Abstract
Pregnancy is associated with a significant decrease in uterine vascular tone and an increase in uterine blood flow. The present study tested the hypothesis that estrogen and progesterone differentially regulate the ERK1/2 and PKC signaling pathways in vascular smooth muscle resulting in a decrease in uterine vascular myogenic tone in pregnancy. Uterine arteries were isolated from nonpregnant (NPUA) and near-term pregnant (PUA) sheep. Chronic treatment (48 h) of NPUA with 17β-estradiol and progesterone caused a significant decrease in PKC-mediated contractions and pressure-induced myogenic tone. In accordance, treatment of PUA for 48 h with ICI 182,780 and RU 486 significantly increased PKC-induced contractions and myogenic tone. In contrast, acute treatment for 30 min had no effects on uterine artery contractility. An ERK1/2 inhibitor PD098059 restored the chronic effect of steroids on PKC-mediated contractions in NPUA. ERK1/2 protein and mRNA levels were greater in PUA as compared with NPUA. 17β-Estradiol and progesterone increased ERK1/2 protein in NPUA. In agreement, ICI 182,780 and RU 486 caused a significant decrease in ERK1/2 protein in PUA. Western blot showed six PKC isozymes, α, βI, βII, δ, ε and ζ in the uterine arteries. 17β-Estradiol and progesterone decreased the particulate-to-cytosolic ratio of PKCα, ε, and ζ, respectively, in NPUA. ICI 182,780 and RU 486 increased them in PUA. The results indicate a direct chronic effect of the steroid hormones in the up-regulation of ERK1/2 expression and down-regulation of PKC signaling pathway, resulting in attenuated myogenic tone of uterine artery in pregnancy.
Keywords: pregnancy, uterine artery, steroids, protein kinase C, ERK, myogenic tone
Introduction
Pregnancy is associated with a significant decrease in uterine vascular tone and an increase in uterine blood flow, which is essential for the growth of the fetus as well as cardiovascular well-being of the mother. Recent studies have demonstrated that pressure-induced myogenic response is significantly decreased in the uterine artery in pregnancy.1-3 The physiological importance of myogenic response in the regulation of uterine blood flow in human pregnancy has been demonstrated in myometrial arteries in term pregnant women.4,5 Given that pressure-dependent myogenic contraction is an important physiological mechanism that regulates basal vascular tone, and is a major contributor to the modulation of organ blood flow, the decreased myogenic tone of the uterine artery is likely to contribute significantly to the adaptation of uterine vascular hemodynamics in pregnancy.
Protein kinase C (PKC) plays an important role in regulating arterial myogenic response.6,7 We have demonstrated that the reduced myogenic tone of resistance-sized uterine arteries in pregnant sheep is mediated primarily by a decreased PKC signaling pathway.1,8,9 In addition, we have demonstrated that extracellular signal-regulated kinase (ERK1/2) functions as an upstream signal in suppressing the PKC activity in pregnant uterine arteries.1,8,9 The inhibition of ERK1/2 increased PKC-mediated contractions and myogenic tone in pregnant uterine arteries,1,8 suggesting a physiological mechanism of ERK1/2 in the increased uterine blood flow by suppressing the basal vascular tone during pregnancy.
The mechanisms in the regulation of PKC/ERK1/2 signaling pathway and myogenic tone of the uterine artery during pregnancy remain undetermined. Previous in vivo studies have suggested an important role of the steroid hormones in the regulation of uterine blood flow during pregnancy. Both estrogen and progesterone receptors have been identified in uterine artery vascular smooth muscle.10,11 Studies in ovariectomized and pregnant ewes have demonstrated a key role of 17β-estradiol (E2β) in the regulation of uterine blood flow.12-14 The effect of progesterone in regulating uterine blood flow during pregnancy is less clear and appears controversial, possibly due to a relative difficulty of in vivo studies with prolonged treatment of a progesterone receptor antagonist in pregnant animals. Nonetheless, most in vivo studies to date have focused primarily on acute and nongenomic effects of the steroid hormones on relaxation of the uterine artery, observed at the concentrations substantially higher than physiological concentrations.12,14-16 The chronic action of physiologically relevant concentrations of the steroid hormones on uterine artery contractility and myogenic tone, and their adaptation to pregnancy remain poorly understood. The differences between pharmacological and physiological responses of uterine blood flow to estrogen have been recognized.17
The present study investigated the effects of estrogen and progesterone on pressure-dependent myogenic tone of the resistance-sized uterine arteries obtained from nonpregnant and near-term pregnant ewes in an ex vivo tissue culture model system. We hypothesized that the steroid hormones have direct and chronic effects in the up-regulation of ERK1/2 expression and down-regulation of PKC signaling pathway in vascular smooth muscle, resulting in attenuated myogenic tone of the uterine artery in pregnancy.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement at http://hyper.ahajournals.org.
Tissue preparation and treatment
Uterine arteries were isolated from nonpregnant and near-term pregnant (∼140 days gestation) sheep, and arterial preparations were incubated in phenol red-free DMEM (Mediatech Cellgro) with 1% charcoal-stripped FBS for 48 h at 37 °C in a humidified incubator with 5% CO2/95% air in the absence or presence of E2β, progesterone, ICI 182,780, and RU 486, respectively. All procedures and protocols were approved by the Institutional Animal Care and Use Committee guidelines.
