Abstract
Absorption of a photon by a vertebrate opsin pigment induces 11-cis to all-trans isomerization of its retinaldehyde chromophore. Restoration of light sensitivity to the bleached opsin requires chemical re-isomerization of the chromophore via an enzyme pathway called the visual cycle. The retinoid isomerase in this pathway is Rpe65, a membrane-associated protein in the retinal pigment epithelium (RPE) with no predicted membranespanning segments. It has been suggested that Rpe65 is S-palmitoylated by lecithin:retinol acyl transferase (LRAT) on Cys231, Cys329, and Cys330, and that this palmitoylation is required for isomerase activity and the association of Rpe65 with membranes. Here we show that the affinity of Rpe65 for membranes is similar in wild-type and lrat-/- mice. The isomerase activity of Rpe65 is also similar in both strains when all-trans-retinyl palmitate is used as substrate. With all-trans-retinol substrate, isomerase activity is present in wild-type but undetectable in RPE homogenates from lrat-/- mice. Substitution of Cys231, Cys329, and Cys330 with Ser or Ala did not affect the affinity of Rpe65 for membranes. Further, these Cys residues are not palmitoylated in Rpe65 by mass spectrometric analysis. Global inhibition of protein palmitoylation by 2-bromopalmitate did not affect the solubility or isomerase activity of Rpe65. Finally, we show that soluble and membrane-associated Rpe65 possesses similar isomerase specific activities. These results indicate that LRAT is not required for isomerase activity beyond synthesis of retinyl-ester substrate, and that the association of Rpe65 with membranes is neither dependent upon LRAT nor the result of S-palmitoylation. The affinity of Rpe65 for membranes is probably an intrinsic feature of this protein.
Vertebrate retinas contain light sensitive proteins called opsins, which are members of the G protein-coupled receptor superfamily. The light-absorbing chromophore in most vertebrate opsins is 11-cis-retinaldehyde (11-cis-RAL).2 Capture of a photon by an opsin pigment induces 11-cis to all-trans isomerization of the retinaldehyde chromophore. After a brief period of activation, the bleached pigment dissociates to yield apoopsin and free all-trans-retinaldehyde (all-trans-RAL). Restoration of light sensitivity to the apo-opsin requires chemical regeneration of 11-cis-RAL via an enzyme pathway called the visual cycle (Fig. 1). Most steps in this pathway take place within cells of the retinal pigment epithelium (RPE), which directly overlay the photoreceptors. Following its release by photoreceptors, all-trans-retinol (all-trans-ROL) is taken up by RPE cells and esterified to a fatty acid from phosphatidylcholine in internal membranes by lecithin:retinol acyl transferase (LRAT) (1). The retinoid isomerase uses an all-trans-retinyl ester (all-trans-RE), such as all-trans-retinyl palmitate (all-trans-RP) as substrate (2-4), harnessing the energy of ester hydrolysis to drive the endothermic (ΔG = +4.1 kcal/mol) isomerization reaction (5). The final catalytic step in the visual cycle involves oxidizing the 11-cis-retinol (11-cis-ROL) product of the isomerase to 11-cis-RAL (6, 7). Newly synthesized 11-cis-RAL is released by the RPE and taken up by photoreceptors where it combines with apo-opsin to form a new light sensitive pigment molecule.
FIGURE 1. Visual cycle in RPE cells.
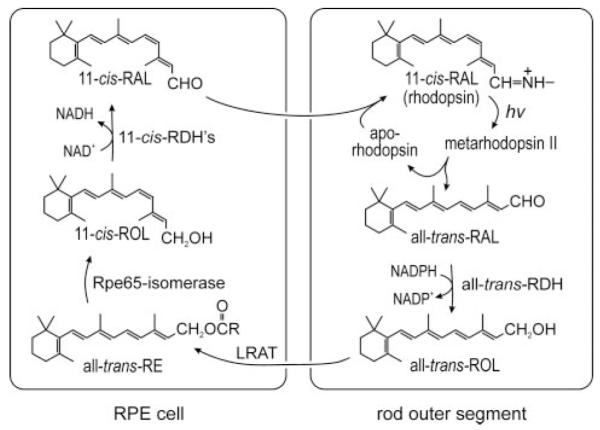
Absorption of a photon (hv) by a rhodopsin pigment molecule induces isomerization of 11-cis-RAL to all-trans-RAL resulting in activated metarhodopsin II. Decay of metarhodopsin II yields apoopsin and free all-trans-RAL, which is reduced to all-trans-ROL by one of several all-trans-ROL dehydrogenases (all-trans-RDH). The all-trans-ROL is released by photoreceptors and taken up by RPE cells where it is esterified to a fatty acid from phosphatidylcholine by LRAT. Rpe65 isomerase uses all-trans-RE as substrate to form 11-cis-ROL plus a free fatty acid. 11-cis-ROL is oxidized to 11-cis-RAL by one of several 11-cis-ROL dehydrogenases (11-cis-RDH). 11-cis-RAL is released by the RPE and taken up by the photoreceptor where it combines with apo-opsin to re-form rhodopsin.
The retinoid isomerase was recently identified as an abundant 63-kDa protein in RPE cells called Rpe65 (8-10). Rpe65 is homologous to β-carotene 15,15′-oxygenase in mammals (11), and apo-carotene oxygenase in cyanobacteria (12). By x-ray diffraction analysis, apo-carotene oxygenase is a seven-bladed β-propeller (12). A similar structure is predicted for Rpe65 (13). Rpe65 associates with membranes, although it contains no predicted transmembrane segments (14). This association was shown to result from S-palmitoylation of residues Cys231, Cys329, and Cys330 (15), which are on or near the protein surface (13). The acyl-transferase responsible for palmitoylation of Rpe65 is thought to be LRAT (15). A “palmitoylation switch” mechanism for the regulation of isomerase activity was proposed whereby binding of Rpe65 to its all-trans-RP substrate is mediated by reversible S-palmitoylation of these three Cys residues (15).
Mice with a knock-out mutation in the gene for LRAT contained only trace levels of all-trans-RE in the eye (16, 17). Although blood levels were nearly normal, all-trans-ROL was dramatically reduced in the eyes of lrat-/- mice, and the other visual retinoids, including 11-cis-ROL and 11-cis-RAL, were undetectable (16, 17). These results establish that LRAT is required for the uptake of all-trans-ROL from blood into ocular tissues. The absence of 11-cis-retinoids in these animals is also consistent with the hypothesis that isomerase activity is dependent on LRAT. In the current study we examined the role of LRAT on isomerase activity and the solubility of Rpe65. We show that isomerase activity is normal in lrat-/- mice. Further, we show that the association of Rpe65 with membranes is not dependent upon LRAT, and that the soluble and membrane-associated forms of Rpe65 possess similar isomerase activities. Finally, we show that the association of Rpe65 with membranes is not affected by amino acid substitutions that block palmitoylation at positions 231, 329, and/or 330.
EXPERIMENTAL PROCEDURES
Mice and Bovine Eyes
Mice with a knock-out mutation in the gene for LRAT (16) were generously provided by Krzysztof Palczewski. Wild-type (WT) mice (strain 129S2/Sv) were purchased from Charles River Laboratories. We determined the rpe65 genotype at codon 450 by DNA sequencing. Only WT and lrat-/- mice homozygous for the normal (Leu450) allele of rpe65 were used in this study. All animal experiments followed procedures approved by the UCLA Animal Research Committee. Mice were maintained in 12-h cyclic light at ∼30 lux. Bovine eyes were from freshly slaughtered cattle (<4 h)and kept on ice until dissection.
