Abstract
Chronic unpredictable mild stress (CMS), an animal model of depression, downregulates hippocampal CB1 receptors in adult male rats. Given that endocannabinoids are implicated in modulating stress and anxiety and that women are vulnerable to stress-related disorders, we tested the effects of CMS on both female and male rats. Gonadectomized (gndx) and gonadally intact male and female rats were exposed to a three-week chronic stress protocol. Following CMS, CB1 receptor and fatty-acid-amide-hydrolase (FAAH) expression levels in the hippocampus were assessed by western blot analysis. CMS reliably produced a downregulation of CB1 receptors (∼50%) in the hippocampus of both gndx and intact males. This effect was more robust in the dorsal than in the ventral hippocampus. Conversely, CMS produced an upregulation of CB1 receptors (∼150%) in the hippocampus of both gndx and intact females. This upregulation was only observed in the dorsal hippocampus of female animals. CMS produced an upregulation of FAAH levels in both male and female animals. In non-stress control animals, males were observed to have higher CB1 levels than females, but no differences in FAAH were found. These findings suggest that the endocannabinoid (eCB) system is preferentially organized in male and female animals to respond differentially to chronic stress. These sex differences in the eCB system may help development of novel treatments for stress and depression that are designed specifically for women and men.
Keywords: Endocannabinoids, Depression, Sex differences, Hippocampus
1. Introduction
Women are more vulnerable to stress-related mental disorders than men [1]. Specifically, prevalence rates for depressive disorders are twice as high for women than men [2,3]. Epidemiological studies in humans and experimental studies in rodents [4] suggest that sexual dimorphisms in the hypothalamic–pituitary–adrenal (HPA) axis stress response are responsible for these disparities. In particular, modulation of the HPA axis by sex steroids (e.g. estrogen, testosterone) is implicated in sex-specific stress responses (see for review [5]). Despite these reports, there remains a paucity of data on the etiology and pathophysiology of sex-differences in depressive disorders.
The plant-derived cannabinoid, Δ9-THC (the active ingredient in marijuana) and the endocannabinoids (eCBs), anandamide (AEA) and 2-arachidonolyglcerol (2-AG), modulate a variety of behavioral phenomena including mood, stress, anxiety, learning and memory [6–8]. Although the precise mechanisms remain unclear, cannabinoids appear to exert much of their action through the two known cannabinoid receptors (CB1, CB2). It is should be noted that AEA also activates the endovallanoid-gatd calcium channel (TRPV1;[34]). CB1 receptors, the focus of this study, are the most abundant G-protein-coupled receptors in the central nervous system and are densely located in brain nuclei of the HPA axis including the hippocampus. In animal models of anxiety, either genetic or pharmacological disruption of CB1 receptor signaling enhances anxiety-like responses [7,9–11]. For example, decreases in open arm entries (anxiogenic) in the elevated-plus-maze were observed for both animals injected with the CB1 receptor antagonist SR 141617A and CB1−/− mice [7,9,10]. Inhibiting the breakdown of AEA by the enzyme fatty acid amide hydrolase (FAAH) also produces anxiolytic responses [12] presumably by increasing endogenous AEA. However, other studies either report anxiolytic-like responses following CB1 receptor antagonism in animal models of anxiety [13,14] or anxiogenic-like responses only in particular contexts [9,15].
Chronic mild stress (CMS) is a valid animal model widely used to study the neurobiological underpinnings of depression [16]. Interestingly, Martin et al. [7] reported that CB1−/− mice have enhanced vulnerability to the depressive effects of CMS compared to wild-type littermates. More recently, Hill et al. [17] demonstrated that CB1 receptor levels in the hippocampus of male rats were decreased 50% following three weeks of CMS. Follow-up studies reported that CMS decreased CB1 receptor binding in the hippocampus, hypothalamus and ventral striatum, but increased binding in the prefrontal cortex [18]. This investigation also observed that AEA was significantly decreased in all brain areas studied. In agreement, administration of the FAAH inhibitor URB597 increased AEA and prevented the effects of CMS in rodents [19]. These studies suggest a link between the endocannabinoid system and depression [20]. Moreover, serum 2-AG content is significantly decreased in female patients diagnosed with major depression and the magnitude of the decrease is significantly correlated with the length and severity of the depressive episode [21]. Despite these findings, effects on eCB signaling in females remain relatively unexplored.
Therefore, in the present study we explored the effects of CMS on eCB signaling in both female and male rats in order to compare their relative responsiveness. Given that sex-differences in the stress response occur in both humans and animals, we hypothesized that CMS exposure would differentially affect CB1 receptor levels in the brains of male and female rats. We also investigated the impact of endocrine status on stress-induced alterations of eCB signaling.
2. Materials and methods
2.1. Subjects
All experimental procedures were carried out in accordance with protocols established by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. Upon arrival in the University animal facility, Sprague–Dawley rats (Charles River, Boston, MA) were group caged in same sex groups (3 per cage) for 5–10 days. All animals were between seven and eight weeks old at the beginning of experimental procedures and maintained on a 12 h/12 h light–dark cycle with lights on at 8:00 a.m. Food and water were available ad libitum in the home cages, unless otherwise noted.
