Abstract
It is well established that the immune response to sepsis is mediated by leukocytes associated with the innate immune system. However, there is an emerging view that T lymphocytes can also mediate this response. Here, we observed a significant depletion of both CD4 and CD8 T cells in human patients after blunt trauma. To determine what effect the loss of these cells may have during a subsequent infection, we obtained CD4- and CD8-deficient mice and subjected them to cecal ligation and puncture (CLP). We observed that CD4 knockout (KO) mice showed increased CLP-induced mortality compared with CD8-deficient and wild-type (WT) mice especially within the first 30 h of injury. CD4 KO mice also exhibited significantly increased IL-6 concentrations after the CLP. The CD4 KO mice had an increased concentration of bacteremia as compared with WT mice. Antibiotic treatment decreased mortality in the CD4 KO mice as compared with no changes in the wild mice after CLP. Neutrophils isolated from septic CD4 KO mice showed decreased spontaneous oxidative burst compared with neutrophils taken from septic controls. We examined the role of IFN-γ by using mice deficient in this cytokine and found these mice to have significantly higher mortality as compared with WT mice. Finally, we detected a 2-fold increase in CD11b+ cells that exhibited intracellular IFN-γ staining in the peritoneum of WT mice after CLP. The data suggest that CD4+ cells may facilitate the early clearance of bacteria by regulating neutrophils function possibly through an IFN-γ–dependent mechanism.
Keywords: Interferon-γ, neutrophils, bacteremia, cecal ligation and puncture
INTRODUCTION
Inflammation mediated by the immune system after trauma, burns, or invading microorganisms must be tightly regulated to prevent the development of systemic inflammatory response syndrome or sepsis, which can lead to septic shock, multiorgan dysfunction, and death. More than 200,000 patients die annually from sepsis. Despite extensive research into treatment of sepsis, mortality is still between 30% and 50%, with an annual health care cost of more than $16 billion (1). The epidemiology of sepsis in children shows more than 42,000 cases of pediatric sepsis per year in the United States, resulting in an estimated annual cost of $2 billion (2).
It has been established that lymphocytes play an important role in host defense during sepsis. Rag-1 knockout mice, which lack mature T and B cells, show a 10-fold increase in bacteremia and an increase in mortality after sepsis induced by cecal ligation and puncture (CLP) compared with wild-type (WT) mice (3). Reconstitution of the T and B compartment(s) by adoptive transfer restores the resistance to overwhelming infection (4). Previously, CD8+ cells have been shown to augment the proinflammatory response and promote mortality after sepsis (5, 6). However, the role of CD4+ cells has not been fully elucidated. Therefore, we sought to understand the contribution of CD4+ cells to the response to sepsis, particularly early during the induction of sepsis.
An important aspect of the response to infection is clearance of bacteria from the site of infection. Interferon γ plays a key role in this process because it can activate macrophages and neutrophils, which are granulocytes that are instrumental in clearing bacteria. However, whether IFN-γ plays a beneficial or detrimental role during sepsis is still somewhat controversial (7-10). The appropriate regulation of IFN-γ during the time course of the infection is likely critical to ensure appropriate clearance of the bacteria while preventing an excessive systemic response.
Here, we determined that shortly after blunt trauma, human patients have decreased numbers of circulating CD4 and CD8 T cells. Using the mice deficient in these cells, we determined that CD4 cells play an important role during the early phases of sepsis. Furthermore, we observed that within the first 30 h of sepsis, CD4+-deficient mice had increased mortality, increased bacterial load, and decreased neutrophil activity as compared with WT mice. Surprisingly, we did not observe intracellular IFN-γ expressing CD4+ cells after CLP. However, peritoneal CD11b+ cells did express intracellular IFN-γ, and the number of such cells was diminished in CD4 knockout (KO) mice. The data suggest that CD4+ cells are unexpectedly active in the initial immune response to polymicrobial sepsis such that they promote early bacterial clearance, possibly by influencing neutrophils in an IFN-γ–dependent manner.
MATERIALS AND METHODS
Patient selection
In this study, 7 male blunt trauma patients and 12 healthy male subjects were included. Informed consent from patients and healthy volunteers was obtained (institutional review board no. 06-03-07-06). Patients were selected on the following criteria: (1) no clinical suspicion of sepsis, (2) in the surgical intensive care unit or hospital, and (3) no evidence of immunosuppression and no immunosuppressive medication. Injured patients 18 years or older were within 50.2 ± 12.5 h of the blunt trauma. There was no significant age difference between the trauma patients (39.1 ± 12.3) and the healthy controls (34.1 ± 7.0).