Measurement of myogenic tone
Pressure-dependent myogenic tone of resistance-sized uterine arteries were measured as described previously.1
Contraction studies
Isometric tensions were measured in tissue baths at 37 °C, as described previously.18
Measurement of ERK1/2 mRNA levels
ERK1/2 mRNA was quantified by coupled RT-PCR amplification in a single tube assay as described previously.19
Western immunoblotting analysis
ERK1/2 protein abundance was measured in freshly isolated uterine arteries and after the hormonal and/or antagonist treatments by Western blot analysis.
Measurement of PKC isozyme translocation
After the treatments, tissues were homogenized in an ice-cold lysis buffer. The cytosolic and particulate fractions were separated as previously described.20 Proteins from cytosolic and particulate fractions were subjected to electrophoresis on 10% SDS-PAGE. PKCα, βI, βII, δ, ε, and ζ were detected and analyzed as previously described.20
Data analysis
Data were expressed as means ± SEM obtained from the number (n) of experimental animals given. Differences were evaluated for statistical significance (P < 0.05) by ANOVA or t-test, where appropriate.
Results
Effect of steroid hormones on pressure-dependent myogenic tone
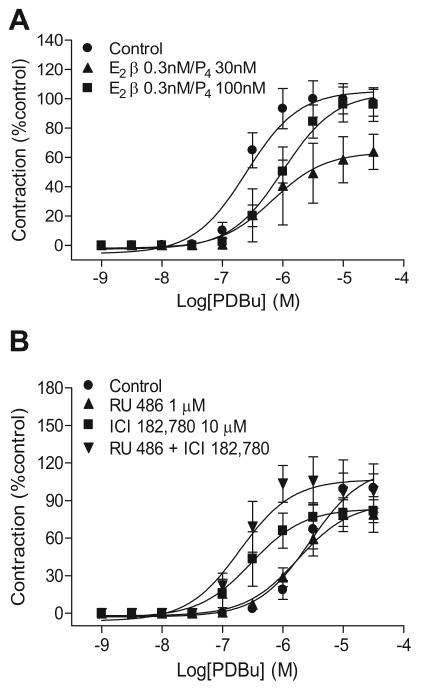
As shown in Figure 1, pressure-dependent myogenic tone was significantly less in the uterine artery of pregnant sheep as compared with that of nonpregnant animals. E2β (0.3 nM) and progesterone (100 nM) treatment for 48 h resulted in significant decreases in myogenic responses in nonpregnant uterine arteries (Figure 1A), and abolished the difference in pressure-induced myogenic tone between nonpregnant and pregnant uterine arteries (Figure 1). In accordance, the chronic treatment with an estrogen receptor antagonist ICI 182,780 (10 μM) and a progesterone receptor antagonist RU 486 (1 μM) caused significant increases in myogenic responses in pregnant uterine arteries (Figure 1B).
Figure 1. Effect of steroid hormones on pressure-dependent myogenic tone.
Panel A: Myogenic tone was determined in nonpregnant uterine arteries after pretreatment with 17β-estradiol (E2β, 0.3 nM) plus progesterone (P4, 100 nM) or vehicle control for 48 h at given pressures. Panel B: Myogenic tone was determined in pregnant uterine arteries after pretreatment with ICI 182,780 (10 μM ) plus RU 486 (1 μM) or vehicle control for 48 h in the presence of E2β (0.3 nM) plus P4 (100 nM) at given pressures. Myogenic tone was determined in the presence of 100 μM L-NNA. Data are means ± SEM of 4-5 animals. * P < 0.05, significant difference between control and treatment groups, as determined by repeated-measures, two-way ANOVA.
Effect of steroid hormones on PKC-mediated contractions
PDBu-induced contractions were significantly attenuated in uterine arteries from pregnant (pD2: 5.52 ± 0.05; Emax: 66.7 ± 7.8 %KCl maximum), as compared with nonpregnant (pD2: 6.61 ± 0.12, P < 0.05; Emax: 136.8 ± 6.9 %KCl maximum, P < 0.05) ewes. Neither E2β (0.3 nM) and progesterone (30 nM) nor ICI 182,780 (10 μM) and RU 486 (1 μM) had acute (30 min pretreatment) effect on PDBu-induced contractions in uterine arteries from nonpregnant or pregnant sheep (data not shown).
