Abstract
Background/Aims
Our previous work suggested an important role for the peptidyl-prolyl isomerase, Pin1, in hepatic NF-κB activation and liver injury during ischemia/reperfusion (I/R). In this study, we sought to determine the function of Pin1 in the injury response to hepatic I/R.
Methods
Wild-type and Pin1-/- mice were subjected to partial hepatic I/R. In addition, hepatocytes and Kupffer cells were isolated from these mice.
Results
Pin1-/- mice had reduced hepatic NF-κB activation and more liver injury after I/R than wild-type mice. The increased injury was not a result of enhanced inflammation as Pin1-/- mice had the same level of proinflammatory cytokine production and less neutrophil accumulation in the liver. The reduced NF-κB activation was not a result of a defect in nuclear translocation of NF-κB. In fact, hepatic nuclear p65 protein expression was higher in Pin1-/- mice than wild-type mice. This suggests that Pin1 is important for NF-κB-DNA binding. This effect was specific to hepatocytes as isolated Kupffer cells from wild-type and Pin1-/- mice were identical in their activation of NF-κB and production of cytokines after stimulation. In contrast, hepatocytes stimulated with TNFα had greatly reduced NF-κB activation, reduced production of the CXC chemokine, MIP-2, and increased cell death.
Conclusions
These data suggest that Pin1 is a critical regulator of NF-κB activation in hepatocytes and its role in these cells appears to confer direct protective effects.
Keywords: liver, hepatocytes, ischemia/reperfusion, Pin1, NF-κB
Introduction
Ischemia/reperfusion (I/R) of the liver is a primary complication of liver resection surgery, transplantation, and trauma (1). Extended periods of hepatic ischemia and subsequent reperfusion can lead to hepatocellular damage and organ dysfunction through the initiation of a biphasic inflammatory response (2). The initial phase of this response is characterized by activation of Kupffer cells and their subsequent production and release of reactive oxygen species, leading to mild hepatocellular injury (3,4). Kupffer cell-induced oxidative stress leads to activation of redox-sensitive transcription factors such as nuclear factor κB (NF-κB) that regulate the expression of many pro-inflammatory mediators, including tumor necrosis factor-α (TNFα) (5,6). TNFα propagates the inflammatory response by inducing the production of CXC chemokines and endothelial cell adhesion molecules that facilitate the adhesion and transmigration of neutrophils from the vascular space into the hepatic parenchyma (7-10). These activated neutrophils then directly injure hepatocytes and vascular endothelial cells through their release of oxidants and proteases (11).
The phosphorylation of proteins on Ser/Thr residues is an important cellular signaling mechanism to induce their functional activity (12). The peptidyl-prolyl isomerase, Pin1, is a key regulatory mediator of phosphorylation-induced protein activation (13,14). Pin1 specifically recognizes pSer/pThr-Pro motifs and induces conformational changes in target proteins that control their function (15-17). It has been reported that Pin1 increases cyclin D1 expression and induces cell proliferation by mediating the progression through G0 to S phase (18). Other studies have shown that Pin1 prevents cell death induced by oxidative stress or DNA damage, and increases cell survival (19,20). Furthermore, Pin1 appears to regulate allergic inflammatory responses, as Pin1 blockade reduces CXC chemokine expression and subsequent eosinophil infiltration and increases eosinophil apoptosis (21).
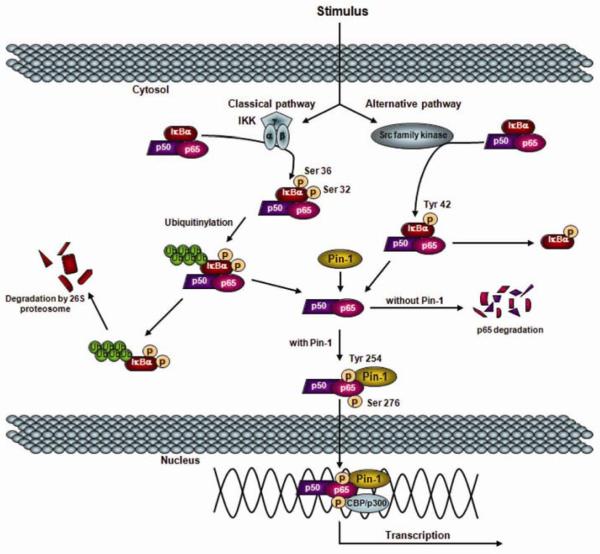
In addition to these functions, Pin1 is known to regulate the activity of several transcriptional factors, such as p53, activator protein-1, and NF-κB. In regards to NF-κB, Pin1 has been shown to specifically bind to the pThr254-Pro motif in p65, inhibit its binding to inhibitory κB-alpha (IκBα), facilitate its nuclear translocation and enhance p65 stability (22; Figure 1). This study further demonstrated that Pin1-deficient mice were refractory to NF-κB activation by TNFα stimulation and unable to transactivate NF-κB target genes, which resulted in cell apoptosis (22). We have recently demonstrated that NF-κB activation in hepatocytes, which is augmented by ischemic hypothermia, is hepatoprotective during I/R injury (23). We have further shown that Pin1, which is degraded during normothermic I/R, is maintained during hypothermic I/R, binds to p65 and inhibits its degradation. Moreover, we have found that concurrent degradation of Pin1 and p65 occurred in hepatocytes, but not in Kupffer cells. These circumstantial data implicate an important role for Pin1 in the regulation of NF-κB during I/R injury. In the present study, we directly examined the function of Pin1 during hepatic I/R injury using Pin 1-knockout mice.