Peripheral blood isolation
Venous blood was drawn from patients and volunteers into syringes containing 15% EDTA. Red blood cells were removed by lysis buffer (BD Pharmingen, San Diego, Calif). After lysis, cells were prepared for flow cytometry as described below.
Mice
Breeding pairs of 6- to 8-week-old C57BL/6J WT, CD4 KO (B6.129S2-Cd4tm1Mac/J [8 backcrosses]), CD8 KO (B6.129S2-Cd8tm1Mac/J [8 backcrosses]), and IFN-γ KO (B6.129S7-Ifngtm1Ts/J [13 backcrosses]) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). All mice used were bred in-house.
Cecal ligation and puncture
Male mice between 6 and 10 weeks old (20–26 g) were used. All experiments involving animals were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Polymicrobial sepsis was induced similarly as described (11). Briefly, the CLP operations were always performed between 8 AM and 1 PM. Normally fed mice were anesthetized to effect by 2% isoflurane in oxygen via facemask. The skin was shaved and disinfected. After a 1-cm laparotomy, the latter 80% of the cecum was ligated with a 3-0 silk tie (Ethicon, Inc., Somerville, NJ) and punctured once on the antimesenterial side with a 23-gauge needle. A small amount of the bowel contents was extruded through the puncture hole to assure a full-thickness perforation. Care was taken not to obstruct the bowel, and this was tested after the animals’ death. The cecum was replaced in its original location, and the midline incision was closed by two-layer suture with 4-0 silk (Schein, Inc., Melville, NY). The animals were resuscitated with 1 mL of sterile saline (s.c.) and kept on a heating blanket with additional oxygen supply for 1 h. Sham-treated controls underwent the same surgical procedures (i.e., laparotomy and resuscitation), but the cecum was neither ligated nor punctured.
Antibiotic treatment
Wild-type and CD4 KO mice were treated with either Primaxin or an equal volume of saline immediately after CLP, followed by additional antibiotic or saline injections every 12 h for 72 h as previously described (12).
Enzyme-linked immunosorbent assay
Peritoneal fluid was harvested from mice by peritoneal lavage after aseptic preparation of the abdominal wall followed by injection of 9 mL of sterile saline into the peritoneal cavity and aspiration of peritoneal fluid. Serum was collected by cardiac puncture. IL-6 levels in the peritoneal fluid and serum were analyzed using enzyme-linked immunosorbent assay according to the manufacturer’s protocol (PreproTech Inc., Rocky Hill, NJ, and BioSource Inc., Camarillo, Calif).
Adoptive transfer of CD4+ lymphocytes
CD4+ cells were isolated from the spleens of male WT mice by negative selection using fluorescein-conjugated monoclonal antibody to B220, CD8, FcRII (BD Pharmingen) followed by incubation with magnetic beads coated with anti-fluorescein isothiocyanate antibodies and separation on an AutoMACS as described by the manufacturer (Miltenyi Biotec, Auburn, Calif). The purity of the CD4+ cells was determined to be greater than 90% by flow cytometry. Purified CD4+ cells were washed extensively and resuspended in sterile phosphate-buffered saline (PBS). CD4+ cells (2 × 107) were injected into CD4−/− mice via the tail vein 18 h before the CLP.
Bacterial counts
Bacterial counts were performed on aseptically harvested blood by cardiac puncture. Samples were serially diluted in sterile saline and cultured on tryptic soy agar pour plates. Plates were incubated at 37°C for 24 h, and colony counts were performed.
Flow cytometry
Analyses of cell surface antigen expression and of cytokine expression in situ were performed as described earlier (13). Flow cytometry data acquisition and analysis were performed on LSR II using CellQuestPro or fluorescence-activated cell sorter Diva software (Becton Dickinson, Mountain View, Calif). All antibodies used for flow cytometry were obtained from BD Pharmingen.
Oxidative burst measurement
For measurement of spontaneous hydrogen peroxide production by oxidation of dihydrorhodamine to fluorescent rhodamine, cells were either left on ice or incubated at 37°C for 30 min before returning cells to ice. Cells were washed twice with ice-cold PBS containing bovine albumin (0.5%). In parallel experiments, cells were incubated at 37°C for 15 min and then activated for another 15 min with 10−5 M of the chemotactic tripeptide formyl-Met-Leu-Phe (fMLP). Activation was stopped by putting cells on ice. FcgII/III receptors were blocked by antimouse CD16/CD32 (10 min; 1 mg/mL), and cells were incubated with PE-antimouse Ly6G (GR-1) (15 min; 0.5 mg/mL) and APC-antimouse CD11b (1 mg/mL). After washing with PBS, cells were analyzed by flow cytometry. Hydrogen peroxide production was determined by the fluorescence intensity of rhodamine on fluorescence channel 1.
