:4853–4858. doi: 10.1167/iovs.03-0204. [DOI] [PubMed] [Google Scholar]
- 24.Grant WM. Tonographic method for measuring the facility and rate of aqueous flow in human eyes. Arch Ophthalmol. 1950;44:204–214. doi: 10.1001/archopht.1950.00910020209003. [DOI] [PubMed] [Google Scholar]
- 25.Liu JH, Kripke DF, Hoffman RE, et al. Elevation of human intraocular pressure at night under moderate illumination. Invest Oph-thalmol Vis Sci. 1999;40:2439–2442. [PubMed] [Google Scholar]
- 26.Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol. 1960;64:494–501. doi: 10.1001/archopht.1960.01840010496004. [DOI] [PubMed] [Google Scholar]
- 27.Kitazawa Y, Horie T. Diurnal variation of intraocular pressure in primary open-angle glaucoma. Am J Ophthalmol. 1975;79:557–566. doi: 10.1016/0002-9394(75)90792-8. [DOI] [PubMed] [Google Scholar]
- 28.Konstas AG, Mantziris DA, Cate EA, Stewart WC. Effect of timolol on the diurnal intraocular pressure in exfoliation and primary open-angle glaucoma. Arch Ophthalmol. 1997;115:975–979. doi: 10.1001/archopht.1997.01100160145002. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson SF. The uveoscleral outflow routes. Eye. 1997;11:149–154. doi: 10.1038/eye.1997.43. [DOI] [PubMed] [Google Scholar]
- 30.Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999;127:407–412. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- 31.Asamoto A, Yablonski ME. Physiology and measurement of episcleral venous pressure. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. St. Louis: Mosby-Year Book, Inc.; 1996. pp. 345–355. [Google Scholar]
- 32.Townsend DJ, Brubaker RF. Immediate effect of epinephrine on aqueous formation in the normal human eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci. 1980;19:256–266. [PubMed] [Google Scholar]
- 33.Bill A, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res. 1971;12:275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- 34.Brubaker RF. Clinical measurements of aqueous dynamics: implications for addressing glaucoma. In: Civan MM, editor. The Eye’s Aqueous Humor: from Secretion to Glaucoma. San Diego, CA: Academic Press Inc.; 1998. pp. 233–284. [Google Scholar]
- 35.Brown B, Burton P, Mann S, Parisi A. Fluctuations in intra-ocular pressure with sleep: II. Time course of IOP decrease after waking from sleep. Ophthalmic Physiol Opt. 1988;8:249–252. doi: 10.1016/0275-5408(88)90175-5. [DOI] [PubMed] [Google Scholar]
- 36.Goichot B, Weibel L, Chapotot F, Gronfier C, Piquard F, Brandenberger G. Effect of the shift of the sleep-wake cycle on three robust endocrine markers of the circadian clock. Am J Physiol. 1998;275:E243–E248. doi: 10.1152/ajpendo.1998.275.2.E243. [DOI] [PubMed] [Google Scholar]
- 37.Bursztyn M, Mekler J, Wachtel N, Ben-Ishay D. Siesta and ambulatory blood pressure monitoring. Comparability of the afternoon nap and night sleep. Am J Hypertens. 1994;7:217–221. doi: 10.1093/ajh/7.3.217. [DOI] [PubMed] [Google Scholar]
- 38.Brubaker RF. Targeting outflow facility in glaucoma management. Surv Ophthalmol. 2003;48(Suppl 1):S17–S20. doi: 10.1016/s0039-6257(03)00003-1. [DOI] [PubMed] [Google Scholar]