Preparation of Cell Homogenates, Membrane Pellets, and Membrane-free Supernatants from Mouse and Bovine RPE
After removal of the anterior section, vitreous, and neural retina, the RPE and choroid of mouse or bovine eyes were homogenized in 20 mM HEPES buffer, pH 7.5 (60 μl per eye for mouse and 1.0 ml per eye for cow). Samples were centrifuged 10 min at 400 × g and 4 °C to remove cell debris. The resulting low speed supernatants represent RPE cell homogenates. The homogenates were diluted with buffer to yield protein concentrations of 1–3 mg/ml. A portion of each homogenate was centrifuged for 1 h at 100,000 × g, 4 °C to pellet the membranes. The resulting ultra-supernatants represent membrane-free supernatant fractions. The membrane pellets were resuspended in same volume of HEPES buffer containing 0.1% SDS. All samples were irradiated for 10 min on ice with 365-nm light from a Spectroline Model EN-140L ultraviolet light source to destroy endogenous retinoids. The protein contents in the preparations were determined using the Micro BCA Protein Assay kit (Pierce). Proteins (50 ng to 5.0 μg) from the homogenate and the identical volume of membrane-free supernatant were analyzed by immunoblot analysis using an antibody against bovine Rpe65 (see below).
Cell Culture and Transfection
HEK-293T (293T) cells stably expressing LRAT (293T-L) or LRAT plus CRALBP (293T-LC) (8) were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (100 units per ml penicillin G and 100 μg per ml streptomycin) at 37 °C under 5% CO2. The 293T, 293T-L, and 293T-LC cells were transfected using PolyFect reagent (Qiagen) according to the manufacturer’s procedure.
Site-directed Mutagenesis of Bovine RPE65 cDNA
The complete coding region of the bovine RPE65 cDNA was subcloned into the mammalian expression vector, pRK5. Site-directed mutagenesis was done using the QuikChange XL site-directed mutagenesis kit (Stratagene) to yield cDNAs for Rpe65 with the indicated Cys-to-Ser or Cys-to-Ala substitutions. Following mutagenesis, all plasmid inserts were sequenced in both directions on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems/Hitachi) using DYEnamic ET Terminator Cycle Sequencing Kits (Amersham Biosciences). Plasmids for mammalian cell transfection were prepared using the PureLink Hipure plasmid miniprep or midiprep kit (Invitrogen).
Assay for Membrane Association of Rpe65s in 293T and 293T-L Cells
Two days post-transfection with plasmids encoding normal or mutated Rpe65s, 293T and 293T-L cells from 100-mm culture dishes were washed twice in 10 ml of PBS, resuspended in 0.4 ml of ice-cold 10 mM HEPES buffer (pH 7.5) containing 280 mM sucrose, 10 mM MgCl2 and protease-inhibitor mixture (Roche Applied Science), and homogenized in a Dounce homogenizer. Protein concentration of each cell homogenate was adjusted to 1–3 mg/ml. A portion of each homogenate was centrifuged for 20 min at 1000 × g. The supernatants were collected and centrifuged for 1 h at 100,000 × g. The resulting ultra-supernatant represents the membrane-free fraction. The ultra-pellets were rinsed in the same buffer and suspended in the same volume of 10 mM HEPES buffer (pH 7.5) containing 6 mM sodium cholate. 500 ng to 12 μg of proteins in the total cell homogenates and the identical volumes of the membrane-free supernatants were analyzed by immunoblotting with antisera against Rpe65.
Extraction of Rpe65 Proteins from 293T Cell Membranes
The 293T cells grown in 100-mm dishes were transfected with expression plasmids for normal and Y368H-substituted Rpe65s. At 2-day post-transfection, the cells (from four dishes per plasmid construct) were washed twice in 40 ml of PBS by low speed centrifugation and frozen for 15 min at -85 °C. The cells were homogenized in 20 mM HEPES buffer (pH 7.5) containing EDTA-free protease mixture (Roche Applied Science) and centrifuged for 30 min at 250,000 × g. After removing the supernatant, the pellets were washed three times in the same buffer by homogenization and repeat ultracentrifugation. Pel-lets from the final centrifugation were resuspended in 20 mM HEPES buffer containing 0.45 M NaCl and 0–10 mM sodium cholate (as indicated), and incubated on ice for 15 min to extract Rpe65 from the membranes. After centrifugation for 45 min at 250,000 × g, the supernatants were used for in vitro isomerase assays. Supernatants were also analyzed by immuno-blotting (500 ng protein per lane) using antisera against Rpe65.
Immunoblot Analysis
Proteins in Laemmli sample buffer were heated for 10 min at 75 °C, separated by SDS-PAGE in a 10% polyacrylamide gel, and transferred to an Immobilon-P membrane (Millipore). The membrane was incubated in blocking buffer (PBS pH 7.4, 5% nonfat milk) for 2 h at 37 °C, then overnight at 4 °C with rabbit polyclonal antiserum against the Rpe65 peptide, NFITKINPETLETIK (residues 150–163) (4). In some experiments, polyclonal antiserum against human Fyn kinase (Abcam) was used. After washing three times in PBS for 10 min, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories) or with IRDye 800CW-conjugated goat anti-rabbit IgG (LI-COR Biosciences) for 1 h and washed again. Rpe65 that reacted with the antibodies was visualized with the enhanced ECL-Plus Western blot detection system (Amersham Biosciences), or by scanning the membrane in an Odyssey Infrared Imaging System (LI-COR Biosciences). The fluorescence intensity of each band was measured using Odyssey 2.1 software (LI-COR Biosciences).
Preparation of Acrylamide-modified Bovine Rpe65
Microsomal proteins from bovine RPE, prepared as previously described (3) were dissolved in 50 mM Tris-HCl buffer (pH 8.0) containing 0.1% SDS (2.25 μg of protein/μl). To a 50-μl aliquot we added 20 μl of 50 mM dithiothreitol (DTT) in 50 mM ammonium bicarbonate (pH 8.4), and the solution was incubated at 70 °C for 3 h. To another 50-μl aliquot, we added 20 μl of 50 mM tributylphosphine (TBP) in 50 mM ammonium bicarbonate (pH 8.4), and the solution was incubated at 37 °C for 30 min. The DTT sample was then incubated with 20 μl of 250 mM acrylamide (AA-d0) in 50 mM ammonium bicarbonate (pH 8.4) at 25 °C for 3 h, while the TBP sample was incubated under the same conditions with 2,3,3-acrylamide-d3 (AA-d3) (Cambridge Isotope Lab) instead. These samples were pooled and separated by SDS-PAGE. We identified the prominent Rpe65 band (∼20 μg) in the lane by Rpe65-immunoblot analysis of a duplicate gel.
In-gel Proteolytic Digestion of Rpe65
After dissecting the Rpe65-containing bands from the SDS gels, the gel slices were dehydrated in acetonitrile, following established procedures (18). An enzyme solution containing 200 ng modified trypsin (Promega) or 100 ng of endoproteinase Glu-C (Roche Applied Science) in 50 mM ammonium bicarbonate (pH 8.0) was added to the gel slices and the samples incubated at 37 °C for 15 h.
Analysis of Peptides by Liquid Chromatography and Electrospray Ionization Mass Spectrometry
Peptide samples following proteolytic digestion were separated by reverse-phase high performance liquid chromatography (HPLC) on an Agilent Zorbax 300SB-C18 column (250 H 0.5 mm, 5.0 μm) using an Agilent 1100 capillary liquid chromatograph. Eluent A was an aqueous solution of 0.1% formic acid and 0.005% heptafluorobutyric acid (HFBA), and eluent B was an acetonitrile solution of 0.1% formic acid and 0.005% HFBA. The flow rate was 10 μl/min, and the gradient program was 5% B to 10% B for 7 min; 10% B to 40% B for 53 min; 40% B to 80% B for 30 min; 80% B for 10 min; 80% B to 5% B for 5 min. The injection volume was 20 μl. The elution profile was monitored by a Thermo Finnigan LCQ Deca XP ion trap mass spectrometer (MS) using electrospray ionization (ESI) in positive-ion mode with nitrogen as the sheath gas. The heated capillary temperature was 200 °C, the electrospray voltage was 5.20 KeV, and the capillary voltage was 44 V. Three scan events were used: (i) 500–2000 m/z full scan MS; (ii) data-dependent zoom scan on the most intensive ion from the MS full scan spectrum, and (iii) data-dependent full scan MS/MS on the most intensive ion from the MS full scan. The MS/MS collision energy was set to 35 V. When an m/z ratio for an ion was selected for data-dependent scan it was placed in a list and dynamically excluded for 1 min for further fragmentation.
