Abstract
Sepsis is a difficult condition to treat and is associated with a high mortality rate. Sepsis is known to cause a marked depletion of lymphocytes, although the function of different lymphocyte subsets in the response to sepsis is unclear. γδ T cells are found largely in epithelial-rich tissues, and previous studies of γδ T cells in models of sepsis have yielded divergent results. In the present study, we examined the function of γδ T cells during sepsis in mice using cecal ligation and puncture (CLP). Mice deficient in γδ T cells had decreased survival times and increased tissue damage after CLP compared with wild-type mice. Furthermore, bacterial load was increased in γδ T cell-deficient mice, yet antibiotic treatment did not change mortality. Additionally, we found that recruitment of neutrophils and myeloid suppressor cells to the site of infection was diminished in γδ T cell-deficient mice. Finally, we found that circulating levels of IFN-γ were increased, and systemic levels of IL-10 were decreased in γδ T cell-deficient mice after CLP compared with wild-type mice. γδ T cell-deficient mice also had increased intestinal permeability after CLP compared with wild-type mice. Neutralization of IFN-γ abrogated the increase in intestinal permeability in γδ T cell-deficient mice. The intestines taken from γδ T cell-deficient mice had decreased myeloperoxidase yet had increased tissue damage as compared with wild-type mice. Collectively, our data suggest that γδ T cells modulate the response to sepsis and may be a potential therapeutic target.
Keywords: IFN-γ, intestine, myeloid suppressor cells, neutrophils, bacteremia
INTRODUCTION
In contrast to conventional T cells, γδ TCR-bearing cells or γδ T cells constitute only a small proportion (1–5%) of the lymphocytes that circulate in the blood and peripheral organs [1]. γδ T cells are more widespread within epithelial-rich tissues, such as the skin, intestine, and reproductive tract, where they can comprise up to 50% of T cells. Antigen recognition by γδ T cells is limited as compared with conventional T cells, and the repertoire of natural ligands is not well developed [2, 3].
γδ T cells isolated from the peritoneum have been shown to respond differentially to pathogenic insults by secreting IFN-γ, IL-4, IL-10, and TNF-α [4]. In contrast, γδ T cells isolated from the spleen predominantly produce IFN-γ and TNF-α when activated by Con A [5]. Previously, two reports used γδ T cell-deficient mice to determine their role during bacterial infections. Skeen et al. [6] infected mice i.p. with the gram-positive bacterium Listeria monocytogenes, resulting in infection of the intestinal epithelium. Moore et al. [7] infected mice intratracheally with the gram-negative bacteria Klebsiella pneumoniae, resulting in a pulmonary infection. In both models, mortality was increased in the mice deficient in γδ T cells. However, in the Klebsiella model, it was found that the rapid up-regulation of IFN-γ and TNF-α was impaired in the lung and liver tissue from the γδ T cell-deficient mice. In contrast, in the Listeria model, it was found that the systemic levels of proinflammatory cytokines were higher in the γδ T cell-deficient mice. Thus, it is likely that γδ T cells can differentially regulate the inflammatory response based on the type or site of infection.
Recently, two reports have used the same γδ T cell-deficient mice to determine their role during cecal ligation and puncture (CLP)-induced sepsis [8, 9]. Both laboratories conducted survival curves. Enoh et al. [9] afflicted a severe CLP model (wild-type mean survival time was ~36 h with a 100% mortality) and saw no significant differences between the γδ T cell-deficient and wild-type mice. Further, no differences in bacterial clearance or cytokine production were observed. In contrast, Chung et al. [8] used a less-severe model (wild-type mean survival time was ~48 h with 60% mortality) and observed that the wild-type mice had decreased mortality and increased systemic inflammatory cytokines as compared with γδ T cell-deficient mice. It was further observed that upon ex vivo stimulation of splenic T cells or peritoneal macrophages from septic mice that IFN-γ and IL-12 accumulation was increased in samples taken from the γδ T cell-deficient mice. Altogether, the differing severity of the model used could explain the differences observed from the two reports.
There is a paucity of data examining the presence or absence of γδ T cells in patients predisposed to sepsis, and previous studies of γδ T cells in sepsis have produced contrasting results. In the present study, we examined the kinetics of γδ T cells in trauma patients and their function during the response to sepsis using a murine model of sepsis induced by CLP.
MATERIALS AND METHODS
Patient selection
Seven male blunt-trauma patients and seven healthy male subjects were included in this study. Informed consent from patients and healthy volunteers was obtained (IRB #06-03-07-06). Patients were selected on the following criteria: no clinical suspicion of sepsis; in the surgical intensive care unit or hospital; no evidence of immunosuppression and no immunosuppressive medication. Injured patients, 18 years or older, were within 66.5 ± 18.4 h of the blunt trauma. The average injury severity score was 25.7 ± 13.1. There was no significant age difference between the trauma patients (31.8±12.8) and the healthy controls (37.7±5.1). Data are the average ± SD.
