Abstract
The cytochrome P450 (CYP) enzymes CYP2C8, CYP2C9, and CYP2J2 metabolize arachidonic acid to epoxyeicosatrienoic acids (EETs), which are known to be vital in regulation of vascular tone and cardiovascular homeostasis. Since there is limited information regarding the relative expression of these CYP enzymes in cardiovascular tissues, this study examined the expression of CYP2C8, CYP2C9 and CYP2J2 mRNA and protein in human heart, aorta and coronary artery samples by real-time PCR, immunoblotting and immunohistochemistry. CYP2J2 and CYP2C9 mRNA levels were highly variable in human hearts, while CYP2C8 mRNA was present in lower abundance. CYP2J2 mRNA was approximately 103 times higher than CYP2C9 or CYP2C8 in human heart. However, CYP2C9 mRNA was more abundant than CYP2J2 or CYP2C8 in one ischemic heart. In human aorta, mean CYP2C9 mRNA levels were ~ 50 times higher than CYP2J2 and 5-fold higher than CYP2C8. In human coronary artery, mean values for CYP2C9 mRNA were ~ 2-fold higher than CYP2J2 mRNA and 6-fold higher than CYP2C8 mRNA. Immunoblotting results show relatively high levels of CYP2J2 and CYP2C8 protein in human hearts, which was confirmed by immunohistochemistry. CYP2C9 protein was also detected at high levels in one ischemic heart by immunoblotting. CYP2C9 was present at higher levels than CYPJ2 in aorta and coronary artery, while CYP2C8 protein was below the limits of detection. The expression of CYP2J2 and CYP2C8 in human heart, and CYPC9 and CYP2J2 in aorta and coronary artery is consistent with a physiological role for these enzymes in these tissues.
Coronary heart disease, including myocardial infarction, is one of the leading causes of morbidity and mortality in North America (Link and Tanner, 2001). It is now known that the endothelium, a single layer of cells lining the vascular wall, plays a significant role in regulation of vascular tone and homeostasis (Busse and Fleming, 2003). Disruption of endothelial cell homeostasis can lead to hypertension, atherosclerosis and cardiovascular diseases (Cines et al., 1998; Russo et al., 2002; Voetsch et al., 2004). Nitric oxide (NO) has been shown to play a significant role in endothelial dysfunction by its interaction with superoxide (O2−) to form reactive oxygen species (ROS). A number of studies indicate that the cytochrome P450 enzymes (CYP) produce arachidonic acid metabolites as well as generate ROS, both of which are involved in the regulation of inflammation and cardiovascular physiology. CYP enzymes are also important in the regulation of post-ischemic cardiac contractility and vasodilation in coronary arteries and aorta (Campbell and Harder, 1999; Seubert et al., 2004).
CYP enzymes comprise the third pathway of arachidonic acid metabolism (in addition to cyclooxygenase and lipoxygenase pathways). Arachidonic acid is important in maintaining normal physiological function of cell membranes, such as signal transduction, ion transport and hormonal regulation (Capdevila et al., 2000). CYP enzymes metabolize arachidonic acid to produce epoxyeicosatrienoic acids (EETs), which are believed to represent endothelium-derived hyperpolarizing factors (EDHFs) involved in vasodilation. EETs contribute to vascular smooth muscle hyperpolarization, regulation of blood pressure (Sinal et al., 2000) and hypertension (Imig et al., 2002). CYP2C enzymes (CYP2C8/CYP2C9) have been proposed as likely candidates for EDHF synthases (Fisslthaler, 1999). However, due to the high homology of these enzymes, most studies have not been able to distinguish between CYP2C8 and CYP2C9.
CYP and CYP2J2 enzymes have prominent roles in vascular regulation and homeostasis in humans (Fleming, 2001). CYP2J2 has been widely studied in human heart and has been found to be constitutively expressed with a large degree of interindividual variation (Wu et al., 1996). CYP2J2 is also localized in the endothelium of both large and small coronary arteries (Node et al., 1999). CYP2C9 and CYP2C8 mRNAs have been reported in human myocardium (Enayetallah et al., 2004) as well as endothelial cells (Lin et al., 1996). More specifically, polymorphisms in CYP2J2, CYP2C8 and CYP2C9 have been implicated in cardiovascular disease (Yasar et al., 2003; Spiecker et al., 2004). One study (Yasar et al., 2003) suggested a link between myocardial infarctions (MI) and CYP2C9*2 and CYP2C9*3 polymorphisms in female patients as well as CYP2C8*3 polymorphisms in male and female patients.
It has been hypothesized that during reperfusion, following a long period of ischemia, the heart undergoes damage brought about by the presence of CYP enzymes in this tissue (Gottlieb, 2003). This damage occurs by the activation of cardiac CYP enzymes which lead to the production of ROS (Gottlieb, 2003). ROS is believed to contribute to myocardial infarction as well as vascular dysfunction by damaging DNA, proteins and lipids. In contrast, CYP2J2 overexpression has been found to be cardioprotective following hypoxia/reoxygenation injury (Yang et al., 2001; Seubert et al., 2004). Inhibitors of CYPs have also been shown to decrease post-ischemic infarct size in rats and rabbits (Granville et al., 2004; Gross et al., 2004; Hunter et al., 2005). More specifically, sulfaphenazole, a specific inhibitor of CYP2C9, has been shown to have a protective effect and has been proposed to be a possible therapeutic agent in patients with ischemic heart disease (Hunter et al., 2005).