2.2. Surgical procedures
All surgeries were performed under Ketamine (75 mg/kg BW) anesthesia supplemented with Acepromazine (2.5 mg/kg BW). For gonadectomies, the vas deferens was ligated bilaterally and the testes removed. Ovariectomies were performed by removing the gonads through ligation of the blood supply, suturing of the abdominal wall in females, and closing of the incision with sterile surgical staple. Sham surgeries for both procedures were performed similarly; however the gonads were not removed or damaged. Post-operative procedures included treating the incision with a topical antibiotic and injecting Buprenorphine (0.015 mg/kg BW, i.m.) to reduce post-operative pain. Animals were approximately six weeks old at the time of surgery and were allowed to recover for at least 7–10 days prior to the start of any experimental procedures.
2.3. Chronic mild stress protocol (CMS)
Animals were subjected to either the CMS protocol or the no-stress protocol (handled daily). Each day, 1–3 stressors were administered according to a set schedule. The complete regimen lasted 7 days/week for three weeks. Individually, no stressor was severe, and the unpredictability of the protocol was thought to constitute much of the stress [16]. The stressors were: (1) 5min swim in 20 °C water, (2) cage rotation (social stress), (3) social isolation with damp bedding, (4) 14 h food deprivation, 14 h water or 14 h food and water deprivation, (5) 30 min physical restraint, and (6) 30 min strobe light exposure. During Experiment 1, a two-bottle sucrose-to-water preference test was given during once a week during a social isolation for the stressed animals. Non-stressed control animals were also socially isolated once a week to perform this test. To minimize stress on the control animals, they were acclimated to social isolation prior to testing.
Body weight was measured daily as an index of health. One animal lost ≥15% of its body weight over the course of the stress protocol and was removed from the study. After three weeks, the animals were euthanized (CO2) in the a.m. of day 22 and the brains were dissected. For Experiments 1 and 2, the hippocampus, hypothalamus and cortex were isolated for Western blot analysis. The cortical tissue was used to optimize the CB1 antibody and the level of CB1 receptors in the hypothalamus was too low for reliable detection. Therefore, only the hippocampus was dissected for Experiments 3 and 4; whole brains were frozen and sectioned into 350 µM coronal slices on a Leica cryostat. These slices were then micropunched using the technique of Palkovits and Brownstein [37] to isolate the dorsal and ventral hippocampi (see Fig. 1) for Western analysis.
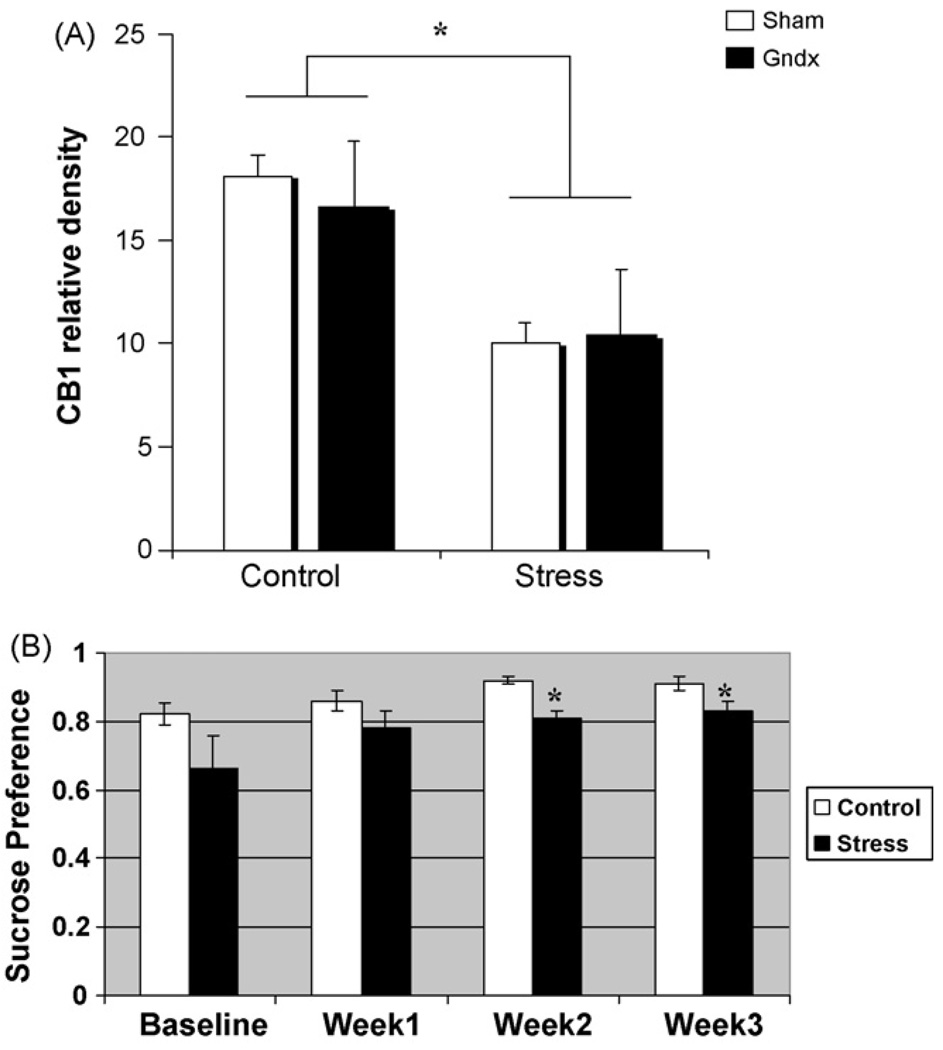
Fig. 1.