Figure 1.
Proposed model of Pin1 interaction with NF-κB derived from cell culture experiments.
Materials and methods
Mice
Male wild-type (Jackson Laboratory, Bar Harbor, ME) and Pin1-/- mice on a C57BL/6J background were used in this study and housed under normal conditions. Pin1-/- mice were obtained from Dr. Anthony Means (Duke University Medical Center, Durham, NC). These mice had the Pin1 gene deletion transferred into an isogenic C57BL/6J background using marker-assisted speed congenic protocols by the Jackson Laboratory (24). All mice weighed 22-28g. This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines.
Hepatic ischemia/reperfusion injury model
Wild-type and Pin1-/- mice underwent either sham surgery or partial hepatic ischemia as described previously (9). Briefly, mice were anaesthetized with sodium pentobarbital (60 mg/kg, i.p.). A midline laparotomy was performed and an atraumatic clip was used to interrupt blood supply to the left lateral and median lobes of the liver. After 90 minutes of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Sham control mice underwent the same protocol without vascular occlusion. Mice were sacrificed after 1, 4 or 8 hours of reperfusion and blood and samples of the left lateral lobe were taken for analysis. Experimental group sizes ranged from n=4 to n=11 and are indicated in the figure legends.
Electrophoretic mobility shift assay
Nuclear extracts of liver tissue were prepared by the method of Deryckere and Gannon (25), and analyzed by electrophoretic mobility shift assay (EMSA). Briefly, double-stranded consensus oligonucleotides to NF-κB (Promega, Madison, WI) were end-labeled with γ[32P] ATP(3,000 Ci/mmol at 10mCi/ml, Perkin Elmer, Waltham, MA). Binding reactions (total volume 15 μl) containing equal amounts of nuclear protein extract (20 μg) and 35 fmols (∼50,000 cpm, Cherenkov counting) of oligonucleotide and were incubated at room temperature for 30 minutes. Binding reaction products were separated in a 4% polyacrylamide gel and analyzed by autoradiography.
Western blot analyses
Liver lysates or nuclear extracts containing equal amounts of protein in equal volumes of sample buffer were separated in a denaturing 10% polyacrylimide gel and transferred to a 0.45 μm pore polyvinylidene difluoride membrane. Nonspecific binding sites were blocked with TBS (40 mM Tris, pH 7.6; 300 mM NaCl) containing 5% non-fat dry milk for 12 hours at 4°C. Membranes were then incubated with antibodies to Pin1, p65, IκBα or β-actin (Cell signaling Technology, Danvers, MA) in TBS with 0.1% Tween 20 (TBST), washed and incubated with secondary antibodies conjugated to horseradish peroxidase. Immunoreactive proteins were detected by enhanced chemiluminescence.
Liver neutrophil accumulation
Liver myeloperoxidase (MPO) content was assessed by methods described elsewhere (26). Neutrophil esterase staining was performed on liver sections using a Naphthol AS-D Chloroacetate Esterase Kit (Sigma, St. Louis, MO) per manufacturer’s instructions.
Blood and tissue analysis
Blood was obtained by cardiac puncture. Serum was analyzed for alanine aminotransferase (ALT) by bioassay (Wiener Laboratories, Rosario, Argentina) as an index of hepatocellular injury. Serum levels of TNFα and macrophage inflammatory protein-2 (MIP-2) were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Liver tissues were fixed in 10% neutral-buffered formalin, processed and then embedded in paraffin for light microscopy. Sections were stained with hematoxylin and eosin for histological examination.