Recent reports have provided limited information on the relative levels of the CYP2C enzymes in cardiovascular tissues (Thum and Borlak, 2000) (Larsen et al., 2006). Because of their extensive homology, more specific antibodies and oligonucleotide primers are needed to determine the levels of the individual CYP2C8/CYP2C9 proteins and mRNAs. This study is the first to utilize specific quantitative and qualitative techniques to localize CYP2C8, CYP2C9 and CYP2J2 in human cardiovascular tissues. The findings provide evidence of the relative expression of these enzymes at the mRNA and protein level in cardiovascular tissues. Our data are consistent with the hypothesis that these enzymes may play a significant role in the regulation of cardiovascular homeostasis.
METHODS
Heart Tissues
Paraffin blocks and frozen tissue of two human hearts (G and H) were obtained from the Cooperative Human Tissue Network (CHTN). Five hearts (coded A–E) were obtained from the National Disease Research Interchange (NDRI). One additional heart (F) and five coronary artery and five aorta tissues were obtained from discarded heart transplant tissues at Duke University Hospital. These tissues were considered exempt by the NIEHS IRB since discarded tissues with no patient identifiers were used. Most heart samples were from the left ventricle; a list of heart samples and diagnoses of condition at the time of retrieval is given in Table 1. Available clinical information on coronary artery and aorta tissues from five patients (numbered 1–5) are shown in Table 2. Due to the availability of appropriate samples, coronary and aorta samples were obtained from the same patients, while heart samples originated from different individuals.
TABLE 1.
Human Heart Tissues
| Heart Tissues | Ventricle | Age | Sex | Race | Diagnosis |
|---|---|---|---|---|---|
| Heart A | Left | 33 | Female | African American | CVA |
| Heart B | Left | 66 | Female | White | ICH |
| Heart C | Left | 74 | Female | White | ICH |
| Heart D | Left | 54 | Male | White | CVA |
| Heart E | Left | 67 | Male | White | ICH |
| Heart F | No information | 55 | Male | White | Brain Dead Donor |
| Heart G | Left | 35 | Female | African American | Subaracnoid hematoma |
| Heart H | Left | 62 | Male | White (Hispanic) | Ischemic Heart Disease |
ICH = intra-cranial hemorrhage; CVA=cerebral-vascular accident (stroke)
TABLE 2.
Clinical information for human coronary and aorta tissues
| Coronary Tissues | Diagnosis |
|---|---|
| Coronary 1 | No atherosclerosis |
| Coronary 2 | Minimal atherosclerosis |
| Coronary 3 | No atherosclerosis |
| Coronary 4 | Severe atherosclerosis |
| Coronary 5 | Moderate atherosclerosis |
| Aorta Tissues | Diagnosis |
| Aorta 1 | No atherosclerosis |
| Aorta 2 | Minimal atherosclerosis |
| Aorta 3 | No atherosclerosis |
| Aorta 4 | No atherosclerosis |
| Aorta 5 | No atherosclerosis |
Isolation of total RNA and Preparation of cDNA
Total RNA was extracted from human tissues using RNeasyfrom Qiagen (Valencia, CA) following the manufacturer’s protocol. RNA (200 ng) was transcribed to cDNA using 200 units of Superscript™ II reverse transcriptase from Invitrogen (Carlsbad, CA), 100 ng random hexamers and 10 mM each of dGTP, dATP, dTTP and dCTP in 1X buffer in a total volume of 20 μl following the manufacturer’s protocol. The reaction was initially incubated at 25°C for 2 minutes and then at 25°C for 10 min after addition of Superscript™ with a final incubation at 42°C for 50 min.
Quantitative real-time PCR
Quantitative real-time PCR was performed using the following primer and probe sets from Applied Biosystems (Foster City, CA): CYP2C8, catalog # Hs00426387_m1, CYP2C9, catalog # Hs00426397_m1, CYP2J2, catalog # Hs00356035_m1 and GAPDH, catalog # Hs99999905_m1. The TaqMan® probe and primer sets used for CYP2C8, CYP2C9, CYP2J2 and GAPDH mRNA quantitation have been reported to have equal efficiencies (100 ±10 %) (http://docs.appliedbiosystems.com/pebiodocs/00113186.pdf). The cDNAs from each reversed transcribed reaction were diluted 4 fold, mixed with Taqman® 2X Universal PCR Master Mix No AmpErase® UNG and amplified using cycling conditions including a denaturation step at 94°C for 10 min, another denaturation step at 95°C for 15 sec and an annealing step at 60°C for 60 sec for 45 cycles. Reactions were run in an ABI Prism™ 7900 Sequence Detector from Applied Biosystems (Foster City, CA). Relative mRNA expression of CYP2C8, CYP2C9 and CYP2J2 was determined using the ΔCt method (value obtained by subtracting the Ct value of GAPDH mRNA from the Ct value of the target mRNA). Data was expressed as the ratio (calculated using 2−(ΔCt)) of target mRNA to GAPDH mRNA (Livak and Schmittgen, 2001). The studies were performed in triplicate at least three times and the data was summarized as mean ± standard error (SE) values of all values.
