Summary
Insects such as the fruit fly, Drosophila melanogaster, rely on contact chemosensation to detect nutrient-rich foods, to avoid consuming toxic chemicals, and to select mates and hospitable zones to deposit eggs. Flies sense tastants and non-volatile pheromones through gustatory bristles and pegs distributed on multiple body parts including the proboscis, wing margins, legs and ovipositor. The sensilla house gustatory receptor neurons, which express members of the family of 68 gustatory receptors (GRs). In contrast to mammalian chemosensation or Drosophila olfaction, which are initiated by receptors composed of dimers of one or two receptor types, the functional Drosophila GRs may include three or more subunits. Several GRs appear to be expressed in multiple cell types that are not associated with contact chemosensation raising the possibility that these proteins may have roles that extend beyond the detection of tastants and pheromones.
Introduction
The decision by insects to initiate courtship and mating, lay eggs or consume plants and other foods is influenced heavily by direct contact with non-volatile compounds. Mammals and insects such as the fruit fly, Drosophila melanogaster, respond to many of the same tastants, including sugars and bitter compounds. However, in contrast to mammals, which taste primarily through taste buds on the tongue, fruit flies are able to sample tastants through receptor cells on many parts of the body. The wide distribution of taste organs contributes to the various behaviors initiated by gustatory input and to animal survival.
Contact chemosensation in insects is also exploited by plants to avoid destruction. To prevent feeding by insects, plants produce deterrent compounds with low volatility, referred to as antifeedants, including aristocholic acid, caffeine, quinine and many others, all of which taste bitter to humans. Despite the critical role of contact chemosensation in the control of insect behavior, our understanding of the molecular mechanisms involved in the detection of gustatory stimuli in insects has lagged behind vision and olfaction.
A critical advance in insect contact chemosensation was the identification of a family of gustatory receptors (GRs) in the fruit fly, Drosophila melanogaster [1,2]. However, until recently only one of these receptors, GR5a, was linked to a specific tastant [3-5], although more recent data described below indicates that it is likely to be part of multi-subunit complex. During the last few years there has been significant progress defining the repertoire of GRs required for attractive and aversive tastants and for courtship behavior. The advances have emerged through a combined application of behavioral, genetic, cell biological and electrophysiological approaches, which are readily available using Drosophila as a model organism. The characterization of the GRs had led to unexpected complexities and differences from mammalian taste receptors.
The distribution and structure of gustatory sensilla
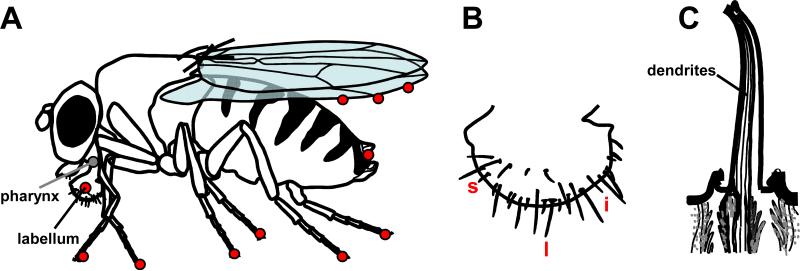
Drosophila and many other insects detect tastants on multiple body parts through hairlike projections referred to as gustatory sensilla (Figure 1; Table 1) [6-8]. The proboscis includes both external and internal sensilla and might be considered the functional equivalent of the mammalian tongue. Thirtyone external sensilla are situated in four rows on each of two labelial palps or labella, along with shorter and simpler taste structures, the taste pegs. The internal sensilla in the pharyngeal portion of the proboscis permit the flies to evaluate food immediately following ingestion but before it is transferred to the digestive system. Sensilla on the labella, anterior wing margins and legs enable the flies to sample potential foods without consuming them. This ability contributes to survival since the animals can taste attractive nutrients or toxic chemicals before deciding whether or not to extend the proboscis and ingest the food. Other sensilla surround the ovipositer [7], thereby allowing the females to identify locations with the preferred nutrient-conditions for depositing their eggs [9*]. Males have more sensilla on their forelegs than females (50 versus 37), which allow them to detect non-volatile pheromones that promote courtship and mating with females [10].
Figure 1.