Statistics and image analyses
Statistical comparisons were performed using ANOVA testing on all data except survival analyses. Survival assessment was determined by log-rank analyses. StatView (SAS Institute) and GraphPad Prism 3.0 were used for statistical analyses. The mean and standard error of the mean were calculated in experiments with multiple data points. Paired data were analyzed using a paired t test. A value of P < 0.05 was considered statistically significant.
RESULTS
CD4 and CD8 T-cell numbers are decreased after blunt trauma
It has been reported that after trauma and during sepsis, there is a decrease in circulating lymphocytes (14). More specifically, we examined blood drawn shortly (50.25 ± 7.5 h) after blunt trauma. Total numbers of white blood cells increased 57% in trauma patients as compared with healthy controls. However, the total number of lymphocytes was decreased 43% in the trauma patients. In Figure 1, we show that the total proportion of CD4+/CD3+ and CD8+/CD3+ T cells decreased from 12.5% and 7.1% in healthy controls to 2.7% and 1.3% in the trauma patients, respectively. Combining the lymphocyte counts with the flow cytometric analysis, numbers of CD4 T and CD8 T cells dropped from 0.7 and 0.4 million cells/mL in healthy controls to 0.3 and 0.1 million cells/mL in trauma patients, respectively. Thus, the absolute concentrations of circulating T cells are markedly lower in patients shortly after injury.
Fig. 1. Human circulating CD4 and CD8 T cells are decreased after blunt trauma.

Flow cytometric analysis of PBMCs taken from healthy volunteers (A) or blunt trauma patients (B). Cells were gated first for viability by forward and side scatter. The percentage of positive cells represents the mean ± SD of 7 trauma or 12 healthy humans. PBMC indicates peripheral blood mononuclear cell.
A recent report has shown that patients with blunt injuries (84%) and penetrating injuries (16%) developed a nosocomial infection in 45.4% of the cases (15). To determine whether decreased numbers of CD4- or CD8-expressing cells play a role in a subsequent infection, we continued our studies with CD4- or CD8-deficient mice using a physiologically relevant model of sepsis.
CD4-deficient mice have increased mortality 30 h after CLP
To determine the impact of the specific deletion of either CD4- or CD8-expressing cells during sepsis, we used mice deficient in these cells. Flow cytometric analysis of both the spleens and peritoneum of the mutant mice demonstrated no expression of the deleted receptor (data not shown).
To explore the contribution of CD4 and CD8 T cells to CLP-induced mortality, the survival of CD4 KO, CD8 KO, and WT mice was assessed over an 8-day period (Fig. 2A). The log-rank test using Kaplan-Meier survival analysis showed overall significant differences in survival among the CD4 KO, CD8 KO, and WT mice (P = 0.0218). Of the three possible pairwise comparisons, only one was statistically significant, CD4 KO versus the CD8 KO (P = 0.0093). Survival in WT mice was 45%, whereas mice lacking CD8 cells had improved survival after CLP (60%), as previously shown (5). In contrast, CD4 KO mice had decreased survival (25%) after CLP. The mean survival time of WT mice was 109.5 h, nearly 2.5 times longer than that of the CD4 KO mice (45.0 h). Because the divergence in survival between groups occurred mainly within the first 30 h, we analyzed the survival of WT and CD4 KO mice 30 h after CLP (Fig. 2B). After 30 h, the survival rate was significantly (P = 0.019) lower in CD4 KO mice (65%) compared with WT control mice (90%). IL-6 concentrations after CLP are known to be predictive of outcome (16), so IL-6 in serum and peritoneal fluid of WT and CD4 KO mice were measured after 18 h after CLP. As shown in Figure 2C, the serum IL-6 concentration was significantly higher in CD4 KO mice (2,614.9 ± 651.3 pg/mL) as compared with the WT control group (951.9 ± 268.5 pg/mL). Similarly, the concentration of IL-6 in the peritoneal fluid of CD4 KO mice (10,424.2 ± 2,852.5 pg/mL) was significantly higher than WT control mice (4,123.5 ± 1,384.4 pg/mL) (n = 4 per group; P = 0.0073).
Fig. 2. CD4 cells increase early survivability after CLP.