Peripheral blood isolation
Venous blood was drawn from patients and volunteers into syringes containing 15% EDTA. RBCs were removed by lysis buffer (BD PharMingen, San Diego, CA, USA). Following lysis, cells were prepared for flow cytometry as described below.
Mice
Breeding pairs of 6- to 8-week-old C57BL/6J wild-type and TCR-δ-deficient (B6.129P2-Tcrdtm1Mom/J) mice [10] were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mice have 12 backcrosses on the C57BL/6J strain. All mice used were bred in-house.
CLP
Male mice between 6 and 10 weeks of age (20–26 g) were used. All experiments involving animals were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati (Cincinnati, OH, USA). Polymicrobial sepsis was induced similarly as described [11]. Briefly, the CLP operations were always performed between 8 a.m. and 1 p.m. Normally fed mice were anesthetized to effect by 2% isoflurane in oxygen via facemask. The skin was shaved and disinfected. After a 1-cm laparatomy, the latter 80% of the cecum was ligated with a 3–0 silk tie (Ethicon, Inc., Somerville, NJ, USA) and punctured once on the antimesenterial side with a 23-gauge needle. A small amount of the bowel contents was extruded through the puncture hole to assure a full-thickness perforation. Care was taken not to obstruct the bowel, and this was tested after the animals’ death. The cecum was replaced in its original location, and the midline incision was closed by a two-layer suture with 4–0 silk (Schein, Inc., Melville, NY, USA). The animals were resuscitated with 1 mL sterile saline s.c. and kept on a heating blanket with additional oxygen supply for 1 h. Sham-treated controls underwent the same surgical procedures (i.e., laparotomy and resuscitation), but the cecum was neither ligated nor punctured.
Intestinal lymphocyte isolation
The small intestine was placed in 4°C RPMI 1640. Mesenteric fat and Peyer’s patches were removed. The intestine was opened longitudinally with mucus and fecal material removed. The intestine was subsequently cut into 5-mm pieces, washed three times with Ca2+- and Mg2+-free PBS, placed in extraction buffer (5 mM EDTA, 2 mM 2-ME in PBS), and incubated with continuous, brisk stirring at 37°C for 30 min. The tissue slurry was then passed through a 70-μ filter to remove undigested tissue pieces. The cells were then collected and prepared for flow cytometric analysis.
Flow cytometry for surface staining
Cells were resuspended in FACS buffer (PBS with 1% BSA and 0.1% azide). Nonspecific binding to cells was controlled by adding 5% rat serum (Caltag, Burlingame, CA, USA) and 1 μg/tube Fc block (BD PharMingen) to the FACS buffer. Cells were stained in a three-color configuration using FITC-, PE-, or allophycocyanin (APC)-labeled antibodies. Samples were run on a Becton Dickinson LSR using the 488 line of the argon (FITC, PE) and the 633 line of the helium neon laser (APC). Myeloid cells were surface-stained with the following antibodies: APC-labeled CD11b (clone M1/70.15, BD PharMingen); FITC-labeled 7/4 (clone 7/4, Caltag); and PE-labeled Gr-1 (clone RB6-8C5). For intestinal T cells, cells were surface-stained with APC-labeled TCR-β (clone H57-597, BD PharMingen) and FITC-labeled TCR-δ (clone GL3, Caltag).
Antibiotic treatment
Wild-type and γδ T cell-deficient mice were treated with Primaxin or an equal volume of saline immediately after CLP, followed by additional antibiotic or saline injections every 12 h for 72 h as described previously [12].
ELISA
Peritoneal fluid was harvested from mice by peritoneal lavage after aseptic preparation of the abdominal wall followed by injection of 9 ml sterile saline into the peritoneal cavity and aspiration of peritoneal fluid. Serum was collected by cardiac puncture. IL-6 levels in the peritoneal fluid and serum were analyzed using ELISA, according to the manufacturers’ protocols (PeproTech Inc., Rocky Hill, NJ, USA, and BioSource Inc., Camarillo, CA, USA).
Bacterial counts
Bacterial counts were performed on aseptically harvested blood by cardiac puncture. Samples were serially diluted in sterile saline and cultured on tryptic soy agar pour plates, which were incubated at 37°C for 24 h, and colony counts were performed.
Intestinal permeability in vitro
In vitro permeability was measured in segments from the distal ileum mounted in Ussing chambers. Segments of intestine (2–3 cm) were opened along the mesenteric border, rinsed with Krebs-Ringer bicarbonate buffer (pH 7.4), and pinned as intact sheets between siliconized Ussing half-chambers (0.5 cm2 exposed surface area). The Ussing chambers were equipped with two calomel voltage-sensitive electrodes and two Ag-AgCl current-passing electrodes (Easy Mount Diffusion Chamber System, Physiologic Instruments, San Diego, CA, USA).
