Abstract
The complex structure of the lung is developed sequentially, initially by epithelial tube branching and later by septation of terminal air sacs with accompanying coordinated growth of a variety of lung epithelial and mesenchymal cells. Groups of transcriptional factors, peptide growth factors and their intracellular signaling regulators, as well as extracellular matrix proteins are programmed to be expressed at appropriate levels in the right place at the right time to control normal lung formation. Studies of lung development and lung repair/fibrosis to date have discovered that many of the same factors that control normal development are also key players in lung injury repair and fibrosis. TGF-β family peptide signaling is a prime example. Lack of TGFβ signaling results in abnormal lung branching morphogenesis and alveolarization during development, while excessive amounts of TGF-β signaling cause severe hypoplasia in the immature lung and fibrosis in mature lung. This leads us to propose the “Goldilocks” hypothesis of regulatory signaling in lung development and injury repair: that everything must be done just right!
Keywords: Lung development, lung stem cell, lung progenitor cells, branching morphogenesis, alveolarization, lung injury repair, lung fibrosis, lung regeneration, growth factor, transcription factor, extracellular matrix protein
Introduction
The functional mammalian lung has a honeycomb like structure, containing extensively branched, perfectly matched conduits for air and blood. This configuration maximizes the gas exchange surface area, supports effective ventilation and gas exchange between air and blood, and facilitates maximally efficient placement within the chest cavity. In humans, the gas exchange membrane (about 1 μm thick) consists of alveolar epithelial cells, basement membrane, and endothelial cells, while the total surface area is about 70m2. This complex structure is developed sequentially by early epithelial tube branching and later by septation of terminal air sacs with accompanying coordinated growth of a variety of lung cells. Alteration of cell proliferation, differentiation, apoptosis, migration and shape of one or more cell types will directly and/or indirectly perturb the developmental process, resulting in abnormal lung structure and hence impairment of gas exchange function. This complexity of lung structure makes it difficult to restore respiratory units to their original functional state if severe lung injury and/or inappropriate repair processes occur in the developed lung. Instead, fibrotic tissues may fill in the damaged spot as a consequence of abnormal wound healing. Therefore, fundamental knowledge about these basic developmental processes and the underlying molecular and cell biology is essential to understand the pathobiology of lung development, repair, and fibrosis, and thus to design novel therapeutic strategies to prevent and treat the related lung diseases.
Lung Developmental Process
The lung originates from the ventral surface of the primitive foregut at 5 weeks gestation in human. The “lung anlage” emerges as the laryngo-tracheal groove, located in the ventral foregut endoderm, which invaginates into the surrounding splanchnic mesenchyme 1;2. The respiratory tree then develops by branching morphogenesis, in which reiterated outgrowth, elongation and subdivision of epithelial buds occurs, possibly using proximal-distal domain branching, planar bifurcation, and orthogonal bifurcation modes, as seen in mouse lung formation, to generate complicated bronchial trees 3;4. Three lobes on the right side and two lobes on the left side are formed in human lung with 23 generations of airway branching. The first sixteen generations of branching are stereotypically reproducible and are completed by 16 weeks, while the remaining seven generations are random and are completed by about 24 weeks. Alveolarization begins around 38 weeks of pregnancy in human, and is completed postnatally, continuing up to at least 7 years of age.
Histologically, lung development and maturation has been divided into four stages.
(1) the pseudoglandular stage (5 to 17 weeks of human pregnancy, E9.5-16.6 days in mouse embryo). This is the earliest lung development stage, in which the embryonic lung undergoes branching morphogenesis, developing epithelial tubular structures lined with cuboidal epithelial cells that resemble an exocrine gland. However, this fluid containing respiratory tree structure is too immature to perform gas exchange.
(2) the canalicular stage (16 to 25 weeks of human pregnancy, E16.6-17.4 days in mouse embryo). The cranial part of the lung develops relatively faster than the caudal part, resulting in partial overlap between this stage and the previous stage. During the canalicular stage, the respiratory tree is further expanded in diameter and length, accompanied by vascularization and angiogenesis along the airway. A massive increase in the number of capillaries occurs. The terminal bronchioles are then divided into respiratory bronchioles and alveolar ducts, and the airway epithelial cells are differentiated into peripheral squamous cells and proximal cuboidal cells.
(3) The terminal sac stage (24 weeks to late fetal period in human, E17.4 to postnatal day 5 (P5) in mouse). There is substantial thinning of the interstitium during the terminal sac stage. This results from apoptosis as well as ongoing differentiation of mesenchymal cells 5;6. Additionally, at this stage, the alveolar epithelial cells are more clearly differentiated into mature squamous Type I alveolar epithelial cells and secretory rounded Type II alveolar epithelial cells. The capillaries also grow rapidly in the mesenchyme surrounding the alveoli to form a complex double network. In addition, the lymphatic network in lung tissue becomes well developed during this stage. Towards the end of this stage, the fetal lung can support relatively inefficient gas-exchange, but sufficient to maintain the life of prematurely born neonates. Although human premature infants can breathe with lungs that have developed to the end of the terminal sac stage, the immature lung is nevertheless vulnerable to hyperoxic injury and barotrauma, resulting in the alveolar hypoplasia phenotypes termed bronchopulmonary dysplasia. Maturation of surfactant synthesis and secretion is a key factor in determining whether the newborn lung can sustain gas exchange without collapsing. Another key factor is the rapid switch from chloride ion driven fluid secretion into the airway to sodium driven uptake of fluid out of the airway. This switch is driven by the response of the adrenergic system to cord cutting at birth.