Preparation of Heart Microsomes and Aorta and Coronary Tissue Lysates
Heart tissue was homogenized in 50 mM Tris-HCl, pH 7.4/1 mM EDTA/1.15% KCl/0.5 mM phenylmethylsulfonyl floride (PMSF) using an Omni tissue grinder on ice, then centrifuged at 800 × g for 10 minutes. The resulting supernatant was centrifuged at 10,000 × g for 30 minutes two times. The 10,000g supernatant was then recentrifuged at 100,000 × g for 70 minutes. The pellet was washed in 100 mM sodium pyrophosphate, pH 7.4/0.1 mM EDTA, and recentrifuged at 100,000 × g for 70 minutes. All procedures were performed at 4°C. The final microsomal pellet was resuspended in 10 mM KPO4, pH 7.4/1 mM EDTA/20% glycerol and stored at −80°C.
To prepare aorta and coronary artery lysates, frozen tissues (0.01 mg to 0.1 mg) were added to 1 ml of Lysis Buffer (50mM Tris-HCL (pH 7.4), 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM PMSF (0.25 M stock), 0.25% sodium deoxycholate, 1 μg/ml aprotinin, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mM sodium orthovanadate, 1 mM sodium fluoride) and homogenized immediately on wet ice. The samples were transferred to dry ice for 5 minutes and then thawed on wet ice. After thawing, the samples were vortexed and centrifuged for 5 minutes @ 13,400 × g at 4°C. The supernatants were sonicated and the resulting cell lysates stored at −80°C.
Protein Immunoblotting
Polyclonal rabbit anti-CYP2C8 and anti-CYP2C9 were raised to bacterially expressed human CYP2C8 or CYP2C9 (Covance, Denver, Pa), respectively, using a standard NIEHS rabbit antibody production protocol. Recombinant CYP2C protein standards were from a yeast cDNA expression system since no N-terminal modification of the cDNA is used for expression of cytochrome P450s in yeast (Romkes et al., 1991). Therefore, the mobility of the yeast expressed standards is identical to those of the human CYP proteins expressed in vivo. The CYP2J2-specific peptide antibody HMDQNFGNRPVTPMR (anti-CYP2J2pep1) was prepared as described (Wu et al., 1996). CYP2J2 recombinant protein expressed in baculovirus was prepared as described (Wu et al., 1996).
Aorta and coronary artery lysates, heart microsomes, and recombinant CYP proteins were electrophoresed in SDS-10% (w/v) polyacrylamide gels and transferred to PVDF membranes. Membranes were immunostained with rabbit anti-CYP2C8, rabbit anti-CYP2C9 and rabbit anti-CYP2J2 and goat anti-rabbit IgG conjugated to horseradish peroxidase from Pierce (Rockford, IL). Polypeptide bands were visualized using SuperSignal West Femto Chemiluminescent Substrate from Pierce (Rockford, IL) and a SynGene GeneGnome chemiluminescence detection system from Synoptics (Cambridge, UK). Polypeptide bands representing authentic recombinant CYP standard proteins were quantitated with GeneTools version 3.06 (Synoptics) using manual band quantification and the quadratic curve-fitting option. Immunoreactive polypeptide bands in lysates and microsomes with electrophoretic mobility identical to those of the recombinant CYP standards were quantified by comparison to the standard curves for the recombinant proteins.
Immunohistochemistry
Immunohistochemistry was performed using the avidin-biotin-peroxidase method (Vector Laboratories, Burlingame, CA). Sections were stained with one of the following antibodies at the respective dilution: rabbit anti-CYP2J2 (1:50), rabbit anti-CYP2C8 (1:50) or rabbit anti-CYP2C9 (1:75). Slides were deparaffinized in xylene and hydrated through a series of graded alcohols to 1X Automation Buffer™ (Biomeda, Foster City, CA). Antigen unmasking was accomplished by heating sections to 95°C in 200 ml of citrate buffer (pH 6.0) (Biocare Medical, Walnut Creek, CA) using a Decloaker™ (Biocare) for 30 minutes. Slides were then allowed to cool for 10 min. Immunohistochemical staining was completed using the automated NeXes™ instrument (Ventana Medical Instruments, Tucson, AZ). A titration run with additional blocking (Blocker A and Blocker B, Ventana) was performed on the instrument with a 32 min incubation period for all titrated primary antibodies. All slides were counterstained using a modified Harris hematoxylin (Harelco, Gibbstown, NJ) for 45 sec, dehydrated through a graded series of alcohols to xylene and coverslipped with Permount (Surgipath, Richmond, IL).
Statistical Analyses
Pairwise comparisons or individual tissues were performed using Mann-Whitney test with one-sided p-values. A p-value of <0.05 was considered statistically significant; no correction was made for multiple testing. No significant differences were found among at least three experiments done in triplicates, so replicates from all experiments were pooled for the statistical analyses. Friedman’s analysis was used to calculate p-values for comparisons of mean ΔCt values relative to GAPDH within each tissue. A p-value of <0.05 was considered statistically significant.