The mucosal and serosal reservoirs of the chamber contained 5 ml oxygenated Krebs-Ringer bicarbonate buffer (pH 7.4). The buffer was oxygenated (95% O2–5% CO2) continuously during the experiment and kept at 37°C by a water-jacketed system. After the preparations had stabilized for 20 min, and baseline potential difference and resistance had been established, HRP (0.1 mg/ml) was added to the mucosal reservoir. Samples (0.22 ml) were taken (and replaced with identical volumes of fresh medium) at 30 min intervals during 180 min from the serosal reservoir for measurement of HRP.
In vivo IFN-γ capture assay
Briefly, mice are injected with a biotin-labeled, neutralizing anti-IFN-γ antibody, which binds secreted IFN-γ and prevents its excretion, use, or degradation. The biotin–anti-IFN-γ antibody–cytokine complex is detected by ELISA, using an antibody to a second epitope on the same cytokine molecule to bind the biotin–anti-IFN-γ antibody–IFN-γ complex and enzyme linked to streptavidin, followed by a chromogenic substrate for that enzyme to detect the bound complex. The kit to conduct this assay is commercially available (BD PharMingen) [13].
IFN-γ neutralization
Two doses of 250 μg anti-IFN-γ (clone XMG 1.2, generously provided by Dr. Fred Finkelman, University of Cincinnati) were injected i.p., 18 h prior to and immediately after CLP. The IgG1 κ isotype control (clone R3-34, BD PharMingen) was used in control experiments.
Myeloperoxidase (MPO) assay
Intestine tissue (100 mg) was homogenized in 2 mL buffer A (3.4 mM KH2HPO4 and 16 mM Na2HPO4, pH 7.4). After centrifugation for 20 min at 10,000 g, the pellet was resuspended in 10 vol buffer B (43.2 mM KH2HPO4, 6.5 mM Na2HPO4, 10 mM EDTA, and 0.5% hexadecyltrimethylammonium, pH 6.0) and was sonicated for 10 s. After heating for 2 h at 60°C, the supernatant was reacted with 3,3′,5,5′-tetramethylbenzidine (Sigma Chemical Co., St. Louis, MO, USA), and OD was determined at 655 nm.
Tissue analysis
Intestinal tissue was fixed in buffered formalin before embedding in paraffin. Intestines were sectioned and stained with H&E.
Statistics
Statistical comparisons were performed using Kaplan Meier LogRank (survival), Student’s t-test (two groups), or Tukey’s test and ANOVA (more than two groups). StatView (SAS Institute, Cary, NC, USA) and GraphPad Prism 3.0 (GraphPad Software, San Diego, CA, USA) were used for statistical analyses. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
γδ T cell numbers are decreased following blunt trauma
It has been reported that following trauma and during sepsis, there is a decrease in circulating lymphocytes [14]. As information regarding γδ T cells is lacking in these patient populations, we examined lymphocytes in human blood drawn 66.5 ± 18.4 h after blunt trauma. The relative percentage of γδ T cells (of total lymphocytes) in circulation did not change significantly after trauma. However, there was a marked decrease in the concentration of circulating lymphocytes, consistent with previous reports [14]. As shown in Figure 1, the absolute number of γδ+/CD3+ T cells decreased 47% in trauma patients as compared with healthy controls. Thus, the absolute number of circulating γδ T cells decreases significantly after blunt trauma.
Fig. 1.
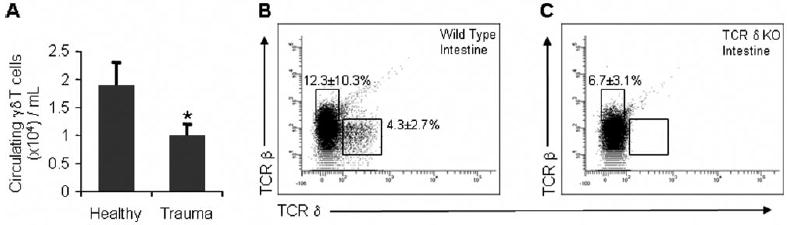
Human circulating γδ T cell numbers are decreased following blunt trauma. Absolute numbers of peripheral γδ T cells taken from (A) healthy volunteers or blunt trauma patients. Cell numbers represent the mean ± SD of seven trauma or seven healthy humans. Proportions of αβ or γδ T cells from the intestines of (B) wild-type or (C) γδ T cell-deficient mice. The cell samples were first tightly gated on lymphocytes, as determined by the forward-scatter and side-scatter. The intestine sample size equals three for each group, and values represent the average ± SD. *, P ≤ 0.05, as compared with healthy controls. KO, Knockout.