In nonpregnant uterine arteries, the chronic treatment with E2β produced a concentration-dependent attenuation of PDBu-induced contractions (Figure S1A). Treatment with 0.3 nM E2β significantly decreased the pD2 value (6.6 ± 0.1 vs. 5.9 ± 0.1, P < 0.05) but not the Emax. However, 10 nM E2β significantly decreased the Emax (105.5 ± 5.3 vs. 71.2 ± 7.2% control response, P < 0.05) but not the pD2 value. Similarly, progesterone also produced a concentration-dependent decrease in PDBu-mediated contractions (Figure S1B). Chronic treatment with 30 nM progesterone significantly reduced the Emax (105.5 ± 5.3 vs. 76.7 ± 7.4% control response, P < 0.05) but not the pD2 value. Higher concentration of 100 nM progesterone decreased the pD2 value (6.6 ± 0.1 vs. 5.8 ± 0.1, P < 0.05) but not the Emax. Additionally, the combined treatment of E2β plus progesterone significantly inhibited PDBu-induced contractions (Figure 2A). Combination of 0.3 nM E2β and 30 nM progesterone significantly attenuated the Emax (105.5 ± 5.3 vs. 63.6 ± 9.0% control response, P < 0.05) but not the pD2 value. Combination of 0.3 nM E2β and 100 nM progesterone significantly decreased the pD2 value (6.6 ± 0.1 vs. 6.0 ± 0.1, P < 0.05) but not the Emax. Unlike uterine arteries, the same concentrations of the steroid hormones had no significant effects on PDBu-induced contractions in mesenteric arteries (Figure S2).
Figure 2. Effect of steroid hormones on PDBu-induced contractions.
Panel A: Nonpregnant uterine arteries were treated with 17β-estradiol (E2β) plus progesterone (P4) or vehicle control for 48 h. Panel B: Pregnant uterine arteries were treated with ICI 182,780 or/and RU 486 or vehicle control for 48 h in the presence of E2β (0.3 nM) plus P4 (100 nM). Data are means ± SEM of 4-11 animals. pD2 and Emax values are presented in Results.
In contrast to PDBu-induced contractions, neither acute (30 min) nor chronic (48 h) pretreatments with E2β (0.3, 10 nM), progesterone (30 nM, 100 nM) or their combinations had effects on KCl-induced contractions (data not shown).
In pregnant uterine arteries, the chronic treatment with ICI 182,780 significantly increased the pD2 value of PDBu-induced contractions from 5.6 ± 0.2 to 6.5 ± 0.2 (P < 0.05) (Figure 2B). On the other hand, RU 486 alone had no significant effect on PDBu-induced contractions. However, the combined treatment with RU 486 and ICI 182,780 significantly increased the pD2 value (5.6 ± 0.2 vs. 6.7 ± 0.2, P < 0.05) (Figure 2B). To determine whether the endothelium plays a role in the steroid hormones-mediated effect, the studies were repeated in endothelium-denuded uterine arteries. As shown in Figure S3, the effects of the steroid hormone receptor antagonists on PDBu-mediated contractions were similar as those determined in the endothelium intact preparations, with the combined treatment of ICI 182,780 plus RU 486 increasing the pD2 values from 5.3 ± 0.1 to 6.4 ± 0.1 (P < 0.05).
Given that RU 486 blocks glucocorticoid receptors as well as progesterone receptors, we determined a possible involvement of glucocorticoid receptors in progesterone-mediated effects in uterine arteries, using a potent and selective glucocorticoid receptor antagonist 21-hydroxy-6,19-epoxyprogesterone (5b).21 As shown in Figure S4, 10 μM 5b had no effect on progesterone-mediated inhibition of PDBu-induced contractions in the uterine arteries. We further tested its effect on glucocorticoid receptor-mediated responses in the uterine arteries. Consistent with our previous studies,18 chronic treatment with cortisol significantly enhanced norepinephrine-induced contractions in nonpregnant uterine arteries, which was blocked by 10 μM 5b (Figure S5).
Effect of ERK1/2 in steroid hormones-suppressed PKC-mediated contractions
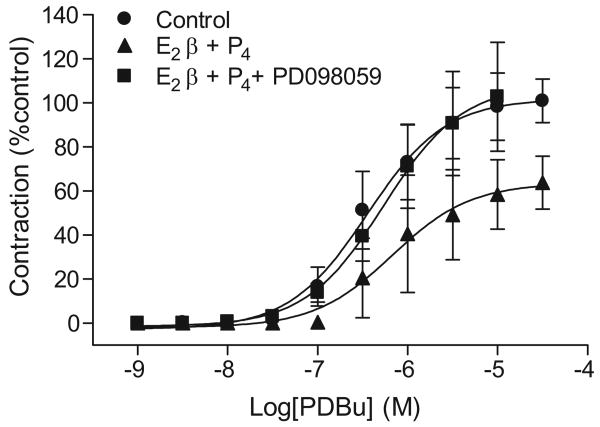
As shown in Figure 3, an ERK inhibitor PD098059 reversed the effect of E2β plus progesterone on PDBu-induced contractions in nonpregnant uterine arteries. ERK1/2 protein and mRNA abundance was determined in uterine artery vascular smooth muscle. Western blot analysis showed a significant increase in ERK1/2 protein abundance in pregnant, as compared with nonpregnant, uterine arteries (Figure 4A). In accordance, there were significantly higher levels of phospho-ERK1/2 in pregnant uterine arteries (Figure 4A). However, the ratio of phospho-ERK1/2/total-ERK1/2 was not significantly different in the uterine arteries between nonpregnant and pregnant ewes. In agreement with the increased protein abundance, ERK1/2 mRNA levels were also significantly greater in pregnant, as compared with nonpregnant, uterine arteries (Figure S6).
Figure 3. Effect of ERK on steroids-suppressed PKC-mediated contractions.