In Vivo Isomerase Assay in Cultured Cells
To analyze 11-cis-ROL synthesis in 293T-LC cells from all-trans-ROL substrate added to the medium, cells grown in 12-well culture plates (Corning) were transfected with expression plasmids for normal and mutated Rpe65 proteins using PolyFect transfection reagent (Qiagen). The amount of plasmid was adjusted to yield similar levels of the normal and substituted Rpe65 proteins (0.2–0.4 μg of DNA for normal and 0.8–1.6 μg for mutant plasmids). Thirty hours after transfection, cell media were replaced with fresh media containing 15% fetal bovine serum, 0.5% bovine serum albumin, and 25 mM HEPES buffer (pH 7.5). Under dim red light, all-trans-ROL in ethanol was added to the medium (5.0 μM final concentration), and the cells were incubated for an additional 18 h. After removing the medium, the cells were washed in 1.0 ml of PBS, pelleted by low speed centrifugation, and lysed in 0.4 ml of lysis buffer (0.2% SDS in 10 mM HEPES buffer, pH 7.5). Ten micrograms of protein from the cell lysates were analyzed by immunoblotting with the anti-Rpe65 antibody, as described above. For HPLC analysis, retinoids were extracted with hexane as previously described (8).
In Vitro Isomerase Assay
The isomerase assay mixtures contained 20 mM HEPES (pH 7.5), 150 mM NaCl, 6% bovine serum albumin, 10 μM all-trans-ROL, and 50–500 μg of protein. In some experiments, isomerase was assayed in the same buffer without added NaCl with no change in the observed activities. When all-trans-RP was used as a substrate, 6.0 mM sodium cholate was added to the reaction mixture. 50 μg of protein from bovine RPE homogenates and 150 μg of protein from mouse RPE homogenates were used in the isomerase assay with all-trans-ROL. 150 μg of protein from bovine RPE homogenates and 450 μg of protein from mouse RPE homogenates were used in assays with all-trans-RP substrate. 100–170 μg of protein solubilized from membranes of 293T cells expressing unsubstituted or Y368H-substituted Rpe65s were used for the isomerase assay. The final volume for each reaction was 400 μl. After incubation for 2 h in the dark at 37 °C, the reactions were quenched by adding 0.2% SDS and 2 volumes of methanol. Retinoids were extracted with hexane and analyzed by HPLC, as described below. Specific isomerase activities were calculated after subtracting endogenous 11-cis-ROL, determined by hexane extraction of representative reaction mixtures before addition of substrate. To determine the effect of LRAT on the substrate kinetics of Rpe65 isomerase, we performed the in vitro isomerase assay on RPE homogenates from WT and lrat-/- mice. The assay mixtures were as described above except that they contained 450 μg of mouse RPE protein, 9.0 mM sodium cholate, and 0–64 μM all-trans-RP substrate. Reaction mixtures were incubated for 60 min at 37 °C.
Inhibition of Protein Palmitoylation with 2-Bromopalmitate
After transfecting with the plasmid for bovine Rpe65, 293T-LC cells were incubated in medium containing 100 μM 2-bromopalmitate (2BP) for 8 h, as previously described (19, 20). Control cells were treated similarly without addition of 2BP. The cells were washed in PBS, and homogenized in 20 mM HEPES buffer (pH 7.5). Aliquots of the homogenates containing 500 μg total protein were assayed for isomerase activity. To determine the effect of 2BP on Rpe65 and Fyn kinase solubility, total cell homogenates and membrane-free supernatants were prepared from Rpe65-expressing 293T-LC cells cultured in the presence or absence of 100 μM 2BP. Cell homogenates and membrane-free supernatants were prepared from these cells as described above, and identical fractions were analyzed by immunoblotting using antisera against bovine Rpe65 and human Fyn kinase, as described above.
In Vitro LRAT Activity Assay
The activity of LRAT was determined by monitoring the formation of all-trans-RE from added all-trans-ROL. The 293T-L and 293T cells were homogenized in ice-cold 20 mM HEPES buffer (pH 7.5). The reaction mixture (volume 200 μl) contained 400 μg of protein, 2.0 mM MgCl2, 2.0 mM CaCl2, 1.0 mM DTT, 1% bovine serum albumin, and 5.0 μM all-trans-ROL. The mixtures were incubated in the dark for 30 min at 37 °C. After quenching the reaction with addition of 4.0 μl of 10% SDS and 400 μl methanol, retinyl esters were extracted with 2.0 ml of hexane and analyzed by HPLC as described below.
HPLC Analysis of Retinoids
Retinoids were analyzed by normal-phase HPLC as previously described (21). In brief, samples were dissolved in 200 μl of hexane, and retinoids were separated by chromatography on a silica column (Supelcosil LS-SI 5 μm, 4.6 × 250 mm ID) by gradient elution of mobile phase (0.2–10% dioxane in hexane, 2.0 ml per min flow rate) in an Agilent 1100 liquid chromatograph equipped with a UV photodiode-array detector. Identified peaks were confirmed by spectral analysis and co-elution with authentic retinoid standards. Representative chromatograms and UV spectra acquired for 11-cis-ROL are shown in Fig. 2, D and E.
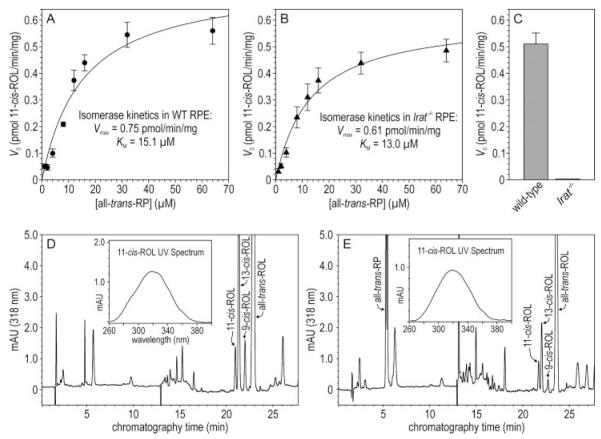
FIGURE 2. Isomerase kinetics in RPE from WT and lrat-/- mice.
A, Michaelis-Menten plot showing the initial rates of 11-cis-ROL synthesis (V0) versus concentrations of all-trans-RP substrate using RPE homogenates from WT mice as an enzyme source. B, similar plot as in Fig. 2A except RPE homogenates from lrat-/- mice were used as an enzyme source. Eadie-Hofstee transformations of the data in A and B yielded the kinetic parameters, Vmax and Km. C, 11-cis-ROL synthesis from all-trans-ROL substrate by WT and lrat-/- mouse RPE homogenates. Error bars show S.D. (n = 3). D, representative HPLC chromatogram of retinoids extracted following an in vitro isomerase assay of a WT mouse RPE-homogenate using all-trans-ROL substrate. The 11-cis-, 13-cis-, 9-cis-, and all-trans-ROL peaks are labeled. Inset shows the UV-spectrum acquired from the 11-cis-ROL peak. E, representative HPLC chromatogram of retinoids extracted following an in vitro isomerase assay of an lrat-/- mouse RPE-homogenate using all-trans-RP substrate. Peaks showing all-trans-RP and 11-cis-, 13-cis-, 9-cis-, and all-trans-ROL are labeled. Inset shows the UV-spectrum acquired from the 11-cis-ROL peak.