Taste sensilla. (A) Taste sensilla are distributed on the labellum, pharynx, legs and wing margins. (B) A labellum showing l-, s- and i-type sensilla. (C) Structure of a sensillum with four dendrites extending above the surface of the cuticle. The sensillum has a terminal pore.
Table 1.
Number and distribution of sensilla and GRNs that function in contant chemosensation. The number and type of sensilla or taste pegs and the number of GRNs and mechanosensitive neurons (MSNs) per sensilla or taste pegs are indicated [7,56].
| Body location |
Organ name |
# gustatory sensilla/pegs |
Sensilla type |
GRNs |
MSNs |
|---|---|---|---|---|---|
| External head | Labella palps (or labella) | 31 sensilla total | |||
| 9 sensilla | Long (l) | 4 | 1 | ||
| 10 sensilla | Intermediate (i) | 2 | 1 | ||
| 12 sensilla | Short (s) | 4 | 1 | ||
| |
|
∼30 pegs |
|
1 |
|
| Internal head: pharynx | LSO labial sense ogan | 3 sensilla | 7 | 8 | |
| 8 | 1 | 1 | |||
| 9 | 1 | 1 | |||
| VCSO ventral cibarial sense organs | 3 sensilla | Proximal | 2 | ||
| Middle | 2 | ||||
| Distal | 4 | ||||
| |
DCSO dorsal cibarial sense organs |
|
|
|
|
| 2 sensilla | Posterior | 3 | |||
| Anterior | 3 | ||||
| Leg Tarsi | |||||
| male-first leg | ∼50 sensilla | 2−4 | 1 | ||
| female-first leg | ∼37 sensilla | 2−4 | 1 | ||
| second leg | 30 sensilla | 2−4 | 1 | ||
| third leg |
|
32 sensilla |
|
2−4 |
1 |
|
Wing Margins |
|
∼40 sensilla |
|
4 |
1 |
| |
|
|
|
|
|
| Ovipositer | ∼10 sensilla | ? | ? |
The external gustatory sensilla contain a single pore for receiving non-volatile compounds and multiple cell types that facilitate the detection of attractive and repulsive compounds [7]. These include two to four gustatory receptor cells, one mechanosensory neuron and several types of accessory cells. In flies the cells that detect the tastants are bona fide neurons rather than neuroepithelial cells found in mammalian taste buds. Each gustatory receptor neuron (GRN) is a bipolar cell with a cell body that lies beneath the surface of the cuticle and includes a single dendrite that extends to the tip of the sensilla and one axon that projects to the subesophageal ganglion region of the brain.
The three types of sensilla on the labellum are defined based on their length [long (l-type), intermediate (i-type) and short (s-type); Figure 1B] [11]. The four GRNs in the l- and s-type sensilla respond to sugars (S cell), water (W cell), low concentrations of salt (L1) and high salt concentrations (L2). In the labellum, bitter compounds are detected primarily in s-type sensilla via the L2 cell. Bitter compounds also inhibit activity of the S cells and W cells [12]. Thus, chemicals that elicit inhibitory behavioral responses, such as high salt and bitter compounds, stimulate L2 cells. The i-type sensilla include two GRNs, one of which is stimulated by attractive agents, sugars and low salt, while the other is activated by aversive chemicals, including bitter compounds and high salt [13].
The relative responses of the different types of sensilla to attractive and repulsive compounds are not the same. In general, the l-type sensilla produce stronger responses to sugars than the i- and s-type sensilla, although the s-type respond more robustly to sucrose than to other sugars [11]. Different types of sensilla also respond differently to bitter compounds [12]. These variations in sensitivities to different sweet and bitter compounds suggest diversity in expression patterns of the receptors required for sensing non-volatile compounds.
GRs and the detection of sweet and bitter taste
In mammals, most chemosensory receptors are classic seven transmembrane G protein-coupled receptors (GPCRs), including those involved in smell and in detecting three taste qualities: sweet, bitter and umami (the taste of monosodium glutamate) [14,15]. Thus, it was anticipated that the Drosophila taste receptors might also be GPCRs. Indeed, the 19 original members of the Drosophila GR family were successfully identified based on a bioinformatic screen for seven transmembrane receptors, 18 of which were expressed in the labellum [1]. Following completion of the Drosophila genome project, the GR family was expanded to 68 members (Figure 2A) [2,16,17]. The amino acid conservation among members of these proteins is as low as <10%, although it is considerably higher in cases in which the corresponding genes appear to have undergone relatively recent gene duplications [17]. Related GR families are encoded in other insects including Anopheles gambiae and Aedes aegypti, which are the vectors for malaria and yellow fever respectively [18-20]. Nevertheless, many of the GRs encoded by the Drosophila, Anopheles and Aedes genomes do not have recognizable homologs in the other insects suggesting significant differences in ligand specificities or sensitivities.