Nonpregnant uterine arteries were treated with 17β-estradiol (E2β) plus progesterone (P4) or vehicle control for 48 h in the presence or absence of PD098059 (30 μM) and then submitted to the cumulative additions of PDBu in the tissue bath. Data are means ± SEM of 3-9 animals.
Figure 4. Effect of pregnancy and steroid hormones on ERK1/2 protein abundance.
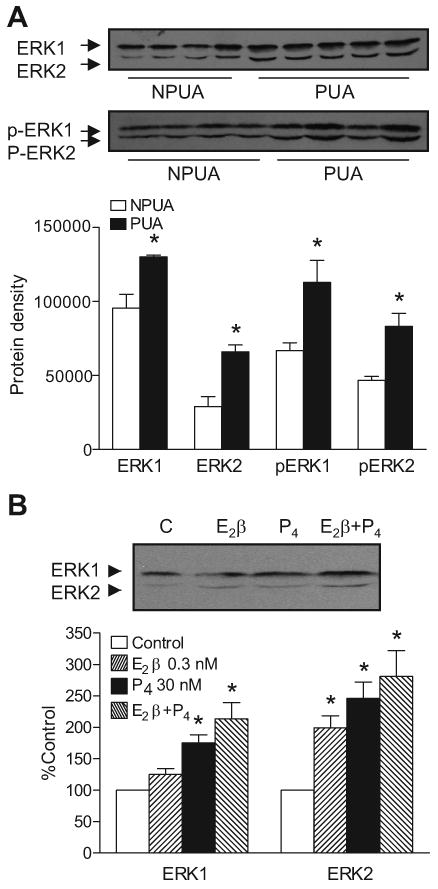
Panel A. Uterine arteries were isolated from pregnant (PUA) and nonpregnant (NPUA) sheep. Panel B. Nonpregnant uterine arteries were treated with 17β-estradiol (E2β) or/and progesterone (P4) or vehicle control for 48 h. Total and phosphorylated ERK1/2 protein abundance was determined by Western blotting. Data are means ± SEM of 4-5 animals. * P < 0.05, vs. NPUA (Panel A) or vs. control (Panel B).
As shown in Figure 4B, the chronic treatment with 0.3 nM E2β produced a significant increase in ERK2 but not ERK1 in nonpregnant uterine arteries. The treatments with progesterone (30 nM) or combined E2β plus progesterone resulted in significant increases in both ERK1 and ERK2 (Figure 4B). In accordance, the chronic treatment with 10 μM ICI 182,780 decreased ERK2 but not ERK1 in pregnant uterine arteries (Figure S7). RU 486 (1 μM) or combined ICI 182,780 and RU 486 produced significant decreases in both ERK1 and ERK2 (Figure S7).
Effect of steroid hormones on PKC isozymes translocation
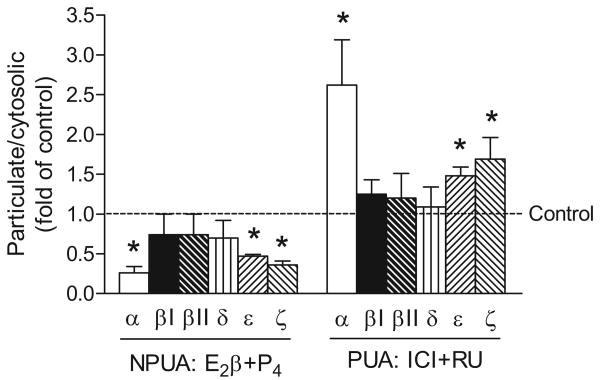
The subcellular distribution of PKC isozymes in cytosolic and particulate fractions in uterine artery vascular smooth muscle after the chronic treatments with the steroid hormones or their receptor antagonists is shown in Figure 5. Six isozymes of PKC, α, βI, βII, δ, ε, and ζ were determined in the uterine arteries. In nonpregnant uterine arteries, the combined treatment of E2β plus progesterone produced significant decreases in the particulate-to-cytosolic (P/S) ratio of PKCα, ε, and ζ (Figure 5). The subcellular distribution of PKCβI, βII, δ was not significantly affected. In accordance, in pregnant uterine arteries, the combined ICI 182,780 plus RU 486 significantly increased the P/S ratio of PKCα, ε, and ζ (Figure 5).
Figure 5. Effect of steroid hormones and antagonists on PKC activities.
Nonpregnant uterine arteries (NPUA) were treated with 17β-estradiol (E2β, 0.3 nM) plus progesterone (P4, 100 nM) or vehicle control for 48 h. Pregnant uterine arteries (PUA) were treated with ICI 182,780 (ICI, 10 μM) plus RU 486 (RU, 1 μM) or vehicle control for 48 h in the presence of E2β (0.3 nM) plus P4 (100 nM). The cytosolic and particulate fractions were prepared as described in Methods. PKC isozymes were determined by Western blotting and expressed as ratio of particulate/cytosolic fractions. Data are means ± SEM of 4 animals. * P < 0.05, vs. control.