Results
Real-time PCR
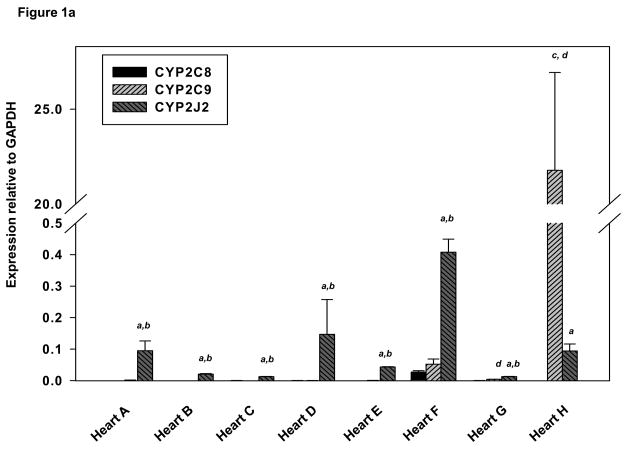
The expression of CYP2C8, CYP2C9 and CYP2J2 mRNA in cardiovascular tissues was expressed relative to GAPDH utilizing real time-PCR analysis. CYP2J2 mRNA expression was relatively abundant in all hearts. With the exception of heart H, CYP2J2 mRNA expression was greater than CYP2C9 (p<0.01) or CYP2C8 (p<0.05) in each of the remaining seven hearts (Fig. 1A). Mean values for expression of each mRNA relative to GAPDH are shown in Table 3. The mean for CYP2C9: GAPDH excluded heart H since the level of CYP2C9 mRNA in this heart was ~800 times greater than the mean for the remaining samples. Mean values for CYP2J2 mRNA were ~ 900 times higher than CYP2C9 (p<0.0001) in the remaining seven hearts and 1600 times higher than CYP2C8 (p<0.0001). In contrast, CYP2C9 mRNA expression was 200-fold greater than that of CYP2J2 (p<0.05) and ~ 1000-fold greater than CYP2C8 (p<0.05) in Heart H from an individual who died from ischemic heart disease (Fig. 1A).
Figure 1.
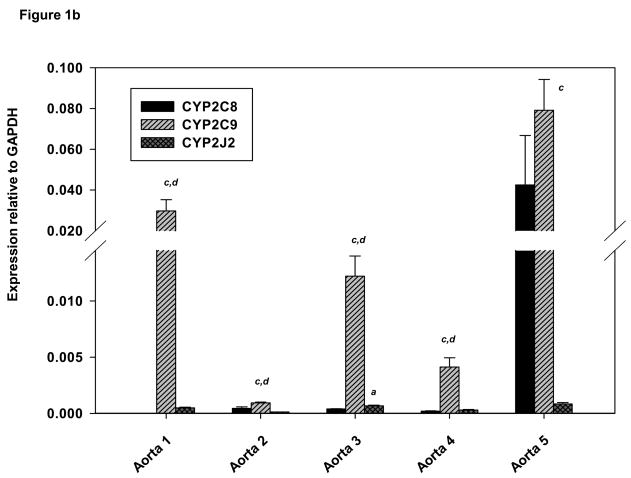
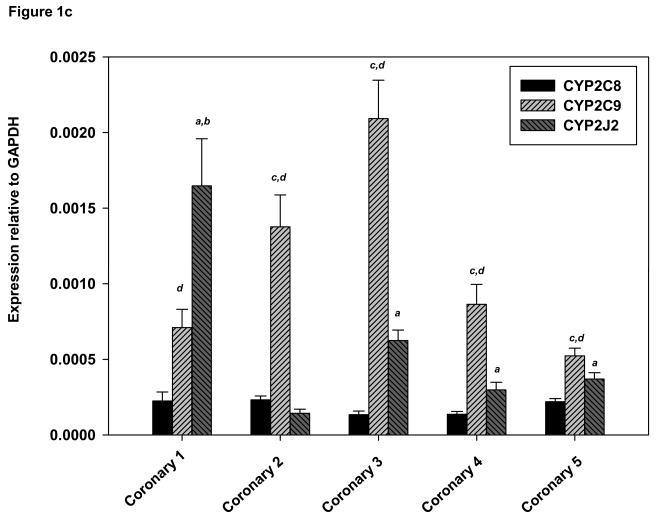
(A) Expression of CYP2C8, CYP2C9 and CYP2J2 in human heart by real–time PCR. GAPDH was used as an internal control to normalize all target values. Derivations of triplicate samples are shown and experimental data are mean ± SE. (B) Expression of CYP2C8, CYP2C9 and CYP2J2 in human aorta by real–time PCR. (C) Expression of CYP2C8, CYP2C9 and CYP2J2 human coronary artery tissues by real–time PCR. aCYP2J2 greater than CYP2C8, p<0.05; bCYP2J2 greater than CYP2C9 p<0.05, cCYP2C9 greater than CYP2J2 p<0.05; dCYP2C9 greater than CYP2C8, p<0.05 (pairwise comparisons were performed using Mann-Whitney one-sided p-values).
TABLE 3.
Mean relative target mRNA/GAPDH ratios in human hearta
| CYP2C8:GAPDH × 10−3 | CYP2C9:GAPDH × 10−3 | CYP2J2:GAPDH × 10−3 | |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| Aorta | 4.30 ± 3.3 | 23.6 ± 11.6 | 0.44 ± 0.09 |
| Coronary | 0.18 ± 0.01 | 1.1 ± 0.28 | 0.61 ± 0.26 |
| Heart | 13.7 ± 3.5 | 25.7b ± 9 | 22,336 ± 9,728 |
Data are expressed as the ratio of Ct values for target mRNA to GAPDH mRNA. Values shown are the means ± SE of triplicate analyses in at least three independent experiments; SE’s: estimate between-patient variability and were calculated from analyses of variance.
CYP2C9:GAPDH values for Heart H were excluded from the mean, since they differed dramatically from the other values (22,000 ± 5,000 × 10−3).