(4) The alveolar stage (38 weeks to childhood in human, P5-P30 in mouse). Alveolarization is the last step of lung development. The majority of the gas exchange surface is formed during this stage. Alveolarization can be positively and negatively influenced by many exogenous factors including inspired oxygen concentration, stretch in fetal airway, dexamethasone and retinoic acid. Formation of new septa within terminal sacs is the key step for differentiation of the saccules into alveoli. This involves a complex interaction between myofibroblasts, adjacent airway epithelial cells, and vascular endothelial cells. Since the myofibroblasts produce elastin matrix within the mesenchyme, controlled proliferation and differentiation as well as migration of the myofibroblast progenitors cells within terminal sac walls are therefore important for formation of new alveolar septa. Myofibroblasts, which are precursors of smooth muscle cells with the morphology of fibroblasts, migrate to the proper position within nascent alveolar septa, and synthesize and deposit elastin 7;8. Two additional processes are necessary in septum maturation for appropriate morphology and function. One is the thinning out of the septal mesenchyme and the other is the maturation of the capillary bed. Thinning of the mesenchymal tissue involves apoptosis of “unwanted” cells in the postnatal lung mesenchyme. There is a substantial reduction in the number of interstitial myofibroblasts resulting from increased apoptosis during this phase of rapid alveolarization 9;10. The immature lung contains at least two morphologically distinct fibroblast populations, lipid-filled interstitial fibroblasts (LFIF) and non-LFIF (NLFIF). Apoptosis during alveolarization occurs preferentially in the LFIF 11. This thinning of the previously thickened immature interstitium occurs simultaneously with the ongoing capillary remodeling from duplicated circuits into a single network. Finally the new septum epithelial differentiates into a functional respiratory membrane that consists of type I alveolar epithelial cells, basement membrane and capillary endothelial cells. The respiratory membrane provides a short distance for gas diffusion and thus facilitates optimal gas exchange. It is estimated that about 50 million alveoli are present in neonatal lung. However, by age 7 to 8 years, when the alveolarization is substantially complete, the number of alveolar units in the lung has grown six times to about 300 million alveoli.
Lung cell lineages and their stem/progenitor cells
More than 40 specific types of cells are differentiated during embryonic lung development.
The composition of the lung epithelium changes significantly along the proximal-distal axis. In proximal airway epithelium, there are two major cell components, pseudostratified ciliated columnar cells and mucous (goblet) cells. Both of them derive from basal cells that express keratins K5/K14, but ciliated cells predominate in number. Goblet cells release mucus granules into the bronchial lumen to prevent drying of the walls, and trap particulate matter. Mucous cells begin to mature around 13 weeks gestation in humans, when the mature ciliated columnar cells are already present. The beating of cilia results in a cephalad movement of the mucus blanket, thereby cleaning and protecting the airway. There are also three different types of cells in bronchial submucosal glands. Myoepithelial cells surround the gland, while mucous cells (pale cytoplasm) and serous cells (basophilic cytoplasm) produce mucins. Cartilage lies outside the submucosa and decreases in amount as the caliber of the bronchi decreases. Cartilage is present in the bronchi, but is not present in the bronchioles.
Pulmonary neuroendocrine cells (PNEC) are identified by calcitonin gene-related peptide (CGRP) positive staining on the airway surface next to submucosal glands. A cluster of PNECs in an innervated location is also referred to as neuroendocrine body (NEB), which may also be one of the epithelial stem cell niches. PNEC produce a variety of peptide hormones such as serotonin and calcitonin. Certain lung neoplasms (i.e. small cell carcinoma and carcinoid tumors) in adult lungs may originate from PNECs. Clara cells are found in the distal bronchiolar airway epithelium that normally lacks mucous cells, including NEB and bronchoalveolar duct junction (BADJ). Their most important cellular marker is Clara cell-specific protein (CCSP). Clara cells begin to mature during the 19th week in humans, and produce a mucus-poor, watery proteinaceous secretion, and assist with clearance and detoxification, as well as reduction of surface tension in small airways. Recently, subsets of CCSP+ cells located within the localized anatomic niches of NEB (CCSP+) and BADJ (CCSP+/SP-C+) have been reported to possess airway epithelial regenerative capacity 12.
The majority of the alveolar surface is normally covered by type I alveolar epithelial cells (AECI). These flat cells are believed to be terminally differentiated cells, expressing several specific molecular markers, such as T1α and aquaporin 5. AECI account for only 40% of the total airway epithelial cells, even though 95% of the surface area of the alveolar wall is covered by this cell type. The other 60% of the alveolar epithelial cells are rounded cells that cover only 3% of the alveolar surface, named type II alveolar epithelial cells (AECII). The AECII is plump or cubical and has a finely stippled cytoplasm and surface microvilli. They manufacture and package surfactant phospholipids and proteins into lamellar bodies, and secrete them onto the alveolar surface to reduce the surface tension in the lung, therefore, stabilizing and maintaining the alveoli in an open position despite the variation in alveolar size. AECII are also capable of regenerating and replacing AECI after lung injury. A commonly used cellular marker of type II cells is surfactant protein C (SP-C).
Developing lung mesenchyme is comprised of fibroblasts, myofibroblasts, smooth muscle cells (airways and blood vessels), endothelial cells, vascular pericytes, chondrocytes in large airways, and a variety of extracellular matrixes. Embryonic lateral splanchnic mesoderm is considered to be the major source for these lung mesenchymal cells. However, about 30% of the smooth muscle cells within the blood vessel walls as well as some mesenchymal cells outside the blood vessel walls are derived from mesothelial cells, as shown in mouse lung 13. In addition, alveolar macrophages constitute a small percentage of the cells in alveoli, but they represent a major cellular sentinel of the host defense mechanism in the alveolar space. They are part of the mononuclear phagocyte system and are derived primarily from blood monocytes.
Respiratory stem and progenitor cells have important functions in repairing damaged trachea, bronchi, bronchioles or alveoli. However, the precise identification of lung stem/progenitor cells remains uncertain. The large surface area and highly branched and folded geography of the lung dictates that there must be several kinds of stem or progenitor cells in the respiratory system. As mentioned above, in the trachea and bronchi, certain basal cells and mucus-gland duct cells are believed to be stem/progenitor cells. Subsets of Clara cells and AECII such as BADJ cells are also thought to function as stem/progenitor cells in bronchioles and alveoli, respectively. Lung mesenchymal stem/progenitor cells are as yet relatively poorly characterized, although Summer et al, reported that a candidate mesenchymal progenitor could be isolated from the adult lung using flow cytometry by taking advantage of stem cells' capacity to efflux vital fluorescent dyes 14. Finally, whether bone marrow derived cells contribute directly to the structure of airways or alveoli after lung injury is still controversial, but it is likely that circulating bone marrow stem cells may provide significant support directly or stimulate local progenitor cells by paracrine mechanisms to repair injured lung structure 15. Research on both systemically derived as well as resident lung stem or progenitor cells may hold keys to understanding lung morphogenesis, lung regeneration, and pathogenesis of lung diseases. Moreover, in human idiopathic pulmonary fibrosis (IPF), bone marrow derived fibrocytes that express collagen I can be found as a high proportion of the buffy coat in peripheral blood and likely contribute to ongoing fibrosis. High numbers of these cells in the peripheral blood buffy coat are associated with exacerbation or poor prognosis in IPF 16;17.