Figure 2.
Drosophila gustatory receptors. (A) A dendrogram of the Drosophila GR subfamily adapted from Robertson et al. [17]. The GRs that have been characterized functionally are indicated. Green and red lettering highlight GRs that are required for the responses to sugars and bitter compounds respectively. The blue boxes indicate GRs that function in male courtship towards females or in avoiding courtship with other males. The two GRs that are required for sensing CO2 gas in ORNs are indicated. (B) Tastants or pheromones detected by GRs shown to function in contact chemosensation. (C) The Gr64 cluster. The six genes appear to include a single promoter and one polyadenylation site. The rectangles indicate exons. The genes that have been subjected to functional analyses (Gr64a and Gr64f) are highlighted in green.
During the last few years, the expectation that GRs function broadly in contact chemosensation has been confirmed. These include roles in sweet and bitter taste and in the detection of non-volatile pheromones as summarized below. However, there have been surprises. At least four members of the “GR” family are expressed in olfactory receptor neurons [2,16], including two highly related GRs (GR21a and GR63a) that function in the detection of CO2 [21,22]. Although the 68 fly GRs have no sequence homology with mammalian taste receptors or other GPCRs, they are distantly related to the second family of Drosophila chemosensory receptors comprised of 62 olfactory receptors (ORs). These latter proteins have a topology opposite to GPCRs [23,24], with cytoplasmic N-termini and extracellular C-termini. Surprisingly, the ORs are cation channels [25**,26**]. According to one report the ORs are strictly ligand-gated channels [25*], while a second study concludes that the ORs are both ligand gated channels and GPCRs [26*]. This results in production of cyclic nucleotides, which also activates the channels. Nevertheless, these findings raise the unresolved question as to whether the fly GRs are also cation channels.
Many GRs are co-expressed in the same GRNs, which is reminiscent of the finding multiple bitter receptors (T2Rs) are expressed in single mammalian taste receptor cells [27]. Gr66a is detected primarily in one GRN per s- and i-type sensilla and genetic ablation of GR66a-expressing GRNs eliminates the response to bitter compounds but not to sugars [28,29]. Thus, GR66a broadly marks bitter-responsive GRNs. Two additional GRs (GR93a and GR33a) appear to be co-expressed in most if not all Gr66a-expressing GRNs in the labella [30,31*]. 10 other GRs are present in at least a subset of Gr66a-expressing GRNs [28,29,32*]. Some of these additional Grs are produced in half or more of the Gr66a-expressing GRNs. Since the expression patterns of most GRs have not been investigated (48 out of 68), the full complement of receptors present in single bitter-responsive GRNs is not known. Nevertheless, it appears that a minimum of four GRs is expressed in most labellal GRNs that sense aversive compounds, and it is possible that some may express as many as 10 or more GRs.