It has been recently reported that ~45% of patients suffering blunt or penetrating trauma develop nosocomial infections [15]. To determine whether decreased numbers of γδ T cells mediate the immune response during a subsequent infection, we examined the response of γδ T cell-deficient mice in a physiologically relevant model of sepsis. It has been reported that these mice have normal patterns of CD4+ and CD8+ αβ T cells [9]. Here, we show that these mice have no expression of the γδ TCR, as assessed by flow cytometric analysis of cells from the intestine (Fig. 1, B and C), blood, and spleen (data not shown).
γδ T cell-deficient mice have increased mortality following CLP
To explore whether the function of γδ T cells affects CLP-induced mortality, the survival of γδ T cell-deficient and wild-type mice was assessed over a 10-day period after CLP. Wild-type mice had a significantly higher (46%) survival than mice lacking γδ T cells (14%) following CLP (Fig. 2A). The medial survival time was higher in wild-type mice (100 h) as compared with the γδ T cell-deficient mice (71 h; data not shown). As the level of IL-6 in the circulation 6 h after CLP is known to be predictive of outcome [16], serum from wild-type and γδ T cell-deficient mice was measured for IL-6 6 h after CLP. As shown in Figure 2B, levels of IL-6 were significantly higher in γδ T cell-deficient mice (11.6±1.8 ng/ml) as compared with the wild-type control group (3.6±0.5 ng/ml). Twenty hours after CLP, we found significantly higher amount of liver damage, as determined by serum ALT, in the γδ T cell-deficient mice compared with wild-type mice (Fig. 2C). However, we found no significant differences in systemic AST, a nonspecific marker of tissue injury. These data suggest that γδ T cells can alter survival and liver damage during sepsis.
Fig. 2.
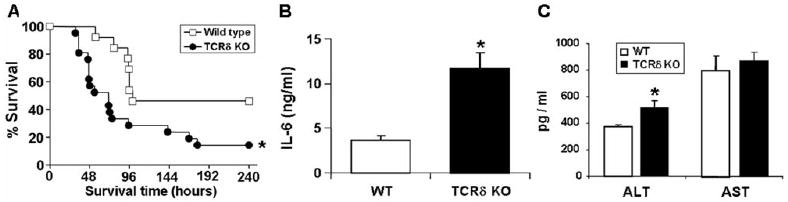
γδ T cells increase survivability following CLP. (A) Wild type (n=13) and γδ T cell-deficient mice (n=21) underwent CLP and were monitored for survival for 10 days. The survival curve combines data from two independent experiments. (B) Serum IL-6 levels 6 h after CLP. IL-6 cytokine levels (ng/ml) were determined using ELISA. The sample size equals four to six per group, and values represent the mean ± SD. Serum was collected 20 h after CLP. (C) Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations were determined 20 h following CLP. The sample size equals five to six per group, and values represent the average ± SD. *, P < 0.05, as compared with wild-type (WT).
γδ T cell-deficient mice have increased bacteremia following CLP
We next investigated the extent of bacteremia in wild-type and γδ T cell-deficient mice. We assessed bacteria in the blood 18 h after CLP, prior to any mortality in either of the groups. Blood was cultured for 24 h, and the amount of viable bacteria present was quantified. Colony counts were ~3 logs higher in the γδ T cell-deficient mice than in wild-type mice (Fig. 3A). In control experiments, no bacteria were observed in the blood of sham mice from either group (data not shown). To determine if the observed, high bacterial load contributed to the increased mortality of γδ T cell-deficient mice, we tested whether treatment with the antibiotic Primaxin increased survival following CLP. Wild-type and γδ T cell-deficient mice were treated with Primaxin or an equal volume of normal saline immediately after CLP and every 12 h thereafter for 3 days. As shown in Figure 3, B and C, treatment with Primaxin did not significantly change survival in wild-type (P=0.8110) or γδ T cell-deficient mice (P=0.7087). In control experiments, we found that Primaxin reduced bacteremia 92% in the γδ T cell-deficient mice and 100% in the wild-type mice (data not shown). Thus, our data suggest that γδ T cells are involved in controlling bacterial load, yet the increased bacterial load in γδ T cell-deficient mice does not contribute to the increased mortality observed in these mice.
Fig. 3.
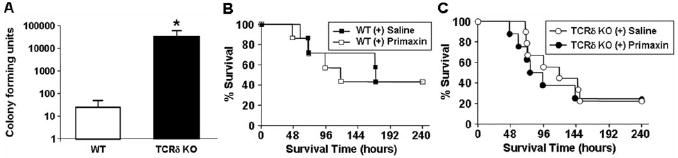
Bacterial counts are elevated in the blood of the γδ T cell-deficient mice. (A) Bacterial counts 20 h after CLP. The blood was plated, and bacterial CFU were calculated in γδ T cell-deficient (n=6) and wild-type mice (n=5). γδ T cell-deficient and wild-type mice underwent CLP and were treated every 12 h with the antibiotic Primaxin (50 μg, s.c.) or saline for 72 h (n=7–9 in each group). Survival was monitored over 240 h for (B) wild-type mice ± antibiotic or (C) γδ T cell-deficient ± antibiotic.