Regulatory mechanisms of lung development
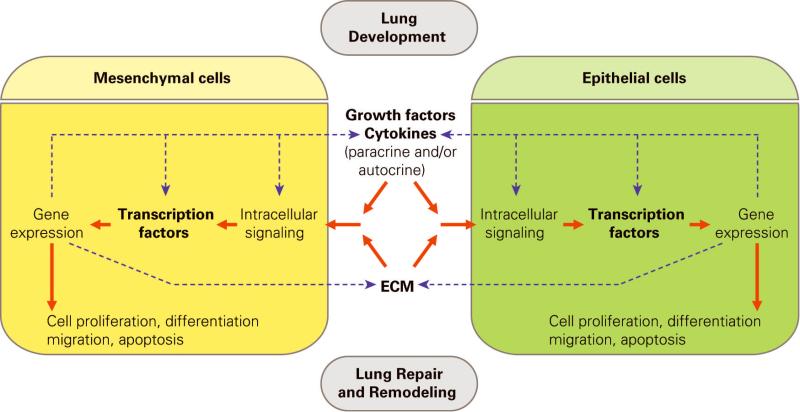
Normal lung development is controlled by many genes as well as by physical and chemical factors, including intraluminal hydraulic pressure, relative hypoxia, and calcium concentration. Genetic factors include (1) transcription factors that directly modulate gene expression in the cell nucleus; (2) peptide growth factors and cytokines as well as their related intracellular signaling components that mediate cell proliferation, differentiation, migration, and cell-cell interaction; (3) extracellular matrix that provides important environmental cues for developing lung cells to differentiate. The specifics of all these integrated regulatory mechanisms are still being explored, but the interaction between epithelium and mesenchyme compartments has long been known to play a critical role during airway branching morphogenesis and lung maturation (Fig. 1).
Figure 1.
Lung development is regulated by epithelial-mesenchymal cell interaction. Solid line: regulatory processes between cells; Dot line: molecular cross talks at multiple levels.
Transcription factors
Transcription factors are a group of nuclear proteins that are directly involved in regulation of gene expression. Both lung developmental and lung injury repair processes are mediated by dynamic changes in gene expression through modulating and coordinating transcription factor activities. Details on transcription factors in regulating lung development have been reviewed elsewhere 18. Nkx2.1 and some members of the forkhead box family will be briefly discussed herein. Gli will be discussed under the Sonic Hedgehog (SHH) pathway.
NKX2.1 (or thyroid-specific transcription factor, TTF1), is one of most important genes in lung development 19;20. Abrogation of Nkx2.1 in mice results in full absence of distal airway branches, while two main bronchial stems are still formed, suggesting lung development is arrested at a very early stage 21. In contrast, increased expression of Nkx2.1 causes dose-dependent morphological alterations in postnatal lung. For example, modest overexpression of Nkx2.1 causes type II alveolar epithelial cell hyperplasia and increased levels of SP-B. Higher expression level of Nkx2.1 disrupts alveolar septation, causing emphysema due to alveolar hypoplasia. The highest overexpression of Nkx2.1 in transgenic mice causes severe pulmonary inflammation, fibrosis, and respiratory failure, associated with eosinophil infiltration as well as increased expression of eotaxin and IL-6 22. Nkx2.1 expression can be activated by other transcription factors such as FOXA2 and GATA-6 during lung morphogenesis 23;24.
FOX (Forkhead box) proteins are a family of transcription factors sharing a winged helix DNA binding domain, and play important roles in regulating the expression of genes involved in cell growth, proliferation, differentiation. Members of FOX proteins have differential expression patterns in lung during development. For example, FOXA1 and FOXA2 are co-expressed in developing lung epithelia, while FOXF1 is expressed in lung mesenchyme 25. Blockade of both FOXA1 and FOXA2 disrupts mouse lung branching morphogenesis, resulting in hypoplastic lung formation with severe defects in epithelial and smooth muscle cell differentiation 26. Interestingly, SHH expression in epithelial cells is also downregualted in FOXA1/FOXA2 double knockout lung, while SHH from lung epithelial cells is required for formation of bronchial and vascular smooth muscle. Therefore, FOXA1/FOXA2 may be important factors in mediating epithelialmesenchymal cell interaction through regulating several pathways such as SHH. In addition, reduced FOXF1 expression levels in FOXF1 heterozygous knockout mice result in pulmonary hemorrhage and peripheral microvascular defects, with downregulation of many genes including Notch-2 receptor and Notch downstream target hairy enhancer of split-1 (HES-1). Moreover, reduced FOXF1 in surviving heterozygous FOXF1 knockout mice makes the lung more susceptible to injury in adulthood 27.
Peptide growth factors that regulate lung development
Experiments in vitro and in vivo indicate that the embryonic lung mesenchymal and epithelial cells interact through autocrine or paracrine factors. Peptide growth factors and their signal pathways play important regulatory roles in controlling lung morphogenesis 28. Many of them, including Wnt, FGF, TGF/BMP, SHH, PDGF, also play important roles in adult lung injury repair and related lung pathology, such as pulmonary fibrosis. Wnt signals are transduced through seven-transmembrane-type Wnt receptors encoded by Frizzled (Fzd) genes to activate canonical β-catenin-TCF pathway, and JNK or Ca2+-releasing non-canonical pathways. In mice, null mutation of Wnt7b, a ligand for Wnt canonical pathway, results in markedly hypoplastic lung and perinatal lethality accompanied by reduced cell replication in both epithelial and mesenchymal cells. Effects on vascular smooth muscle cell differentiation are controversial 29;30. In contrast, deletion of Wnt5a, a ligand for Wnt non-canonical pathway, causes overexpansion of distal airways and thickened intersaccular interstitium, with increased cell proliferation in both epithelial and mesenchymal cells 31. In addition, blockade of Wnt-canonical signaling by specifically deleting β-catenin in lung epithelial cells alone disrupts proximal-distal axis of the lung 32, while abrogation of β-catenin in lung mesenchyme also reduces early lung epithelial branching and mesenchymal growth 33. Interestingly, constitutive activation of Wnt canonical pathway in embryonic lung epithelium makes these cells transdifferentiate into intestinal epithelial lineages 34. Therefore, an appropriate level of Wnt signaling in the right place at the right time is essential for normal lung organogenesis.