RESULTS
Isomerase Activity Is Normal in Mice Lacking LRAT
It was previously shown that 11-cis-retinoids are undetectable in the eyes of lrat-/- mice (16). This suggests that the isomerase, which synthesizes 11-cis-ROL (Fig. 1), requires LRAT for catalytic activity. To test this possibility, we measured isomerase kinetics in homogenates of WT and lrat-/- RPE using all-trans-RP as substrate. The maximum rate of 11-cis-ROL synthesis (Vmax) was 0.75 in WT and 0.61 pmol/min/mg-protein in lrat-/- RPE (Fig. 2, A and B). Thus, the presence of LRAT mildly stimulates isomerase activity. The measured Km of the isomerase was slightly higher in WT versus lrat-/- RPE (Fig. 2, A and B). This may reflect depletion of all-trans-RP substrate by LRAT through the reverse estersynthase reaction (22). We observed no synthesis of 11-cis-ROL from all-trans-ROL substrate by lrat-/- RPE homogenates, while WT RPE-homogenates synthesized 0.51 pmol/min/mg-protein (Fig. 2C). These results confirm that all-trans-RP is the substrate for the isomerase, and that LRAT is required to synthesize all-trans-RP from all-trans-ROL. In the absence of LRAT, however, isomerase activity is nearly normal.
Affinity of Rpe65 for Membranes Is Not Dependent on LRAT
To determine the role of LRAT in the association of Rpe65 with membranes, we performed quantitative immunoblot analysis on 1.0-μl samples of total homogenates (1.0 μg/μl) from WT and lrat-/- mouse RPE. We compared the Rpe65 signals using a Licor Odyssey Infrared Fluorescent Imaging System (Table 1). The fraction of total Rpe65 associated with membranes was similar in WT and lrat-/- RPE (83.8% and 81.3%, respectively). Fig. 3, A and B show representative immunoblots from this experiment using ECL detection. To corroborate these results, we also did immunoblot analysis on membrane-free supernatants and total homogenates from WT and lrat-/- mouse RPE. The Rpe65 signals in lanes containing 5.0 μl of membrane-free supernatants were intermediate between the signals in lanes containing 0.5 and 4.0 μl of total RPE homogenates (1.0 μg/μl) from both WT and lrat-/- mice (Fig. 3, C and D). By this analysis, ∼15% of total Rpe65 was soluble in WT and lrat-/- mice. These results agree with the quantitative analysis of the membrane fractions, and show that the ratio of soluble to membrane-associated Rpe65 is similar in WT and lrat-/- mice.
TABLE 1. Quantitative immunoblot analysis of Rpe65 in mouse RPE fractions.
Fluorescence intensity shown for the indicated protein samples. After separation of by SDS-PAGE and transfer to Immobilon-P, protein blots were reacted with rabbit anti-Rpe65 serum and IRDye 800CW-conjugated goat anti-rabbit IgG. Rpe65 immunoreactivity was measured by scanning the membranes in an Odyssey Infrared Imaging System using 780-nm excitation and 800-nm detection wavelengths. Triplicate blots were quantitated with Odyssey 2.1 software.
| lrat -/- mice | Wild-type mice | |
|---|---|---|
| RPE total homogenate (1.0 μg) | 15.2 ± 4.4 | 11.3 ± 3.2 |
| RPE membranes (1.0 μg) | 12.4 ± 1.6 | 9.5 ± 1.6 |
| RPE membranes (1.5 μg) | 20.2 ± 2.1 | 15.3 ± 5.8 |
FIGURE 3. LRAT-independent association of Rpe65 with membranes in mouse RPE.
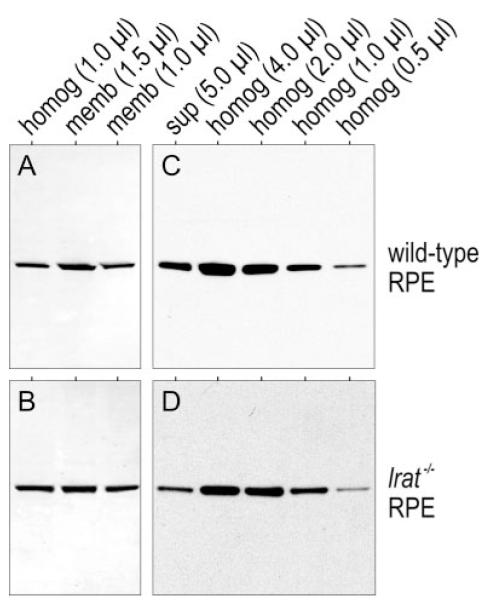
A and B, representative immunoblot analysis of Rpe65 in total RPE homogenates and membrane pellets. Lanes of the gel contained 1.0 μl of total homogenate (homog) (1.0 μg/μl), and 1.0 or 1.5 μl of suspended membrane pellet (memb) from WT (A) or lrat-/- (B) RPE. C and D, representative immunoblot analysis of Rpe65 in 5.0 μl of membrane-free supernatants (sup), and 4.0, 2.0, 1.0, or 0.5 μl of total homogenates (homog) (1.0 μg/μl) from WT (C) or lrat-/- (D) RPE. The Rpe65 bands were visualized by ECL detection.
Palmitoylation of Residues Cys231, Cys329, orCys330 Is Not Required for the Association of Rpe65 with Membranes
Rpe65 was suggested to undergo reversible, LRAT-mediated S-palmitoylation of residues Cys231, Cys329, and Cys330 (15). As a test of this hypothesis, we substituted these residues with Ser, individually and in all combinations, by site-directed mutagenesis. Plasmids encoding the Cys-to-Ser substituted Rpe65 were transfected into 293T and 293T-L cells. LRAT is stably expressed in 293T-L cells (specific activity = 6.1 ± 0.5 pmol/min/mg protein) and undetectable in 293T cells (8). We assayed for membrane association of the substituted Rpe65 by immunoblot analysis of total homogenates and washed membrane fractions prepared from expressing 293T and 293T-L cells. If the association of Rpe65 with membranes is dependent upon S-palmitoylation of residues Cys231, Cys329, and/or Cys330, the Ser-substituted Rpe65 should be reduced significantly in the washed membrane fractions. Instead, we observed similar Rpe65 immunoblot signals in membranes from cells expressing the unsubstituted and Ser-substituted Rpe65 (Fig. 4).
FIGURE 4. Membrane association of Rpe65 after substitution of the putatively S-palmitoylated Cys residues.
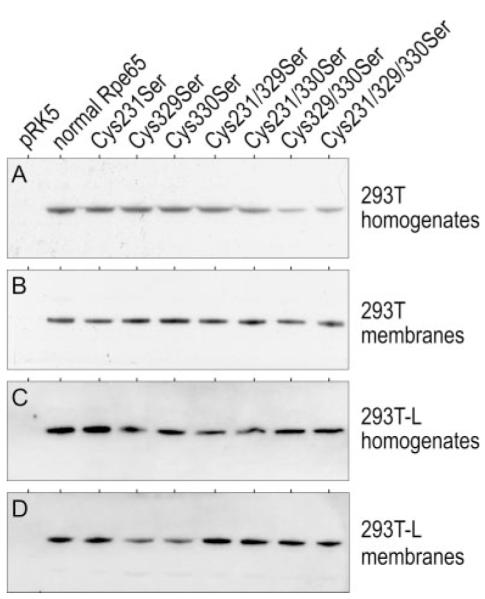
Rpe65 immunoblots of total homogenates and washed membranes from 293T (A) and (B) and 293T-L cells (C) and (D) expressing normal and the indicated Cys-to-Ser substituted Rpe65. Negative control samples were prepared from 293T or 293T-L cells transfected with non-recombinant pRK5 vector. Each lane contains 10 μg of protein. Rpe65 bands were visualized by ECL detection. Note the similar Rpe65 immunoreactivities in membrane samples from cells expressing unsubstituted and substituted Rpe65.