Effect of gonadal status on hippocampal CB1 receptor level in control males and males subjected to three weeks unpredictable stress. (A) There was a significant reduction in the level of CB1 protein detected in whole hippocampus by western blot in both gonadally intact males (n = 3) and gonadectomized males (n = 3) that underwent the stress paradigm (two-way ANOVA; *p = 0.02, data represent the mean ± S.E.M.). There was also no effect of gonadal status in the unstressed males (p > 0.20; n = 4 gndx, n = 3 sham). (B) Stressed animals (n = 6) exhibit a significant reduction in sucrose preference compared to control animals (n = 6). Asterisks indicate p < 0.05; gdnx and sham animals were pooled for this analysis.
2.4. Western blot analysis
Membrane homogenates from dissected hippocampal sections were homogenized in RIPA buffer (Tris, NaCl, H20, Igepal CA630, deoxycholic acid, EDTA, protease inhibitor, phosphotase inhibitor). Homogenates were centrifuged at 5000 × g for 30 min at 4 °C. A Bradford assay was used to determine the final protein concentrations in the resulting supernatant. Protein (10 µg/µL) was run on an SDS–PAGE gel using a mini-cell system (Hoefer) and transferred onto a PVDF membrane. Membranes were washed in T-TBS, blocked in 5% or 10% milk for 1 h and probed with a rabbit anti-CB1 receptor polyclonal antibody (Calbiochem) at a 1:1000 dilution overnight at 4 °C. This CB1 receptor antibody recognizes the first 77 amino acids of the 60 kDa rat CB1 receptor. Some membranes were probed with a rabbit anti-FAAH (1:500, Cayman Chemicals) polyclonal antibody (amino acids 561–579) overnight at 4 °C. Membranes were then washed 3× in T-TBS, exposed to HRP-conjugated anti-rabbit IgG (1:3000) for 30 min and then washed and developed using a chemiluminescent detection system. Membranes were then probed with Ponceau S as a loading control [22]. The densitometry values from the samples were normalized to their respective Ponceau S densitometry values. Densitometry values were acquired using NIH Image. Statistical comparisons were performed using SPSS and consisted of one-way and two-way ANOVAs with post hoc Tukey analyses with a p-value of less than 0.05 required to reach significance.
2.5. Plasma corticosterone measurements
Trunk blood was collected for both stressed and non-stressed animals at the time of sacrifice (0900–1200). This analysis was performed only with animals from Experiment 3. Blood for each animal was collected in heparin-coated tubes kept on ice. The tubes were quickly centrifuged at 1900 × g at 4 °C for 15 min. The plasma was collected and stored at −80 °C and then shipped on dry ice to the University of Virginia, Center for Research in Reproduction for corticosterone analysis by RIA. University of Virginia uses a commercially available RIA kit (DPC Commercial Kits, Los Angeles, CA) with a sensitivity of 20 ng/ml and an average reportable range of 20–550 ng/ml.
3. Results
3.1. Experiment 1: effect of gonadal status on stress-induced down regulation of CBI receptors in male rats
Gonadectomized (gndx) males and sham surgery males were subject to chronic unpredictable stress or handled daily (control) for three weeks before quantification by western blot of CB1 receptor levels in hippocampus. A two-way ANOVA (stress x gonadal status) revealed a significant effect of stress (F[1,9] = 7.70; p = 0.02) but no effect of gonadal status and no interaction between gonadal status and stress (p> 0.7; Fig. 1A and Fig. 2B). Thus, in control animals not subjected to stress, there was no difference in CB1 receptor protein levels assessed by western blot in whole hippocampus of males that were gonadally intact versus gonadectomized males. Conversely, in males subjected to three weeks of chronic stress, there was a significant decrease in CB1 receptor protein, but this decrease was to the same degree in gonadally intact versus gonadectomized males. To assess the anhedonic effects [16] of our stress protocol, we administered a sucrose preference (sucrose vs. water intake) during each week of stress. As seen in Fig. 1B, stressed animals showed significantly lower sucrose preference scores during weeks 2 and 3 of the stress regimen (two-way repeated measures ANOVA, F[3,30] = 4.02; p = 0.02). It is important to note that gdnx and sham data were pooled in this analysis.