Hepatocyte and Kupffer cell isolation
Hepatocytes and Kupffer cells were isolated as described previously (23). Cells were distributed onto 24-well flat-bottomed plates (Trasadingen, Switzerland) at a concentration of 2.0 × 105 cells/500 μl/well, and incubated overnight to allow cell adherence. Hepatocytes and Kupffer cells were treated with 1 μg/ml lipopolysaccharide (LPS) for 1 hour. In separate experiments, hepatocytes were treated with 50 ng/ml TNFα for 4 hours. Culture media was collected and analyzed via ELISA for TNFα or MIP-2 (R&D Systems). Hepatocyte cytotoxicity was determined by lactate dehydrogenase (LDH) assay according to the manufacturer’s instructions (Roche, Mannheim, Germany). Nuclear extracts were also prepared from isolated hepatocytes after 30 minutes of treatment with 50 ng/ml TNFα or Kupffer cells after 1 hour of treatment with 1 μg/ml LPS.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Data were analyzed with a one-way analysis of variance with subsequent Student-Newman-Keuls test. Differences were considered significant when P<0.05.
Results
Pin1-deficiency augments liver injury after ischemia/reperfusion
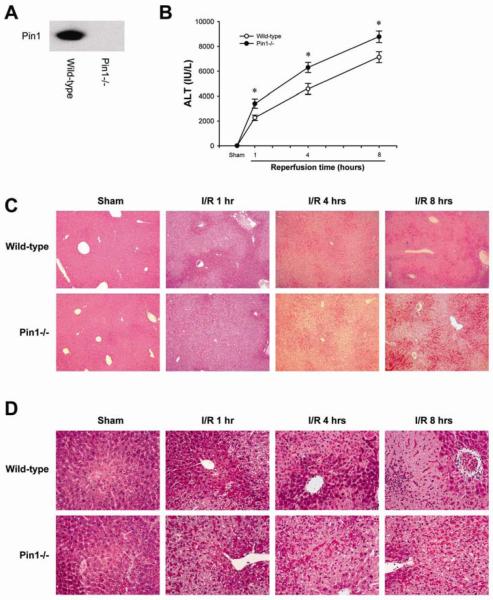
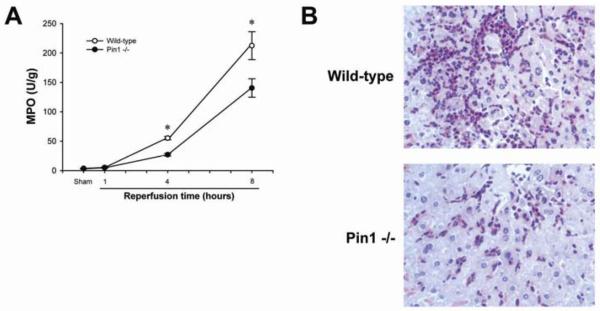
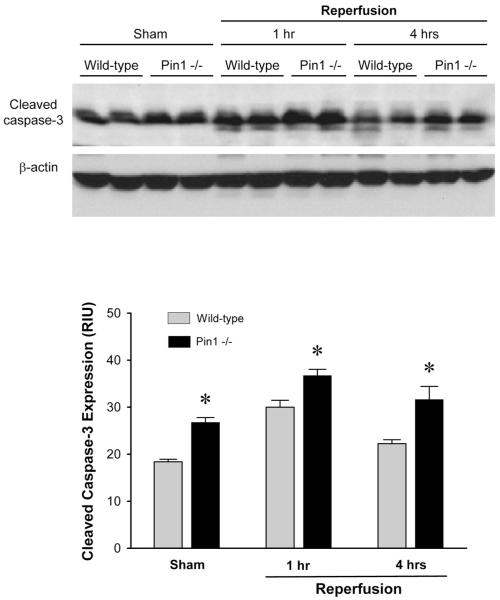
Because we have previously reported that Pin1 binds to p65 and inhibits its degradation during hepatic I/R injury (23), we first assessed the response of wild-type and Pin1-/- mice to hepatic I/R injury. Deletion of Pin1 in the livers of Pin1-/- mice was confirmed by Western blot analysis (Figure 2A). In response to ischemia and 1, 4 and 8 hours of reperfusion, Pin1-/- mice had significantly more liver injury as determined by serum levels of ALT (Figure 2B). These findings were confirmed by histological examination (Figures 2C and D). Both wild-type and Pin1-/- mice undergoing sham surgery had normal liver architecture. After 1 hour of reperfusion, wild-type mice still had large areas of normal liver architecture whereas Pin1-/- mice had markedly higher hepatocellular necrosis. Moreover, after 4 and 8 hours of reperfusion, Pin1-/- mice had significantly more hepatocellular necrosis. In contrast to the increase in liver injury, Pin1-/- mice had significantly less neutrophil accumulation after 4 and 8 hours of reperfusion, as measured by hepatic MPO content (Figure 3A). These findings were confirmed by immunohistochemical staining for esterase (Figure 3B). Because Pin1 has been linked to maintenance of programmed cell death machinery, we evaluated the level of apoptosis in the liver of wild-type and Pin1-/- mice after I/R. To assess hepatocellular apoptosis, we measured the expression of cleaved caspase-3, a terminal event in the apoptotic pathway. Expression of cleaved caspase-3 was higher in Pin1-/- mice compared to wild-type mice after sham operation and I/R (Figure 4). This suggests that there is a general increase in apoptotic signaling in Pin1-/- mice compared to wild-type mice that may contribute to the increased liver injury observed.