Individual human aortas exhibited varying levels of all three mRNAs. CYP2C9 mRNA was considerably higher than CYP2J2 (p<0.05) in all five aortas and CYP2C8 mRNA in four of the aortas (p<0.05) (Fig 1B). CYP2C8 mRNA was also more abundant than CYP2J2 in aorta 5 (Fig. 1B). Mean values of CYP2C9 mRNA relative to GAPDH were ~50 times higher than those of CYP2J2 (p<0.0001) and 5-fold higher than those of CYP2C8 (p<0.0001) (Table 3). Individual coronary samples also exhibited considerable interindividual variability in expression of the three mRNAs. CYP2C9 mRNA expression was generally greater than CYP2J2 or CYP2C8 (Fig. 1C). Specifically, CYP2C9 mRNA was higher than CYP2J2 in four out of five coronary artery samples (p<0.02), and CYP2J2 was higher than CYP2C8 in (p<0.01) in all samples except coronary 2. Mean values for CYP2C9 mRNA relative to GAPDH were ~two times higher those of CYP2J2 (p<0.0001) and six fold higher than those of CYP2C8 (p<0.0001) (Table 3).
Western blotting
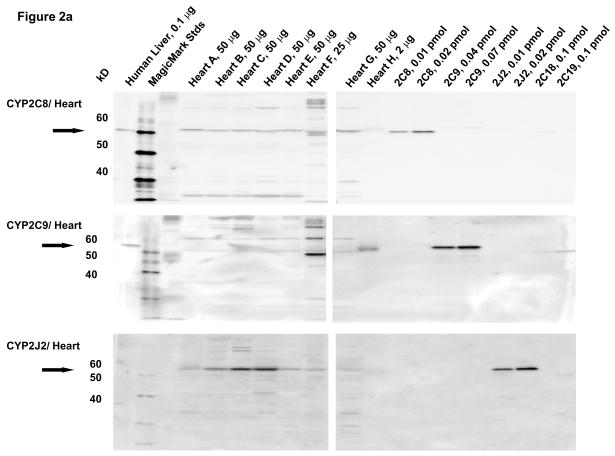
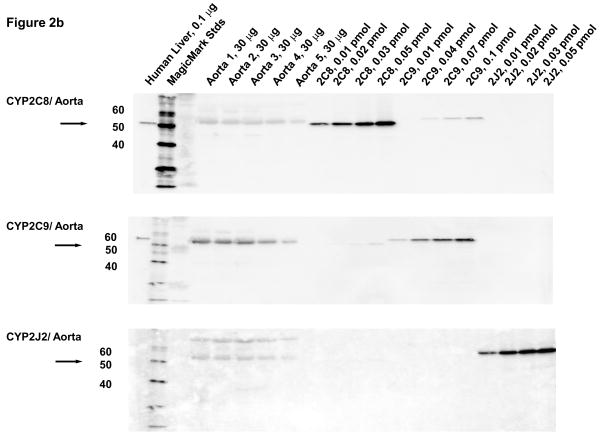
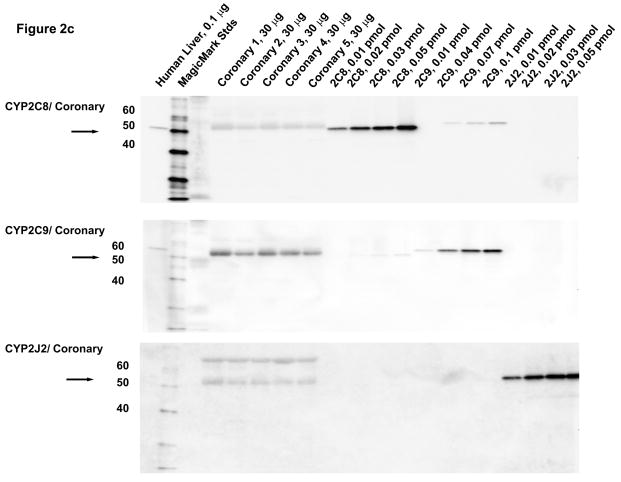
Microsomal fractions of human liver and heart, and lysates of coronary artery and aorta were separated by electrophoresis and subjected to immunoblotting using antibodies to CYP2C8, CYP2C9, and CYP2J2. Recombinant human CYP2C8, CYP2C9, CYP2J2, CYP2C18 and CYP2C19 proteins were used as standards. A polyclonal antibody to CYP2C8 detected recombinant CYP2C8 as an immunoreactive band at 55 kD, as shown by the arrow (Fig. 2A). The CYP2C8 antibody did not cross-react with recombinant CYP2C18, CYP2C19 or CYP2J2 proteins. Although the CYP2C8 antibody cross-reacts slightly with recombinant CYP2C9, the two proteins can be distinguished by their electrophoretic mobilities. An immunoreactive polypeptide band with molecular mobility identical to that of recombinant CYP2C8 protein was detected at comparable levels in all of the human heart microsomal samples except for heart H, where limited amounts of heart microsomes probably prevented detection of this protein. The amounts of CYP2C8 protein in the heart microsomes were estimated to be 0.20 ± 0.02 pmol/mg tissue by comparison with recombinant standards. The CYP2C9 antibody did not cross-react with recombinant CYP2C8 or CYP2J2 protein. Although the CYP2C9 antibody cross reacted slightly with recombinant CYPC19, the two proteins can be distinguished by their mobilities. CYP2C9 protein was below the level of detection in immunoblots of most heart microsomes with the exception of heart H. Despite the limited amount of this heart sample, CYP2C9 was clearly detected at relatively high levels (~5.5 pmol/mg). Notably, heart H also contained high amounts of CYP2C9 mRNA (Fig. 2A). The CYP2J2 antibody did not cross-react with recombinant CYP2C8, CYP2C9, CYP2C18 proteins. CYP2J2 protein was detected in at relatively high levels in individual heart microsomes, but showed some interindividual variability (0.05–0.4 pmol/mg; mean = 0.17 ± 0.05 pmol/mg) (Fig. 2A).