A, Wild-type, CD4 KO, and CD8 KO mice underwent CLP (sample size, 20 per group). Survival rates were monitored over 192 h. B, Early survival rates (30 h) after CLP of CD4 KO mice compared with WT (n = 20 per group; P = 0.019). C, Peritoneal fluids and serum were collected 18 h after CLP. IL-6 cytokine levels (nanograms per milliliter) were determined using enzyme-linked immunosorbent assay. The sample size equals four per group, whereas values represent the mean ± SD. *P < 0.05 as compared with WT; †P = 0.0093 as compared with CD8 KO group.
Interestingly, the adoptive transfer of 20 million purified splenic CD4-expressing T cells into CD4 KO mice did not alter post-CLP survival to the level of WT control mice (data not shown). The inability of these lymphoid-derived, mostly naive CD4+ T cells to abrogate the survival defect of the CD4 KO mice suggests that such cells might not play a role in the very early events of sepsis. Therefore, we analyzed the peritoneal T cells at the site of CLP-induced infection. Approximately 400,000 CD4+ T cells were isolated from the peritoneal fluid of WT mice. These CD4+ cells were analyzed by flow cytometry, and greater than 90% were found to be nonnaive or memory T cells (CD62Llow and CD44high). However, the difficulties isolating and purifying of these cells prevented their use for further adoptive transfer studies.
CD4 KO mice have higher incidence and levels of bacteremia
To investigate the extent of the bacteremia in WT and CD4 KO mice at an early time point post-CLP, we collected blood from these mice 18 h after CLP. Blood was cultured for 24 h, and the amount of viable bacteria present was quantified (Fig. 3). Colony counts were 2 logs higher in the CD4 KO mice than in WT mice. Furthermore, in the WT control group, bacteremia was observed in two of eight mice, as compared with six of the seven CD4 KO mice. In control experiments, no bacteremia was observed in the sham groups for either WT or CD4 KO mice (data not shown).
Fig. 3. Bacterial counts are elevated in the blood of the CD4-deficient mice.
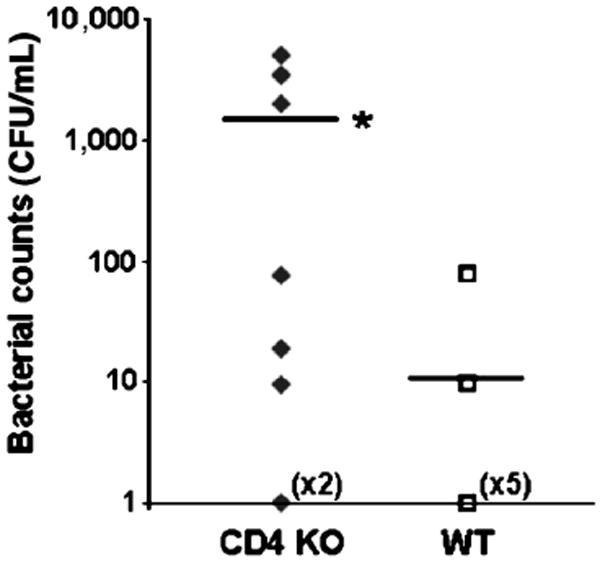
Blood was collected by cardiac puncture 18 h after CLP. The blood was plated, and bacterial CFUs were calculated in CD4 KO and WT mice (n = 7–8/group;). *P < 0.05 as compared with WT. CFU indicates colony-forming unit.
Antibiotic treatment selectively improves survival in CD4-deficient mice
To determine if the observed high bacterial load contributed to the mortality of CD4 KO mice, we tested whether treatment with the antibiotic Primaxin increased survival rate after CLP. Wild-type and CD4 KO mice were treated with either Primaxin or an equal volume of isotonic sodium chloride solution immediately after CLP, followed by additional antibiotic or saline injections every 12 h for 72 h. As shown in Figure 4A, the survival of CD4 KO mice treated with Primaxin was significantly higher (80%) within the first 24 h compared with the CD4 KO control group (20%). Similar treatment of WT mice with Primaxin did not result in an increase in survival compared with WT mice injected with saline (Fig. 4B).
Fig. 4. Antibiotic treatment enhances survival only in CD4-deficient mice.