Figure 2.
Response of Pin1-/- mice to hepatic ischemia/reperfusion. (A) Pin1 protein expression was determined in liver lysates by Western blot. (B) Liver injury was measured by serum levels of alanine aminotransferase (ALT). Data for sham groups were: wild-type, 11 ± 1 IU/L; Pin1-/-, 15 ± 2 IU/L. Data are mean ± SEM with n=5-11 per group. *P<0.05 compared to wild-type mice. Liver histology after ischemia/reperfusion in wild-type and Pin1-/- mice was examined at 5X (C) and 50X (D). Normal hepatic architecture was observed in sham-operated wild-type mice and Pin1-/- mice. After 1 hour of reperfusion, livers from Pin1-/- mice had large areas of necrosis, whereas livers from wild-type mice showed less evidence of necrosis. After 4 and 8 hours of reperfusion, livers from wild-type mice after ischemia/reperfusion had the usual degree of hepatocellular necrosis and neutrophil infiltration, whereas Pin1-/- mice had significantly more hepatocellular necrosis with significantly less evidence of neutrophil infiltration.
Figure 3.
Effect of Pin1-knockout on liver recruitment of neutrophils after ischemia/reperfusion. (A) Neutrophil accumulation was determined by liver content of myeloperoxidase (MPO). Data are mean ± SEM with n=4-9 per group. *P<0.05 compared to Pin1-/- mice. (B) Neutrophil recruitment was examined by esterase staining of liver sections from mice after ischemia and 8 hours of reperfusion. Original magnification, 50X.
Figure 4.
Effect of Pin1-knockout on hepatocellular apoptosis after ischemia/reperfusion. Liver apoptosis was determined by expression of cleaved caspase-3 in liver tissues. Expression of cleaved caspase-3 was determined by Western blot. Results were quantitated by image analysis of chemiluminescence films. Data are mean ± SEM with n=4 per group.
Activation of NF-κB is decreased in Pin1-/- mice after I/R
NF-κB activation is known to be mediated by Pin1 (22), and our previous data suggest that hepatocyte NF-κB activation is hepatoprotective during I/R (23). Therefore, we assessed NF-κB activation in wild-type and Pin1-/- mice. In sham-operated mice, there was no difference in NF-κB activation between wild-type and Pin1-/- mice (Figure 5A). Interestingly, NF-κB activation was significantly decreased in Pin1-/- mice compared to wild-type mice after 1 and 8 hours of reperfusion. Competition and supershift assays revealed that the NF-κB complex was composed of p50 and p65 (Figure 5B). These data suggest that Pin1 is an important mediator of hepatic NF-κB activation during I/R.
Figure 5.
Effect of Pin1-knockout on liver NF-κB activation after ischemia/reperfusion. (A) Liver nuclear extracts were analyzed by EMSA. Results were quantitated by image analysis of autoradiograms. Data are mean ± SEM with n=4 per group. *P<0.05 compared to wild-type mice. (B) Liver nuclear extracts from wild-type mice undergoing ischemia and 1 hour of reperfusion were subjected to competition and supershift assays. The arrowhead indicates the supershift band of p65.