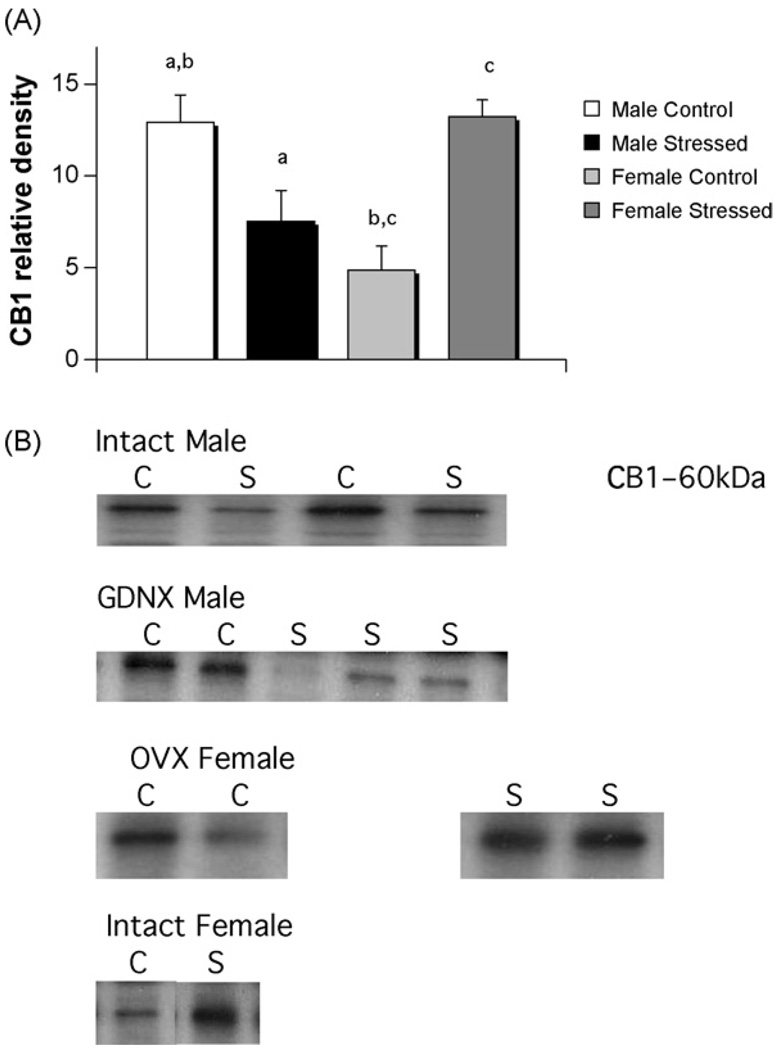
Fig. 2.
A sex difference and opposite effects of stress on CB1 receptors in hippocampus of males versus females. (A) There was a significant difference in the level of CB1 receptors of males (n = 12) and females (n = 11), with females having lower levels, in unstressed controls (two-way ANOVA; p < 0.03). Males (n = 6) subjected to three weeks of unpredictable stress exhibited significantly reduced levels of CB1 receptor protein (Tukey; p < 0.05), whereas females (n = 5) had the opposite response, showing increased CB1 receptor protein (Tukey: p< 0.004, groups sharing the same letter (a–c) are significantly different from each other. Data represent the mean ± S.E.M.). (B) Representative photomicrographs of western blots showing visualization of bands for CB1 receptors. C = control; S = stressed.
3.2. Experiment 2: comparison of gonadally intact and gonadectomized males and females following chronic unpredictable stress
A separate group of animals were either gonadectomized or underwent sham surgery prior to three weeks of chronic unpredictable stress (males: n = 3 for each gndx and sham, females: n = 3 for each gndx and sham) or daily handling (control) (males: n = 4 for gndx, n = 2 sham, females: n = 3 for gndx and n = 2 for sham). There was no effect of gonadal status on CB1 receptor level in whole hippocampus of control males and females nor was there an effect on gonadal status in those experiencing three weeks of chronic unpredictable stress, which is consistent with results for males in Experiment 1 (individual t-tests, p > 0.5; Fig. 3B). We therefore pooled gonadally intact and gonadectomized animals. A two-way ANOVA indicated a significant interaction between sex and stress (F[1,23] = 24.86; p < 0.001). There was a sex difference in CB1 receptor levels in control males and females with densities being higher in males (post hoc Tukey, p < 0.03) and there was again a significant decrease in hippocampal CB1 receptors following stress in males (post hoc Tukey, p < 0.05), although to a lesser degree than that seen in Experiment 1. In contrast, there was a significant increase in CB1 receptor levels in the hippocampus of stressed females compared to control females (post hoc Tukey, p < 0.004; Fig. 3).