It was recently suggested that LRAT may catalyze O-palmitoylation of Rpe65 (23). Therefore, a possible explanation for the observed association of Cys-to-Ser substituted Rpe65 with membranes (Fig. 4) is O-palmitoylation of one or more of the newly introduced Ser residues. To control for this possibility, we transfected a plasmid encoding Rpe65 with triple Cys-to-Ala substitutions at positions 231, 329, and 330 into 293T and 293T-L cells. We compared the immunoblot signal-intensities of unsubstituted and Cys-to-Ala-substituted Rpe65 in membrane-free supernatants and total homogenates from these cells. We adjusted the amount of cellular proteins loaded in this experiment to compensate for the 3-fold lower expression of triple Cys-to-Ala-substituted versus unsubstituted Rpe65. For unsubstituted Rpe65, the immunoblot signal intensities were similar in lanes containing 5 μl of supernatant and 1 μl of total homogenate (1.0 μg/μl) from both 293T and 293T-L cells (Fig. 5, A and B). For Cys-to-Ala-substituted Rpe65, the signal intensities were similar in lanes containing 15 μl of supernatant and 3 μl of total homogenate (1.0 μg/μl) from expressing 293T and 293T-L cells (Fig. 5, C and D). Therefore, the soluble fraction of Rpe65 is ∼20% for both the unsubstituted and triple Cys-to-Ala substituted forms. As with RPE from WT and lrat-/- mice, the fractional association of LRAT with membranes was unchanged in the presence or absence of LRAT. These data show that the association of Rpe65 with membranes is not dependent on palmitoylation of Cys231, Cys329, or Cys330.
FIGURE 5. Fraction of normal and C231A/C329A/C330A-substituted Rpe65 in membrane-free supernatants.
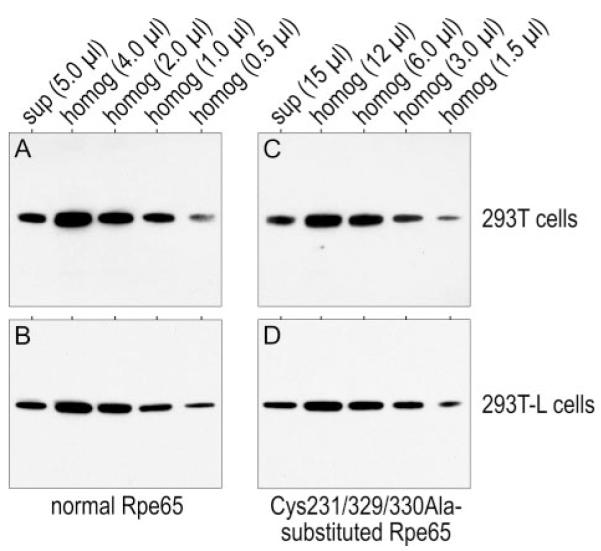
Rpe65 immunoblots of normal (A) and (B) or C231A/C329A/C330A-substituted (C) and (D) Rpe65 expressed in 293T (A) and (C) or 293T-L (B) and (D) cells. Lanes contain the indicated amounts of total protein from membrane-free supernatants (sup) or cell homogenates (homog). Note the similar immunoblot signal-intensities of normal Rpe65 in lanes containing 5.0 μl of supernatant and 1.0 μl of total homogenate (1.0 μg/μl) (A) and (B), and the similar signal intensities of C231A/C329A/C330A-substituted Rpe65 in lanes containing 15 μl of supernatant and 3.0 μl of total homogenate (C) and (D). Thus, for both forms of Rpe65, the soluble fraction is ∼20%.
Residues Cys231, Cys329, and Cys330 Are Not Palmitoylated in Rpe65
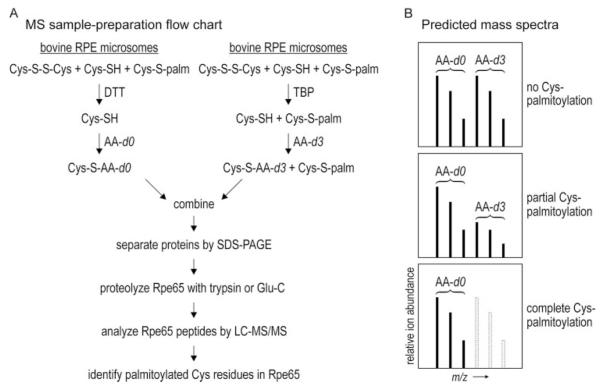
We assayed for palmitoylation of Cys231, Cys329, or Cys330 using mass spectrometry (MS) and isotopic labeling of Rpe65. The disulfide-reducing agent, DTT, efficiently converts S-acylated Cys residues in a protein to the free thiol form during extended incubation (24). We performed extended incubation of bovine RPE microsomes with DTT to reduce and de-acylate Cys residues in Rpe65. Then, we incubated the same RPE microsomes with non-deuterated acrylamide (AA-d0), which efficiently S-alkylates free thiol groups (25). We reacted another aliquot of RPE microsomes with tributylphosphine (TBP), which reduces disulfides but does not react with acylated (e.g. palmitoylated) Cys residues (26). Next, we incubated these microsomes with triple deuterium-labeled acrylamide (AA-d3). Subsequently, we pooled these two microsomal samples, separated the proteins by SDS-PAGE, and isolated gel slices containing Rpe65. The Rpe65 samples were submitted to in-gel proteolysis with trypsin or Glu-C, and the resulting peptides were analyzed by LC-MS. By comparing the ratio of d0 to d3-labeled Rpe65 peptides, we can estimate the extent of palmitoylation at positions Cys231, Cys329, and Cys330 (Fig. 6).
FIGURE 6. Strategy to determine palmitoylation status of Cys231, Cys329, Cys330 in Rpe65.
A, flow chart for the preparation of Rpe65 peptide samples for LC-MS analysis. One aliquot of bovine RPE membranes are incubated with DTT, which reduces disulfides and de-palmitoylates Cys residues in all proteins including Rpe65. The resulting free sulfhydryl groups are alkylated by incubating the microsomes with non-deuterated acrylamide (AA-d0). A second aliquot of RPE microsomes are incubated with TBP to reduce disulfide without de-palmitoylating Cys residues. The resulting free sulfhydryls are alkylated by incubating with triple-deuterated acrylamide (AA-d3). The microsomal samples are combined and the constituent proteins separated by SDS-PAGE. The band containing Rpe65 is digested with trypsin or endopeptidase Glu-C, and the resulting peptides are analyzed by LC-MS/MS. B, schemes of possible mass spectra. If a Cys-containing peptide is not palmitoylated, it would incorporate equal amounts of AA-d0 and AA-d3. Therefore, two sets of native isotopic peaks that differ by three atomic mass units would be equally abundant in the spectrum. If the Cys residue is partially palmitoylated, the triple-deuterated ions would be correspondingly less abundant than the nondeuterated ions. If the Cys residue is fully palmitoylated, no triple-deuterated ions would be represented in the spectrum.
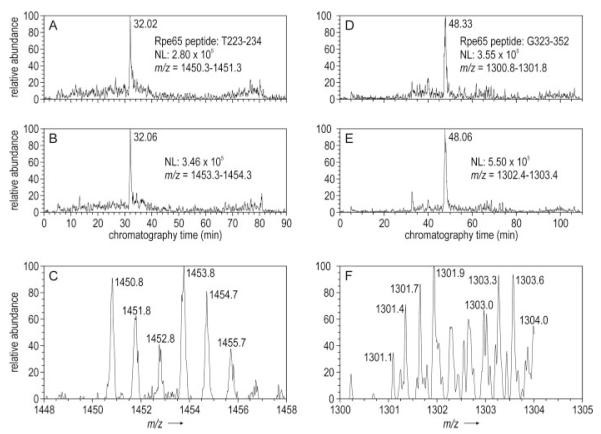
We identified a singly charged ion corresponding to the acrylamide-modified tryptic peptide from bovine Rpe65, T223–234 (SEIVVQFPC*SDR) by MS (Fig. 7, A-C) and MS/MS (not shown). This peptide contains residue Cys231. The corresponding d0- and d3-containing isotopes were of similar abundance, indicating that Cys231 is not palmitoylated. We also identified a triple-charged ion corresponding to the acrylamide-modified GluC peptide from bovine Rpe65, G323–352 (FLIVDLC*C*WKGFEFVYNYSYLANLRENWEE), containing two positions of acrylamide modification (Fig. 7D). This peptide contains residues Cys329 and Cys330. Here again, the corresponding d0 and d3 isotopes of this peptide were of similar abundance (Fig. 7, E and F). These data indicate that Cys329 and Cys330 are also not palmitoylated.