Recruitment of neutrophils and myeloid suppressor cells (MSC) to the site of infection is decreased in γδ T cell-deficient mice
As neutrophils play a key role in eliminating bacteria [17], we examined whether γδ T cells mediate the activation and recruitment of these cells during the early stages of CLP-induced sepsis. We isolated peritoneal neutrophils from γδ T cell-deficient and wild-type mice 20 h after CLP and found no significant differences in spontaneous or fMLP-stimulated oxidative burst activity (data not shown). The absolute counts of all cells from the peritoneal cavity 20 h after CLP were consistently approximately twofold higher in wild-type compared with γδ T cell-deficient mice. Flow cytometric analysis using the combination of mAb 7/4 and Gr-1 identified two populations of myeloid cells. Sorting on the 7/4+ Gr-1hi population (R2) followed by cytospins of sorted populations displayed cells characteristic of murine neutrophils. Combining the leukocyte counts with the flow cytometric analysis (Fig. 4A), we determined that the number of peritoneal neutrophils (R2 gate) was 186% higher in wild-type mice than in γδ T cell-deficient mice (Fig. 4B). The Gr-1int population (R3 gate) had much lower side-scatter but was larger than standard polymorphonuclear cells. The numbers of these cells were twofold higher in wild-type mice when compared with γδ T cell-deficient mice (Fig. 4C). Sorting on the R3 gate and visualization by cytospin revealed a number of ring-shaped cells within this cell population (data not shown), suggesting a MSC phenotype [18]. As expression of Arginase I has been shown to be a specific characteristic of MSC [19], we examined the expression of Arginase I by Western blot of these sorted cells. This cell population had high expression of Arginase I compared with the sorted neutrophils (R2 gate; Fig. 4D). These data suggest that γδ T cells regulate the recruitment of neutrophils and MSC to the peritoneum following CLP.
Fig. 4.
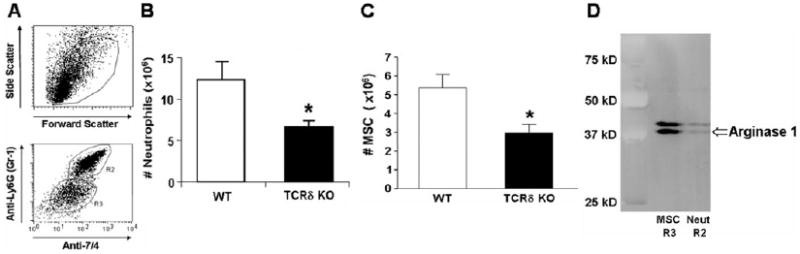
γδ T cells augment recruitment of neutrophils and MSC to the site of infection. γδ T cell-deficient (n=7) and wild-type mice (n=5) underwent CLP. Twenty hours later, cells within the peritoneum were collected by lavage. (A) Representative flow cytometric analysis of peritoneal cells. (B) Absolute numbers of peritoneal neutrophils from γδ T cell-deficient and wild-type mice. (C) Absolute numbers of peritoneal MSC from γδ T cell-deficient and wild-type mice. (D) Immunoblot analysis of Arginase I of cells sorted on the R2 or R3 gate as shown in A, representative of three independent immunoblots. Cell numbers represent the average ± SD. *, P < 0.05, as compared with healthy controls. Neut, Neutrophils.
Increased systemic IFN-γ, intestinal permeability/injury, and decreased IL-10 and intestinal MPO are associated in TCR-δ-deficient mice following CLP
A previous report documented increased ex vivo IFN-γ production by splenic T cells from septic γδ T cell-deficient mice compared with those from wild-type mice [8]. We hypothesized that systemic IFN-γ concentrations would be increased in γδ T cell-deficient mice during sepsis and that this increased IFN-γ contributed to the increased organ injury observed in the γδ T cell-deficient mice. Using an in vivo capture assay [13], serum levels of IFN-γ in γδ T cell-deficient mice were 238% higher than wild-type mice 24 h after CLP (Fig. 5A). In vitro studies have demonstrated that IFN-γ can induce a leaky mucosal barrier (reviewed in ref. [20]). To determine if the increased IFN-γ observed in γδ T cell-deficient mice was responsible for altered intestinal barrier function, we isolated distal ileum segments of intestine 20 h after CLP and determined intestinal permeability ex vivo. In wild-type mice, there was a progressive increase in intestinal permeability to HRP (Fig. 5B). Treatment of wild-type mice with anti-IFN-γ had no effect on the increase in permeability. Distal ileal segments from γδ T cell-deficient mice had significantly higher permeability to HRP (Fig. 5B) when compared with wild-type mice. Treatment of γδ T cell-deficient mice with anti-IFN-γ abrogated the increase in permeability to levels similar to wild-type mice (Fig. 5B). The addition of the anti-IFN-γ isotype control did not significantly alter intestinal permeability in wild-type or γδ T cell-deficient mice as compared with saline (data not shown). As IL-10 and NO have been demonstrated to reduce T cell-mediated IFN-γ production [21], we investigated whether there were systemic differences in these mediators. Wild-type mice had a 15% increase in systemic IL-10 (Fig. 5C) and a threefold increase of IL-10 in the peritoneum (data not shown), and no significant differences were observed in systemic and peritoneal NO concentrations (data not shown).