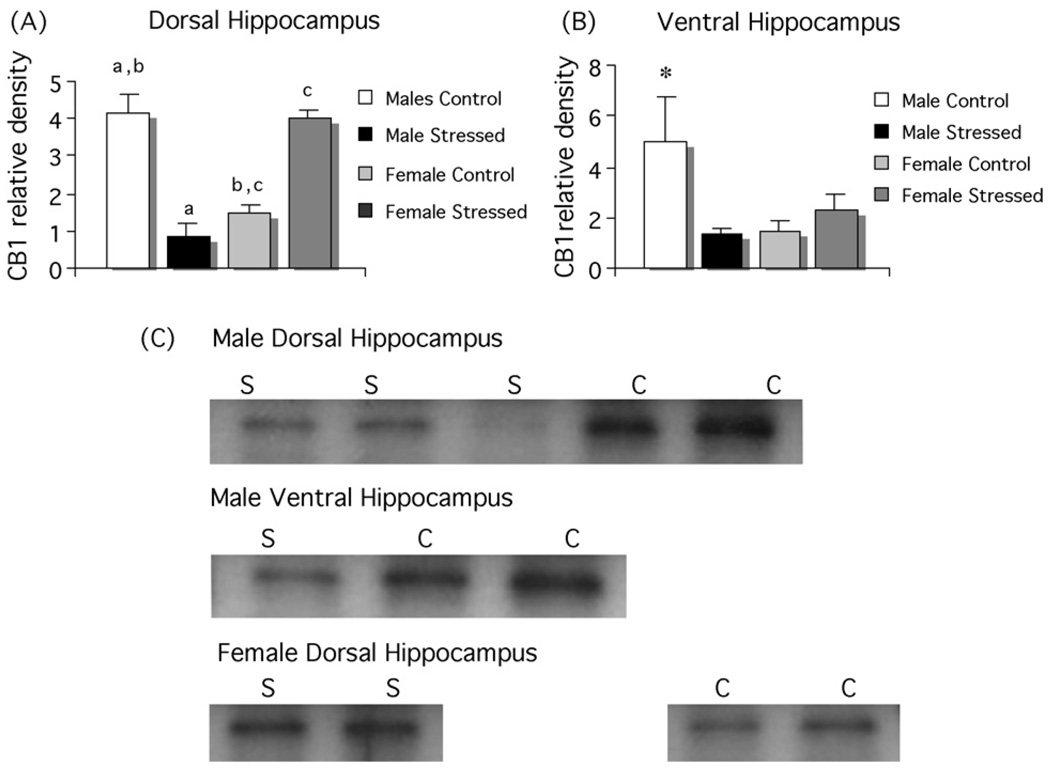
Fig. 3.
Stress decreases CB1 receptors in the dorsal and ventral hippocampus of males and increases CB1 receptor level in female dorsal hippocampus. (A) Levels of CB1 protein were analyzed in micropunches of dorsal hippocampus and there was again a significant decrease in males (n = 6) and a significant increase in females (n = 11) following three weeks of unpredictable stress and control males (n = 6) had significantly higher CB1 levels than control females (n = 6; two-way ANOVA; p < 0.01, groups sharing the same letter are significantly different from each other. (B) In the same animals, CB1 receptors levels in the ventral hippocampus trend toward a reduction in stressed males compared to control males (p < 0.07), but did not change in females. Levels of CB1 receptors in control males were significantly higher than in control or stressed females (*p < 0.05). (C) Representative photomicrographs of western blots showing visualization of bands for CB1. C = control, S = stressed.
3.3. Experiment 3: examination of CB1 receptor in subregions of male and female hippocampus following chronic unpredictable stress
The use of Palkovits micropunches allowed for a comparison of the dorsal and ventral hippocampus. All animals were gonadectomized. Consistent with Experiment 2, there was a significant interaction between stress and sex (F[1,32] = 82.60; p < 0.001) manifested as a decrease in CB1 receptor protein following stress in the dorsal hippocampus of males (post hoc Tukey, p < 0.01) and an increase in the dorsal hippocampus of females (p < 0.001). There was also a sex difference between unstressed males and females (p < 0.001; Fig. 4). In the ventral hippocampus, there was again a significant interaction between stress and sex (F[1,32] = 6.66; p < 0.05) but post hoc analysis indicated this was due to a sex difference in unstressed animals (p < 0.03) with a trend towards a decrease in stressed versus unstressed males (p < 0.07). There was no effect of stress in females (p > 0.8).
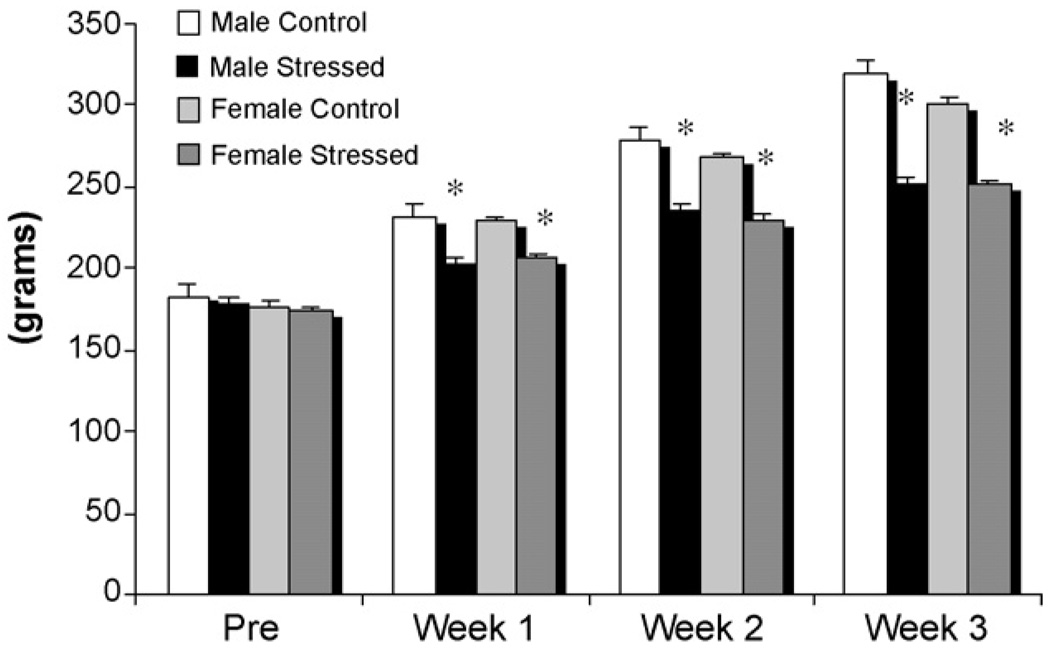
Fig. 4.