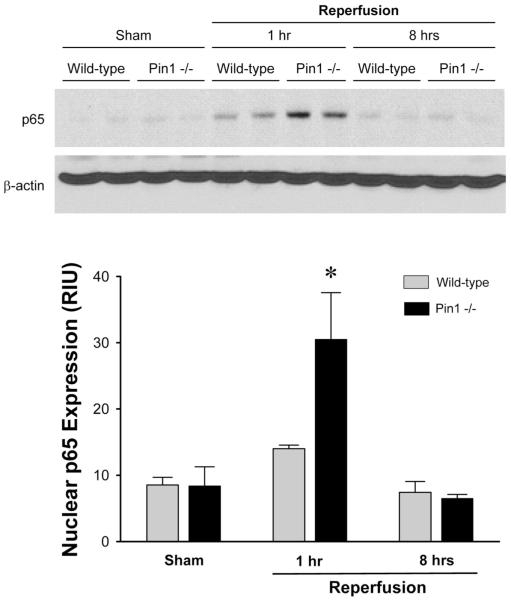
Lack of Pin1 does not alter hepatic expression of p65 or IκBα, but results in increased nuclear accumulation of p65 after I/R
We have previously shown that liver p65 and IκBα proteins are decreased after ischemia and 1 hour of reperfusion (23). Because Pin1-/- mice had reduced hepatic NF-κB activation (Figure 5), we next sought to determine whether this effect was due to decreased protein expression of p65 or IκBα. Western blots for p65 and IκBα were performed on whole liver lysates. After ischemia and 1 hour of reperfusion there was marked degradation of p65 and IκBα in both wild-type and Pin1-/- mice (Figure 6). After 8 hours of reperfusion, p65 and IκBα protein expression recovered slightly, but were still below baseline expression levels observed in sham mice. No differences in p65 or IκBα expression were observed between wild-type and Pin1-/- mice at either time point. Because NF-κB-DNA interactions occur in the nucleus, we next assessed the protein expression of p65 in liver nuclear extracts. Interestingly, nuclear p65 expression after 1 hour of reperfusion was significantly higher in Pin1-/- mice compared to wild-type mice (Figure 7). After 8 hours of reperfusion, hepatic nuclear p65 protein expression returned to basal levels in both wild-type and Pin1-/- mice. These data suggest that Pin1 contributes to NF-κB-DNA binding since higher levels of p65 in the nucleus of Pin1-/- mice were associated with decreased NF-κB activation (Figure 5).
Figure 6.
Effects of Pin1-knockout on protein expression of p65 and IκBα in liver after ischemia/reperfusion. Liver lysates were assessed for p65 or IκBα protein expression by Western blot. β-actin staining served as a loading control. Chemiluminescence films were quantitated by image analysis. Data are mean ± SEM with n=4 per group.
Figure 7.
Effects of Pin1-knockout on hepatic nuclear p65 protein expression after ischemia/reperfusion. Liver nuclear extracts were assessed for p65 protein expression by Western blot. β-actin staining served as a loading control. Chemiluminescence films were quantitated by image analysis. Data are mean ± SEM with n=4 per group. *P<0.05 compared to wild-type mice.
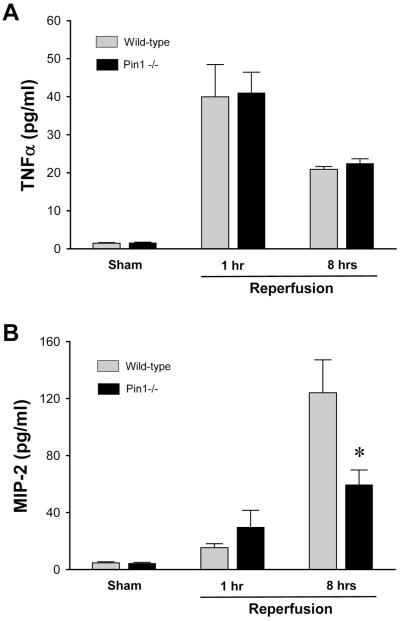
Pin1 is required for full production of MIP-2, but is not relevant to the production of TNFα
NF-κB is the primary transcriptional regulator of the cytokine, TNFα, and the chemokine, MIP-2 (27,28). Both of these mediators are central to the inflammatory response induced by I/R (6,9). To assess the effects of Pin1 deletion on the expression of these mediators, we measured serum levels of TNFα and MIP-2 during I/R injury. Serum TNFα levels were significantly increased after I/R in both wild-type and Pin1-/- mice (Figure 8A). However, no difference was observed in serum TNFα levels after 1 and 8 hours of reperfusion between wild-type and Pin1-/- mice (Figure 8A). In contrast, serum levels of MIP-2 were increased after 8 hours of reperfusion, but were significantly lower in Pin1-/- mice compared to wild-type mice (Figure 8B). These data suggest that the decreased hepatic MPO content and decreased histological evidence of neutrophil accumulation in Pin1-/- mice may be a result of decreased expression of MIP-2 in these animals.
Figure 8.
Effects of Pin1-knockout on serum TNFα and MIP-2 levels after ischemia/reperfusion. (A) TNFα and (B) MIP-2 levels in serum were determined by ELISA. Data are mean ± SEM with n=4-9 per group. *P<0.05 compared to wild-type mice.