Fig. 5.
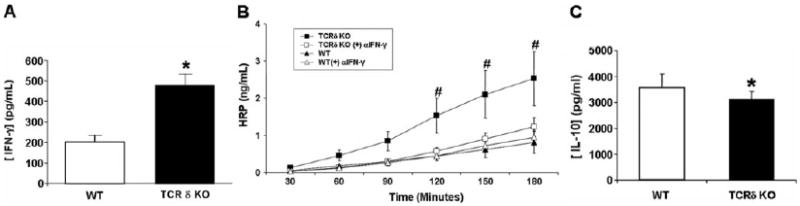
Increased intestinal permeability in γδ T cell-deficient mice is associated with increased IFN-γ. Wild-type (n=4) and γδ T cell-deficient mice (n=3) underwent CLP. (A) Systemic IFN-γ after 24 h was determined by the in vivo capture assay as described in Materials and Methods. Wild type (n=5) and γδ T cell-deficient mice (n=5) underwent CLP, both ± neutralizing IFN-γ antibodies. Permeability of (B) 40 kDa HRP in segments of distal ileum incubated in Ussing chambers. Values represent the average ± SD. (C) Serum IL-10 concentrations 24 h following CLP as determined by ELISA. *, P < 0.05, as compared with wild-type; #, P < 0.05, as compared with TCRδ KO, TCRδ KO + αIFN-γ, wild-type, and wild-type + αIFN-γ.
We hypothesized that changes in intestinal permeability would be associated with altered numbers of neutrophils. Therefore, we measured the intestinal content of MPO, a surrogate marker of neutrophil accumulation. Twenty-four hours following CLP, we found the MPO content within the intestine derived from septic γδ T cell-deficient mice to be approximately one-half that from wild-type mice (Fig. 6A). Analysis of the intestinal histology revealed that the intestines from sham-operated, wild-type and γδ T cell-deficient mice were healthy and not markedly different (Fig. 6, B and C). Analysis of intestines taken from wild-type mice 24 h following CLP revealed increased leukocyte infiltration of the musculara mucos, sloughing of villious tips, as well as evidence of unhealthy crypt cells (Fig. 6D). Examination of the intestines taken from γδ T cell-deficient mice 24 h following CLP revealed decreased leukocyte infiltration, greater reduction of the villious height, and decreased crypt depth (Fig. 6E). However, there was no evidence of edema as determined by intestine wet/dry ratios (data not shown). Thus, the data show that intestinal injury is more severe in septic γδ T cell-deficient mice, and this is independent of neutrophil numbers.
Fig. 6.
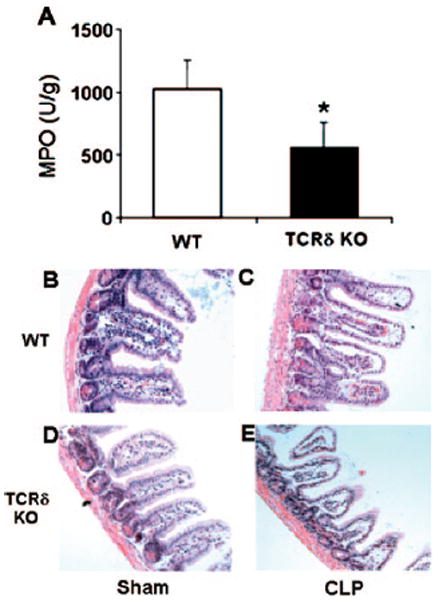
Increased sepsis-induced intestinal injury mediated by γδ T cells is myeloid cell-independent. Intestines were extracted from sham- and CLP-operated mice after 24 h. (A) Intestinal MPO concentration was determined from γδ T cell and wild-type mice as described in Materials and Methods. (B–E) Histology of sham- and CLP-operated ileum after 24 h from γδ T cell and wild-type mice as described in Materials and Methods. *, P < 0.05, as compared with wild-type.