FIGURE 7. ESI-MS data showing similar abundance of AA-d0 and AA-d3 modified peptides containing residues Cys231, Cys329, and Cys330.
A, mass chromatogram of ions with m/z range 1450.3–1451.3. This range contains the single charged tryptic peptide, T223–234 from bovine Rpe65 after modification of Cys231 with AA-d0. NL indicates the ion current of the base peak for abundance normalization. B, mass chromatograms of ions with m/z range 1453.3–1454.3 containing singly charged peptide T223–234 after modification of Cys231 with AA-d3. C, zoom-scan spectrum of the indicated m/z values acquired at 32.13 min. Note duplication of the three native isotopic peaks (dark numbers)at +3 m/z (light numbers). The similar abundance of the corresponding AA-d0 and AA-d3-labeled ions indicates that Cys229 is not palmitoylated. D, mass chromatogram of ions with m/z range 1300.8–1301.8. This range contains the triple charged GluC peptide, G323–352 from bovine Rpe65 after modification of Cys329 and Cys330 with AA-d0. E, mass chromatogram of ions with m/z range 1302.4–1303.4, which contains triple charged peptide G323–352 after modification of Cys329 and Cys330 with AA-d3. F, zoom-scan spectrum of the indicated m/z values acquired at 47.99 min. The native-isotopic peaks (dark numbers) differ by ∼0.3 m/z due to the triple charge on peptide G323–352. Note the duplication of these four peaks at +2 m/z (light numbers). The similar abundance of the corresponding AA-d0 and AA-d3-labeled ions indicates that Cys329 and Cys330 are not palmitoylated.
Triple, but Not Single, Cys-to-Ser and Cys-to-Ala Substitutions Abolish the Isomerase Activity of Rpe65
Reportedly, the binding of Rpe65 to all-trans-RP and its participation in the isomerase reaction are also dependent upon palmitoylation of residues Cys231, Cys329, and Cys330 (15). To test this hypothesis, we did in vivo isomerase assays on the same group of Ser- or Ala-substituted Rpe65 expressed in 293T-LC cells. These cells stably express LRAT and CRALBP (8). The isomerase activity of C231S-substituted Rpe65 was 1.3-fold higher than unsubstituted Rpe65 (Fig. 8). C330S-, C231S/C329S-, and C231S/C330S-substituted Rpe65 retained 60–75% of normal isomerase activity, while isomerase activity was undetectable in C231S/C329S/C330S-substituted Rpe65 (Fig. 8). The isomerase activities of C329S- and C329S/C330S-substituted Rpe65 were 30% of normal. Thus, substitution of the individual Cys residues, and two combinations of paired Cys residues, did not abolish isomerase activity. Isomerase activity was abolished with triple Cys-to-Ser- or Cys-to-Ala-substitutions. As discussed above, this loss of activity is not correlated with reduced membrane association of Rpe65.
FIGURE 8. Isomerase activities of normal and substituted Rpe65 that eliminate the putative S-palmitoylation sites.
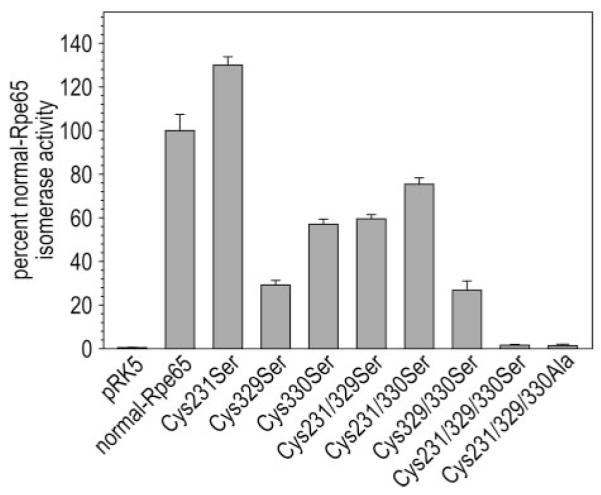
Synthesis of 11-cis-ROL by 293T-LC cells expressing the indicated Rpe65 from all-trans-ROL substrate added to the medium. Isomerase activities are expressed as the percent 11-cis-ROL synthesized by unsubstituted Rpe65 assayed in 293T-LC cells (pmol of 11-cis-ROL per mg protein). No synthesis of 11-cis-ROL by 293T-LC cells transfected with nonrecombinant pRK5 (negative control). Error bars show S.D. (n = 3).
Soluble and Membrane-associated Rpe65 Possess Similar Isomerase-specific Activities
Rpe65 associates reversibly with phospholipid membranes (14). It was recently suggested that the membrane-associated but not the soluble form of Rpe65 binds all-trans-RP and participates in the isomerase reaction (15). To test this hypothesis, we measured the isomerase specific activities in membrane-free supernatants and total homogenates of bovine RPE. As before, we first determined the relative levels of Rpe65 in these fractions of bovine RPE by immunoblotting. Immunoblot signals of similar intensity were observed in lanes containing 0.2 μl of RPE total homogenate (1.0 μg/μl) and 1.2 μl of membrane-free supernatant (Fig. 9A), showing that ∼17% of Rpe65 is soluble. The total homogenate contained 6.5-fold higher isomerase activity than the supernatant, indicating that ∼84% of isomerase activity is associated with membranes and 16% is in the soluble fraction of bovine RPE (Fig. 9B). Dividing the relative isomerase activities by the relative concentrations of Rpe65 showed that the specific activities of membrane-associated and soluble Rpe65 are similar (Fig. 9B).
FIGURE 9. Isomerase activities of soluble and membrane-associated Rpe65.
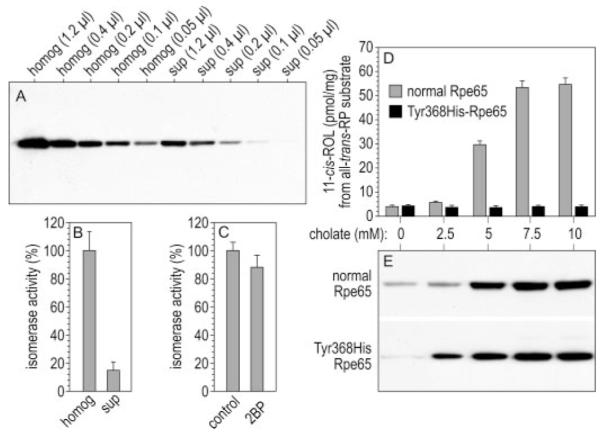
A, immunoblot analysis of Rpe65 in total homogenates (homog) (1.0 μg/μl) and membrane-free supernatants (sup) of bovine RPE. The amount of total protein loaded is shown for each lane. Note the similar Rpe65 immunoreactivity in lanes containing 0.2 μl of homogenate and 1.2 μl of supernatant, indicating that ∼17% of Rpe65 is soluble in bovine RPE. B, isomerase activities of bovine RPE homogenates and membrane-free supernatants, shown as percent of the isomerase activity in bovine RPE homogenates. C, isomerase activities of homogenates from 293T-LC cells expressing Rpe65 that were untreated (control) or incubated with 100 μM 2BP in the culture medium for eight hours (2BP). Shown is percent of the isomerase activity in control cells. Error bars show S.D. (n = 3). D, isomerase activities of normal or Y368H-substituted Rpe65 following extraction from expressing 293T-cell membranes with the indicated concentrations of sodium cholate. Activities are expressed as pmol of 11-cis-ROL synthesized per mg of protein during a 2-h incubation. The substrate was all-trans-RP. E, immunoblot analysis of Rpe65 in the same supernatant samples as D. Note the strong correlation of isomerase activity and Rpe65 immunoreactivity in the supernatant fractions of cells expressing normal Rpe65.