The regulatory functions of fibroblast growth factor (FGF) ligand family members during respiratory organogenesis are very well conserved from Drosophila to mammals 35;36. Based on their protein sequence homology, FGFs have been divided into several subgroups. Similarly, their cognate transmembrane protein tyrosine kinase receptors are classified into several different types, contributing to the specificity of FGF ligand binding 37. Heparin or heparan sulfate proteoglycan, an extracellular matrix protein, has been reported to be essential for FGF ligand-receptor binding and activation 38-40. FGF10 is expressed in the mesenchyme of E11-12 mouse lungs, adjacent to distal epithelial tubules. These sites of expression change dynamically in a pattern that is compatible with the concept that FGF10 appears in the mesenchyme at prospective sites of bud formation 41. Blockade of FGF10 function results in complete absence of distal embryonic lung, despite the formation of larynx and trachea 42. Therefore, FGF10 is believed to be a major growth factor that induces lung epithelial branching. FGF10-mediated activity can also be negatively regulated through several antagonists including Spry2/4. Murine Spry2 is expressed in the distal tip of embryonic lung epithelial branches, but is down regulated between the sites of new bud formation. Overexpression of Spry2 or Spry4 can inhibit lung branching morphogenesis through reducing epithelial cell proliferation 43-45;45. Another FGF family member FGF9 also regulates branching morphogenesis. In E10.5 mouse lung, FGF9 is expressed in the visceral pleural membrane that lines the outside of the lung bud as well as in the epithelium of the developing bronchi. At E12.5 and E14.5, FGF9 expression persists in the mesothelium of the visceral pleura, but is no longer detected in airway epithelium 46. FGF9 null mice exhibit reduced mesenchyme and decreased branching of the airways, but show significant distal airspace formation and alveolar epithelial cell differentiation. The reduction in the amount of mesenchyme in FGF9 knockout lungs limits expression of mesenchymal FGF10 47, which in turn affects lung epithelial cells.
The transforming growth factor-β (TGF-β) superfamily comprises a large number of structurally related polypeptide growth factors including TGF-β, bone morphogenetic protein (BMP), and activin subfamilies. TGF-β ligands bind to their cognate receptors on the cell surface, and activate downstream Smad proteins, which translocate into the nucleus and modulate target gene expression 48;49. There are three TGF-β isoforms in mammals, TGF-β1, 2, 3, which play unique and nonredundant roles during embryonic development. Mice lacking TGF-β1 develop severe pulmonary inflammation 50, whereas TGF-β2 null mutation results in embryonic lethality around E14.5 with abnormally developed lung 51. TGF-β3 null mutant mice display cleft palate, retarded lung development, and neonatal lethality 52;53. However, overexpression of TGF-β1 in embryonic lung epithelium also results in arrest of embryonic lung growth and epithelial cell differentiation, as well as inhibition of pulmonary vasculogenesis 54;55. Thus, the activity of TGF-β signaling is regulated precisely at multiple levels. For example, integrin β6, latent TGF-β binding proteins (LTBPs), and thrombospondin are involved in regulating the release of TGF-β mature signaling peptide, while betaglycan/endoglin or decorin influence the affinity of TGF-β receptor binding. Mutation of the above genes results in phenotypes similar to those seen with altered TGF-β signaling. For example, null mutation of LTBP-3 or LTBP-4 causes profound defects in elastin fiber structure and lung alveolarization, similar to the phenotypic changes observed in Smad3 knockout mouse lung 56-58. In addition, TGF-β signaling in mesenchymal cells versus epithelial cells of developing mouse lung regulates lung branching morphogenesis and alveolarization in vivo, respectively. Selective blockade of endogenous TGF-β signaling in embryonic lung mesenchymal cells results in retarded lung branching after midgestation, while abrogation of epithelial cell-specific TGF-β signaling causes abnormal postnatal lung alveolarization, but does not appear to have a significant impact on prenatal lung development 59.
BMPs, with more than 20 family members, have been shown to regulate many developmental processes including lung development 60. Transgenic overexpression of BMP4 in the distal endoderm of fetal mouse lung, driven by a 3.7 kb human surfactant protein C (SP-C) promoter, causes abnormal lung morphogenesis with cystic terminal sacs 61. In contrast, SP-C promoter-driven overexpression of either the BMP antagonist Xnoggin or Gremlin to block BMP signaling, results in severely reduced numbers of distal epithelial cell phenotypes and increased proximal cell phenotypes in the lungs of transgenic mice 62;63. Interestingly, blockade of endogenous BMP4 in embryonic mouse lung epithelial cells using a gene conditional knockout approach results in abnormal lung development with dilated terminal sacs, similar to those seen in the BMP4 transgenic mouse lung 64, suggesting that an appropriate level of BMP4 is essential for normal lung development. Among three cognate BMP type I receptors (Alk2, Alk3, and Alk6), Alk3 expresses predominantly in distal airway epithelial cells during mouse lung development. Abrogation of Alk3 in mouse lung epithelium starting from either early organogenesis or late gestation resulted in similar neonatal respiratory distress phenotypes, accompanied by collapsed lungs 65. Early-induction of Alk3 knockout in lung epithelial cells causes retardation of early lung branching morphogenesis, and reduced cell proliferation and differentiation, while late gestation induction of Alk3 knockout also causes significant epithelial apoptosis accompanied by lack of surfactant secretion 65. Furthermore, canonical Wnt signaling is perturbed, possibly through reduced WIF-1 expression in Alk3 knockout lungs 65. Therefore, deficiency of appropriate BMP signaling in lung epithelial cells results in prenatal lung malformation, neonatal atelectasis and respiratory failure. In addition, BMP signaling is also important in lung vasculogenesis and angiogenesis. Mutations of BMP type II receptor (BMPRII) and changes in the expression level of BMP antagonist Gremlin are associated with primary pulmonary hypertension 66;67.