DISCUSSION
Following trauma, the immune response is characterized by changes in circulating leukocytes. All patients in our human study showed a decrease in the absolute number of circulating γδ T cells after blunt trauma. It is unclear whether the decrease in the number of lymphocytes in the trauma patients is a result of apoptosis, recruitment from the circulation to the peripheral tissues, or changes from transfusion of blood products. Based on the published literature (ref. [14] and reviewed in ref. [22]), as well as on our own unpublished data, we speculate that the decrease is largely a result of apoptosis. Decreases in lymphocyte numbers are thought to contribute to the immunosuppression observed following trauma and therefore, the increased susceptibility to infection in this patient population. Many of the previous studies investigating the role of T cells in sepsis have used athymic or Rag-1-deficient mice. A limitation to these studies is that CD4, CD8, γδ, and NK T cells are absent in these mice.
Our present report demonstrates that shortly following blunt trauma, human patients have decreased numbers of circulating γδ T cells. To investigate the significance of that observation, we examined the response of γδ T cell-deficient mice in a clinically relevant model of sepsis induced by CLP. We found that γδ T cell-deficient mice had increased mortality, systemic IL-6, and liver damage as compared with wild-type mice following CLP. The bacterial load was increased significantly in γδ T cell-deficient mice, yet antibiotic treatment did not significantly change mortality. A variety of other studies has shown that imipenem (Primaxin) treatment increases survival in mice that have undergone CLP [23-25]. Here, we show Primaxin treatment did not confer any survival benefit for treatment of our septic mice. Other reports have shown that imipenem treatment does not alter survival in wild-type mice [9, 26], which is in agreement with our current data. We cannot explain the different results seen with this antibiotic during sepsis. However, we speculate that the differences in strains of mice, imipenem treatment modality, and CLP model used may contribute to the differences observed in the various studies. Even in the presence of antibiotics, inflammation-increasing mediators such as endotoxins and superantigens are likely still present. We speculate that γδ T cells beneficially alter the response to these agents.
MSC were first identified in cancer studies [27]. They were named as a result of their ability to inhibit T cell proliferation and IFN-γ production [28]. That MSC are present and play a role during sepsis and following trauma has only recently been determined. During sepsis, the elegant characterization of splenic MSC during CLP-induced sepsis was conducted [29]. Here, it was found that sepsis induced an increase in splenic CD11b+ and Gr-1+ MSC. It was also found that LPS stimulation of MSC taken from septic mice resulted in the robust production of IL-10 [29]. We recently characterized splenic MSC isolated from thermally injured mice. These cells were also identified by CD11b and Gr-1 expression and by their ability to suppress T cell proliferation [30]. Here, we identify MSC in the peritoneum of septic mice. We observed that recruitment of MSC to the site of infection was diminished significantly in γδ T cell-deficient mice. The data show that γδ T cells did not alter the proportion of MSC recruited to the site of infection. However, MSC absolute numbers were decreased in the absence of γδ T cells. This may be a result of the reduced production of a MSC-specific chemokine or by reduced production of cytokines that generate MSC, possibly G-CSF. We further found that systemic IL-10 is higher in wild-type mice. We hypothesize that in wild-type mice, this increased IL-10 is associated with the increased numbers of MSC, and this may be, in part, responsible for the decreased IFN-γ concentration.
We show that neutrophils were reduced in the peritoneal wash as well as from the intestine. It is possible that γδ T cells augment the neutrophil-attracting chemokine, keratinocyte-derived chemokine (data not shown). We are currently examining whether γδ T cells augment the production of the relevant chemokines directly or through effects on other cell types. Activation of the neutrophil’s antibacterial and inflammatory functions is often not specific to the target cell and can lead to robust bystander cell injury. This is thought to precipitate in organ dysfunction and failure. In general, a bacterial infection in the lung results in the increase of proinflammatory cytokines such as TNF-α and IL-6. In contrast, a more anti-inflammatory response is observed in the gut [31]. We speculate that γδ T cells located in the gut contribute to the anti-inflammatory milieu by mediating production of anti-inflammatory cytokines, such as IL-4, IL-10, TGF-β, or PGE2. Further, this may reduce the neutrophil’s nonspecific tissue damage and enhance bacterial clearance. Altogether, our data suggest that γδ T cells enhance bacterial clearance and decrease liver and intestine tissue damage.
Finally, we found that systemic levels of IFN-γ were increased in γδ T cell-deficient mice during the first 24 h of sepsis and that this was associated with an increase in intestinal permeability. We found that neutralization of IFN-γ abrogated the increase in intestinal permeability in γδ T cell-deficient mice. Other reports have shown that increased IFN-γ levels are associated with increased bacteremia. It has further been shown that decreased IFN-γ enhances fibrin deposits in the peritoneum [32]. It appears that the negative regulation of IFN-γ production by γδ T cells limits intestinal permeability. This along with the ability of γδ T cells to augment neutrophil recruitment to the site of infection likely account for the increased bacteremia observed in γδ T cell-deficient mice after CLP.
In summary, our study demonstrates that γδ T cells enhance survival in the CLP model of sepsis. γδ T cells are required to reduce bacteremia and enhance neutrophil recruitment to the site of infection. Finally, γδ T cells play a key regulatory role in maintaining intestinal permeability during sepsis and accomplish this by modulating systemic IFN-γ concentrations.