Global Inhibition of Protein Palmitoylation Does Not Affect Rpe65 Solubility and Isomerase Catalytic Activity
Does palmitoylation of Rpe65 at any positions determine its affinity for membranes? To address this question, we incubated 293T-LC cells expressing Rpe65 with the global inhibitor of protein palmitoylation, 2BP (19, 20). We compared the content of Rpe65 in total homogenates and membrane-free supernatants of these cells by quantitative immunoblotting. We observed no significant difference in the fraction of soluble Rpe65 between control and 2BP-treated cells (Table 2). As a positive control, we compared the fraction of soluble endogenous Fyn kinase (p59fyn) in the same cell homogenates. Reversible S-palmitoylation of Fyn kinase confers its affinity for plasma membranes (27). Incubation of Rpe65-expressing 293T-LC cells with 2BP increased by nearly 2-fold the soluble fraction of Fyn kinase (Table 2), confirming the efficacy of 2BP-inhibition of protein S-palmitoylation. We also tested the effect of inhibiting palmitoylation on Rpe65 isomerase activity. We observed no significant difference in the rates of 11-cis-ROL synthesis from all-trans-RP substrate using homogenates of 2BP-treated or control 293T-LC cells expressing Rpe65 (Fig. 9C). Also, 10 μM 2BP in the assay mixture had no effect on isomerase activity (not shown). These data suggest that palmitoylation of Rpe65 does not affect the affinity of this protein for membranes or its isomerase activity.
TABLE 2. Quantitative immunoblot analysis showing percent soluble Rpe65 and Fyn kinase after incubation with an inhibitor of protein palmitoylation.
Rpe65-expressing 293T-LC cells were incubated in the presence or absence of 2BP protein-palmitoylation inhibitor (19, 20). Membrane-free supernatants were prepared from homogenates of these cells and analyzed by quantitative immunoblotting using antisera against bovine Rpe65 or human Fyn kinase. The results are represented as the percent Rpe65 or Fyn kinase immunoreactivity in the soluble (supernatant) fraction ± S.D. (n = 3).
| Rpe65 | Fyn kinase | |
|---|---|---|
| 2BP | 18.9 ± 2.7 | 26.2 ± 6.7 |
| Control | 16.1 ± 0.76 | 15.2 ± 1.8 |
To confirm this result, we expressed normal and Y368H-substituted Rpe65 in 293T cells. The Y368H substitution is associated with the blinding disease, Leber congenital amaurosis (28), and was shown previously to be expressed at normal levels but to possess no isomerase activity (8). Membrane pellets from expressing 293T cells were suspended in buffers containing 0.45 M NaCl and increasing concentrations of sodium cholate (0–10 mM). As expected, the amount of normal and Y368H-substituted Rpe65 increased with increasing concentration of sodium cholate in the extraction buffer (Fig. 9E). When we measured 11-cis-ROL production from all-trans-RP substrate by these soluble fractions, we observed a tight correlation between the amount of unsubstituted Rpe65 protein and isomerase catalytic activity (Fig. 9D). No isomerase activity was detectable in detergent extracts from the control Y368H-expressing membranes, although the amount of Rpe65 protein in these samples was similar to unsubstituted Rpe65. These results show that the membrane-associated form of Rpe65 retains isomerase activity after dissociation from membranes.
DISCUSSION
This study investigated the role of LRAT on retinoid isomerization in RPE cells. The Rpe65-isomerase uses fatty acyl esters of all-trans-ROL as substrate (2-4). These all-trans-RE are water insoluble and confined to phospholipid membranes or lipid droplets inside RPE cells (29, 30). It was recently suggested that the affinity of Rpe65 for membranes is modulated by reversible palmitoylation by LRAT (15). Further, LRAT-mediated palmitoylation of Rpe65 was suggested to “switch” Rpe65 into an active form that binds all-trans-RP substrate (15). The reported absence of 11-cis-retinoids in lrat-/- mice (16) suggests that 11-cis-ROL synthesis may be dependent on the presence of LRAT. One possibility is that LRAT is required for isomerase catalytic activity, perhaps through LRAT-mediated palmitoylation of Rpe65. Alternatively, LRAT may only be required to synthesize the isomerase substrate, all-trans-RE. To distinguish between these possibilities, we measured the kinetics of 11-cis-ROL synthesis by RPE homogenates from WT and lrat-/- mice. The calculated Vmax of 11-cis-ROL synthesis and Km for all-trans-RP substrate were similar with the WT and lrat-/- homogenates (Fig. 2, A and B). In contrast, we observed no synthesis of 11-cis-ROL from all-trans-ROL by the lrat-/- RPE homogenates, while the WT homogenate synthesized abundant 11-cis-ROL from the same substrate (Fig. 2C). These results are consistent with the latter explanation and establish that LRAT is not required for isomerase catalytic activity.
An alternative possibility is that LRAT modulates the affinity of Rpe65 for membranes, possibly through S-palmitoylation of Cys residues, and that this modulation has no effect on the intrinsic isomerase activity of Rpe65. To test this possibility, we performed quantitative immunoblot analysis of Rpe65 in membrane pellets, membrane-free supernatants, and total homogenates of RPE from WT and lrat-/- mice. The ratios of soluble to membrane-associated Rpe65 from WT and lrat-/- RPE were similar (Fig. 3 and Table 1). Further, we observed similar ratios of soluble to total Rpe65 expressed in 293T versus 293T-L cells (Fig. 5, A and B). As discussed, LRAT is stably expressed in 293T-L cells but not in 293T cells (8). The fraction of soluble Rpe65 was 15–20%. These results suggest that LRAT has little influence on the affinity of Rpe65 for membranes.
Reportedly, Rpe65 is S-palmitoylated on residues Cys231, Cys329, and Cys330, which may explain its affinity for membranes (15). The results discussed above rule-out LRAT as the palmitoyl transferase. However, another acyl transferase may perform this function. To test the role of S-palmitoylation at Cys231, Cys329, and Cys330, we substituted these residues with Ser in all combinations. Unexpectedly, we observed no difference in the fraction of Ser-substituted versus unsubstituted Rpe65 in the membrane fractions (Fig. 4, B and D). The persistent association with membranes of Cys-to-Ser substituted Rpe65 could be explained by promiscuous O-palmitoylation of Ser at one or more of these residues. Accordingly, we repeated this experiment using triple Cys-to-Ala substituted Rpe65 at positions 231, 329, and 330. Here again, the soluble fraction was ∼20% for both Cys-to-Ala substituted and unsubstituted Rpe65. Finally, we established that neither Cys231, Cys329, nor Cys330 are palmitoylated in Rpe65 from bovine RPE by MS analysis (Figs. 6 and 7). Thus, the association of Rpe65 with membranes is not dependent on palmitoylation of Cys231, Cys329, or Cys330.
Another possibility was that the association of Rpe65 with membranes is mediated by palmitoylation of other residues besides Cys231, Cys329, or Cys330. We tested this by treating Rpe65-expressing 293T-LC cells with 2BP, which globally inhibits protein palmitoylation (19, 20). We observed no significant difference in the solubility or isomerase activity compared with Rpe65 expressed in untreated cells (Table 2 and Fig. 9C). In contrast, the solubility of Fyn kinase increased almost 2-fold in the same homogenates after treating the cells with 2BP (Table 2). Although these results do not rule-out palmitoylation at residues besides Cys231, Cys329, or Cys330, such modifications would not affect the affinity of Rpe65 for membranes or its isomerase activity.