Discussion
The present study demonstrated for the first time that physiologically relevant concentrations of E2β and progesterone as observed in ovine pregnancy,22 had direct chronic effects on down-regulating pressure-dependent myogenic tone in the uterine artery. The finding that the steroids eliminated the difference in myogenic contractions between nonpregnant and pregnant uterine arteries suggests a major role of the hormones in the down-regulation of basal vascular tone of the uterine artery in pregnancy. This is further supported by the finding that hormonal receptor antagonists significantly increased pressure-induced myogenic tone in the uterine artery of pregnant animals. The concentration of ICI 182,780 (10 μM) used in the present study is consistent with the previous studies showing that 10 μM ICI 182,780 selectively blocked estrogen-mediated responses.23-25 In agreement with the present study, the similar temporal response of the rise in uterine blood flow has been demonstrated in ovary-intact ewes, showing the maximal increase of uterine blood flow at approximately 45–55 h in animals treated physiologically with the steroid hormones.17
Myogenic tone is an intrinsic property of smooth muscle and is independent of neural, metabolic and endothelial influences. In the present study, the hormonal effects on the myogenic reactivity were determined in the presence of an eNOS inhibitor, suggesting a smooth muscle effect of the hormones. Although a possible role of prostacyclin may not be excluded in the present study, it has been shown that the inhibition of prostaglandin synthesis dose not alter basal uterine blood flow.26,27 Consistent with the present finding, a recent study in resistance arteries of subcutaneous biopsies from postmenopausal women receiving 3-month therapy with estradiol (E2), medroxyprogesterone acetate (MPA), or E2 plus MPA, demonstrated that although E2 alone improved flow-mediated vasodilatation, pressure-dependent myogenic tone was significantly reduced only in patients receiving combined E2 plus MPA.28 An earlier study showed that in vitro treatment of subcutaneous resistance arteries from postmenopausal women with a high concentration of E2 (100 nM) for 3 h, which avoided the confounding influence of acute endothelial effects of E2, decreased myogenic tone, and this effect was blocked by ICI 182,780.29 Further findings that E2 and propyl pyrazole triol, a selective ERα agonist, but not ERβ selective genistein decreased myogenic tone ex vivo implicated the importance of ERα in the vascular responses.29 This genomic effect of ERα was supported by a following study in ERβ knockout mouse.30
In the present study, PKC-mediated contractions were significantly attenuated in nonpregnant uterine arteries following the chronic treatment with physiological concentrations of combined E2β plus progesterone. In contrast, the acute treatment had no significant effect. PKC plays an important role in the regulation of pressure-dependent myogenic response of resistance arteries, 1,6,7 and a decrease in the PKC signaling pathway accounts for the attenuated myogenic tone of the uterine artery in pregnancy.1,8,9,31,32 These findings suggest that the chronic action of the steroids in attenuating myogenic tone of the uterine artery is mediated by down-regulating the PKC signaling pathway in vascular smooth muscle. This is supported by the finding that a blockade of the hormonal receptors significantly increased PKC-mediated contractions in the uterine artery of pregnant animals. Given that RU 486 blocks glucocorticoid receptors as well as progesterone receptors, and that there is no highly selective progesterone receptor antagonist available, the present finding that a potent and selective glucocorticoid receptor antagonist 21-hydroxy-6,19-epoxyprogesterone21 blocked cortisol-induced effects but had no significant effect on the progesterone-mediated response in the uterine arteries, suggests a minimum role of glucocorticoid receptors in progesterone's action. The finding that the effects of hormonal receptor antagonists were the same in the endothelium-denuded arteries is consistent with the results of hormonal effects on myogenic tone, and thus further supports a smooth muscle action of the steroids in the uterine artery. In agreement, previous studies in ovariectomized monkeys have demonstrated that physiological concentrations of estrogen and progesterone have inherent effects in vascular smooth muscle cells and suppress the PKC activity and contractility of the coronary artery.33,34 The present finding of steroids' effects on vascular smooth muscle in the regulation of uterine artery myogenic tone provides a mechanism additional to estrogen-mediated endothelium-dependent vasodilatation in the uterine artery that accounts only in part for increased uterine blood flow during pregnancy in sheep.13,17
Consistent with the present finding, it has been shown that PDBu-mediated contractions and PKC activity are significantly greater in ovariectomized female rats than intact female animals.35 Additionally, treatment of ovariectomized females with 17β-estradiol, but not 17α-estradiol, caused significant reduction in PDBu-induced contractions and PKC activity in endothelium-denuded aortic strips, which was blocked by ICI 182,780.35 Together with the findings in human subcutaneous arteries28,29 and monkey coronary arteries,33,34 these studies suggest a systemic effect of estrogen on vascular tone. In the present study, the same concentrations of the steroids had no significant effects on PDBu-induced contractions in mesenteric arteries. This is probably due to the differences in the tissue sensitivity and estrogen receptor density between uterine and mesenteric arteries. The chronic action of estrogen in suppressing PKC activity and vascular tone is consistent with its role in the regulation of uterine blood flow demonstrated in ovariectomized and pregnant ewes. The effect of progesterone in regulating uterine blood flow is less clear, and appears controversial in animal studies between the ovarian cycle and the pregnancy. Studies in ewes have shown that during the estrous cycle and when hormones are given, changes in uterine blood flow directly relate with the ratio of concentrations of estrogen to progesterone and indirectly with the concentrations of progesterone alone.36 However, in the second half of pregnancy and at term, changes in uterine blood flow directly related with progesterone concentrations and even more prominently with the sum of progesterone and estrogen.37,38 The present study provided evidence of a direct chronic effect of progesterone in attenuating PKC-mediated vascular tone in the uterine artery. Similar findings were obtained in primate coronary vascular smooth muscle.34 In contrast, other studies demonstrated an acute effect of progesterone in sensitizing α1-adrenoceptor-mediated contractions of the uterine arteries.38,39 Taken together, these findings suggest that progesterone may have a dual role in the regulation of uterine artery contractility, i.e. sensitization of the phasic contraction but down-regulation of basal vascular tone, which may be of physiological importance during pregnancy in maintaining low myogenic tone in response to increased uterine blood flow, as well as sustaining tissue reactivity and allowing a redistribution of blood by contracting the uterine artery in response to circulating catecholamine under stress.