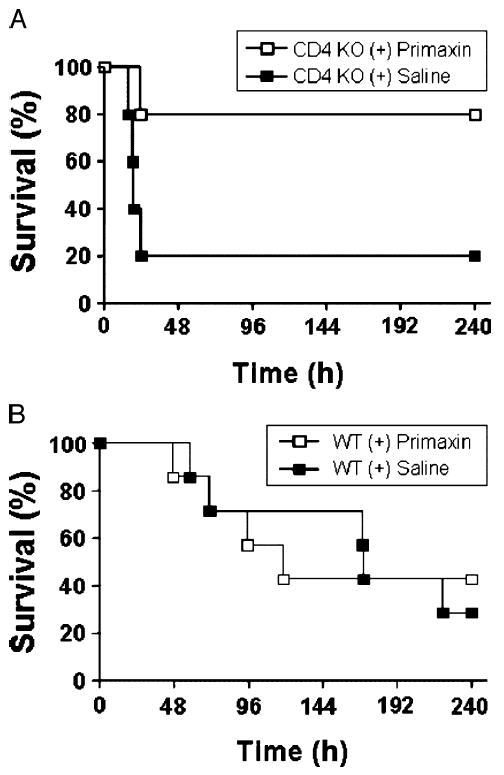
A, CD4 KO mice underwent CLP and were treated every 12 h with the antibiotic Primaxin (50 μg, s.c.) or saline 0.9% for 72 h. Survival rate was monitored over 240 h. B, Wild-type mice underwent CLP and were similarly treated. *P < 0.05 as compared with WT.
Peritoneal neutrophils taken from septic WT mice have greater activity as compared with those isolated from CD4 KO mice
As neutrophils play a key role in eliminating bacteria (17), we examined whether CD4-expressing cells mediate the activation of these cells during the early stages of sepsis. As shown in Figure 5, we detected decreased spontaneous oxidative burst activity in peritoneal neutrophils isolated from CD4 KO mice (285.4 mean fluorescent intensity [MFI]) compared with WT mice (513.1 MFI) 18 h after CLP. Interestingly, activation of neutrophils with fMLP did not result in any further significant increase in oxidative burst activity in neutrophils from both CD4 KO and WT mice (612.1 MFI and 808 MFI; n = 13 per group; P < 0.01). In peritoneal neutrophils from sham mice, there was no difference in the spontaneous oxidative burst activity between CD4 KO and WT mice. After stimulation with fMLP, only a moderate increase in oxidative burst activity was detected in neutrophils isolated from either CD4 KO or WT mice with no difference between the groups.
Fig. 5. Decreased oxidative burst capacity of neutrophils isolated from CD4 KO as compared with WT mice.

A, Spontaneous activity of sham mice and activity after stimulation with fMLP for 20 min (n = 4 per group). B, Spontaneous activity of septic mice 18 h after CLP and activity after stimulation with fMLP for 20 min (n = 4/group). *P < 0.05 WT as compared with WT.
IFN-γ–deficient mice exhibit increased mortality after CLP
Because IFN-γ elicits potent antimicrobial activity that is in part executed through activation of neutrophils (18), it was next tested whether IFN-γ promoted survival after CLP. To determine differences in CLP-induced mortality, WT and IFN-γ KO mice underwent CLP, and the survival of these mice was observed over 10 days (Fig. 6). The IFN-γ KO mice exhibited higher mortality (90%) compared with the WT control group (60%) after the CLP. Interestingly, within the first 30 h, similarly to the CD4 KO mice, IFN-γ KO mice exhibited a significantly (P = 0.0221) higher mortality rate (50%) compared with WT control mice (10%).
Fig. 6. Mice deficient in IFN-γ have decreased survival rate as compared with WT mice.

Interferon γ KO and WT mice underwent CLP. Survival rate was monitored over 240 h. Data are combined from two independent experiments. The sample size is 10 per group. *P < 0.05 as compared with WT.
Peritoneal CD11b cells from septic WT mice show increased amounts of intracellular IFN-γ as compared with CD4 KO–derived cells
Given that the decreased survival of IFN-γ KO mice in the early time points after CLP exhibited a pattern similar to that seen in CD4 KO mice, we hypothesized that CD4+ cells promote production of IFN-γ after CLP. To test this, we isolated peritoneal cells and analyzed for intracellular IFN-γ in CD4 cells. Although we found that there was a sizeable number of cells positive for intracellular IFN-γ, none of the cells had CD4 expression. Subsequent experiments showed that the cells positive for intracellular IFN-γ were CD11b expressing. Furthermore, there was a higher proportion of the CD11b+ cells with intracellular IFN-γ taken from WT mice as compared with CD4-deficient mice (Fig. 7). Furthermore, analysis has shown that these CD11b, intracellular IFN-γ–expressing cells are also Gr-1 and 7/4 bright, strongly suggesting that these cells are neutrophils (19).