The membrane-associated form of Rpe65 was reported to bind all-trans-RP with 26-fold higher affinity than the soluble form (15). This observation suggests that the isomerase activity of the membrane-associated form should be much higher. To test this prediction, we measured the isomerase specific activities in membrane-free fractions and total homogenates of bovine RPE. To this end, we compared the ratios of isomerase activity to Rpe65 immunoreactivity in these samples (Fig. 9, A and B). This analysis yielded the unexpected result that soluble and membrane-associated Rpe65 possess similar catalytic activity. The supernatants contained 17% of Rpe65 protein by quantitative immunoblotting and 16% isomerase activity by enzyme assay using all-trans-RP as substrate. We also observed a tight correlation of Rpe65 content and isomerase activity in supernatant fractions when membranes pellets from expressing 293T cells were extracted with increasing concentrations of sodium cholate detergent (Fig. 9, D and E). These data confirm that Rpe65 is the retinoid isomerase, and establish that the specific activities of soluble and membrane-associated Rpe65 are similar.
The results presented here are in disagreement with several features of the palmitoylation switch hypothesis, proposed as a regulatory mechanism for the isomerase in RPE cells (15). Xue et al. (15) suggested that Rpe65 undergoes LRAT-mediated S-palmitoylation of residues Cys231, Cys329, or Cys330. They suggested further that palmitoylation causes Rpe65 to bind membranes, and greatly increases its affinity for all-trans-RP. Finally, the authors (and other investigators at that time) understood Rpe65 to be an all-trans-RE-binding protein required for isomerase activity but not itself the isomerase (4, 15, 31). It was subsequently shown by several laboratories that Rpe65 possesses intrinsic isomerase activity and catalyzes the conversion of all-trans-RP to 11-cis-ROL (8-10). In the current work we show (i) that the presence or absence of LRAT in mouse-RPE or cultured 293T-cell homogenates does not affect the retinoid isomerase activity of Rpe65; (ii) the presence or absence of LRAT in mouse RPE or cultured cells does not affect the affinity of Rpe65 for membranes; (iii) that the association of Rpe65 with membranes is not dependent on palmitoylation of residues Cys231, Cys329, and/or Cys330 by any acyl-transferase including LRAT; (iv) that Rpe65 from bovine RPE is not palmitoylated on residues Cys231, Cys329, or Cys330; and (v) that the soluble and membrane-associated forms of Rpe65 possess identical isomerase specific activities. In view of these results, Rpe65 may still undergo reversible palmitoylation at residues other than Cys231, Cys329, or Cys330. However this modification would have to be catalyzed by an acyl-transferase other than LRAT, and would not affect the affinity of Rpe65 for membranes or its isomerase activity. More likely, the affinity of Rpe65 for membranes is mediated by intrinsic structural features rather than post-translational modification.
Acknowledgments
We thank Joanna Kaylor and Roxana Radu for their valuable insights and useful comments on the manuscript. We thank Krzysztof Palczewski for providing the lrat-/- knock-out mice.
Footnotes
This work was supported by grants from the National Eye Institute and the Foundation Fighting Blindness.
- 11-cis-RAL
- 11-cis-retinaldehyde
- RPE
- retinal pigment epithelium
- LRAT
- lecithin:retinol acyl transferase
- WT
- wild-type
- 11-cis-ROL
- 11-cis-retinol
- all-trans-ROL
- all-trans-retinol
- all-trans-RP
- all-trans-retinyl palmitate
- all-trans-RE
- all-trans-retinyl ester
- all-trans-RAL
- all-trans-retinaldehyde
- LCA
- Leber congenital amaurosis
- PBS
- phosphatebuffered saline
- DTT
- dithiothreitol
- HPLC
- high performance liquid chromatography
REFERENCES
- 1.Saari JC, Bredberg DL. J. Biol. Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- 2.Gollapalli DR, Rando RR. Biochemistry. 2003;42:5809–5818. doi: 10.1021/bi0341004. [DOI] [PubMed] [Google Scholar]
- 3.Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr., Redmond TM, Ma JX. Biochemistry. 2003;42:2229–2238. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 4.Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. J. Biol. Chem. 2004;279:635–643. doi: 10.1074/jbc.M310042200. [DOI] [PubMed] [Google Scholar]
- 5.Rando RR. Biochemistry. 1991;30:595–602. doi: 10.1021/bi00217a001. [DOI] [PubMed] [Google Scholar]
- 6.Driessen CA, Janssen BP, Winkens HJ, van Vugt AH, de Leeuw TL, Janssen JJ. Invest. Ophthalmol. Vis. Sci. 1995;36:1988–1996. [PubMed] [Google Scholar]
- 7.Kim TS, Maeda A, Maeda T, Heinlein C, Kedishvili N, Palczewski K, Nelson PS. J. Biol. Chem. 2005;280:8694–8704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr. J. Biol. Chem. 2001;276:6560–6565. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 12.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. Science. 2005;308:267–269. doi: 10.1126/science.1108965. [DOI] [PubMed] [Google Scholar]
- 13.Guo H, Zheng C, Gaillard ER. J. Theor. Biol. 2006;242:117–122. doi: 10.1016/j.jtbi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Tsilou E, Hamel CP, Yu S, Redmond TM. Arch. Biochem. Biophys. 1997;346:21–27. doi: 10.1006/abbi.1997.0276. [DOI] [PubMed] [Google Scholar]
- 15.Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. Cell. 2004;117:761–771. doi: 10.1016/j.cell.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. J. Biol. Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Gudas LJ. J. Biol. Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez CR, Huang L, Qiu Y, Burlingame AL. In: Current Protocols in Protein Science. Coligan JE, editor. Vol. 2. Wiley; New York: 2002. pp. 16.14.11–16.14.15. [Google Scholar]
- 19.Webb Y, Hermida-Matsumoto L, Resh MD. J. Biol. Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 20.Resh MD. Methods. 2006;40:191–197. doi: 10.1016/j.ymeth.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mata NL, Radu RA, Clemmons R, Travis GH. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saari JC, Bredberg DL, Farrell DF. Biochem. J. 1993;291:697–700. doi: 10.1042/bj2910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue L, Jahng WJ, Gollapalli D, Rando RR. Biochemistry. 2006;45:10710–10718. doi: 10.1021/bi060897y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson CS, Magee AI. In: Current Protocols in Protein Science. Coligan JE, editor. Vol. 2. Wiley; New York: 2002. pp. 14.12.11–14.12.19. [Google Scholar]
- 25.Brune DC. Anal. Biochem. 1992;207:285–290. doi: 10.1016/0003-2697(92)90013-w. [DOI] [PubMed] [Google Scholar]
- 26.Ruegg UT, Rudinger J. Methods Enzymol. 1977;47:111–116. doi: 10.1016/0076-6879(77)47012-5. [DOI] [PubMed] [Google Scholar]
- 27.Wolven A, Okamura H, Rosenblatt Y, Resh MD. Mol. Biol. Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felius J, Thompson DA, Khan NW, Bingham EL, Jamison JA, Kemp JA, Sieving PA. Arch. Ophthalmol. 2002;120:55–61. doi: 10.1001/archopht.120.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Ho MT, Pownall HJ, Hollyfield JG. J. Biol. Chem. 1989;264:17759–17763. [PubMed] [Google Scholar]
- 30.Imanishi Y, Gerke V, Palczewski K. J. Cell Biol. 2004;166:447–453. doi: 10.1083/jcb.200405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gollapalli DR, Maiti P, Rando RR. Biochemistry. 2003;42:11824–11830. doi: 10.1021/bi035227w. [DOI] [PubMed] [Google Scholar]