The question arises as to how the steroids might affect PKC activity in uterine artery vascular smooth muscle. Our previous studies have demonstrated that pregnancy-increased ERK1/2 act as an upstream signal in suppressing PKC-mediated contractions and pressure-dependent myogenic tone in the uterine arteries, suggesting a physiological mechanism of ERK1/2 in the increased uterine blood flow by suppressing the basal vascular tone during pregnancy.1,8 In ovine uterine arteries, an ERK1/2 inhibitor PD098059 inhibited phosphorylation and activation of ERK1/2.8,9 In the present study, we demonstrated that the inhibition of ERK1/2 by PD098059 restored the steroids-mediated attenuation of PDBu-induced contractions in the uterine arteries. This indicates a key role of ERK1/2 activation in the hormone-mediated suppression of PKC activity. Consistent with this finding, protein levels of total and phosphorylated ERK1/2 were significantly greater in pregnant, as compared with nonpregnant, uterine arteries. The finding that the ratio of phospho-ERK1/2/total-ERK1/2 was not significantly different between pregnant and nonpregnant uterine arteries suggests that pregnancy increases the expression of ERK1/2 resulting in elevated phospho-ERK1/2, rather than stimulates their activities per se. The increased expression of ERK1/2 in pregnant uterine arteries is likely mediated through a direct action of the steroids as it has been demonstrated in the present study that E2β plus progesterone significantly increases ERK1/2 protein abundance in nonpregnant uterine arteries. This is further supported by the finding that the ICI 182,780 and RU 486 decreased ERK1/2 in pregnant uterine arteries. Unlike progesterone that increased both ERK1 and ERK2, the finding that E2β up-regulated only ERK2 is intriguing, and suggests that ERK2 may be involved in the steroid-mediated attenuation of PKC activity and myogenic tone in the uterine artery. While many previous studies showed that both E2β and progesterone increased ERK1/2 activity acutely, few examined the effect of the steroids on the transcriptional control of ERK1/2. Future studies of the transcriptional mechanisms are needed.
The present study demonstrated further that the steroids differentially regulated the sub-cellular distribution of PKC isozymes in uterine artery vascular smooth muscle. Six PKC isozymes, α, βI, βII, δ, ε, and ζ have been detected in the smooth muscle of the uterine artery.20 E2β plus progesterone significantly reduced PKCα, ε, and ζ in the particulate fraction in nonpregnant uterine arteries, suggesting an attenuation of basal activities of these isozymes. This is supported by the finding that ICI 182,780 plus RU 486 significantly increased the particulate fractions of PKCα, ε, and ζ in pregnant uterine arteries. Both PKCα and PKCε have been implicated in contractions of vascular smooth muscle through increasing the Ca2+ sensitivity.40,41 Our recent studies demonstrated that PDBu-induced activation of PKCα was similar in the uterine arteries between nonpregnant and pregnant ewes, in the absence or presence of PD098059.42 In contrast, PDBu-induced PKCε activation was significantly attenuated in pregnant uterine arteries, which was restored by PD098059.42 Taken together, these studies suggest that the steroid-mediated decrease in the activity of PKCε, but not PKCα, may be involved in the attenuation of PKC-mediated contractions in pregnancy. Although PKCζ is an atypical PKC isozyme that is not activated by Ca2+, DAG, or phorbol esters, and it may be less likely to be involved in the PDBu-induced contractions, its role in pressure-dependent myogenic contractions of the uterine artery may not be excluded. In agreement with the present study, it has been shown that a gender-related decrease in PKC-mediated vascular smooth muscle contractions in female rats is associated with reductions in the activity of PKCα, δ, and ζ.35
Perspectives
The present study has demonstrated a direct chronic effect of the steroid hormones in the up-regulation of ERK1/2 expression and down-regulation of PKC signaling pathway resulting in a reduced myogenic tone of the uterine artery in pregnancy. Given that pressure-dependent myogenic contraction is an important physiological mechanism that regulates basal vascular resistance and is a major contributor to the modulation of organ blood flow, dysregulation of myogenic tone is likely to contribute significantly to the maladaptation of uterine vascular hemodynamics in pregnancy and an increased risk of preeclampsia. Not only do the present findings provide an understanding of the mechanisms of the steroid hormone-mediated adaptation of uterine artery contractility to pregnancy, but they also offer insights into the mechanisms in the hormonal regulation of myogenic tone of resistance arteries in general, and improve our understanding of vascular benefits of hormone replacement therapy in postmenopausal women, given the well established finding that premenopausal women are at lower risk of developing hypertension and coronary heart disease than men of the same age and that the cardiovascular risk increases only after the cessation of ovarian function.