Sonic hedgehog (SHH) is a vertebrate homolog of hedgehog (HH), a gene that patterns the segment, leg, wing, eye and brain in Drosophila. Hh binds to patched (Ptc), a transmembrane protein, and thus releases the inhibitory effect of Ptc on downstream smoothened (Smo), which is a G protein-coupled 7-span transmembrane protein. This leads to the activation of cubitus interruptus (Ci), a 155KD transcription factor that is usually cleaved to form a 75KD transcription inhibitor in cytosol. Elements of the Drosophila HH signaling pathway and their general functions in the pathway are highly conserved in vertebrates, albeit with increased levels of complexity. Gli1, 2, and 3 are the three vertebrate Ci gene orthologues 68. The SHH signal transduction pathway plays important roles in mesenchyme-epithelium interaction. Null mutation of SHH produces profound lung hypoplasia and failure of trachea-esophageal septation, although proximal-distal differentiation of epithelial airway is still preserved 69;70. Lung specific SHH overexpression results in severe alveolar hypoplasia and a significant increase in interstitial tissue caused by increased proliferation of both epithelium and mesenchyme 71. Defective hedgehog signaling may lead to esophageal atresia and tracheoesophageal fistula 72. HIP1, a membrane-bound protein, attenuates HH signaling by directly binding all mammalian Hedgehog (HH) proteins 73. Targeted disruption of HIP1 results in neonatal lethality with respiratory failure. The initial stereotyped branching from the two primary buds is absent in HIP1 knockout lungs, although asymmetry in their growth was conserved. Moreover, null mutation of Gli2 plus Gli3 genes results in total absence of lung. Mice with Gli3 deficiency only are viable, but lung is smaller and the shape of the lung is also altered 74. In Gli2 null mutant mice, the right and left lungs are not separated but exist as a single lobe with a reduced size, and the primary branching in right lung is defective. In addition, both trachea and esophagus are hypoplastic, though they are separated from each other, and proximal-distal differentiation is normal 75. Therefore, Gli2 plays an important role in the asymmetric patterning of the lung.
Platelet-derived growth factor (PDGF) consists of four different peptides. PDGF-A and PDGF-B can form either homodimers (AA or BB) or heterodimers (AB). Two types of PDGF receptors, α and β, are present in embryonic mouse lung, and are differentially regulated in fetal rat lung epithelial cells versus fibroblasts 76. PDGF-A homozygous null mutant mice are perinatally lethal. The pulmonary phenotypes include lack of lung alveolar smooth muscle cells (SMC), reduced deposition of elastin fibers in the lung parenchyma, and hence alveolar hypoplasia due to complete failure of alveogenesis 7;77. Moreover, PDGF-B and its receptor are crucial for vascular growth and integrity during the alveolar phase 8.
Extra cellular matrix and lung development
Extracellular matrix (ECM) includes the interstitial matrix and the basement membrane, which has multiple functions, such as providing support and anchorage for cells, forming a tissue scaffold, and regulating intercellular communication. Interstitial matrix is composed of polysaccharides and fibrous proteins filled in the intercellular spaces, while basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. The protein components of basement membrane, laminin, entactin/nidogen, type IV collagen, perlecan, SPARC and fibromodulin, are important in mediating cell-cell and cell-extracellular matrix (ECM) interaction during fetal lung morphogenesis. These structural proteins may not only provide the support for tissue architecture, but may also play an active role in modulation of cell proliferation and differentiation during lung development 78. Absence or inhibition of the interaction of epithelial cells with the basement membrane results in failure of either normal lung development or lung injury repair 79.
Laminin is a group of glycoproteins involved in cell adhesion, migration, proliferation and differentiation during tissue development and remodeling. Laminin is composed of three chains, one central (α) and two laterals (β and γ) that are linked by disulfide bonds to form a cross-shaped molecule 80. To date five α, four β and three γ chain isoforms have been identified, which suggests that their combination can lead to many variants of laminin 81. Expression of laminin α1 is restricted to the first trimester, and found mainly in epithelial cells 82. Laminin α2 and α4 are produced by mesenchymal cells, while laminin α3 and α5 are produced by epithelial cells 83. In lung explant culture, laminin α1 has been shown to be important for lung branching morphogenesis and bronchial smooth muscle cell formation 84;85. Moreover, studies using laminin α5 mutant mice suggest that laminin α5 is essential for normal lobar septation in early lung development, and alveolarization and maturation in late lung development 86;87.
Nidogen (150 kDa) is a constituent of the basement membranes that binds to the γ1 and γ3 chains of laminin, and forms a link between laminin and collagen IV 88-90. Although blocking the interaction of Nidogen with laminin affects the progression of lung development in vitro 88;91;92, null mutation of Nidogen does not have significant impact on lung formation 93
Fibronectin also plays important roles in branching morphogenesis, in which repetitive epithelial cleft and bud formation create the complex three-dimensional branching structures in several organs. For example, fibronectin is essential for cleft formation during the initiation of epithelial branching in salivary gland. Fluorescent immunostaining of fibronectin during early branching of lung and kidney also shows an accumulation of fibronectin at sites of epithelial constriction and indentation 94, supporting possible roles for fibronectin in branching morphogenesis 95. Direct examination of the role of fibronectin, by treatment of developing lung rudiments with anti-fibronectin antibody or siRNA, inhibited branching morphogenesis, while fibronectin supplementation promoted branching of lung 94. The EIIIA segment of fibronectin is one of the major alternatively spliced segments and modulates the cell proliferative potential of fibronectin in vitro. The EIIIA-containing fibronectin isoform localized to both the epithelium and mesenchyme. Its expression gradually decreases from the pseudoglandular stage to the saccular stage and then slightly increases from the saccular stage to the alveolar stage. This change in expression pattern of EIIIA-containing fibronectin correlates well with the numbers of distal pulmonary PCNA-positive cells throughout lung development 96.
Lung injury repair and fibrosis
The lung, with its vast surface area and unique gas exchange function, is the frontline of defense against harmful environmental challenges including physical, chemical, and biological factors. Therefore, injury to large conducting airways through to terminal air exchange alveoli is frequent throughout life, and related repair and remodeling processes are key steps to restore normal lung function.