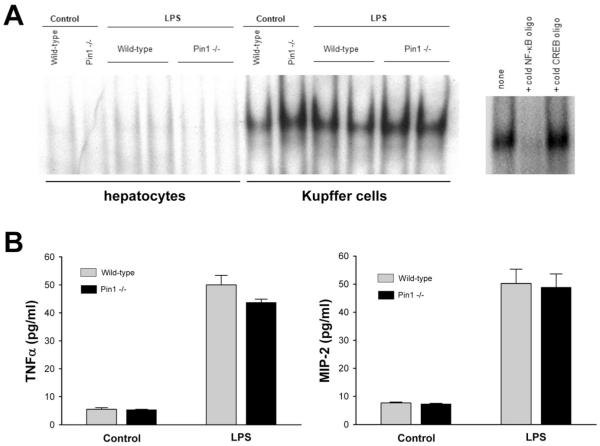
Pin1 does not regulate NF-κB activation or cytokine expression in Kupffer cells
To further discern the function of Pin1 on hepatic I/R injury, we next examined the role of this protein in specific cell types. We first examined the function of Pin1 during LPS-induced stimulation of hepatocytes and Kupffer cells. Cells were isolated from wild-type and Pin1-/- mice and treated with 1 μg/ml LPS for 1 hour. LPS was used as a stimulus because it is a known activator of NF-κB and allows for the measurement of TNFα and MIP-2. LPS treatment had no effect on NF-κB activation in hepatocytes (Figure 9A). However, LPS induced the activation of NF-κB to a similar degree in Kupffer cells from both wild-type and Pin1-/- mice (Figure 9A). The specificity of the NF-κB band was confirmed with competition assay. Consistent with the NF-κB assay, LPS stimulated the production of both TNFα and MIP-2 in Kupffer cells from wild-type and Pin1-/- mice (Figure 9B). No differences in TNFα or MIP-2 production were observed between Kupffer cells from wild-type or Pin1-/- mice. These data suggest that Pin1 does not regulate NF-κB activation or cytokine/chemokine production in Kupffer cells.
Figure 9.
Effects of Pin1-knockout on LPS-induced stimulation of hepatocytes and Kupffer cells in vitro. (A) Hepatocytes and Kupffer cells were isolated from wild-type and Pin1-/- mice and treated with 1 μg/ml LPS for 1 hour. Nuclear extracts were collected and analyzed by elecrophoretic mobility shift assay for NF-κB. Nuclear extracts of Kupffer cells from wild-type mice treated with LPS were subjected to competition assay for NF-κB. (B) Kupffer cell TNFα and MIP-2 production after LPS treatment was determined by ELISA. Data are mean ± SEM with n=4 per group.
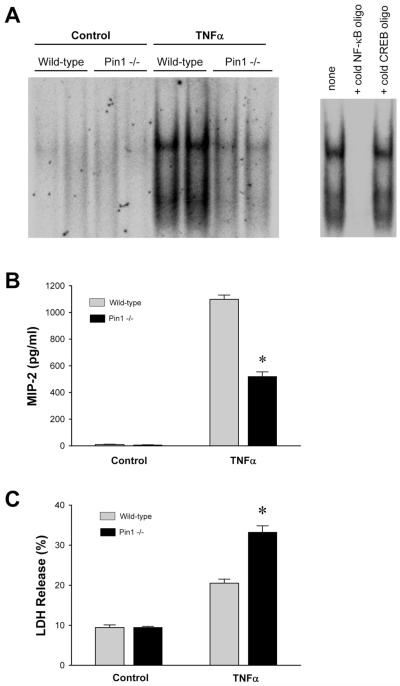
Pin1 mediates hepatocyte NF-κB activation, production of MIP-2, and protects hepatocytes from TNFα-induced cell death
Because our studies with isolated Kupffer cells indicated that Pin1 was not involved in NF-κB activation or cytokine or chemokine production in those cells, and because hepatocytes are resistant to LPS stimulation, we next examined the function of Pin1 in hepatocytes stimulated with TNFα. TNFα is known to be the primary factor that induces hepatocyte NF-κB activation after I/R. Therefore, we treated isolated hepatocytes with 50 ng/ml TNFα for 30 minutes. Treatment with TNFα increased NF-κB activation in wild-type hepatocytes (Figure 10A). However, in hepatocytes from Pin1-/- mice, treatment with TNFα resulted in only a slight increase in NF-κB-DNA binding (Figure 10A). The specificity of NF-κB binding was confirmed in a competition assay. To determine whether the regulatory role of Pin1 on hepatocyte NF-κB altered the response of these cells to TNFα, we stimulated isolated hepatocytes with TNFα for 4 hours and measured production of MIP-2 as well as LDH release. Treatment with TNFα resulted in marked production of MIP-2 by wild-type hepatocytes (Figure 10B). In contrast, Pin1-/- mice produced significantly less MIP-2 than their wild-type counterparts (Figure 10B). Treatment of wild-type hepatocytes with TNFα also increased LDH release, a marker of cell death (Figure 10C). In hepatocytes from Pin1-/- mice, there was significantly higher LDH release compared to wild-type hepatocytes (Figure 10C). These data suggest that Pin1 is critical for hepatocyte activation of NF-κB, production of MIP-2 and protection against cell death.