Fig. 7. Peritoneal CD11b-positive cells from WT mice express more intracellular IFN-γ as compared with CD4-deficient mice.

CD4 KO and WT mice underwent CLP. Peritoneal fluid was collected 18 h after CLP. The amount of intracellular IFN-γ in CD11b+ cells was detected using flow cytometry. The data shown are representative of four independent experiments. *P < 0.05 as compared with WT.
DISCUSSION
This study demonstrates that after blunt trauma, there is a marked decrease in circulating CD4 and CD8 T cells in human patients. To gain insight into what this decrease in T cells may have upon a subsequent infection, we continued the studies with a physiologically relevant model of sepsis using mice deficient in these cells. Our studies show that CD4 cells enhance survival especially during the early stages of sepsis. A lack of these cells is associated with increased IL-6 concentrations, increased bacteremia, and decreased neutrophil activity. Because IFN-γ can play a role in these processes (20-22), we induced sepsis in IFN-γ–deficient mice and found decreased survival in these mice as compared with WT controls. Finally, we found that intracellular IFN-γ levels were higher in the myeloid cells isolated from WT as compared with CD4-deficient mice.
After trauma, the immune response is characterized by changes in circulating leukocytes. All seven patients in our human study showed decreased proportions and numbers of circulating CD4 and CD8 T cells shortly after blunt trauma. It is unclear whether the decrease in the number of lymphocytes in the trauma patients is due to apoptosis, recruitment from the circulation to the peripheral tissues, changes due to transfusion of blood products, or hemodilution from large crystalloid infusions. Based upon published literature (14 and reviewed in 23) and our own unpublished data, we speculate that the decrease is largely due to apoptosis. Decreases in lymphocyte numbers are thought to contribute to the immunosuppression observed after trauma and increased susceptibility to infections. Previous sepsis studies using mice typically used athymic or Rag-1–deficient mice. A limitation to these studies is that CD4, CD8, γδ, and natural killer T cells were all absent. Here, we found that CD4 and CD8 cells have contrasting actions in mediating survival after sepsis. Furthermore, it was found that reconstitution of the CD4-deficient mice with naive CD4 T cells did not ameliorate the increased mortality as compared with WT mice. Thus, it is likely that nonnaive CD4 cells or regulatory T cells are the specific T cells that play a role in survival.
Surprisingly, the lack of CD4 cells altered survival within the first 30 h of sepsis. The traditional view of T-cell actions during infections is that it takes 48 to 96 h for T cells to contribute to an effective defense against extracellular bacteria. However, there is an emerging view that lymphocytes play an early role during acute injuries. That these cells play a key role early likely implicates memory CD4 T cells, which can respond to antigens within an acute time frame (24). Furthermore, because the bacteria released during CLP are endogenous and originate from the gut, it is likely that peritoneal memory CD4 cells may be antigenic toward the bacteria seen during the CLP. If this is the case, and the memory CD4 cells are antigenic toward bacteria, then it is likely they are of a Th1 phenotype.
A variety of other studies have shown that imipenem treatment increases survival in mice that have undergone CLP (20, 25, 26). Here, we show imipenem treatment increased survival only in mice deficient in CD4-expressing cells, whereas WT mice showed no survival improvement. CD4 cells are known to be reactive to inflammation-increasing mediators such as endotoxins and superantigens, which will be observed in the presence of antibiotics. In agreement with other reports (27-29), we speculate that antibiotic treatment, coupled with the elimination of T cells, beneficially mediates the acute inflammatory response after CLP. Finally, others reports have shown that imipenem treatment does not alter survival in WT mice, which is in agreement with the data presented here (27, 29). The mechanism(s) by which imipenem differentially mediates survival remain unknown. However, we speculate that the different (1) strains of mice, (2) imipenem treatment modality, and (3) CLP model used may play role(s) in the differences seen.
It has been reported that nonnaive and unconventional T cells can functionally respond to inflammatory cytokines in a short period (30, 31). Memory Th1 cells can rapidly secrete IFN-γ upon recognition of the processed bacterial antigen within the context of major histocompatibility complex II. Interferon γ has been reported to activate myeloid cells and increase these cells’ ability to clear bacteria (32). Here, we show that mice deficient in CD4 cells have increased bacteremia. We further show that neutrophils taken from CD4-deficient mice have significantly decreased spontaneous oxidative burst. However, the potential oxidative capacity between the neutrophils taken from the two sets of mice shows no significant differences. Thus, at the time of the neutrophil isolation, the lack of CD4 actions either directly or indirectly diminished the neutrophil oxidative burst. CD4 cells can achieve this by receptor-ligand interactions such as CD154/CD40 or CD28/CD80/86 or by the secretion of activating soluble mediators such as IFN-γ or IL-17. Alternatively, CD4 cells can have receptor-ligand interactions or soluble mediator secretions that result in alterations of a second cell, which then alters the neutrophils’ oxidative burst.