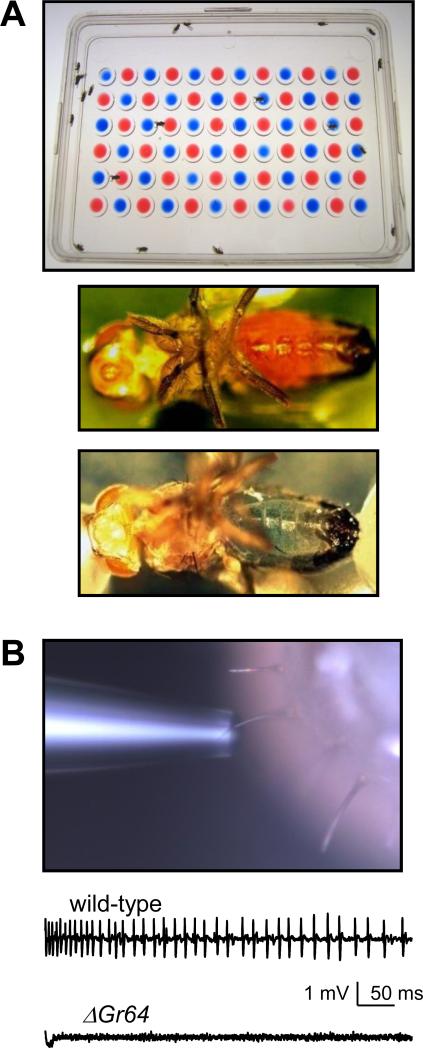
The large assortment of GRs that are co-expressed in GRNs is reflected by co-requirements for multiple GRs for the responses to bitter substances. In Drosophila it is relatively straightforward to generate mutations and to assay the effects on taste using either of two behavioral assays. One of these assays, the two-way choice test, is performed by placing a group of starved flies in a microtiter dish with alternating agar-containing wells mixed with either of two tastants and red or blue food coloring (Figure 3A) [33]. Food preference is assessed by inspecting the color of the abdomens. A second behavioral assay, the proboscis extension response (PER) entails applying tastants to gustatory sensilla on the legs or labellum [34]. In wild-type animals, appealing compounds such as sugars induce an extension of the proboscis, while a PER is inhibited by addition of a noxious chemical to the sugar, such as a bitter compound. If a mutation affects taste in the peripheral receptor cells (the GRNs) rather than neurons in the subesophageal ganglion or in the central brain, there is a change in tastant-induced action potentials, after applying a sugar or bitter compound directly to the gustatory sensilla (Figure 3B) [35].
Figure 3.
Assaying the gustatory response using behavior and electrophysiology. (A) Two-way choice assay. Flies are starved overnight and placed in a microtiter dish containing two types of tastants mixed with either red or blue food coloring (e.g. sugar versus sugar plus a bitter tastants). The colors of the abdomens are assessed by visual inspection. Wild-type flies will choose the sugar without the bitter compound. The dyes are switched to make sure they do not influence the preferences for the tastants. The preference index (PI) is calculated as follows: PI= (NBlue + 0.5Mix)/(NRed + NBlue + NMix) or (NRed + 0.5Mix)/(NRed + NBlue + NMix). (B) Tip recording. The photograph show an electrode placed over a wild-type l-type sensillum on the labellum. Representative traces showing action potentials induced in wild-type and ΔGr64 mutant flies (missing the entire Gr64 locus; Figure 2C) in response to 50 mM sucrose.
Using both behavioral and electrophysiological assays, it appears that GR66a and GR93a are both essential for the responses to the two methylxanthines, caffeine and theophylline, which are present in coffee and tea [30,36]. These receptors are not required for detection of other methylxanthines or compounds that are aversive to flies. While both GR66a and GR93a are crucial for sensing caffeine they do not appear to be sufficient since misexpression of these two receptors in sugar-responsive GRNs does not confer a caffeine-response to these cells [30]. Null mutations in a third receptor, Gr33a, disrupt the ability to detect almost any repulsive compound ranging from caffeine to quinine, denatorium and berberine [31*]. However, misexpression of all three receptors is insufficient to elicit a response to caffeine or other bitter chemicals. These findings raise the possibility that a functional bitter taste receptor might be comprised of four or more distinct subunits. This is in contrast to the Drosophila CO2 receptor, which includes two subunits expressed in ORNs, GR21a and GR63a [21,22]. Unlike OR83b, which is required broadly for trafficking other ORs to the dendrites [37], the immunofluorescent localizations of the GR66a or GR93a proteins were indistinguishable between the Gr33a mutant and wild-type [31*]. Thus, GR33a might be a functional co-receptor subunit but is not required for insertion of other GRs into the dendrites.
Each sugar-responsive GRN also expresses multiple GRs. A Gr5a reporter is expressed in a non-overlapping subset of cells from Gr66a and marks most if not all GRNs in the labella that are stimulated by sweet compounds [28,29]. The seven GRs most closely related to GR5a (GR61a and six GRs encoded by the Gr64 locus, GR64a, GR64b, GR64c, GR64d, GR64e, GR64f, hereafter referred to as GR64a-f) are co-expressed in at least some of the GR5a positive GRNs (Figure 2A) [32*,38*]. The six Gr64a-f genes may be transcribed as a single polycistronic mRNA, which is then processed into individual RNAs (Figure 2C). However, this conclusion is not definitive since a single RT-PCR product cannot be generated that encompasses all six genes. It is also unclear whether the mature mRNAs are all stably expressed in the same GRNs. Gr64f RNA may be produced in all the same GRNs in the labellum as Gr5a [38*]. A subset of the Gr5a/Gr64f positive GRNs also expresses Gr61a [38*]. Since the remaining five Gr64 genes (Gr64a-e) are also produced in at least portions of the Gr5a GRNs [32*], it appears that the sugar-responsive GRNs co-express between three and eight GRs.