Acknowledgments
We are grateful for the gift of 21-hydroxy-6,19-epoxyprogesterone from Dr. Gerardo Burton.
Sources of Funding: This work was supported by National Institutes of Health Grants HL89012 (LZ), HD31226 (LZ), and S06GM073842 (SY).
Footnotes
Disclosures: None.
References
- 1.Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol. 2006;290:H2337–H2343. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- 2.Grandley RE, Conrad KP, McLaughlin MK. Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1–R7. doi: 10.1152/ajpregu.2001.280.1.R1. [DOI] [PubMed] [Google Scholar]
- 3.Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283:H2226–H2233. doi: 10.1152/ajpheart.00593.2002. [DOI] [PubMed] [Google Scholar]
- 4.Kublickiene KR, Cockell AP, Nisell H, Poston L. Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. Am J Obstet Gynecol. 1997;177:1263–1269. doi: 10.1016/s0002-9378(97)70048-6. [DOI] [PubMed] [Google Scholar]
- 5.Kublickiene KR, Kublickas M, Lindblom B, Lunell NO, Nisell H. A comparison of myogenic and endothelial properties of myometerial and omental resistance vessels in late pregnancy. Am J Obstet Gynecol. 1997;176:560–566. doi: 10.1016/s0002-9378(97)70548-9. [DOI] [PubMed] [Google Scholar]
- 6.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 7.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- 8.Xiao D, Zhang L. ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am J Physiol Heart Circ Physiol. 2002;282:H292–H300. doi: 10.1152/ajpheart.2002.282.1.H292. [DOI] [PubMed] [Google Scholar]
- 9.Xiao D, Zhang L. Adaptation of uterine artery thick- and thin- filament regulatory pathway to pregnancy. Am J Physiol Heart Circ Physiol. 2005;288:H142–H148. doi: 10.1152/ajpheart.00655.2004. [DOI] [PubMed] [Google Scholar]
- 10.Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J Physiol. 2005;565:85–99. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrot-Applanat M, Groyer-Picard MT, Garcia E, Lorenzo F, Milgrom E. Immunocytochemical demonstration of estrogen and progesterone receptors in muscle cells of uterine arteries in rabbits and humans. Endocrinology. 1998;123:1511–1519. doi: 10.1210/endo-123-3-1511. [DOI] [PubMed] [Google Scholar]
- 12.Magness RR, Parker CR, Jr, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am J Physiol. 1993;265:E690–E698. doi: 10.1152/ajpendo.1993.265.5.E690. [DOI] [PubMed] [Google Scholar]
- 13.Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol. 2005;565:71–83. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989;256:E536–E542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- 15.Killam AP, Rosenfeld CR, Battaglia FC, Makowski EL, Meschia G. Effect of estrogens on the uterine blood flow of oophorectomized ewes. Am J Obstet Gynecol. 1973;115:1045–1052. doi: 10.1016/0002-9378(73)90552-8. [DOI] [PubMed] [Google Scholar]
- 16.Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. Am J Physiol. 1998;275:H731–H743. doi: 10.1152/ajpheart.1998.275.3.H731. [DOI] [PubMed] [Google Scholar]
- 17.Gibson TC, Phernetton TM, Wiltbank MC, Magness RR. Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycles in sheep. Biol Reprod. 2004;70:1886–1894. doi: 10.1095/biolreprod.103.019901. [DOI] [PubMed] [Google Scholar]
- 18.Xiao D, Huang X, Bae S, Ducsay CA, Zhang L. Cortisol-mediated potentiation of uterine artery contractility: effect of pregnancy. Am J Physiol Heart Circ Physiol. 2002;283:H238–246. doi: 10.1152/ajpheart.00842.2001. [DOI] [PubMed] [Google Scholar]
- 19.Xiao D, Bird IM, Magness RR, Longo LD, Zhang L. Upregulation of eNOS in pregnant ovine uterine arteries by chronic hypoxia. Am J Physiol Heart Circ Physiol. 2001;280:H812–820. doi: 10.1152/ajpheart.2001.280.2.H812. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Xiao DL, Longo L, Zhang L. Regulation of α1-adrenoceptor-mediated contractions of the uterine artery by PKC: effect of pregnancy. Am J Physiol Heart Circ Physiol. 2006;291:H2282–H2289. doi: 10.1152/ajpheart.00321.2006. [DOI] [PubMed] [Google Scholar]
- 21.Pecci A, Alvarez LD, Veleiro AS, Ceballos NR, Lantos CP, Burton G. New lead compounds in the search for pure antiglucocorticoids and the dissociation of antiglucocorticoid effects. J Steroid Biochem Mol Biol. 2009;113:155–162. doi: 10.1016/j.jsbmb.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Catchpole HR. Hormonal mechanisms in pregnancy and parturition. In: Cole HH, Cupps PT, editors. Chapter 13 in Reproduction in Domestic Animals. 3rd. 1977. [Google Scholar]