Figure 10.
Effects of Pin1-knockout on TNFα-induced stimulation of hepatocytes in vitro. (A) Hepatocytes isolated from wild-type and Pin1-/- mice were treated with 50 ng/ml TNFα. Nuclear extracts were collected after 30 minutes of treatment and analyzed by elecrophoretic mobility shift assay for NF-κB. Nuclear extracts of hepatocytes from wild-type mice treated with TNFα were subjected to competition assay for NF-κB. (B) MIP-2 production after 4 hours of TNFα treatment was determined by ELISA. Data are mean ± SEM with n=8 per group. *P<0.05 compared to hepatocytes from wild-type mice. (C) Cell death after 4 hours of TNFα treatment was measured by LDH assay. Data are mean ± SEM with n=8 per group. *P<0.05 compared to hepatocytes from wild-type mice.
Discussion
The present study is the first to critically examine the function of the peptidyl-prolyl isomerase, Pin1, during I/R injury. Our data demonstrate that Pin1 is critical for the activation of NF-κB in the liver and protection against hepatocellular injury. Based on our studies, we propose that these regulatory properties are related primarily to the function of Pin1 in hepatocytes, as we found no evidence that Pin1 is important for NF-κB activation or cytokine/chemokine expression in Kupffer cells.
The results of our studies contrast with some previous reports of Pin1 function conducted in other cell types. For example, a previous study using HeLa cells and murine embryonic fibroblasts have found that Pin1 binds to p65 and thereby prevents interaction with IκBα, promotes nuclear translocation of p65, and mediates p65-DNA binding (22). We reveal that hepatic nuclear p65 protein expression is increased in Pin1-/- mice after 1 hour of reperfusion compared to wild-type mice even though NF-κB activation is suppressed. This suggests that, in hepatocytes, Pin1 is not necessary for p65 translocation, but is required for DNA binding. In our model, IκBα degradation after hepatic I/R results in liberation of the NF-κB complex in cytoplasm which allows for subsequent translocation into the nucleus. However, NF-κB DNA-binding is inhibited in Pin1-/- mice after I/R. This may be a result of Pin1 regulating the conformation and function of phosphorylated proteins (29).
Perhaps more interesting is the apparent cell-specific role of Pin1 in the liver. We previously demonstrated that hepatic ischemia/reperfusion resulted in degradation of Pin1 in hepatocytes but not Kupffer cells (23). Furthermore, those studies supported the concept that Pin1 functioned to stabilize p65 in hepatocytes, but not in Kupffer cells. Our current data extend those findings and show that Pin1 plays no regulatory role in the activation of NF-κB in Kupffer cells, whereas in hepatocytes, Pin1 is essential for NF-κB DNA-binding. This divergent role of Pin1 may well be aligned with our developing concept of divergent roles for NF-κB in different liver cells. We have suggested that NF-κB activation is a protective mechanism in hepatocytes (30) and other, more direct studies have validated this concept (31-33). Our previous studies of ischemic hypothermia and age-dependent effects have shown that increased liver NF-κB activation is associated with hepatoprotection (30,34). Our current studies show that cell death is significantly increased and NF-κB activation is nearly ablated in Pin1-/- hepatocytes after TNFα treatment compared to wild-type hepatocytes. This suggests that Pin1-mediated NF-κB activation may be an important protective mechanism for hepatocytes against cellular stress.
It has been well-described that accumulated neutrophils contribute substantially to hepatocellular injury after I/R (8-11). A number of our recent studies, including this one, have revealed instances of augmented liver injury with reduced neutrophil infiltration (34,35). These studies highlight the importance of non-inflammatory mechanisms of hepatocyte injury after I/R. For example, our current data show that Pin1-/- mice have reduced neutrophil infiltration yet greater liver injury compared to their wild-type counterparts. Consistent with the reduced neutrophil accumulation, and perhaps causative of this effect was the reduced levels of MIP-2 in the serum of Pin1-/- mice compared to wild-type mice. These circulating levels of MIP-2 are reflective more of hepatocyte production, since our studies with isolated Kupffer cells showed no difference in MIP-2 production between wild-type and Pin1-/- cells, while Pin1-/- hepatocytes produced significantly less than wild-type cells. It is not clear whether the decreased hepatocyte production of MIP-2 is due to reduced activation of NF-κB or due to the increased cell death observed in these cells. Regardless, the fact that there is increased hepatocellular injury in Pin1-/- mice despite reduced inflammation underscores the importance of this molecule in directly regulating hepatocyte injury.