Figure 2.
Immunoblotting of human heart microsomes and aorta and coronary artery lysates utilizing CYP2C8, CYP2C9 and CYP2J2 antibodies (A)- human heart microsomes, (B)- human aorta tissue lysates, (C)- human coronary artery tissue lysates.
In lysates from human aorta, CYP2C9 protein was detected at relatively high levels in tissues from all five individuals (1.3 ± 0.3 pmol/mg) (Fig. 2B). CYP2J2 protein was also detected on the immunoblots of the aorta lysates, albeit at significantly lower levels (0.055±0.004 pmol/mg) than CYP2C9 protein (p ≤ .01). CYP2C8 protein was not detected in human aorta by immunoblotting (<0.03 pmol/mg). The faint band detected with the CYP2C8 antibody has a slightly lower electrophoretic mobility (higher molecular weight) than CYP2C8 and probably represent cross-reactivity with CYP2C9 protein.
In lysates from human coronary arteries, CYP2C9 protein was detected in all five samples (1.7 ± 0.2 pmol/mg) (Fig. 2C). Significantly smaller amounts of CYP2J2 (0.06 ± 0.01) pmol/mg) (p=0.001) were also detected in all coronary artery samples, while CYP2C8 protein was not detected in lysates of any of the coronary samples tested (<0.03 pmol/mg). As with aorta, a slightly higher molecular weight immunoreactive band was detected in the coronary artery samples with the antibody to CYP2C8, probably representing cross-reactivity to CYP2C9 protein.
Immunohistochemistry
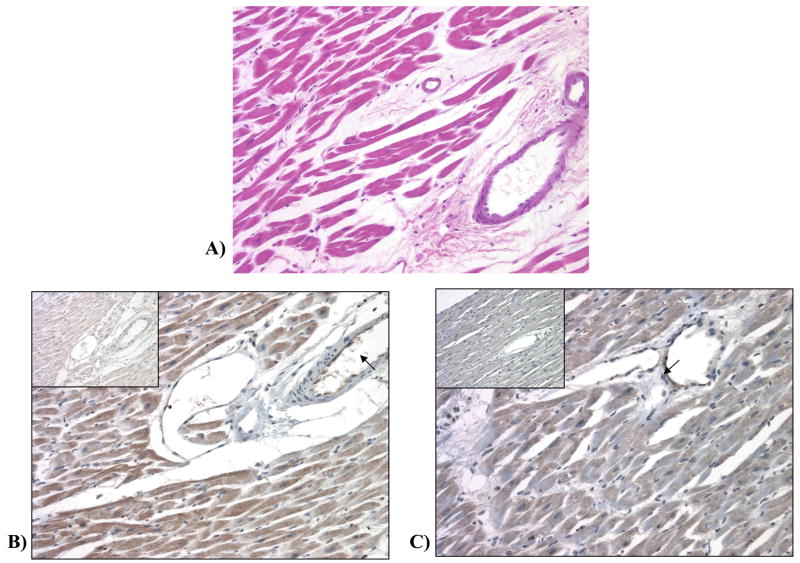
Immunohistochemical staining with the CYP2J2 antibody revealed that CYP2J2 protein was expressed in the cytoplasm of the cardiomyocytes of heart G, as well as in the endothelium of the blood vessels at a 3+ intensity level (out of a 0 to +4 scale). Immunohistochemical staining with the CYP2C8 antibody within heart G revealed more prominent expression of the CYP2C8 protein in the subendocardial zone of the myocardium (2+ intensity) than in the central portion of the myocardium (1+ intensity). Some, but not all, of the blood vessels in the myocardium also exhibited weak (1+ intensity) staining of the endothelium with the CYP2C8 antibody (Fig. 3). CYP2C9 protein could not be detected in heart G or heart H by immunohistochemistry with our antibody to CYP2C9.
Figure 3.
(A) Hematoxylin and Eosin staining of heart G. (B) IHC staining for CYP2J2 in heart (20x). Endothelial cells are stained (arrow) while staining is absent in the negative control, normal immune serum, (inset). (C) IHC staining for CYP2C8 in heart (20x). Patchy cytoplasmic staining is observed in the myocytes. Endothelial cells are also stained (arrow). Staining is absent in the negative control, normal immune serum, (inset).
Discussion
There have been numerous studies in recent years that have shed light on the potential importance of the cytochrome P450 enzyme superfamily in arachidonic acid metabolism and their physiological role in cardiovascular tissues (Roman, 2002; Gottlieb, 2003; Spiecker and Liao, 2005; Elbekai and El-Kadi, 2006). EET metabolites of CYP enzymes have a number of roles in the vasculature including their involvement in the relaxation of smooth muscle via activation of Ca2+-sensitive K+ channels and vasodilation. Despite an increasing amount of information supporting a physiological role of these enzymes in the cardiovascular tissue, studies identifying the individual CYP isoforms present in these tissues have been limited (Thum and Borlak, 2000; Larsen et al., 2006). It is widely accepted that CYP2J2 is the principal cytochrome P450 enzyme expressed in human heart at the mRNA and protein level (Wu et al., 1996; Node et al., 1999). The present study provides evidence for the relative expression of CYP2J2, CYP2C9, and CYP2C8 in cardiovascular tissues using specific PCR primers and antibodies to these isoforms.