Weight gain in stressed and unstressed males and females. Body weight was determined for animals in Experiment 3 prior to the onset of stress and measured daily thereafter and averaged across the week. Males and females subjected to unpredictable stress showed significantly reduced weight gain compared to controls and there was no sex difference in this response (two-way ANOVA; *p < 0.001).
To assess whether males and females in Experiment 3 experienced similar levels of stress in response to the experimental protocol, we measured both weight gain and corticosterone levels. The monitoring of body weight across the three weeks of stress demonstrated a physiological impact of the stress on both males and females, as there was significantly less weight gain within one week of the onset of the stress protocol in both stressed males and females compared to controls (F[1,30] = 70.09, p < 0.0001). By the third week of stress, the differential in weight gain was substantial, with a mean 67 g difference between control and chronically stressed animals (Fig. 5). At completion of the experiment, trunk blood was collected and assayed for corticosterone levels. There was a significant effect of stress, with an overall decrease in circulating corticosterone concentration in both stressed males (49%) and females (18%) compared to controls (F[1,30] = 5.19; p < 0.05; Table 1). These results suggest that the stress protocol effectively impacted physiology through dysregulation the HPA axis [23–25]. However, further analysis revealed that this effect was only significant in males (p < 0.05); thus buttressing the evidence that male and female physiology responds differently to stress. The amount of corticosterone in each animal did not co-vary with the level of CB1 receptors detected (R2 = 0.011).
Fig. 5.
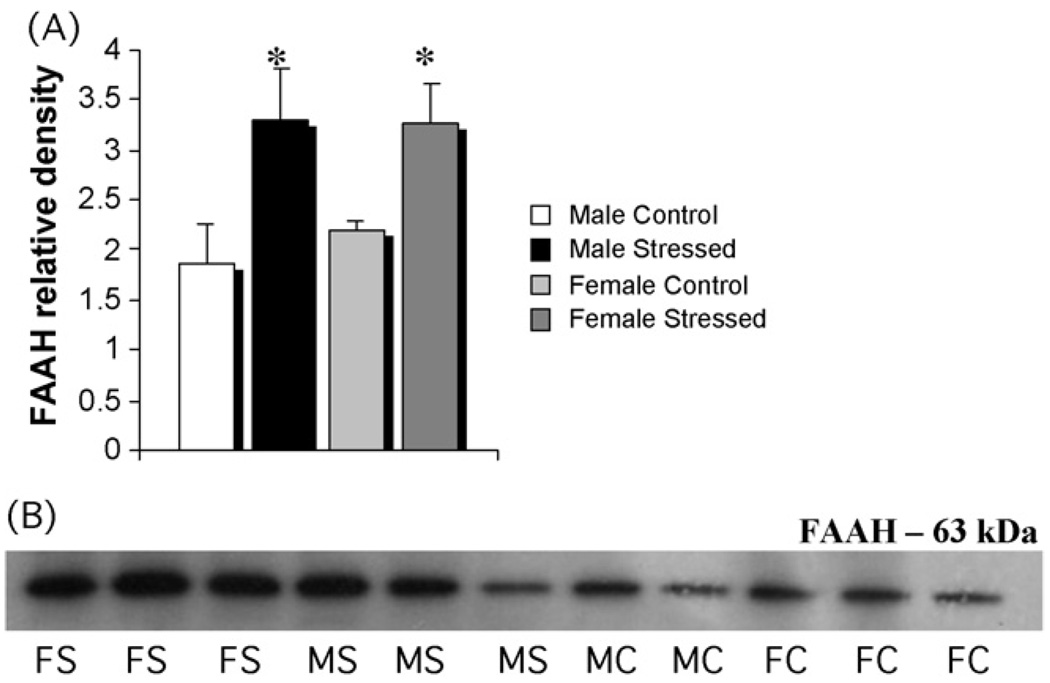
Stress increases FAAH in both males and females in dorsal hippocampus. (A) Tissue from the same animals in Fig. 4 was also assessed for levels of FAAH protein by western blot. There was a significant increase in FAAH in both males and females after three weeks of unpredictable stress (two-way ANOVA; p < 0.03). (B) Representative photomicrographs of western blots showing visualization of bands for FAAH: FS = female stressed, FC = female control, MS = male stressed, MC = male control.
Table 1.