- 23.Xiao S, Gillespie DG, Baylis C, Jackson EK, Dubey PK. Effects of estradiol and its metabolites on glomerular endothelial nitric oxide synthesis and mesangial cell growth. Hypertension. 2001;37:645–650. doi: 10.1161/01.hyp.37.2.645. [DOI] [PubMed] [Google Scholar]
- 24.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81:355–362. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- 25.Gibson LL, Hahner L, Osborne-Lawrence S, German Z, Wu KK, Chambliss KL, Shaul PW. Molecular basis of estrogen-induced cyclooxygenase types 1 upregulation in endothelial cells. Circ Res. 2005;96:518–525. doi: 10.1161/01.RES.0000158967.96231.88. [DOI] [PubMed] [Google Scholar]
- 26.Magness RR, Rosenfeld CR, Faucher DJ, Mitchell MD. Uterine prostaglandin production during ovine pregnancy: Effects of angiotensin II and indomethacin. Am J Physiol Heart Circ Physiol. 1992;263:H188–H197. doi: 10.1152/ajpheart.1992.263.1.H188. [DOI] [PubMed] [Google Scholar]
- 27.Naden RP, Iliya CA, Arant BS, Gant NF, Rosenfeld CR. Hemodynemic effects of indomethacin in chronically-instrumented pregnant sheep. Am J Obstet Gynecol. 1985;151:484–494. doi: 10.1016/0002-9378(85)90275-3. [DOI] [PubMed] [Google Scholar]
- 28.Kublickiene K, Fu XD, Svedas E, Landgren BM, Genazzani AR, Simoncini T. Effects in postmenopausal women of estradiol and medroxyprogesterone alone and combined on resistance artery function and endothelial morphology and movement. J Clin Endocrinol Metab. 2008;93:1874–1883. doi: 10.1210/jc.2007-2651. [DOI] [PubMed] [Google Scholar]
- 29.Kublickiene K, Svedas E, Landgren BM, Crisby M, Nahar N, Nisell H, Poston L. Small artery endothelial dysfunction in postmenopausal women: in vitro function, morphology, and modification by estrogen and selective estrogen receptor modulators. J Clin Endocrinol Metab. 2005;90:6113–6122. doi: 10.1210/jc.2005-0419. [DOI] [PubMed] [Google Scholar]
- 30.Douglas G, Cruz MN, Poston L, Gustafsson JA, Kublickiene K. Functional characterization and sex differences in small mesenteric arteries of the estrogen receptor-beta knockout mouse. Am J Physiol Regul Integr Comp Physiol. 2008;294:R112–R120. doi: 10.1152/ajpregu.00421.2007. [DOI] [PubMed] [Google Scholar]
- 31.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol. 2000;278:R295–R303. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- 32.Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol. 1991;260:E464–E470. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- 33.Miyagawa K, Rösch J, Stanczyk F, Hermsmeyer K. Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm. Nat Med. 1997;3:324–327. doi: 10.1038/nm0397-324. [DOI] [PubMed] [Google Scholar]
- 34.Minshall RD, Miyagawa K, Chadwick CC, Novy MJ, Hermsmeyer K. In vitro modulation of primate coronary vascular muscle cell reactivity by ovarian steroid hormones. FASEB J. 1998;12:1419–1429. doi: 10.1096/fasebj.12.13.1419. [DOI] [PubMed] [Google Scholar]
- 35.Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280:C34–C45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- 36.Ford SP. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J Anim Sci. 1982;55(suppl):32–42. 2. [PubMed] [Google Scholar]
- 37.Caton D, Kalra PS. Endogenous hormones and regulation of uterine blood flow during pregnancy. Am J Physiol. 1986;250:R365–R369. doi: 10.1152/ajpregu.1986.250.3.R365. [DOI] [PubMed] [Google Scholar]
- 38.Ford SP, Reynolds LP, Farley DB, Bhatnagar RK, Van Orden DE. Interaction of ovarian steroids and periarterial alpha 1-adrenergic receptors in altering uterine blood flow during the estrous cycle of gilts. Am J Obstet Gynecol. 1984;150:480–484. doi: 10.1016/s0002-9378(84)90424-1. [DOI] [PubMed] [Google Scholar]
- 39.Ford SP. Control of blood flow to the gravid uterus of domestic livestock species. J Anim Sci. 1995;73:1852–1860. doi: 10.2527/1995.7361852x. [DOI] [PubMed] [Google Scholar]
- 40.Horowitz A, Clement-Chomienne O, Walsh MP, Morgan KG. Epsilon-isoenzyme of protein kinase C induces a Ca2+-independent contraction in vascular smooth muscle. Am J Physiol. 1996;271:C589–C594. doi: 10.1152/ajpcell.1996.271.2.C589. [DOI] [PubMed] [Google Scholar]
- 41.Walsh MP, Andrea JE, Allen BG, Clement-Chomienne O, Collins EM, Morgan KG. Smooth muscle protein kinase C. Can J Physiol Pharmacol. 1994;72:1392–1399. doi: 10.1139/y94-201. [DOI] [PubMed] [Google Scholar]
- 42.Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol. 2009 Apr 17; doi: 10.1152/ajpheart.00090.2009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]