As described above, a variety of epithelial cells lining the airway and alveolar surfaces act as the first line of defense in protecting the lung from external deleterious agents. The protective strategies include mucus secretion, ciliary movement, electrolyte and fluid transportation across respiratory surface membranes, detoxification, surfactant production, etc. During lung injury, damaged epithelial cells are shed from the lining surface, which leaves a denuded epithelial surface with disrupted barrier function. To restore its functions, a regeneration process starts immediately after the lesion has occurred. In distal lung, resident progenitor cells inherited from developing lung cell lineages and/or recruited circulating stem cells migrate, proliferate, and differentiate to re-epithelialize the surface, and restore original cell types and functions if the structural scaffold is not severely damaged. Integrity of underlying ECM may be of primary importance in directing repair of injury, which may provide niches for appropriate cell expansion and differentiation. Studies in lung injury-repair have revealed changes in cell behavior and gene expression that are reminiscent of specific developmental processes in the lung. For example, FGF10, BMP4, TGF-β, PDGF, and Wnt signaling are all involved in certain lung repair processes. However, lung repair has its own unique features in addition to these growth factors, such as involvement of pulmonary inflammation and the secretion of cytokines/chemokines related to the process. Lung fibrosis may be regarded as an abnormal healing process related to failure of resolution of lung damage and restoration of normal structure.
After tissue injury, alpha-smooth muscle actin (αSMA)-positive myofibroblasts are derived from a variety of cell lineages, filling the wound and possibly relieving local mechanical stress. On the other hand, myofibroblasts and excessive ECM production from these cells impair organ structure and function. Activated TGF-β, presence of specialized ECM proteins, and intense extracellular stress are thought to be the driving force for myofibroblast generation 97. As mentioned above, TGF-β-mediated signaling is required for normal embryonic lung mesenchymal and postnatal lung epithelial growth. However, excessive TGF-β signaling in transgenic mouse models also results in hypoplastic lung formation. Therefore, an appropriate level of TGF-β signaling is essential for lung development. Conversely, excessive TGFβ signaling in mature lung is associated with aggressive and progressive fibrosis 98. Moreover, in postnatal lung injury, excessive amounts of activated TGF-β can be released from ECM, alveolar macrophages and other infiltrating inflammatory cells, which in turn up-regulates fibronectin and its integrin receptor in alveolar fibroblasts, stimulating them to differentiate into myofibroblasts. Perivascular and peribronchiolar adventitial fibroblasts, and circulating fibrocytes are also reported to contribute to the myofibroblast population during lung injury 16;17;99;100. In addition, lung myofibroblasts may originate from epithelialmesenchymal transition (EMT) 101, although the relative contribution of EMT remains unclear. Local and transient TGF-β1 overexpression in the pleural cavity induces active pleural fibrosis with extension into lung parenchyma, possibly through a mechanism of mesothelial-fibroblastoid transformation 102. Interestingly, certain lung mesenchymal cells are also found to be derived from mesothelial cells during lung development 13. Therefore, the pathogenic mechanism of myofibroblast expansion during lung fibrosis varies greatly depending on the type of lung injury.
Why is understanding lung development important for unhderstanding the mechanisms of lung repair/fibrosis?
Studies of lung development and lung repair/fibrosis to date have discovered that many of the same factors that control normal development are also key players in fibrosis, with TGF-β family peptide signaling being the prime example. Appropriate amounts of TGF-β signaling are essential for many aspects of normal lung development as well as correct immune function in the lung. However, excessive amounts of TGF-β signaling cause severe hypoplasia in the immature lung, a disease known as bronchopulmonary dysplasia in human prematures, while in the mature lung severe and progressive fibrosis is the pathological hallmark. This leads us to propose the “Goldilocks” hypothesis of signaling in lung development and injury repair: that everything must be done just right! The evolutionary pressure to achieve Goldilocks homeostasis in the TGF-β pathway alone must be massive, when one considers the highly redundant, multilayered regulation that controls latent ligand activation and bioavailability as well as signal amplification. The challenge remains to re-entrain developmental lung processes to quickly achieve epithelial closure, repair and resolution of lung injury, without inducing an excessive damage reaction mediated by excess TGF-β signaling, which culminates in lung hypoplasia or fibrosis.
Acknowledgement
This work was supported by NIH grants HL68597 (WS), HL60231, HL44060, HL44977, HL75773 (DW), Webb Foundation (WS), and the Southern California Environmental Health Sciences Center Pilot Project (WS).
References
- 1.Hogan BL. Morphogenesis. Cell. 1999;96:225–33. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 2.Warburton D, Bellusci S, De Langhe S, et al. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr. Res. 2005;57:26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- 3.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–50. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warburton D. Developmental biology: order in the lung. Nature. 2008;453:733–5. doi: 10.1038/453733a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu MM, Li S, Yang H, Morrisey EE. Foxp4: a novel member of the Foxp subfamily of winged-helix genes co-expressed with Foxp1 and Foxp2 in pulmonary and gut tissues. Gene Expr. Patterns. 2002;2:223–8. doi: 10.1016/s1567-133x(02)00058-3. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto S, Nakano H, Singh G, Katyal S. Expression of Spred and Sprouty in developing rat lung. Gene Expr. Patterns. 2002;2:347–53. doi: 10.1016/s1567-133x(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 7.Bostrom H, Willetts K, Pekny M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–73. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 8.Lindahl P, Karlsson L, Hellstrom M, et al. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–53. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 9.Awonusonu F, Srinivasan S, Strange J, Al-Jumaily W, Bruce MC. Developmental shift in the relative percentages of lung fibroblast subsets: role of apoptosis postseptation. Am. J. Physiol. 1999;277:L848–L859. doi: 10.1152/ajplung.1999.277.4.L848. [DOI] [PubMed] [Google Scholar]
- 10.Schittny JC, Djonov V, Fine A, Burri PH. Programmed cell death contributes to postnatal lung development. Am. J. Respir. Cell Mol. Biol. 1998;18:786–93. doi: 10.1165/ajrcmb.18.6.3031. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan S, Strange J, Awonusonu F, Bruce MC. Insulin-like growth factor I receptor is downregulated after alveolarization in an apoptotic fibroblast subset. Am. J. Physiol Lung Cell Mol. Physiol. 2002;282:L457–L467. doi: 10.1152/ajplung.00050.2001. [DOI] [PubMed] [Google Scholar]