In the present study, considerable interindividual variability was observed in the levels of CYP2J2 CYP2C9 and CYP2C8 mRNA and protein in human heart, coronary artery and aorta tissues by real-time PCR, immunoblotting and immunohistochemistry. The majority of the human hearts used in this study were non-diseased hearts. This study shows that CYP2J2 and CYP2C8 are the principal isoforms expressed in non-diseased human hearts. CYP2J2 mRNA was detected at considerably higher levels than either those of CYP2C8 or CYP2C9 mRNA in the majority of non-diseased human hearts. Despite the low levels of CY2C8 mRNA, CYP2C8 protein was detected in all human hearts. Previous studies have noted discrepancies in expression of mRNA and proteins for CYP enzymes including CYP2J2 which could not be totally explained by genotype (Forrester et al., 1992; Gaedigk et al., 2006). Reasons for discrepancies between mRNA and protein could include the condition of the tissue and integrity of the RNA, differences in pretranslational regulation, stability of the proteins or mRNA, as well as polymorphisms affecting protein expression. Surprisingly, CYP2C9 protein and mRNA were more abundant than those of either CYP2J2 or CYP2C8 in one heart which was from an individual with ischemia. It is interesting to note that hypoxia has recently been shown to increase CYP2C8 and/or CYP9 mRNA and protein expression in human umbilical vein endothelial (HUVEC) cells in culture (Michaelis et al., 2005) as well as increasing transcriptional activity of a transfected CYP2C9 promoter. Our study was originally designed to examine CYP2J2 and CYP2C expression in nonfailing hearts and in aorta and coronary tissues, and thus contained only one diseased heart. Thus it is impossible to know whether the disease state contributed to the high CYP2C9 expression. Additional studies with sufficient numbers of nonfailing and failing hearts will be required to address whether there are differences in the expression of the CYP2C and CYP2J2 genes in heart failure from various etiologies.
EETs have been identified as likely candidates for EDHFs (Campbell et al., 1996; Li and Campbell, 1997). The importance of these EET metabolites generated by the CYP enzymes have previously been reported in cardiovascular tissues (Wu et al., 1996; Node et al., 1999; Roman, 2002; Spiecker and Liao, 2005) and also have been highlighted in reviews (Campbell and Harder, 1999; McGuire et al., 2001). The vital importance of EETs in homeostasis has been seen in a transgenic mouse model, where overexpression of CYP2J2 resulted in increased EET biosynthesis and improved recovery of left ventricular function after cardiac ischemia and reperfusion (Seubert et al., 2004). These mice also exhibited enhancement of cardiomyocyte L-type Ca2+ currents (Xiao et al., 2004). EETs have also been shown to regulate cardiac electrophysiology and vascular tone via their action on KATP channels (Lu et al., 2006). Knowledge of the specific CYP enzymes which generate these EET metabolites has been lacking and selective inhibitors for the various CYP enzymes are limited.
The present study showed that CYP2C9 is the principal cytochrome P450 epoxygenase in aorta and coronary arteries. CYP2J2 protein was also detected at considerably lower levels in aorta, whereas CYP2C8 protein was not detectable by immunoblotting in either coronary artery or aorta samples. CYP2J2 has been previously reported to be localized in the endothelium of both large and small canine and porcine coronary arteries (Zhang et al., 2001) and in our study was found to be localized in cardiac myocytes and in the endothelial cell lining of blood vessels by immunohistochemical staining. Previous reports have shown that overexpression of CYP2C9 in both cultured and native porcine coronary endothelial cells increased both 11,12- and 8,9-EETs (Fleming et al., 2001). The presence of CYP2C9 as well as CYP2J2 in aorta and coronary arteries is consistent with their metabolites acting as potential candidates for EDHF, more over, 11,12-EET, which is produced by CYP2C8, CYP2C9 and CYP2J2, is also known to improve survival of coronary artery endothelial cells in culture (Dhanasekaran et al., 2006). The presence of CYP2C9 as well as CYP2J2 is also consistent with studies showing that EET metabolites are potent coronary artery vasodilators (Campbell et al., 1996). Knowledge of the particular cytochrome P450 isoforms that are present in both the coronary artery and aorta is thus particularly physiologically relevant. For example, polymorphisms resulting in variation in CYP2C9 or CYP2J2 activity or expression in coronary arteries would be predicted to alter the production of EET metabolites. Some redundancy in expression of various CYP isoforms such as CYP2C9 and CYP2CJ2 which produce these EETs could be a protective mechanism.
Our study provides qualitative evidence, using specific primers and immunochemical methods to detect individual CYP isoforms, that CYP2C8, CYP2C9 and CYP2J2 mRNA and protein are present in various amounts in the human heart, coronary artery and aorta tissues. CYP2J2 and CYP2C8 protein appear to predominate in human heart. In contrast, CYP2C9 expression appears to predominate in aorta and coronary arteries, although CYP2J2 is also expressed in these blood vessels. The expression of these CYPs in these tissues is consistent with their proposed role in cardiovascular homeostasis or possibly in cardiovascular pathology. Further investigation into the expression of these enzymes in cardiovascular disease may provide useful information regarding the etiology and treatment of cardiovascular disease, as well as a better understanding of the role of the different CYP enzymes in vascular tone and homeostasis.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. This work was previously presented at the Experimental Biology 2006-ASPET Program.