Interestingly, we observed that neutrophils isolated from the peritoneum of septic mice contained intracellular IFN-γ. Two plausible reasons for this include either that neutrophils are producing IFN-γ or are in the process of consuming the cytokine. Recently, it has been suggested that most IFN-γ–producing cells in the lung were Gr-1 high neutrophils in a bacterial hypersensitivity pneumotitis model (33). Other studies have shown that mouse neutrophils can produce IFN-γ when stimulated with LPS or with a combination of IL-12 and TNF (34), mediators that are likely present at the site of infection during sepsis. However, the mechanisms by which IFN-γ–producing neutrophils in the peritoneum are regulated during sepsis remain unclear. Interferon γ exerts its effects on the surface of target cells through interaction with a specific receptor complex. When IFN-γ is bound, the receptor complex is itself rapidly transported to the nucleus via receptor-mediated endocytosis (35). Antibodies can be used to observe internal IFN-γ for at least 40 min (36). However, the duration of the IFN-γ protein within the cell and its ability to become detected by intracellular cytokine staining is unclear.
We have observed, on average, approximately 400,00 CD4 T cells in the mouse peritoneum. The number of these cells decreases approximately 75% (data not shown) 18 h after CLP. We speculate that these cells either undergo apoptosis and/or migrate out of the peritoneum during these first 18 h. During this time, these cells can secrete IFN-γ or other mediators that increase neutrophil IFN-γ production. Studies within our laboratory are currently being conducted to determine the cell type producing the IFN-γ and whether the cell of interest is producing and/or consuming IFN-γ.
Although this study provides new insights into the role of CD4 cells during sepsis, it has limitations. First, there are multiple subsets of mouse cells that express the CD4 receptor to include naive, effector, memory, regulatory, γδ, and natural killer T cells. Furthermore, these cells have different distributions and numbers within the organism. However, the process to purify these cells in sufficient numbers to reconstitute into the correct compartment of the CD4-deficient mouse is extremely difficult to accomplish. Second, although we hypothesize that IFN-γ produced by CD4 cells is at least partially responsible for the observed differences, the efficacy of exogenous IFN-γ administration has not been shown to affect bacterial clearance or mortality of a nonlethal Pseudomonas challenge 5 days after sublethal CLP. However, differences in doses, schedule, or CLP model (e.g., nonlethal versus lethal) may yield different results.
In summary, our study demonstrates that CD4 cells play an early key role limiting bacteremia in the CLP model of sepsis. CD4 cells seem to be required to reduce bacteremia and enhance neutrophil activity. CD4 cells may accomplish this by modulating IFN-γ production and/or accumulation of this cytokine by myeloid cells.
Acknowledgments
The authors thank Julie M. Caldwell and Kelsey Guanciale for their careful review of the article and Laura E. James for her critical help with the statistical analysis.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 4.Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–129. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 5.Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab Invest. 2004;84:1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 6.Sherwood ER, Lin CY, Tao W, Hatmann CA, Dujon JE, French AJ, Varma TK. Beta 2 microglobulin knockout mice are resistant to lethal intraabdominal sepsis. Am J Respir Crit Care Med. 2003;167:1641–1649. doi: 10.1164/rccm.200208-950OC. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci U S A. 2003;100:6724–6729. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles RH, Paxton TP, Dries DJ, Gamelli RL. Interferon-gamma increases mortality following cecal ligation and puncture. J Trauma. 1994;36:607–611. doi: 10.1097/00005373-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Murphey ED, Sherwood ER. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFN-gamma administration in a murine model of post-CLP immunosuppression. Shock. 2006;26:417–424. doi: 10.1097/01.shk.0000226343.70904.4f. [DOI] [PubMed] [Google Scholar]