Serum corticosterone levels (ng/ml) in males and females left untreated orsubjected to three weeks of unpredictable stress.
| Control | Stressed | |
|---|---|---|
| Male (n = 6) | 301.02 ± 52.8 | 155.44 ± 58.5a |
| Female (n = 11) | 294.73 ± 34.4 | 243.26 ± 33.1 |
ANOVA indicated a significant effect in males only (p < 0.05).
Quantification of the levels of the endocannabinoid degradative enzyme, fatty acid amide hydrolase (FAAH), in the same samples in which CB1 receptors levels were measured indicated a significant increase in the dorsal hippocampus of both males and females subjected to stress compared to controls (F[1,28] = 5.40; p < 0.03). There was no sex difference in the levels of FAAH in control or stressed animals (Fig. 5).
4. Discussion
The present findings demonstrate that chronic stress differentially affects the endocannabinoid system in male and female rats. Specifically, CMS significantly downregulated CB1 receptor levels in the male hippocampus, consistent with previous reports [17] and upregulated CB1 receptor levels in the female hippocampus. This CMS-induced differential effect was observed regardless of gonadal status in whole hippocampi samples; however these particular effects are tempered by the low number of animals in these studies. Subsequent experiments in gonadectomized animals confirmed the CMS-induced sex differences in CB1 receptors densities but showed that the effect was more pronounced in the dorsal hippocampi of male and female animals compared to the ventral hippocampi. These CMS-induced alterations in CB1 receptors were accompanied by concomitant increases in FAAH levels in both male and female animals. We also observed a basal (non-stress) sex difference in hippocampal CB1 receptors levels.
Contrary to our expectation, CMS-induced alterations in CB1 receptors occurred in both intact and gonadectomized males, suggesting the observed sex differences are not a function of circulating testosterone in the adult animal. However, sex hormones induce potent organizational effects during brain development in addition to their activational effects in adulthood [26]. Therefore, we cannot rule out the developmental influence of sex hormones on the eCB system. A recent study by Hill et al. [27] reported that the antidepressant effect of estrogen in animal models of anxiety was prevented by pharmacologically blocking CB1 receptors; thus implicating a modulatory role of estrogen on the eCB system. Future studies will determine the impact of estrogen on stress-induced CB1 receptor alterations. We did confirm that stress equally impaired weight gain in both male and female animals compared to controls, although corticosterone levels were significantly affected only in males. An important point is that our observation of CMS-induced CB1 receptor downregulation in males is consistent with the findings of Hill et al. [17,18,21].
The hippocampus is a critical component of the stress axis and serves to inhibit corticosterone and ACTH secretion under both stressful and basal conditions. Given there are distinct roles for the dorsal and ventral hippocampus in this inhibition [28,35]; we investigated the subregional differences in stress-induced CB1 receptor regulation. Our findings indicate that stress more profoundly affected CB1 receptors in the dorsal hippocampus compared to the ventral hippocampus in both male and female animals. The main role of HPA axis inhibition appears to be mediated by the ventral hippocampus, whereas the dorsal hippocampus is more involved in modulating circadian HPA activities and other biological rhythms with cognitive function [28,35]. Thus, CMS-induced CB1 receptor regulation may primarily affect HPA function by chronically altering basal glucocorticoid rhythms. Interestingly, McLaughlin et al. [29] reported that infusing the CB1 receptor agonist HU-210 into the dorsal hippocampus induced antidepressant-like effects in the Forced Swim Test. In addition, we observed that FAAH levels in the dorsal hippocampus were upregulated in stressed animals regardless of sex. Since FAAH is the main degradative enzyme for the endocannabinoid anandamide (AEA), upregulation of FAAH should result in reduced synaptic levels of AEA. It is well documented that loss or gain of a receptor agonist can cause either a compensatory downregulation or upregulation of a target receptor protein [30]. Since the effects of stress on FAAH were the same in males and females, the bi-directional regulation of CB1 levels between male and female animals appears to reflect a genuine sex difference of the eCB system responsiveness to stress at the level of the receptor. Being that females have lower basal levels of CB1 receptors, it is plausible that endocannabinoid output is also low and may not increase in response to stress. However, based on our observations, the stress-induced increase in FAAH activity may trigger an increase in female CB1 receptor levels. Alternatively, stress may increase both endocannabinoid output and CB1 receptor levels in females and receptor density may remain high in response to the altered FAAH activity. Notably, Hill et al. [17] reported a reduction 2-AG content and not AEA in the male rat hippocampus after three weeks of CMS, but then reported that chronic stress decreased AEA (and not 2-AG) in the hippocampus, hypothalamus, ventral striatum and prefrontal cortex. Additionally, reductions of 2-AG only were observed in human female patients diagnosed with major depression [18,21]. From these findings, it remains unclear how stress impacts individual eCB molecules, although it was recently reported that inhibiting FAAH activity (AEA) prevented the reductions in weight gain and sucrose intake normally caused by CMS but did not affect AEA content in the hippocampus [19].