- 12.Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B. Stem/Progenitor cells in lung development, injury repair, and regeneration. Proc. Am. Thorac. Soc. 2008;5:703–6. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16626–30. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am. J. Respir. Cell Mol. Biol. 2007;37:152–9. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotton DN, Fine A. Lung stem cells. Cell Tissue Res. 2008;331:145–56. doi: 10.1007/s00441-007-0479-2. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J. Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 17.Moeller A, Gilpin SE, Ask K, et al. Circulating Fibrocytes Are an Indicator for Poor Prognosis in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit Care Med. 2009 doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 18.Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–44. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 19.Guazzi S, Price M, De FM, Damante G, Mattei MG, Di LR. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 1990;9:3631–9. doi: 10.1002/j.1460-2075.1990.tb07574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazzaro D, Price M, De FM, Di LR. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 21.Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–9. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 22.Wert SE, Dey CR, Blair PA, Kimura S, Whitsett JA. Increased expression of thyroid transcription factor-1 (TTF-1) in respiratory epithelial cells inhibits alveolarization and causes pulmonary inflammation. Dev. Biol. 2002;242:75–87. doi: 10.1006/dbio.2001.0540. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda K, Shaw-White JR, Wert SE, Whitsett JA. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Mol. Cell Biol. 1996;16:3626–36. doi: 10.1128/mcb.16.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw-White JR, Bruno MD, Whitsett JA. GATA-6 activates transcription of thyroid transcription factor-1. J. Biol. Chem. 1999;274:2658–64. doi: 10.1074/jbc.274.5.2658. [DOI] [PubMed] [Google Scholar]
- 25.Costa RH, Kalinichenko VV, Lim L. Transcription factors in mouse lung development and function. Am. J. Physiol Lung Cell Mol. Physiol. 2001;280:L823–L838. doi: 10.1152/ajplung.2001.280.5.L823. [DOI] [PubMed] [Google Scholar]
- 26.Wan H, Dingle S, Xu Y, et al. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J. Biol. Chem. 2005;280:13809–16. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- 27.Kalinichenko VV, Zhou Y, Shin B, et al. Wild-type levels of the mouse Forkhead Box f1 gene are essential for lung repair. Am. J. Physiol Lung Cell Mol. Physiol. 2002;282:L1253–L1265. doi: 10.1152/ajplung.00463.2001. [DOI] [PubMed] [Google Scholar]
- 28.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech. Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 29.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–42. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopal J, Carroll TJ, Guseh JS, et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–34. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev. Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- 32.Mucenski ML, Wert SE, Nation JM, et al. {beta}-Catenin Is Required for Specification of Proximal/Distal Cell Fate during Lung Morphogenesis. J. Biol. Chem. 2003;278:40231–8. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- 33.Yin Y, White AC, Huh SH, et al. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev. Biol. 2008;319:426–36. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J. Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glazer L, Shilo BZ. The Drosophila FGF-R homolog is expressed in the embryonic tracheal system and appears to be required for directed tracheal cell extension. Genes Dev. 1991;5:697–705. doi: 10.1101/gad.5.4.697. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 37.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izvolsky KI, Zhong L, Wei L, Yu Q, Nugent MA, Cardoso WV. Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am. J. Physiol Lung Cell Mol. Physiol. 2003;285:L838–L846. doi: 10.1152/ajplung.00081.2003. [DOI] [PubMed] [Google Scholar]
- 39.Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev. Biol. 2003;258:185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Buff EM, Perrimon N, Michelson AM. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999;126:3715–23. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- 41.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 42.Min H, Danilenko DM, Scully SA, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–61. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perl AK, Hokuto I, Impagnatiello MA, Christofori G, Whitsett JA. Temporal effects of Sprouty on lung morphogenesis. Dev. Biol. 2003;258:154–68. doi: 10.1016/s0012-1606(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 44.Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell Biol. 1998;18:3966–73. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tefft D, De Langhe SP, Del Moral PM, et al. A novel function for the protein tyrosine phosphatase Shp2 during lung branching morphogenesis. Dev. Biol. 2005;282:422–31. doi: 10.1016/j.ydbio.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Colvin JS, Feldman B, Nadeau JH, Goldfarb M, Ornitz DM. Genomic organization and embryonic expression of the mouse fibroblast growth factor 9 gene. Dev. Dyn. 1999;216:72–88. doi: 10.1002/(SICI)1097-0177(199909)216:1<72::AID-DVDY9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 49.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 50.McLennan IS, Poussart Y, Koishi K. Development of skeletal muscles in transforming growth factor-beta 1 (TGF-beta1) null-mutant mice. Dev. Dyn. 2000;217:250–6. doi: 10.1002/(SICI)1097-0177(200003)217:3<250::AID-DVDY3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 51.Bartram U, Molin DG, Wisse LJ, et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–52. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- 52.Kaartinen V, Voncken JW, Shuler C, et al. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 1995;11:415–21. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 53.Shi W, Heisterkamp N, Groffen J, Zhao J, Warburton D, Kaartinen V. TGF-beta3-null mutation does not abrogate fetal lung maturation in vivo by glucocorticoids. Am. J. Physiol. 1999;277:L1205–L1213. doi: 10.1152/ajplung.1999.277.6.L1205. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L, Dey CR, Wert SE, Whitsett JA. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev. Biol. 1996;175:227–38. doi: 10.1006/dbio.1996.0110. [DOI] [PubMed] [Google Scholar]
- 55.Zeng X, Gray M, Stahlman MT, Whitsett JA. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev. Dyn. 2001;221:289–301. doi: 10.1002/dvdy.1140. [DOI] [PubMed] [Google Scholar]
- 56.Sterner-Kock A, Thorey IS, Koli K, et al. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–73. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colarossi C, Chen Y, Obata H, et al. Lung alveolar septation defects in Ltbp-3-null mice. Am. J. Pathol. 2005;167:419–28. doi: 10.1016/S0002-9440(10)62986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, Sun J, Buckley S, et al. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am. J. Physiol Lung Cell Mol. Physiol. 2005;288:L683–L691. doi: 10.1152/ajplung.00298.2004. [DOI] [PubMed] [Google Scholar]