We would like to thank Dr. Gordon Flake for his pathological expertise and the NIH Fellows Editorial Board for their editorial assistance with this paper. We would like to acknowledge the National Disease Resource Interchange and the Cooperative Human Tissue Network (CHTN) as sources for some of the heart tissues.
Abbreviations
- CYP
Cytochrome P450
- AA
arachidonic acid
- EETs
Epoxyeicosatrienoic acids
- NO
nitric oxide
- EDHF
endothelium derived hyperpolarizing factor
References
- Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci. 2003;24:24–29. doi: 10.1016/s0165-6147(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res. 1999;84:484–488. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- Elbekai RH, El-Kadi AO. Cytochrome P450 enzymes: Central players in cardiovascular health and disease. Pharmacol Ther. 2006 doi: 10.1016/j.pharmthera.2005.05.011. in press. [DOI] [PubMed] [Google Scholar]
- Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem. 2004;52:447–454. doi: 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- Fisslthaler BPR, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthetase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Fleming I. Cytochrome p450 and vascular homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- Forrester LM, Henderson CJ, Glancey MJ, Back DJ, Park BK, Ball SE, Kitteringham NR, McLaren AW, Miles JS, Skett P, et al. Relative expression of cytochrome P450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992;281 (Pt 2):359–368. doi: 10.1042/bj2810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A, Baker DW, Totah RA, Gaedigk R, Pearce RE, Vyhlidal CA, Zeldin DC, Leeder JS. Variability of CYP2J2 expression in human fetal tissues. J Pharmacol Exp Ther. 2006;319:523–532. doi: 10.1124/jpet.106.109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA. Cytochrome P450: major player in reperfusion injury. Arch Biochem Biophys. 2003;420:262–267. doi: 10.1016/j.abb.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, Wentworth P, Jr, Yeager M, Gottlieb RA. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci U S A. 2004;101:1321–1326. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross ER, Nithipatikom K, Hsu AK, Peart JN, Falck JR, Campbell WB, Gross GJ. Cytochrome P450 omega-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. J Mol Cell Cardiol. 2004;37:1245–1249. doi: 10.1016/j.yjmcc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hunter AL, Bai N, Laher I, Granville DJ. Cytochrome p450 2C inhibition reduces post-ischemic vascular dysfunction. Vascul Pharmacol. 2005;43:213–219. doi: 10.1016/j.vph.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- Lin JHC, Kobari Y, Zhu Y, Stemerman MB, Pritchard KAJ. Human umbilical vein endothelial cells express P450 2C8 mRNA:cloning of endothelial P450 epoxygenase. Endothelium. 1996;4:219–229. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, Zeldin DC, Lee HC. Activation of KATP Channels by Endogenous Cytochrome P450 Epoxygenase Metabolites of Arachidonic Acid: Cardiac and Vascular KATP Channels are Activated by Epoxyeicosatrienoic Acids Through Different Mechanisms. J Physiol. 2006;575 (Pt 2):627–644. doi: 10.1113/jphysiol.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JJ, Ding H, Triggle CR. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s) Can J Physiol Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005;118:5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Romkes M, Faletto MB, Blaisdell JA, Raucy JL, Goldstein JA. Cloning and expression of complementary DNAs for multiple members of the human cytochrome P450IIC subfamily [published erratum appears in Biochemistry 1993 Feb 9;32(5):1390] Biochemistry. 1991;30:3247–3255. doi: 10.1021/bi00227a012. [DOI] [PubMed] [Google Scholar]
- Russo G, Leopold JA, Loscalzo J. Vasoactive substances: nitric oxide and endothelial dysfunction in atherosclerosis. Vascul Pharmacol. 2002;38:259–269. doi: 10.1016/s1537-1891(02)00250-1. [DOI] [PubMed] [Google Scholar]
- Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, Node K, Borgel J, Mugge A, Lindpaintner K, Huesing A, Maisch B, Zeldin DC, Liao JK. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110:2132–2136. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433:413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Thum T, Borlak J. Gene expression in distinct regions of the heart. Lancet. 2000;355:979–983. doi: 10.1016/S0140-6736(00)99016-0. [DOI] [PubMed] [Google Scholar]
- Voetsch B, Jin RC, Loscalzo J. Nitric oxide insufficiency and atherothrombosis. Histochem Cell Biol. 2004;122:353–367. doi: 10.1007/s00418-004-0675-z. [DOI] [PubMed] [Google Scholar]
- Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Ke Q, Seubert JM, Bradbury JA, Graves J, Degraff LM, Falck JR, Krausz K, Gelboin HV, Morgan JP, Zeldin DC. Enhancement of cardiac L-type Ca2+ currents in transgenic mice with cardiac-specific overexpression of CYP2J2. Mol Pharmacol. 2004;66:1607–1616. doi: 10.1124/mol.104.004150. [DOI] [PubMed] [Google Scholar]
- Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, Zeldin DC. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–320. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- Yasar U, Bennet AM, Eliasson E, Lundgren S, Wiman B, De Faire U, Rane A. Allelic variants of cytochromes P450 2C modify the risk for acute myocardial infarction. Pharmacogenetics. 2003;13:715–720. doi: 10.1097/00008571-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]