Deletion of the entire Gr64 cluster (ΔGr64) eliminates the responses to all sugars tested except for fructose (Figure 2B) [39*]. The ability to sense sucrose and maltose depends on Gr64a [32*,38*], while Gr5a is required for largely a non-overlapping subset of sugars such as trehalose and melezitose [3-5,38*]. GR64f may be a co-receptor for GR5a and GR64f similar to the requirement for GR33a for bitter responses. Gr64f is required in combination with Gr5a to detect trehahose and in concert with Gr64a to sense sucrose and maltose and glucose [40]. However, expression of GR64f in combination with GR64a or GR5a, either in vitro or in bitter-responsive GRNs, is insufficient to produce any sugar response. Thus, the sugar-receptors may be comprised of at least three independent subunits.
An open question concerns the roles of GRs that are co-expressed in subsets of Gr5a-expressing cells, but are not required for sugar discrimination. Mutation of Gr61a has no apparent effect on the detection of sugars [38*], and reintroduction of Gr64a+ and Gr64f+ transgenes into ΔGr64 flies fully restores normal sugar sensation [40]. Thus, Gr64b-e (Figure 2C) and Gr61a appear to be dispensable for sugar detection. Currently, the receptors required for sensing attractive tastants other than sugars, such as fatty acids and amino acids, are unknown and GR61a and GR64b-e therefore represent candidate detectors for these compounds.
Courtship and mating
Courtship between male and female flies involves a sequence of behaviors that employ visual and auditory cues as well as volatile and nonvolatile pheromones [41,42]. Courtship is initiated when the male orients towards the female [41]. He then taps the female with a foreleg, plays a courtship song by extending and vibrating a wing, licks the genitalia, and attempts to mate. The series of behaviors ends with successful copulation. In males, non-volatile hydrocarbons on the cuticle are detected by sensilla on the tarsi, wing margins and labella, and these pheromones are essential for completing the set of courtship behaviors between males and females, and for inhibiting courtship between males [42].
The first GR shown to participate in mating behavior is GR68a. This receptor appears to be expressed specifically in male-specific sensilla on the forelegs, and inactivation of GR68a-expressing GRNs impairs normal courtship with females [10]. The males with inactivated GR68a GRNs do not exhibit increased courtship to males, suggesting that the reduced interest in females may be due to a perturbation in the reception of a stimulatory pheromone from the females rather than a problem in detecting a male inhibitory pheromone. A reduction in GR68a expression using siRNA reduces male courtship [10]. However, the phenotype is less pronounced than inactivation of the Gr68a-expressing GRNs. This finding raises the possibility that additional GRs may be expressed in the male-specific sensilla and participate in courtship.
Volatile and non-volatile pheromones also contribute to inhibition of male-male courtship. Loss of the GR32a, which is the GR most related to GR68a, increases male-male courtship [43*]. In contrast to wild-type males, which show a diminished propensity to court previously mated females, Gr32a mutant males display similar courtship behavior to virgin and mated females. This latter phenotype might result from a decreased ability to sense an inhibitory pheromone transferred from a male to a female during mating. Some of the GRNs in the leg tarsi that express the Gr32a reporter extend directly into the ventrolateral protocerebrum region of the brain. Since the ventrolateral protocerebrum also receives signals originating from stimulation of auditory neurons and photoreceptor cells, it has been proposed that this region integrates input from multiple sensory modalities that function in courtship behavior [43*].
GR33a, which is essential for bitter taste, is also expressed in Gr32a-expressing GRNs in the tarsi and is required for suppressing male-male courtship [31*]. Thus, GR33a and GR32a may function in concert as subunits of a potentially more complex receptor required for pheromone reception and for controlling sexual preference in males.