In summary, the current data show that Pin1 is required for NF-κB-DNA binding in hepatocytes during I/R injury. In contrast, Pin1 plays no role in the activation of NF-κB or production of cytokines in Kupffer cells. In hepatocytes, Pin1-mediated NF-κB activation appears to protect against cell death. Whether or not Pin1 can be targeted therapeutically remains unclear, but the current studies provide new insights into our understanding of the pathogenesis of hepatic I/R injury.
Abbreviations
- I/R
ischemia/reperfusion
- NF-κB
nuclear factor κB
- TNFα
tumor necrosis factor α
- MIP-2
macrophage inflammatory protein-2
- IκBα
inhibitory κB-alpha
- EMSA
electrophoretic mobility shift assay
- MPO
myeloperoxidase
- ALT
alanine amino transferase
- ELISA
enzyme-linked immunosorbent assay
- LPS
lipopolysaccharide
- LDH
lactate dehydrogenase
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 5.Wanner GA, Ertel W, Muller P, Hofer Y, Leiderer R, Menger MD, et al. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34–40. doi: 10.1097/00024382-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alternations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Colletti LM, Kunkel SL, Waltz A, Burdick MD, Kunkel RG, Wilke CA, et al. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–1803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 12.Lu KP, Liou YC, Zhou XZ. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 13.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Uchida C, Shin RW, Shimazaki K, Uchida T. Prolyl isomerase, Pin1: new findings of post-translational modifications and physiological substrates in cancer, asthma and Alzheimer’s disease. Cell Mol Life Sci. 2008;65:359–375. doi: 10.1007/s00018-007-7270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu PJ, Zhou XZ, Shen M, Lu KP. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- 17.Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- 18.Pang RW, Lee TK, Man K, Poon RT, Fan ST, Kwong YL, et al. PIN1 expression contributes to hepatic carcinogenesis. J Pathol. 2006;210:19–25. doi: 10.1002/path.2024. [DOI] [PubMed] [Google Scholar]
- 19.Ryo A, Hirai A, Nishi M, Liou YC, Perrem K, Lin SC, et al. A suppressive role of the prolyl isomerase Pin1 in cellular apoptosis mediated by the death-associated protein Daxx. J Biol Chem. 2007;282:36671–36681. doi: 10.1074/jbc.M704145200. [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA, Abdul HM, Opii W, Newman SF, Joshi G, Ansari MA, et al. Pin1 in Alzheimer’s disease. J Neurochem. 2006;98:1697–1706. doi: 10.1111/j.1471-4159.2006.03995.x. [DOI] [PubMed] [Google Scholar]
- 21.Esnault S, Rosenthal LA, Shen ZJ, Sedgwick JB, Szakaly RJ, Sorkness RL, et al. A critical role for Pin1 in allergic pulmonary eosinophilia in rats. J Allergy Clin Immunol. 2007;120:1082–1088. doi: 10.1016/j.jaci.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, et al. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 2003;12:1413–1426. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 23.Kuboki S, Okaya T, Schuster R, Blanchard J, Denenberg A, Wong HR, et al. Hepatocyte NF-kB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol. 2007;292:G201–G207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 24.Atchison FW, Capel B, Means AR. Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development. 2003;130:3579–3586. doi: 10.1242/dev.00584. [DOI] [PubMed] [Google Scholar]
- 25.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994;16:405. [PubMed] [Google Scholar]
- 26.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 27.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein B, Baldwin ASJ. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu KP, Liou YC, Zhou XZ. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;2:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 30.Shin T, Kuboki S, Lentsch AB. Roles of nuclear factor-kappaB in postischemic liver. Hepatol Res. 2008;38:429–440. doi: 10.1111/j.1872-034X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 31.Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, et al. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am J Physiol. 1998;75:C1058–C1066. doi: 10.1152/ajpcell.1998.275.4.C1058. [DOI] [PubMed] [Google Scholar]
- 33.Bellas RE, FitzGerald MJ, Fausto N, Sonenshein GE. Inhibition of NF-kappa B activity induces apoptosis in murine hepatocytes. Am J Pathol. 1997;51:891–896. [PMC free article] [PubMed] [Google Scholar]
- 34.Okaya T, Blanchard J, Schuster R, Kuboki S, Husted T, Caldwell CC, et al. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock. 2005;24:421–427. doi: 10.1097/01.shk.0000181282.14050.11. [DOI] [PubMed] [Google Scholar]
- 35.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G969–G976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]