- 59.Chen H, Zhuang F, Liu YH, et al. TGF-{beta} receptor II in Epithelia Versus Mesenchyme Plays Distinct Role in Developing Lung. Eur. Respir. J. 2008;32:285–95. doi: 10.1183/09031936.00165407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–94. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 61.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- 62.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–15. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- 63.Lu MM, Yang H, Zhang L, Shu W, Blair DG, Morrisey EE. The bone morphogenic protein antagonist gremlin regulates proximal-distal patterning of the lung. Dev. Dyn. 2001;222:667–80. doi: 10.1002/dvdy.1231. [DOI] [PubMed] [Google Scholar]
- 64.Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev. Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Sun J, Chen H, Chen C, et al. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am. J. Pathol. 2008;172:571–82. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat. Genet. 2000;26:81–4. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 67.Costello CM, Howell K, Cahill E, et al. Lung Selective Gene Responses to Alveolar Hypoxia: Potential Role for the Bone Morphogenetic Antagonist Gremlin in Pulmonary Hypertension. Am. J. Physiol Lung Cell Mol. Physiol. 2008 doi: 10.1152/ajplung.00358.2007. [DOI] [PubMed] [Google Scholar]
- 68.van TM, Post M. From fruitflies to mammals: mechanisms of signalling via the Sonic hedgehog pathway in lung development. Respir. Res. 2000;1:30–5. doi: 10.1186/rr9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 70.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998;8:1083–6. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 71.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 72.Spilde TL, Bhatia AM, Mehta S, et al. Defective sonic hedgehog signaling in esophageal atresia with tracheoesophageal fistula. Surgery. 2003;134:345–50. doi: 10.1067/msy.2003.243. [DOI] [PubMed] [Google Scholar]
- 73.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–21. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 74.Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev. Biol. 1997;188:337–48. doi: 10.1006/dbio.1997.8644. [DOI] [PubMed] [Google Scholar]
- 75.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat. Genet. 1998;20:54–7. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 76.Buch S, Jassal D, Cannigia I, et al. Ontogeny and regulation of platelet-derived growth factor gene expression in distal fetal rat lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994;11:251–61. doi: 10.1165/ajrcmb.11.3.8086163. [DOI] [PubMed] [Google Scholar]
- 77.Bostrom H, Gritli-Linde A, Betsholtz C. PDGF-A/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev. Dyn. 2002;223:155–62. doi: 10.1002/dvdy.1225. [DOI] [PubMed] [Google Scholar]
- 78.Lwebuga-Mukasa JS. Matrix-driven pneumocyte differentiation. Am. Rev. Respir. Dis. 1991;144:452–7. doi: 10.1164/ajrccm/144.2.452. [DOI] [PubMed] [Google Scholar]
- 79.Hilfer SR. Morphogenesis of the lung: control of embryonic and fetal branching. Annu. Rev. Physiol. 1996;58 doi: 10.1146/annurev.ph.58.030196.000521. 93-113. [DOI] [PubMed] [Google Scholar]
- 80.Burgeson RE, Chiquet M, Deutzmann R, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–11. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen NM, Senior RM. Laminin isoforms and lung development: all isoforms are not equal. Dev. Biol. 2006;294:271–9. doi: 10.1016/j.ydbio.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 82.Pierce RA, Griffin GL, Miner JH, Senior RM. Expression patterns of laminin alpha1 and alpha5 in human lung during development. Am. J. Respir. Cell Mol. Biol. 2000;23:742–7. doi: 10.1165/ajrcmb.23.6.4202. [DOI] [PubMed] [Google Scholar]
- 83.Ekblom M, Falk M, Salmivirta K, Durbeej M, Ekblom P. Laminin isoforms and epithelial development. Ann. N. Y. Acad. Sci. 1998;857:194–211. doi: 10.1111/j.1749-6632.1998.tb10117.x. [DOI] [PubMed] [Google Scholar]
- 84.Schuger L, O'Shea S, Rheinheimer J, Varani J. Laminin in lung development: effects of anti-laminin antibody in murine lung morphogenesis. Dev. Biol. 1990;137:26–32. doi: 10.1016/0012-1606(90)90004-3. [DOI] [PubMed] [Google Scholar]
- 85.Schuger L, Skubitz AP, Zhang J, Sorokin L, He L. Laminin alpha1 chain synthesis in the mouse developing lung: requirement for epithelial-mesenchymal contact and possible role in bronchial smooth muscle development. J. Cell Biol. 1997;139:553–62. doi: 10.1083/jcb.139.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev. Biol. 2005;282:111–25. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen NM, Miner JH, Pierce RA, Senior RM. Laminin alpha 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev. Biol. 2002;246:231–44. doi: 10.1006/dbio.2002.0658. [DOI] [PubMed] [Google Scholar]
- 88.Dziadek M. Role of laminin-nidogen complexes in basement membrane formation during embryonic development. Experientia. 1995;51:901–13. doi: 10.1007/BF01921740. [DOI] [PubMed] [Google Scholar]
- 89.Koch M, Olson PF, Albus A, et al. Characterization and expression of the laminin gamma3 chain: a novel, non-basement membrane-associated, laminin chain. J. Cell Biol. 1999;145:605–18. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reinhardt D, Mann K, Nischt R, et al. Mapping of nidogen binding sites for collagen type IV, heparan sulfate proteoglycan, and zinc. J. Biol. Chem. 1993;268:10881–7. [PubMed] [Google Scholar]
- 91.Ekblom P, Ekblom M, Fecker L, et al. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;120:2003–14. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- 92.Senior RM, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Pierce RA. Entactin expression by rat lung and rat alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 1996;14:239–47. doi: 10.1165/ajrcmb.14.3.8845174. [DOI] [PubMed] [Google Scholar]
- 93.Dong L, Chen Y, Lewis M, et al. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest. 2002;82:1617–30. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- 94.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;%19(423):876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 95.Roman J. Fibronectin and fibronectin receptors in lung development. Exp. Lung Res. 1997;23:147–59. doi: 10.3109/01902149709074027. [DOI] [PubMed] [Google Scholar]
- 96.Kikuchi W, Arai H, Ishida A, Takahashi Y, Takada G. Distal pulmonary cell proliferation is associated with the expression of EIIIA+ fibronectin in the developing rat lung. Exp. Lung Res. 2003;29:135–47. doi: 10.1080/01902140303774. [DOI] [PubMed] [Google Scholar]
- 97.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 98.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997;100:768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am. J. Pathol. 1994;145:114–25. [PMC free article] [PubMed] [Google Scholar]
- 101.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Decologne N, Kolb M, Margetts PJ, et al. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J. Immunol. 2007;179:6043–51. doi: 10.4049/jimmunol.179.9.6043. [DOI] [PubMed] [Google Scholar]