Future perspectives
Despite the recent progress on the characterization of the receptors that initiate Drosophila contact chemosensation, the field is still in the early stages. We still do not understand the minimum subunit composition of the GRs, which appears to include three or more subunits. According to one report, expression of GR5a in Drosophila S2 cells is sufficient for trehalose-induced Ca2+ influx [5]. However, there is no in vivo demonstration that misexpression of a GR in neurons that normally do not respond to these compounds confers responsiveness to any sugar, bitter compound, or soluble pheromone. In view of the inverse topology [23,24] and channel activity of the fly ORs [25**,26**], the question arises as to whether the GRs also display an orientation opposite to GPCRs and are cation channels. It is not known whether GRs sense compounds such as amino acids, fatty acids, low pH, water or carbonation [44**], the latter of which is molecularly and functionally distinct from olfactory detection of CO2 in the antenna by the GR21a/GR63a CO2 receptor [21,22]. Furthermore, the GRs that function in the selection of preferred zones for egg deposition have not been identified, although Gr5a-expressing neurons contribute to this behavior [9*].
It is also unclear whether all tastants are detected directly through gustatory receptors. A Drosophila TRP channel referred to as Painless is required in late-stage larvae for avoiding fructose [45] in adults for avoiding wasabi [46]. However, it is not known if Painless acts in GRNs or whether it functions as a direct sensor for wasabi, rather than downstream of another receptor such as a GR. Nevertheless, TRP channels may be direct sensors for some tastants as multiple TRP channels are activated directly by botanical compounds [47] and TRPP channels have been implicated as a sour sensors in mammals [48-50]. Other candidate receptors for contact chemoreception are relatives of Drosophila ionotropic glutamate receptors, since some ionotropic receptors respond directly to odorants [51**].
An intriguing question is whether GRs have functions unrelated to their established role in chemoreception in peripheral neurons. A recent study indicates that four of the six genes that comprise the Gr28 cluster are expressed in neuroendocrine cells in the brain, hygro- and thermosensory neurons of the arista, proprioceptive neurons, the Johnston's organ, which functions in hearing, and abdominal multidendritic neurons [52*]. These findings, which are reminiscent of the reports that some mammalian taste receptors are expressed and act in the gut [53-55], raise the exciting possibility that Drosophila GRs may function in metabolism, and other sensory modalities such as thermosensation, hygrosensation and proprioception. However, in vivo validation of the expression of and function of GRs outside of conventional chemosensory neurons is required to confirm this hypothesis. Finally, since aversive compounds prevent feeding, the GRs for these repellent compounds provide a potential new avenue for conducting high-throughput screens for more effective antifeedants to reduce insect-induced damage to crops and other plants.
Acknowledgments
I thank Yuchen Jiao for preparation of the figures. The work on contact chemosensation in the author's laboratory is supported by a grant from the NIDCD (DC007864).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- 2.Scott K, Brady R, Jr., Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 3.Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, Yamamoto K, Isono K. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr. Biol. 2001;11:1451–1455. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- 4.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 5.Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. USA. 2003;100:14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk R, Bleiser-Avivi N, Atidia J. Labellar taste organs of Drosophila melanogaster. J. Morph. 1976;150:327–341. doi: 10.1002/jmor.1051500206. [DOI] [PubMed] [Google Scholar]
- 7.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 8.Singh RN. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microsc. Res. Tech. 1997;39:547–563. doi: 10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9*.Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [* of special interestThis paper describes egg laying behavior and the preferred environmental tastants for laying eggs. In contrast to feeding behavior, which is promoted by sucrose and inhibited by bitter compounds such as lobeline, the females prefer to lay eggs in zones containing lobeline over sucrose, but only if the two zones are close together. This behavior is inhibited by hyperpolarizing Gr5a-expressing neurons] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 11.Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog. Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- 12.Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J. Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- 13.Hiroi M, Meunier N, Marion-Poll F, Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J. Neurobiol. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- 14.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 15.Keller A, Vosshall LB. Better smelling through genetics: mammalian odor perception. Curr. Opin. Neurobiol. 2008;18:364–369. doi: 10.1016/j.conb.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 17.Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- 19.Kent LB, Walden KK, Robertson HM. The Gr family of candidate gustatory and olfactory receptors in the yellow-fever mosquito Aedes aegypti. Chem Senses. 