Acknowledgments
This work was supported by funding from the National Institutes of Health R01 GM72760 and by the Shriner’s of North American Project #8560. The authors thank Dr. Fred Finkelman (University of Cincinnati) for the generous donation of the neutralizing IFN-γ antibody. The authors also thank Kelsey Guanciale and Julie M. Caldwell for the careful review of the manuscript as well as Laura E. James for her critical help with the statistical analysis.
Footnotes
The authors have no financial conflict of interest.
References
- 1.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 2.Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 3.Pennington DJ, Vermijlen D, Wise EL, Clarke SL, Tigelaar RE, Hayday AC. The integration of conventional and unconventional T cells that characterizes cell-mediated responses. Adv Immunol. 2005;87:27–59. doi: 10.1016/S0065-2776(05)87002-6. [DOI] [PubMed] [Google Scholar]
- 4.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γ δ T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 5.Duhindan N, Farley AJ, Humphreys S, Parker C, Rossiter B, Brooks CG. Patterns of lymphokine secretion amongst mouse γ δ T cell clones. Eur J Immunol. 1997;27:1704–1712. doi: 10.1002/eji.1830270717. [DOI] [PubMed] [Google Scholar]
- 6.Skeen MJ, Rix EP, Freeman MM, Ziegler HK. Exaggerated proinflammatory and Th1 responses in the absence of γ/δ T cells after infection with Listeria monocytogenes. Infect Immun. 2001;69:7213–7223. doi: 10.1128/IAI.69.12.7213-7223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore TA, Moore BB, Newstead MW, Standiford TJ. γδ-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 8.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of γδ T lymphocytes contributes to mortality and immunosuppression in sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1338–R1343. doi: 10.1152/ajpregu.00283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enoh VT, Lin SH, Lin CY, Toliver-Kinsky T, Murphey ED, Varma TK, Sherwood ER. Mice depleted of αβ but not γδ T cells are resistant to mortality caused by cecal ligation and puncture. Shock. 2007;27:507–519. doi: 10.1097/SHK.0b013e31802b5d9f. [DOI] [PubMed] [Google Scholar]
- 10.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-γ production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21:415–425. doi: 10.1097/00024382-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Benjamim CF, Hogaboam CM, Lukacs NW, Kunkel SL. Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol. 2003;163:2605–2617. doi: 10.1016/S0002-9440(10)63615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse. Int Immunol. 1999;11:1811–1818. doi: 10.1093/intimm/11.11.1811. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 15.Hoover L, Bochicchio GV, Napolitano LM, Joshi M, Bochicchio K, Meyer W, Scalea TM. Systemic inflammatory response syndrome and nosocomial infection in trauma. J Trauma. 2006;61:310–316. doi: 10.1097/01.ta.0000229052.75460.c2. [DOI] [PubMed] [Google Scholar]
- 16.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–1306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 20.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 21.Hanlon AM, Jang S, Salgame P. Signaling from cytokine receptors that affect Th1 responses. Front Biosci. 2002;7:d1247–d1254. doi: 10.2741/hanlon. [DOI] [PubMed] [Google Scholar]
- 22.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 23.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vianna RC, Gomes RN, Bozza FA, Amancio RT, Bozza PT, David CM, Castro-Faria-Neto HC. Antibiotic treatment in a murine model of sepsis: impact on cytokines and endotoxin release. Shock. 2004;21:115–120. doi: 10.1097/01.shk.0000111828.07309.21. [DOI] [PubMed] [Google Scholar]
- 25.Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1048–R1053. doi: 10.1152/ajpregu.00312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enoh VT, Fairchild CD, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on wild-type and NK cell-deficient CD8 knockout mice during acute intra-abdominal injury. Am J Physiol Regul Integr Comp Physiol. 2006;290:R685–R693. doi: 10.1152/ajpregu.00678.2005. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/ CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 29.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noel JG, Osterburg A, Wang Q, Guo X, Byrum D, Schwemberger S, Goetzman H, Caldwell CC, Ogle CK. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock. 2007;28:684–693. doi: 10.1097/shk.0b013e31805362ed. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura M, Fujiyama Y, Niwakawa M, Sasaki T, Bamba T. In vivo cytokine responses in gut-associated lymphoid tissue (GALT) and spleen following oral administration of staphylococcal enterotoxin B. Immunol Lett. 2002;81:77–85. doi: 10.1016/s0165-2478(01)00334-0. [DOI] [PubMed] [Google Scholar]
- 32.Qiu G, Gribbin E, Harrison K, Sinha N, Yin K. Inhibition of γ interferon decreases bacterial load in peritonitis by accelerating peritoneal fibrin deposition and tissue repair. Infect Immun. 2003;71:2766–2774. doi: 10.1128/IAI.71.5.2766-2774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