The literature on the role of CB1 receptors in stress, anxiety and depression overwhelmingly argues that eCBs function to dampen stress/anxiety responses, perhaps to prevent pathological responding (e.g. major depression, see for review [20,31]). The consistent stress-induced downregulation of CB1 receptors in the male hippocampus reported by Hill et al. [17,18] and now by the present study add further support for this hypothesis. Our observations of a stress-induced upregulation in the female hippocampus are somewhat surprising. Given that human [2,3] and animal females [4,32] are more vulnerable to stress and depression, it would be predicted that CMS would result in a more profound downregulation in female hippocampal CB1 receptors. This hypothesis, however, assumes that basal CB1 receptors levels are equal in the male and female hippocampus. Our findings actually indicate there is a basal sex difference in CB1 levels with males having significantly more CB1 receptors than females. This further suggests that the eCB system is developmentally organized to respond to stress in a gender-dependent fashion (e.g. decrease in males; increase in females). It is plausible that CB1 receptor upregulation in females represents a compensatory mechanism to counter a reduction in either AEA or 2-AG. This would in turn, maintain a semi-functional eCB system that may attempt to balance other negative physiological effects of chronic stress. For example, Dalla et al. [32] showed that CMS produced similar behavioral changes (sucrose intake, rearing behavior, etc.) in both male and female rats, but significantly reduced serotonergic activity and increased corticosterone levels only in females. Other studies also report little-to-modest sex differences in CMS-induced behavioral changes [33,36]. Based on these findings, we hypothesize that a higher vulnerability in female rats to the effects of chronic stress is partially ameliorated by an upregulation of CB1 receptors.
In summary, the current findings add to the extensive literature demonstrating a profound responsiveness of the eCB system to stress and anxiety. Our data suggest that the differential stress regulation of CB1 in male and female animals results from a basal sex difference in hippocampal CB1 receptors levels. Interestingly, Suárez et al. [38] reported that hippocampal CB1 immunoreactivity was decreased in neonatal male but not female rats that experienced maternal deprivation. Sex differences in the eCB system could provide a key target for developing novel treatments for stress and depression that are designed specifically for individual women and men.
Acknowledgements
This research was supported by NIH grants RO3 MHO79294-01 to CGR and RO1 NS050525-01A1 to MMM. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. We would like to thank Catherine Reich for her excellent technical assistance.
References
- 1.Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS. Gender differences in the genetic epidemiology of major depression. J Gend Specific Med. 1998;1:28–31. [PubMed] [Google Scholar]
- 3.Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry. 1997;58 Suppl. 15:12–18. [PubMed] [Google Scholar]
- 4.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 8.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 9.Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- 10.Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-speciflc agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- 11.Navarro M, Hernandez E, Munoz RM, Del AI, Villanua MA, Carrera MR, et al. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 12.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 13.Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naive and plus-maze-experienced mice. Behav Pharmacol. 2005;16:405–413. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 16.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 17.Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Down-regulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- 18.Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, et al. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008;106(6):2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62(10):1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41(2):48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olesen KM, Auger AP. Sex differences in Fos protein expression in the neonatal rat brain. J Neuroendocrinol. 2005;17(4):255–261. doi: 10.1111/j.1365-2826.2005.01302.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanke MAC, Fokkema DS, Doornbos B, Postema F, Korf J. Sustained release of corticosterone in rats affects reactivity, but does not affect habituation to immobilization and acoustic stimuli. Life Sci. 2008;83(3–4):135–141. doi: 10.1016/j.lfs.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Bauer ME, Perks P, Lightman SL, Shanks N. Restraint stress is associated with changes in glucocorticoid immunoregulation. Physiol Behav. 2001;73(4):525–532. doi: 10.1016/s0031-9384(01)00503-0. [DOI] [PubMed] [Google Scholar]
- 25.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32(4):350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo–pituitary–adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin RJ, Hill MN, Morrish AC, Gorzalka BB. Local enhancement of cannabinoid CB1 receptor signalling in the dorsal hippocampus elicits an antidepressant-like effect. Behav Pharmacol. 2007;18(5–6):431–438. doi: 10.1097/FBP.0b013e3282ee7b44. [DOI] [PubMed] [Google Scholar]
- 30.Meyer JS, Quenzer LF. Psychopharmacology: drugs, the brain and behavior. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- 31.Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic–pituitary–adrenocortical axis. Progr Brain Res. 2008;170:397–430. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- 32.Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, et al. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 33.Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 2005;179(4):769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- 34.De Petrocellis L, Di Marzo V. Role of endocannabinoids and endovanilloids in Ca(2+) signalling. Cell Calcium. 2009 doi: 10.1016/j.ceca.2009.03.003. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 36.Konkle AT, Baker SL, Kentner AC, Barbagallo LSM, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2000;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Palkovits M, Brownstein M. Microdissection of brain areas by the punch technique. In: Cuello A, editor. Brain microdissection techniques. New York, NY: John Wiley and Sons; 1982. pp. 1–36. [Google Scholar]
- 38.Suárez J, Llorente R, Romero-Zerbo SY, Mateos B, Bermúdez-Silva FJ, de Fonseca FR, et al. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB(1) and CB(2) cannabinoid receptors of neonatal rats. Hippocampus. 2008 doi: 10.1002/hipo.20537. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]