2008;33:79–93. doi: 10.1093/chemse/bjm067. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity. 2009 doi: 10.1038/hdy.2009.55. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 22.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundin C, Kall L, Kreher SA, Kapp K, Sonnhammer EL, Carlson JR, Heijne G, Nilsson I. Membrane topology of the Drosophila OR83b odorant receptor. FEBS Lett. 2007;581:5601–5604. doi: 10.1016/j.febslet.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 26.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 27.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr. Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Moon SJ, Lee Y, Jiao Y, Montell C. A broadly tuned taste receptor essential for aversive contact chemosensation in Drosophila. Curr. Biol. 2009 doi: 10.1016/j.cub.2009.07.061. (in revision) [* of special interestThis report shows that loss of Gr33a impairs the responses to all bitter compounds tested and results in an increase in male-male courtship. These results suggest that GR33a is co-receptor required for sensing all repulsive ligands detected by contact chemosensation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc. Natl. Acad. Sci. USA. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [* of special interestThis work and the papers by Dahanukar et al. (ref. 38) and Slone et al. (ref. 39) demonstrate that the Gr64a-f cluster of six genes encodes at least one sugar receptor, GR64a, which is required in addition to GR5a for the responses to multiple sugars] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanimura T, Isono K, Takamura T, Shimada I. Genetic dimorphism in the taste sensitivity to trehalose in Drosophila melanogaster. J. Comp. Physiol. A. 1982;147:433–437. [Google Scholar]
- 34.Falk R, Atidia J. Mutation affecting taste perception in Drosophila melanogaster. Nature. 1975;254:325–326. doi: 10.1038/254325a0. [DOI] [PubMed] [Google Scholar]
- 35.Hodgson ES, Lettvin JY, Roeder KD. Physiology of a primary chemoreceptor unit. Science. 1955;122:417–418. doi: 10.1126/science.122.3166.417-a. [DOI] [PubMed] [Google Scholar]
- 36.Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr. Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 38*.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [* of special interestThis work and the papers by Jiao et al. (ref. 32) and Slone et al. (ref. 39) demonstrate that the Gr64a-f cluster of six genes encodes at least one sugar receptor, GR64a, which is required in addition to GR5a for the responses to multiple sugars] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr. Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [* of special interestThis work and the papers by Jiao et al. (ref. 32) and Dahanukar et al. (ref. 39) demonstrate that the Gr64 cluster of six genes encodes at least one sugar receptor, which is required in addition to GR5a for the responses to multiple sugars] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 42.Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- 43*.Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [* of special interestThis is the first report of a gene encoding a receptor, Gr32a, required to suppress male-courtship and courtship towards mated females. Due to this phenotype, GR32a may be receptor a for a non-volatile male inhibitory pheromone] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [** of outstanding interestThese workers found that dissolved CO2 is a new taste modality in flies. Unlike CO2 gas, which is detected by the GR21a/GR63a receptor complex in olfactory receptor neurons and is aversive, the CO2 detected by GRNs is attractive. The population of neurons that respond to carbonated water is distinct from those that express either Gr5a or Gr66a] [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Sornborger AT, Lee JK, Shen P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat. Neurosci. 2008;11:676–682. doi: 10.1038/nn.2119. [DOI] [PubMed] [Google Scholar]
- 46.Al-Anzi B, Tracey WD, Jr., Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Venkatachalam K, Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 2006;98:68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- 50.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [** of outstanding interestThe authors showed that several genes encoding proteins related to ionotropic glutamate receptors are expressed in olfactory receptor neurons that do not express members of the OR family. These proteins, referred to as ionotropic receptors (IRs), may be odorant-gated ion channels] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Thorne N, Amrein H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J. Comp. Neurol. 2008;506:548–568. doi: 10.1002/cne.21547. [* of special interestThese workers provide evidence that the spatial localizations of several members of the Gr28 cluster are not restricted to peripheral sensory neurons. These results suggest that GRs have roles unrelated to the detection of gustatory stimuli or CO2 gas] [DOI] [PubMed] [Google Scholar]
- 53.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem. Soc. Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 55.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gendre N, Lüer K, Friche S, Grillenzoni N, Ramaekers A, Technau GM, Stocker RF. Integration of complex larval chemosensory organs into the adult nervous system of Drosophila. Development. 2004;131:83–92. doi: 10.1242/dev.00879. [DOI] [PubMed] [Google Scholar]