- 10.Zantl N, Uebe A, Neumann B, Wagner H, Siewert JR, Holzmann B, Heidecke CD, Pfeffer K. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 1998;66:2300–2309. doi: 10.1128/iai.66.5.2300-2309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21:415–425. doi: 10.1097/00024382-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Benjamim CF, Hogaboam CM, Lukacs NW, Kunkel SL. Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol. 2003;163:2605–2617. doi: 10.1016/S0002-9440(10)63615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 15.Hoover L, Bochicchio GV, Napolitano LM, Joshi M, Bochicchio K, Meyer W, Scalea TM. Systemic inflammatory response syndrome and nosocomial infection in trauma. J Trauma. 2006;61:310–316. doi: 10.1097/01.ta.0000229052.75460.c2. discussion 316–317. [DOI] [PubMed] [Google Scholar]
- 16.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–1306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Ellis TN, Beaman BL. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology. 2004;112:2–12. doi: 10.1111/j.1365-2567.2004.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 20.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallone F, Monteleone G. Mechanisms of tissue damage in inflammatory bowel disease. Curr Opin Gastroenterol. 2001;17:307–312. doi: 10.1097/00001574-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Pearl-Yafe M, Fabian I, Halperin D, Flatau E, Werber S, Shalit I. Interferon-gamma and bacterial lipopolysaccharide act synergistically on human neutrophils enhancing interleukin-8, interleukin-1beta, tumor necrosis factor-alpha, and interleukin-12 p70 secretion and phagocytosis via upregulation of toll-like receptor 4. Shock. 2007;27:226–231. doi: 10.1097/01.shk.0000239765.80033.37. [DOI] [PubMed] [Google Scholar]
- 23.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 24.Stockinger B, Kassiotis G, Bourgeois C. CD4 T-cell memory. Semin Immunol. 2004;16:295–303. doi: 10.1016/j.smim.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Vianna RC, Gomes RN, Bozza FA, Amancio RT, Bozza PT, David CM, Castro-Faria-Neto HC. Antibiotic treatment in a murine model of sepsis: impact on cytokines and endotoxin release. Shock. 2004;21:115–120. doi: 10.1097/01.shk.0000111828.07309.21. [DOI] [PubMed] [Google Scholar]
- 26.Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1048–R1053. doi: 10.1152/ajpregu.00312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enoh VT, Fairchild CD, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on wild-type and NK cell-deficient CD8 knockout mice during acute intra-abdominal injury. Am J Physiol Regul Integr Comp Physiol. 2006;290:R685–R693. doi: 10.1152/ajpregu.00678.2005. [DOI] [PubMed] [Google Scholar]
- 28.Enoh VT, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on injury caused by cecal ligation and puncture in wild-type and NK cell-deficient beta(2)-microgloblin knockout mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G277–G284. doi: 10.1152/ajpgi.00338.2005. [DOI] [PubMed] [Google Scholar]
- 29.Enoh VT, Lin SH, Lin CY, Toliver-Kinsky T, Murphey ED, Varma TK, Sherwood ER. Mice depleted of alphabeta but not gammadelta T cells are resistant to mortality caused by cecal ligation and puncture. Shock. 2007;27:507–519. doi: 10.1097/SHK.0b013e31802b5d9f. [DOI] [PubMed] [Google Scholar]
- 30.Leite-De-Moraes MCHA, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, Dy M. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J Immunol. 1999;163:5871–5876. [PubMed] [Google Scholar]
- 31.Martino GGF, Brambilla E, Codazzi F, Consiglio A, Clementi E, Filippi M, Comi G, Grimaldi LM. Proinflammatory cytokines regulate antigen-independent T-cell activation by two separate calcium-signaling pathways in multiple sclerosis patients. Ann Neurol. 1998;43:340–349. doi: 10.1002/ana.410430312. [DOI] [PubMed] [Google Scholar]
- 32.Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vaccine development. Immunol Rev. 2006;211:58–66. doi: 10.1111/j.0105-2896.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 33.Nance S, Cross R, Yi AK, Fitzpatrick EA. IFN-gamma production by innate immune cells is sufficient for development of hypersensitivity pneumonitis. Eur J Immunol. 2005;35:1928–1938. doi: 10.1002/eji.200425762. [DOI] [PubMed] [Google Scholar]
- 34.Yeaman GR, Collins JE, Currie JK, Guyre PM, Wira CR, Fanger MW. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–5153. [PubMed] [Google Scholar]
- 35.Bader T, Weitzerbin J. Nuclear accumulation of interferon gamma. Proc Natl Acad Sci U S A. 1994;91:11831–11835. doi: 10.1073/pnas.91.25.11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larkin J, 3rd, Johnson HM, Subramaniam PS. Differential nuclear localization of the IFNGR-1 and IFNGR-2 subunits of the IFN-gamma receptor complex following activation by IFN-gamma. J Interferon Cytokine Res. 2000;20:565–576. doi: 10.1089/10799900050044769. [DOI] [PubMed] [Google Scholar]


